Abstract
Social decision making involves the perception and processing of social stimuli, the subsequent evaluation of that information in the context of the individual's internal and external milieus to produce a decision, and then culminates in behavioural output informed by that decision. We examined brain networks in an anuran communication system that relies on acoustic signals to guide simple, stereotyped motor output. We used egr-1 mRNA expression to measure neural activation in male túngara frogs, Physalaemus pustulosus, following exposure to conspecific and heterospecific calls that evoke competitive or aggressive behaviour. We found that acoustically driven activation in auditory brainstem nuclei is transformed into activation related to sensory–motor interactions in the diencephalon, followed by motor-related activation in the telencephalon. Furthermore, under baseline conditions, brain nuclei typically have correlated egr-1 mRNA levels within brain divisions. Hearing conspecific advertisement calls increases correlations between anatomically distant brain divisions; no such effect was observed in response to calls that elicit aggressive behaviour. Neural correlates of social decision making thus take multiple forms: (i) a progressive shift from sensory to motor encoding from lower to higher stages of neural processing and (ii) the emergence of correlated activation patterns among sensory and motor regions in response to behaviourally relevant social cues.
Keywords: immediate-early gene, túngara frog, social decisions, functional connectivity, effective connectivity, sensorimotor integration
1. Introduction
Decision making in a natural social context involves integrating neural systems involved in perception and motor control. Complex decision making requires a nonlinear interaction between neural systems; the neural transformation of sensory inputs to motor output depends on context and can incorporate hormonal state, social environment and previous experience. Most of the studies on the neural bases of social decisions, however, consider only one or a few brain regions and thus provide little information about the functional connectivity among brain networks. In contrast, functional neuroimaging in humans has demonstrated context-specific differences in neural coupling, with attention or emotional relevance modulating connectivity between brain regions (Buchel & Friston 1997; Sergent & Dehaene 2004; Haynes et al. 2005; Smith et al. 2006). These studies on human neural coupling, which are focused primarily on perceptual tasks, suggest a general principle of context-dependent associations between distinct brain regions that may play a central role in sensorimotor integration. In this paper, we identify brain regions participating in sensory and motor processing and examine the emergence of correlations between sensory- and motor-driven brain regions that depends on the behavioural relevance of social cues.
Anuran vocal communication offers a simple system for studying social decision-making behaviour and sensorimotor integration. Acoustic communication in anurans relies on fairly stereotyped signals and responses, and neural mechanisms of auditory processing have been examined extensively (reviewed in Wilczynski & Endepols 2007). Auditory regions in the brainstem are dedicated to acoustic processing. Midbrain auditory regions project to multisensory regions in the thalamus and hypothalamus, and these diencephalic regions form many reciprocal interconnections within those brain divisions as well as with telencephalic nuclei (Wilczynski & Northcutt 1983a,b; Neary & Wilczynski 1986; Hall & Feng 1987; Allison & Wilczynski 1991, 1994; Neary 1995; Endepols & Walkowiak 2001; Endepols et al. 2004). Telencephalic nuclei have wide-ranging connections that include motor output areas (Wilczynski & Northcutt 1983b; Neary 1984; Northcutt & Ronan 1992; Marin et al. 1997a; Moreno & Gonzalez 2003; Endepols et al. 2004; Roth et al. 2004; Roden et al. 2005). One current hypothesis based on anatomical connectivity and limited functional studies is that pure auditory processing may be restricted to the brainstem, with thalamic nuclei integrating multisensory information, hypothalamic nuclei coordinating neuroendocrine functions and telencephalic regions modulating motor output and motivation (Wilczynski & Endepols 2007). Midbrain, thalamic or telencephalic regions have been postulated to be sites of an audiomotor interface (Walkowiak & Luksch 1994; Luksch & Walkowiak 1998; Wilczynski & Endepols 2007). To assess the role of specific forebrain regions in audiomotor integration, we related neural activation to acoustic stimulation and behavioural output in male túngara frogs, Physalaemus pustulosus.
Extensive research has tested behavioural responses of male and female túngara frogs to variation in conspecific and heterospecific calls. The simple advertisement call of males, called a whine, is used to attract mates and compete with other males (Rand & Ryan 1981). Males also produce a complex mating call (the whine–chuck) in addition to another call type, a low-amplitude ‘mew’ used infrequently for short-range aggressive interactions (Ryan 1985). We examined neural responsiveness to two natural conspecific calls (whine and mew) as well as the whine of allopatric Physalaemus enesefae (see figure 1 in the electronic supplementary material). We chose stimuli based on behavioural results in evoked calling experiments: conspecific whines increase production of advertisement calls in male túngara frogs, whereas mews and P. enesefae whines decrease advertisement call number and induce production of mews (Bernal et al. in press). These calls, therefore, are all salient to the frogs but convey different behavioural significance: conspecific whines signal competition, whereas mews and P. enesefae whines trigger aggression.
We compared neural responses throughout the brain in these social contexts using induction of the immediate-early gene egr-1 mRNA as a measure of neuronal activation (Hoke et al. 2004, 2005). We identified brain regions in which egr-1 expression levels corresponded to acoustic stimulus, locomotive responses or a combination in order to localize sites of audiomotor integration in the auditory midbrain, thalamus, hypothalamus and telencephalon. We also examined short- (i.e. two brain regions within a brain division) and long-range (i.e. between two brain regions in different brain divisions) correlations of egr-1 expression in pairs of brain regions to test whether the social context modulated associations between brain regions involved in sensory and motor processing.
2. Material and methods
(a) Exposure of frogs to acoustic stimuli
We collected male P. pustulosus between 19.00 and 21.00 h from natural breeding aggregations near the Smithsonian Tropical Research Institute in Gamboa, Panama (9°07.0′ N, 79°41.9′ W) between 19 June and 8 July 2005. Autoridad Nacional del Ambiente del República de Panamá approved the collection, the animal protocols and the export of tissue (permit number SE/A-37-05). The animals were held in moist plastic bags in the laboratory for 0–5 h prior to transfer to a sound isolation chamber (0.7 m×0.5 m×0.5 m internal measurements) that provided a 35 dB reduction in sound. After 2 h without stimulation, we exposed males to one of the following five acoustic conditions (N=10–11): silence; natural heterospecific P. enesefae call; natural P. pustulosus mew at low amplitude; natural P. pustulosus mew at high amplitude; and natural P. pustulosus whine (see figure 1 in the electronic supplementary material). Since the mew has lower amplitude than the whine, but is used at shorter distances, we used two amplitudes of the mew. Stimulus amplitude was calibrated to 82 dB SPL (re. 20 μP) in the centre of the chamber, 0.35 m from the speaker, with the exception of the low-amplitude mew (10 dB lower).
Stimuli were broadcast alternately from amplified playback speakers (SME-AFS, Saul Mineroff Electronics, Elmont, NY) at opposite sides of an acoustic chamber at the rate of 1 call per 2 s from each speaker for 30 min. Frog locomotion was recorded under infrared illumination using a PC-6EX-2 IR video camera (Supercircuits, Liberty Hill, TX) with a ZR60 miniDV digital camcorder (Canon, Lake Success, NY). The time spent in motion was scored by video analysis with distinct bouts of movement separated by at least 60 s without motion. Animals did not have access to pools of water and thus did not vocalize in response to stimuli.
(b) Analysis of egr-1 expression
We performed radioactive in situ hybridization to visualize egr-1 mRNA as described in Hoke et al. (2004) and used custom automated-counting procedures to measure egr-1 expression in each brain region in digital photomicrographs (Optronics camera, Olympus Bx60 microscope with 100×objective). Our measure of egr-1 mRNA levels was silver grain density, calculated from the ratio of areas covered by silver grains and cell bodies within a standard-sized sampling frame as described (Hoke et al. 2004) with two changes due to lower overall silver grain density: (i) background photos were not subtracted as in Hoke et al. (2004) because silver grain density between tissue sections was uniformly low and (ii) previous measures counted grain number rather than total area covered by grains.
We determined unbiased sampling procedures separately for each brain region shown in figure 2 in the electronic supplementary material. Sections that were torn, missing or angled were excluded from the analysis, resulting in exclusion of some frogs in which no sections were appropriate to measure one or more brain regions. We selected sections and spaced photographs as described (Hoke et al. 2004, 2005) in the following regions: the anterior preoptic area (POA); suprachiasmatic nucleus (SC); dorsal hypothalamus (DH); ventral hypothalamus (VH); lateral hypothalamus (LH); posterior tuberculum (PT); nucleus of the periventricular organ (NP); anterior thalamus (Athal); central thalamus (Cthal); secondary isthmal nucleus (SI); superior olivary nucleus (SO); laminar (Ltor); midline (Mtor); principal (Ptor); and ventral (Vtor) regions of the torus semicircularis. In addition to the previously described 15 brain regions, we analysed nine telencephalic nuclei (nomenclature based on Roden et al. (2005)) and two additional thalamic regions. To do so, we selected nine sections spaced at least 32 μm apart spanning the telencephalon, choosing sections based on the presence of particular telencephalic regions (sections containing each region listed in parentheses below). Three photographs spanned the medial pallium (MP, sections 1–6), with one photograph situated adjacent to the lateral ventricle between 150 and 250 μm ventral relative to the dorsal pallium, a second taken 150 μm medially and a third (sections 2–5 only) taken 100 μm dorsally and adjacent to the ventricle. Owing to their small extent, a single photomicrograph was centred in the dorsal striatum (dST, sections 2–5), ventral striatum (vST, sections 2–5) and nucleus accumbens (NA, sections 2–4). Two images were spaced 200 μm dorsoventrally within the lateral septum (LS, sections 2–4) and one photograph each centred in the dorsal and medial septum was spaced 200 μm (DS and MS, sections 2–4) or 150 μm (sections 7 and 8) mediolaterally. The mediolateral extent of the amygdala was spanned by two photomicrographs taken 200 μm apart in the medial amygdala (MA, sections 7–9) and two in the lateral amygdala spaced by 150 μm (LA, sections 8 and 9). In addition, we imaged the ventromedial thalamus (VMthal) using two photographs 150 μm apart mediolaterally in all three sections containing the Athal and in the most rostral section containing the Cthal. We selected two sections containing the posterior thalamus (Pthal) for photomicrographs spaced by 150 μm in the dorsal to the ventral direction.
(c) Statistics
We used SPSS 11 and R for statistical analyses. We used ANCOVA for each brain region separately to determine how the acoustic stimuli and locomotive behaviour influenced the mean egr-1 expression throughout the brain; acoustic stimulus was the between-subjects factor, and covariates were time spent in motion and an error term representing global activity. To determine global activity, we normalized egr-1 mRNA levels in each brain region by the mean of that brain region. We then averaged normalized egr-1 mRNA levels across the brain for each individual and calculated residuals from this average expression value by removing the effects of stimulus and time in motion. The residuals provided an error term representing average activity throughout the brain unrelated to stimulus or behavioural response. This covariate significantly influenced ANCOVA results in all brain regions except SI and SO. Initial ANCOVAs included the interaction between stimulus condition and movement, and secondary analyses were conducted without this interaction term for interactions with significance less than p=0.2. Most variables were not normally distributed due to positive skew and kurtosis. Data were log-transformed prior to analysis to produce normal distributions. For egr-1 mRNA levels within the auditory system, we further examined stimulus specificity using the following two orthogonal contrasts: (i) one testing for induction by sound (no stimulus compared to all four conspecific and heterospecific calls) and (ii) a second testing for induction based on behavioural relevance of the calls (P. pustulosus whine which increases advertisement call production and the P. enesefae whine or two P. pustulosus mew stimuli which induce aggressive responses). To determine the effects of stimulus condition on motor output, we used ANOVA with acoustic stimulus as the between-subjects factor and time spent in motion as the dependent variable.
We used custom-written R functions for permutation testing to test for whine-induced increase in correlations among egr-1 mRNA levels throughout the brain. We calculated the matrix of Pearson's correlation coefficients for all pair-wise combinations of egr-1 expression levels in the 26 brain regions. We then summarized the overall level of correlation within a group by summing the square of each off-diagonal element in the correlation matrix (abbreviated Σr2). We estimated the increase in correlation in response to whine compared to all other groups by using the following formula:
We then resampled the egr-1 measures for each individual without replacement, assigning the set of egr-1 measures from 10 randomly sampled individuals to the whine and P. enesefae groups, 11 to the silence condition and 9 to each mew condition based on actual sample sizes in each group. We repeated this resampling 2000 times and calculated the cordiff for each bootstrap replicate. We calculated the 95% confidence intervals using the methods of Efron & Tibshirani (1998), and considered the whine as inducing significantly higher correlations than the other groups if the cordiff calculated from the original data was higher than the upper limit of the 95% confidence interval. To identify whether the effects of the whine were on long- or short-distance correlations, we ran two additional versions of the above permutations: one in which only the correlations between brain regions within a division (telencephalon, thalamus, hypothalamus or brainstem) were included and a second based only on correlations between brain regions in different divisions.
3. Results
(a) Activation of brain regions corresponding to sound or movement
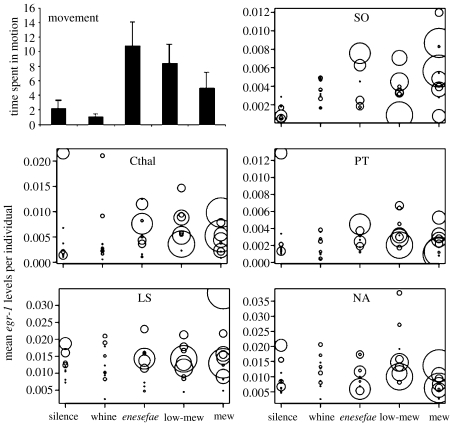
To distinguish between egr-1 mRNA induction related to stimulus and to motor output, we tested effects of stimulus condition on egr-1 expression in 26 brain regions using ANCOVA. For brain regions with no movement-by-stimulus interaction (p>0.2), we omitted the interaction term to increase statistical power to detect effects of stimulus treatment and locomotion (see electronic supplementary material table 1 which contains full statistical results from both the tests and the electronic supplementary figure 3 which depicts results from brain regions not presented in figure 1). Overall, the locomotor activity was significantly influenced by stimulus condition (F4,44=3.78, p=0.01; figure 1).
Figure 1.
Effects of sound on movement and corresponding induction of egr-1 mRNA in male túngara frogs. In bar graph (movement), bars indicate mean±s.e. for each group. Hearing acoustic stimuli increased the amount of time frogs spent moving for a subset of conspecific and heterospecific stimuli. In other panels, circles represent egr-1 expression in a single brain region for individuals in each stimulus condition, with diameter of circle linearly related to amount of movement. Acoustic stimulation increased egr-1 mRNA levels in the superior olivary nucleus. Egr-1 expression in Cthal, PT and NA was associated with an interaction between locomotive behaviour and acoustic stimulation. Motor output is associated with egr-1 levels in LS.
Acoustic stimulation had a statistically significant effect on the induction of egr-1 expression in two auditory brainstem regions: the SO and the Ltor. In neither region was egr-1 mRNA abundance correlated with movement. Egr-1 mRNA levels in the SO were modulated by acoustic stimulation (F4,36=7.03, p<0.001; figure 1), and orthogonal contrasts demonstrated that this activation was a non-specific egr-1 mRNA induction in response to sound in general (t(1)=4.91, p<0.001), as egr-1 levels did not differ in frogs exposed to conspecific advertisement calls and other acoustic stimuli (t(1)=0.487, p=0.555). Egr-1 mRNA levels in the Ltor were also dependent upon acoustic stimulation (F4,38=5.91, p=0.001; see also Hoke et al. 2004). Expression of egr-1 in Ltor was induced by all sounds (t(1)=2.66, p=0.001), but in contrast to the SO, there was selective induction in response to conspecific advertisement calls compared with the other acoustic stimuli (t(1)=1.30, p=0.022). Neither the other toral divisions nor the secondary isthmal nucleus had significant main effects of stimulus condition on egr-1 mRNA levels.
We found that egr-1 mRNA induction in thalamic targets of the auditory system related to both locomotive responses and auditory stimulation, in contrast to simple acoustic responsiveness as in the auditory system. VMthal egr-1 mRNA induction covaried with movement (F1,35=8.026, p=0.007) when interaction terms between calls and locomotion were not included. Egr-1 expression in the Athal corresponded to locomotion (F1,36=10.12, p=0.003) but not the stimulus type. Egr-1 mRNA abundance in the Cthal covaried with movement (F1,36=16.34, p<0.001; figure 1) and acoustic stimulation (F4,36=3.67, p=0.013), with a significant stimulus by movement interaction (F4,36=3.71, p=0.012). Activation in the Pthal was also related to both locomotion and acoustic stimulation (call: F4,35=5.63, p=0.001; movement: F1,35=7.83, p=0.008; call by movement interaction: F4,35=4.30, p=0.006).
Hypothalamic egr-1 expression was predominantly associated with a combination of acoustic stimulus condition and locomotive responses. We found that egr-1 mRNA levels in three caudal hypothalamic nuclei had significant interactions between locomotion and acoustic stimulation (NP: F4,36=5.08, p=0.002; DH: F4,35=4.28, p=0.006; PT: F4,34=3.09, p=0.029; figure 1). In addition, the main effects of stimulus condition and movement were significant in the NP (call: F4,36=3.78, p=0.011; movement: F1,36=24.7, p<0.001) and of call type in DH (F4,35=2.68, p=0.048). Call type influenced VH egr-1 expression (F4,35=2.91, p=0.034) independent of behavioural response. Egr-1 mRNA levels in the LH were not significantly related to either movement or acoustic stimulation (see table 1 in the electronic supplementary material). Both rostral hypothalamic nuclei had significant effects of call type and stimulus-by-movement interactions on egr-1 mRNA levels (POA call: F4,38=3.54, p=0.015; POA interaction: F4,38=3.18, p=0.024; SC call: F4,35=2.91, p=0.036; SC interaction: F4,35=3.25, p=0.023) and egr-1 covariation with movement was significant in the POA (F1,38=13.7, p=0.001).
Egr-1 mRNA induction in the telencephalon was related to either motor output or an interaction of locomotion and acoustic condition. We found that the NA, MS and dST have significant interactions between movement and acoustic condition (NA: F4,38=5.10, p=0.002; MS: F4,38=3.35, p=0.019; dST: F4,38=2.66, p=0.048; figure 1). In addition, the NA had a main effect of call (F4,38=9.99, p<0.001) and locomotion (F1,38=6.18, p=0.017), whereas MS egr-1 mRNA levels covaried with motion (F1,38=5.69, p=0.022). Egr-1 mRNA levels in the LS, MA and MP were associated with locomotive behaviour (LS: F1,42=16.9, p<0.001; MA: F1,42=10.7, p=0.002; MP: F1,42=4.66, p=0.037; figure 1). Neither acoustic stimulation nor motor output corresponded to changes in egr-1 expression in DS, LA and vST (see table 1 in the electronic supplementary material).
(b) Correlations among brain regions in egr-1 activation
Acoustic stimulation and consequent behavioural responses were associated with differences not only in the mean egr-1 mRNA levels of many brain regions but also in the covariance structure among brain regions. Egr-1 expression levels were correlated within brain divisions (brainstem, thalamus, hypothalamus and telencephalon) in animals not receiving acoustic stimulation, conspecific mew or heterospecific whine stimuli, whereas frogs hearing whines had widespread correlations across the brain (figure 2). To test for a significant change in connectivity in the brains of animals hearing whines, we used permutation tests to determine if correlations in brains of animals were significantly higher when hearing whines compared to other stimuli. This was the case; frogs hearing whines had significantly higher correlations throughout the brain than those hearing all other stimuli (cordiff for whine=412, outside the 95% confidence interval for permutations assigning individuals at random to each group (−240 to 365)). Moreover, the whine-stimulated group differed from other conditions in having higher correlations of egr-1 mRNA levels across different brain divisions, but not within brain divisions; that is, egr-1 mRNA levels were more strongly associated between telencephalic, brainstem and diencephalic regions following stimulation with conspecific whines compared to stimulation with the other acoustic stimuli (cordiff between divisions: whine=311, permutations 95% confidence interval ranged from −177 to 283; cordiff within divisions: whine=100, permutations −76 to 105). The same patterns emerged when we controlled for the relationship between movement and egr-1 mRNA levels in each brain region (not shown).
Figure 2.
Long-distance coupling in brain activation increased by conspecific advertisement calls. This visual representation of the symmetric correlation matrix shows Pearson's correlation coefficients between all pairs of brain regions with yellow indicating positive correlation and blue marking negative relationships. Regions are arranged roughly rostrocaudally, with brain division marked by shade of bar above or to the left of the correlation matrix, and order of brain regions as in table 1 in the electronic supplementary material. Nuclei within a brain division are typically correlated in all stimulus conditions, as seen by red squares near the diagonal. Animals hearing the conspecific whine stimulus had increased correlation in the off-diagonal regions, showing greater coupling between telencephalic regions and both auditory brainstem and diencephalic nuclei.
4. Discussion
We measured neural activation throughout the brain to gain insights into the neural substrates involved in social decision making. Acoustic social cues induced widespread changes in neural activation as assessed by both mean egr-1 expression and correlations in expression between brain regions. The expression patterns suggest a caudal-to-rostral progression in neural responsiveness in the frog brain: auditory processing in the brainstem to audiomotor integration in the diencephalon to premotor modulation or motivation in the telencephalon. Furthermore, exposure to the conspecifics whine, but not other calls, induced correlated egr-1 responses across brain divisions.
(a) Widespread sensorimotor integration in the forebrain
Our results nominate a distributed set of diencephalic nuclei as participating in integrating sensory information to modulate motor output. Cells involved in audiomotor integration should respond to acoustic stimuli and modulate motor output. In this study, brain regions would be implicated in audiomotor integration if the egr-1 mRNA levels increased in response to hearing calls and covaried with movement generated. Based on the current understanding of egr-1 induction (Clayton 2000; Jarvis 2004; Knapska & Kaczmarek 2004), we interpret such results as indicating that cells were depolarized by auditory inputs in response to calls, and that electrical activity in these cells influenced both egr-1 expression and motor output. Regions with egr-1 responses only to sound probably lack a direct influence on motor output. Regions with egr-1 mRNA levels covarying only with movement are probable targets of audiomotor regions that do not receive direct auditory input, but may directly modulate behaviour. Note that covariation between egr-1 expression and movement could either implicate a brain region in regulating movement or reflect sensory inputs as a consequence of movement. Given the anatomical connections of the brain regions in question, we interpret covariation as an indication that the brain region influenced movement, but additional work is necessary to confirm this conclusion. We propose that auditory brainstem regions send auditory inputs to sensorimotor integrators in the hypothalamus and thalamus, and that these in turn influence telencephalic nuclei with direct effects on motor systems. Our results corroborate the proposal by Wilczynski & Endepols (2007) that the midbrain torus semicircularis, homologous to the inferior colliculus in mammals, is the most rostral brain region dedicated principally to auditory processing, and that forebrain regions in frogs perform multisensory or integrative tasks. The prevalence of motor or audiomotor egr-1 responsiveness in the thalamus, hypothalamus and limbic telencephalon is concordant with the widespread anatomical connections in the anuran forebrain and raises the question of the specific or overlapping functional roles each of these brain regions plays in directing behavioural and physiological responses to social cues. We discuss our findings in the light of previous functional work to propose roles for these brain regions.
Anatomical data suggest a major role of the torus semicircularis in audiomotor integration (Walkowiak & Luksch 1994; Luksch & Walkowiak 1998), and connections certainly indicate that the torus distributes auditory information to motor centres and in turn receives input from them (Endepols & Walkowiak 2006). However, we do not see egr-1 mRNA induction in any toral region that mirrors stimulus-induced motor responses in males, as would be expected by a model in which the torus directs motor behaviour. We do not conclude that the torus is an unnecessary component of behavioural responses to calls, and in fact, lesions studies demonstrate the importance of the torus in phonotaxis in female frogs (Schmidt 1988; Endepols et al. 2003). The torus's role in distributing call-related information to the forebrain makes it an essential component of sensorimotor integration in the broader sense even though egr-1 mRNA responses within the torus itself were not directly correlated with movement. Furthermore, the lack of a significant relationship between movement and egr-1 mRNA levels does not rule out electrical activity related to motor behaviours. Egr-1 is one of the several immediate-early genes that sometimes show different response patterns (e.g. Yamada et al. 1999; Sockman et al. 2005); thus, levels of other immediate-early genes in the torus might covary with motor output. Moreover, egr-1 mRNA activation reflects average depolarization by inputs and thus does not provide information about inhibitory inputs or temporal patterns of firing. In sum, our results corroborate the extensive previous evidence that the laminar nucleus of the torus semicircularis receives excitatory inputs conveying auditory information. These laminar neurons may in turn directly activate the premotor areas that control behavioural responses and thus play a role as an audiomotor interface by regulating motor responses to calls as proposed previously (Walkowiak & Luksch 1994; Luksch & Walkowiak 1998). The same premotor areas probably also receive modulatory input from forebrain regions in which neural activation, as measured by egr-1 expression, covaried with movement. Lesioning thalamic nuclei, for example, alters the frequency and timing of phonotaxis behaviour in females but does not completely obliterate it (Endepols et al. 2003), whereas lesions in the striatum and septum retard or prevent phonotaxis (Walkowiak et al. 1999), consistent with a modulatory role for forebrain regions in behavioural responses. Future research combining complementary approaches such as subtle lesioning experiments, electrophysiological recordings from freely moving frogs and comprehensive analyses of immediately early gene expression patterns may further clarify the interactions between specific brain regions in regulating behaviours.
Auditory brainstem regions responded to acoustic stimulation with either broad acoustic responsiveness or specificity for conspecific advertisement calls regardless of the motor output induced by stimuli. The SO receives direct projections from the primary auditory brainstem region and is dedicated to basic auditory processing of sounds (Will et al. 1985; Feng 1986), substantiating our finding of broad responsiveness in the SO to all acoustic stimuli. We found signal selectivity in the Ltor, a higher stage of processing in which conspecific whines induced higher egr-1 mRNA levels than did mews or P. enesefae whines. Cells in the torus typically are responsive to complex signal properties characteristic of species-specific calls (Diekamp & Schneider 1988; Fuzessery 1988; Walkowiak 1988; Feng et al. 1990; Walkowiak & Luksch 1994; Penna et al. 1997; Edwards et al. 2002), although we would expect auditory responsiveness in the other toral regions as well. As in Hoke et al. (2004), we found that animals kept in silence had the lowest average levels of egr-1 expression in the Mtor, Ptor and Vtor subdivisions, with patterns of activation similar in Mtor and Ptor but lacking significant effects of stimulus condition. Similarly, acoustic responsiveness has been demonstrated in the SI (Bibikov 2003) and we find that although egr-1 mRNA levels are consistently low in the SI of unstimulated animals, the acoustic stimuli used here did not induce sufficiently robust egr-1 expression to achieve statistical significance. While we could not detail the acoustic specificity at progressive stages of auditory processing, we can state that an early stage of processing (SO) had low selectivity and that a late stage (Ltor) had higher selectivity in responsiveness among natural calls.
The relationship between movement and egr-1 mRNA levels in the thalamus is the first functional evidence supporting the conjecture that, in anurans, the thalamic nuclei are not sensory relay nuclei dedicated to auditory processing but rather participate in audiomotor integration (Wilczynski & Endepols 2007). The Cthal and Pthal respond selectively to acoustic features of species-specific calls (Mudry et al. 1977; Fuzessery & Feng 1983; Hall & Feng 1987; Mudry & Capranica 1987), and thalamic lesions in females decrease the phonotaxis behaviour (Endepols et al. 2003, but see also Schmidt 1988). These functional studies, however, could not distinguish whether the thalamic nuclei act as auditory relay nuclei, as do nuclei in the mammalian thalamus, or as multimodal associative regions, as anatomical connections suggest (Endepols et al. 2003; Roth et al. 2003; Westhoff et al. 2004; Wilczynski & Endepols 2007). The consistent covariance we found between thalamic egr-1 expression and movement along with the elevation in egr-1 mRNA in response to sound in two dorsal nuclei nominates the anuran thalamus as a major contributor to sensorimotor integration.
Interactions between acoustic input and motor output in driving hypothalamic nuclei are consistent with the hypothesis that the hypothalamus may integrate internal and external environments to guide physiological and behavioural responses to social cues. Acoustic responsiveness has been demonstrated using electrophysiology in the POA (Urano & Gorbman 1981; Wilczynski & Allison 1989; Allison & Wilczynski 1991; Allison 1992) and VH (Wilczynski & Allison 1989; Allison & Wilczynski 1991; Allison 1992). We report concordant results in which POA and VH egr-1 expression depended on acoustic stimulus type, and further that POA egr-1 levels covaried with motor responses as well. As further support for a general role for the POA in acoustically guided behaviours, lesioning the anterior POA interferes with phonotaxis in female toads (Schmidt 1989). Previous work in female túngara frogs found egr-1 mRNA induction in response to advertisement calls in the LH, PT and SC, but not NP or DH (Hoke et al. 2005). Our current finding that egr-1 expression levels in the PT and SC were associated with both acoustic stimulus and locomotion adds insight into the possible roles of these regions in females, for locomotion was not measured in the earlier experiment. In fact, the only other assessment of PT function in frogs implicates dopaminergic PT neurons in a premotor circuit required for phonotaxis behaviour (Endepols et al. 2004). The lack of significant effects of sound in the NP and DH in females (Hoke et al. 2005) suggests that motor output may be a critical covariate in explaining the hypothalamic neuronal activation or that sex differences exist in hypothalamic responses. The differences in acoustic responsiveness in the LH in females (Hoke et al. 2005) and males in this study may be a result of either sex differences or in the particular the acoustic stimuli used in each case.
Limbic activation during social behaviour is credible given the proposed function of the limbic system in motivational or emotional aspects of social behaviour in other vertebrates, but no published work has characterized the response patterns of limbic nuclei in frogs. Limbic areas do project to premotor regions (e.g. Marin et al. 1997b; Endepols et al. 2005) and septal lesions decrease phonotaxis behaviour in female Hyla versicolor (Walkowiak et al. 1999). Our results showed widespread activation in limbic areas associated with stimulus-induced movement, with activation in some regions additionally varying based on the stimulus type. Based on anatomical connections of the striatum (Wilczynski & Northcutt 1983a,b; Northcutt & Ronan 1992; Marin et al. 1997a,b; Westhoff & Roth 2002; Roth et al. 2004), one report of acoustic responsiveness (Mudry & Capranica 1980) and one lesioning study (Walkowiak et al. 1999), we would expect that either movement or acoustic stimulation could drive striatal egr-1 activation. We find such evidence only in the dST. We do see trends in activation in the vST, and perhaps examining females or eliciting behavioural responses using other acoustic stimuli would reveal egr-1 mRNA induction in this region. Further studies of telencephalic responsiveness in frogs are required to determine whether limbic activation accompanies all motivated behaviours or is specific to aggressive or reproductive social interactions.
(b) Emergence of long-distance correlations
The pattern of covariance between egr-1 mRNA levels in distinct brain regions of túngara frogs depends on the behavioural significance of social cues, both in this study and a previous one (Hoke et al. 2005). In this case, hearing the conspecific whine induced higher correlations in activation throughout the brain compared with hearing other sounds. Moreover, when we considered only correlations of egr-1 mRNA levels within a brain division (brainstem, thalamus, hypothalamus or telencephalon), conspecific whines did not significantly increase correlations between brain regions. In contrast, hearing whines did induce long-range coupling of brain regions in different divisions. The positive correlations between the telencephalon and auditory or audiomotor regions that emerged in response to hearing conspecific whines are particularly surprising in that analysing mean expression levels in these animals indicated that the whine did not increase telencephalic egr-1 mRNA abundance (figure 1 and see figure 3 in the electronic supplementary material). Comparing mean egr-1 mRNA levels and correlations in egr-1 expression therefore gave qualitatively different interpretations: in the former case, egr-1 activation was related to movement induced primarily in response to mew or heterospecific calls, and in the latter, egr-1 induction was related to activation patterns throughout the brain when the animal heard conspecific whines. This difference relied on the fact that we compared within-stimulus covariation in the functional connectivity analyses, variance that is orthogonal to mean effects of treatment across stimulus conditions. In our connectivity analyses, the covariation between egr-1 levels in two brain regions probably arose from the following four main sources: direct unidirectional or reciprocal connections between the two regions themselves; indirect connections by which activation of one brain region reached the second; activation by shared inputs; and the global variation throughout the brain that generally influenced activation levels. Group differences in covariation between two regions could reflect changes in the strength of influence of any of these sources. The finding that between-division correlations as a whole were elevated in response to whines suggests either that global activity was more coherent and thus the brain as a whole was more tightly coupled perhaps as a result of widespread neuromodulatory changes or that a few long-distance connections were more functionally active and that the tight coupling within local networks resulted in widespread increases in correlations. Since within-division coupling was not significantly elevated, the latter mechanism seems more probable, with the caveat that lack of statistical significance does not prove cross-group equivalence. We suggest that audiomotor integration in these frogs involves a complex interaction among distinct neural systems, with coupling between systems modulated by the behavioural relevance of the stimulus.
Human fMRI studies have demonstrated that the emotional or attentional context of a stimulus regulates correlations in neural activation patterns of anatomically distant brain regions (Buchel & Friston 1997; Sergent & Dehaene 2004; Haynes et al. 2005; Smith et al. 2006). For example, emotional relevance of visual stimuli in a contextual memory task modulates the coupling between amygdala and hippocampus (Smith et al. 2006). Thus, one proposed mechanism by which the brain integrates diverse information to form a unified percept is through transient correlations (Sergent & Dehaene 2004). We find evidence that modulation of similar long-range correlations may regulate audiomotor integration. Unlike the previous studies in humans, our egr-1 experiments did not provide temporal information; thus, our measure of static or average correlation is distinct from analyses of synchronous activation. Nonetheless, the basic result appears surprisingly general: context of the sensory stimulus or the social decision determines the form and degree of correlations among brain regions. Perhaps, the coupling between brain divisions reflects the social or behavioural significance of sensory inputs in the context of internal and external environments.
List of abbreviations.
| MP | medial pallium |
| dST | dorsal striatum |
| vST | ventral striatum |
| LS | lateral septum |
| DS | dorsal septum |
| MS | medial septum |
| NA | nucleus accumbens |
| MA | medial amygdala |
| LA | lateral amygdala |
| POA | anterior preoptic area |
| SC | suprachiasmatic nucleus |
| VH | ventral hypothalamus |
| LH | lateral hypothalamus |
| NP | nucleus of the periventricular organ |
| DH | dorsal hypothalamus |
| PT | posterior tuberculum |
| Athal | anterior thalamic nucleus |
| Cthal | central thalamic nucleus |
| Pthal | posterior thalamic nucleus |
| VMthal | ventromedial thalamic nucleus |
| Mtor | torus semicircularis—midline region |
| Ltor | torus semicircularis—laminar nucleus |
| Ptor | torus semicircularis—principal nucleus |
| Vtor | torus semicircularis—ventral region |
| SI | secondary isthmal nucleus |
| SO | superior olivary nucleus |
Acknowledgments
The authors thank Glennis Julian for behavioural analysis, Lawrence Cormack and an anonymous referee for their helpful suggestions for statistical analyses and NSF IBN 9816564, NIH MH 057066 and NIH T32 MH65728 for funding.
Supplementary Material
ANCOVA table showing tests of stimulus- and movement-related egr-1 levels in each brain region
Recordings of natural mating calls used as acoustic stimuli and photomicrographs showing boundaries of brain regions
Additional results graphs showing dependence of egr-1 mRNA levels in each brain region on stimulus condition and movement
References
- Allison J.D. Acoustic modulation of neural activity in the preoptic area and ventral hypothalamus of the green treefrog (Hyla cinerea) J. Comp. Physiol. A. 1992;171:387–395. doi: 10.1007/BF00223968. doi:10.1007/BF00223968 [DOI] [PubMed] [Google Scholar]
- Allison J.D, Wilczynski W. Thalamic and midbrain auditory projections to the preoptic area and ventral hypothalamus in the green treefrog (Hyla cinerea) Brain Behav. Evol. 1991;38:322–331. doi: 10.1159/000114398. [DOI] [PubMed] [Google Scholar]
- Allison J.D, Wilczynski W. Efferents from the suprachiasmatic nucleus to basal forebrain nuclei in the green treefrog (Hyla cinerea) Brain Behav. Evol. 1994;43:129–139. doi: 10.1159/000113630. [DOI] [PubMed] [Google Scholar]
- Bernal, X. E., Rand, A. S. & Ryan, M. J. In press. Sexual differences in receiver permissiveness to advertisement calls in túngara frogs, Physalaemus pustulosus Anim. Behav
- Bibikov N.G. Auditory responses in the isthmal region of the frog. Brain Behav. Evol. 2003;62:169. [Google Scholar]
- Buchel C, Friston K.J. Modulation of connectivity in visual pathways by attention: cortical interactions evaluated with structural equation modeling and fMRI. Cerebral Cortex. 1997;7:768–778. doi: 10.1093/cercor/7.8.768. doi:10.1093/cercor/7.8.768 [DOI] [PubMed] [Google Scholar]
- Clayton D.F. The genomic action potential. Neurobiol. Learn. Mem. 2000;74:185–216. doi: 10.1006/nlme.2000.3967. doi:10.1006/nlme.2000.3967 [DOI] [PubMed] [Google Scholar]
- Diekamp B, Schneider H. Neuronal processing of conspecific and related calls in the torus semicircularis of Rana r. ridibunda Pall. (Anura): single-unit recordings. J. Comp. Physiol. A. 1988;163:301–315. doi: 10.1007/BF00604006. doi:10.1007/BF00604006 [DOI] [PubMed] [Google Scholar]
- Edwards C.J, Alder T.B, Rose G.J. Auditory midbrain neurons that count. Nat. Neurosci. 2002;5:934–936. doi: 10.1038/nn916. doi:10.1038/nn916 [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R.J. Chapman & Hall/CRC; New York, NY: 1998. An introduction to the bootstrap. [Google Scholar]
- Endepols H, Walkowiak W. Integration of ascending and descending inputs in the auditory midbrain of anurans. J. Comp. Physiol. A. 2001;186:1119–1133. doi: 10.1007/s003590000159. doi:10.1007/s003590000159 [DOI] [PubMed] [Google Scholar]
- Endepols H, Feng A.S, Gerhardt H.C, Schul J, Walkowiak W. Roles of the auditory midbrain and thalamus in selective phonotaxis in female gray treefrogs (Hyla versicolor) Behav. Brain Res. 2003;145:63–77. doi: 10.1016/s0166-4328(03)00098-6. doi:10.1016/S0166-4328(03)00098-6 [DOI] [PubMed] [Google Scholar]
- Endepols H, Roden K, Luksch H, Dicke U, Walkowiak W. Dorsal striatopallidal system in anurans. J. Comp. Neurol. 2004;468:299–310. doi: 10.1002/cne.11006. doi:10.1002/cne.11006 [DOI] [PubMed] [Google Scholar]
- Endepols H, Roden K, Walkowiak W. Hodological characterization of the septum in anuran amphibians: II. Efferent connections. J. Comp. Neurol. 2005;483:437–457. doi: 10.1002/cne.20455. doi:10.1002/cne.20455 [DOI] [PubMed] [Google Scholar]
- Feng A.S. Afferent and efferent innervation patterns of the superior olivary nucleus of the leopard frog. Brain Res. 1986;364:167–171. doi: 10.1016/0006-8993(86)90998-4. doi:10.1016/0006-8993(86)90998-4 [DOI] [PubMed] [Google Scholar]
- Feng A.S, Hall J.C, Gooler D.M. Neural basis of sound pattern recognition in anurans. Prog. Neurobiol. 1990;34:313–329. doi: 10.1016/0301-0082(90)90008-5. doi:10.1016/0301-0082(90)90008-5 [DOI] [PubMed] [Google Scholar]
- Fuzessery Z.M. Frequency tuning in the anuran central auditory system. In: Fritzsch B, Ryan M.J, Wilczynski W, Hetherington T.E, Walkowiak W, editors. The evolution of the amphibian auditory system. Wiley; New York, NY: 1988. pp. 253–273. [Google Scholar]
- Fuzessery Z.M, Feng A.S. Mating call selectivity in the thalamus and midbrain of the leopard frog (Rana p. pipiens): single and multiunit responses. J. Comp. Physiol. 1983;150:333–344. doi:10.1007/BF00605023 [Google Scholar]
- Hall J.C, Feng A.S. Evidence for parallel processing in the frog's auditory thalamus. J. Comp. Neurol. 1987;258:407–419. doi: 10.1002/cne.902580309. doi:10.1002/cne.902580309 [DOI] [PubMed] [Google Scholar]
- Haynes J.D, Tregellas J, Rees G. Attentional integration between anatomically distinct stimulus representations in early visual cortex. Proc. Natl Acad. Sci. USA. 2005;41:14 925–14 930. doi: 10.1073/pnas.0501684102. doi:10.1073/pnas.0501684102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke K.L, Burmeister S.S, Fernald R.D, Rand A.S, Ryan M.J, Wilczynski W. Functional mapping of the auditory midbrain during mate call reception. J. Neurosci. 2004;24:11 264–11 272. doi: 10.1523/JNEUROSCI.2079-04.2004. doi:10.1523/JNEUROSCI.2079-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke K.L, Ryan M.J, Wilczynski W. Social cues shift functional connectivity in the hypothalamus. Proc. Natl Acad. Sci. USA. 2005;102:10 712–10 717. doi: 10.1073/pnas.0502361102. doi:10.1073/pnas.0502361102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis E.D. Brains and birdsong. In: Marler P, Slabberkoorn H, editors. Nature's music: the science of birdsong. Elsevier-Academic Press; New York, NY: 2004. pp. 239–275. [Google Scholar]
- Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog. Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. doi:10.1016/j.pneurobio.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Luksch H, Walkowiak W. Morphology and axonal projection patterns of auditory neurons in the midbrain of the painted frog, Discoglossus pictus. Hearing Res. 1998;122:1–17. doi: 10.1016/s0378-5955(98)00081-1. doi:10.1016/S0378-5955(98)00081-1 [DOI] [PubMed] [Google Scholar]
- Marin O, Gonzalez A, Smeets W.J. Basal ganglia organization in amphibians: afferent connections to the striatum and the nucleus accumbens. J. Comp. Neurol. 1997a;378:16–49. doi: 10.1002/(sici)1096-9861(19970203)378:1<16::aid-cne2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Marin O, Gonzalez A, Smeets W.J. Basal ganglia organization in amphibians: efferent connections of the striatum and the nucleus accumbens. J. Comp. Neurol. 1997b;380:23–50. [PubMed] [Google Scholar]
- Moreno N, Gonzalez A. Hodological characterization of the medial amygdala in anuran amphibians. J. Comp. Neurol. 2003;466:389–408. doi: 10.1002/cne.10887. doi:10.1002/cne.10887 [DOI] [PubMed] [Google Scholar]
- Mudry K.M, Capranica R.R. Evoked auditory activity within the telencephalon of the bullfrog (Rana catesbeiana) Brain Res. 1980;182:303–311. doi: 10.1016/0006-8993(80)91190-7. doi:10.1016/0006-8993(80)91190-7 [DOI] [PubMed] [Google Scholar]
- Mudry K.M, Capranica R.R. Correlation between auditory evoked responses in the thalamus and species-specific call characteristics. I. Rana catesbeiana (Anura: Ranidae) J. Comp. Physiol. A. 1987;160:477–489. doi: 10.1007/BF00615081. doi:10.1007/BF00615081 [DOI] [PubMed] [Google Scholar]
- Mudry K.M, Constantine-Paton M, Capranica R.R. Auditory sensitivity of the diencephalon of the leopard frog, Rana pipiens. J. Comp. Physiol. 1977;114:1–14. doi:10.1007/BF00656805 [Google Scholar]
- Neary T.J. Anterior thalamic nucleus projections to the dorsal pallium in ranid frogs. Neurosci. Lett. 1984;51:213–218. doi: 10.1016/0304-3940(84)90553-6. doi:10.1016/0304-3940(84)90553-6 [DOI] [PubMed] [Google Scholar]
- Neary T.J. Afferent projections to the hypothalamus in ranid frogs. Brain Behav. Evol. 1995;46:1–13. doi: 10.1159/000113254. [DOI] [PubMed] [Google Scholar]
- Neary T.J, Wilczynski W. Auditory pathways to the hypothalamus in ranid frogs. Neurosci. Lett. 1986;71:142–146. doi: 10.1016/0304-3940(86)90548-3. doi:10.1016/0304-3940(86)90548-3 [DOI] [PubMed] [Google Scholar]
- Northcutt R.G, Ronan M. Afferent and efferent connections of the bullfrog medial pallium. Brain Behav. Evol. 1992;40:1–16. doi: 10.1159/000113898. [DOI] [PubMed] [Google Scholar]
- Penna M, Lin W.Y, Feng A.S. Temporal selectivity for complex signals by single neurons in the torus semicircularis of Pleurodema thaul (Amphibia:Leptodactylidae) J. Comp. Physiol. A. 1997;180:313–328. doi: 10.1007/s003590050051. doi:10.1007/s003590050051 [DOI] [PubMed] [Google Scholar]
- Rand A.S, Ryan M.J. The adaptive significance of a complex vocal repertoire in a neotropical frog Physalaemus pustulosus. Zeitschr. Tierpsychol. 1981;57:209–214. [Google Scholar]
- Roden K, Endepols H, Walkowiak W. Hodological characterization of the septum in anuran amphibians: I. Afferent connections. J. Comp. Neurol. 2005;483:415–436. doi: 10.1002/cne.20454. doi:10.1002/cne.20454 [DOI] [PubMed] [Google Scholar]
- Roth G, Grunwald W, Dicke U. Morphology, axonal projection pattern, and responses to optic nerve stimulation of thalamic neurons in the fire-bellied toad Bombina orientalis. J. Comp. Neurol. 2003;461:91–110. doi: 10.1002/cne.10670. [DOI] [PubMed] [Google Scholar]
- Roth G, Muhlenbrock-Lenter S, Grunwald W, Laberge F. Morphology and axonal projection pattern of neurons in the telencephalon of the fire-bellied toad Bombina orientalis: an anterograde, retrograde, and intracellular biocytin labeling study. J. Comp. Neurol. 2004;478:35–61. doi: 10.1002/cne.20265. doi:10.1002/cne.20265 [DOI] [PubMed] [Google Scholar]
- Ryan M.J. University of Chicago Press; Chicago, IL: 1985. The túngara frog: a study in sexual selection and communication. [Google Scholar]
- Schmidt R.S. Mating call phonotaxis in American toads: lesions of central auditory system. Brain Behav. Evol. 1988;32:119–128. doi: 10.1159/000116539. [DOI] [PubMed] [Google Scholar]
- Schmidt R.S. Mating call phonotaxis in female American toad: lesions of the anterior preoptic nucleus. Horm. Behav. 1989;23:1–9. doi: 10.1016/0018-506x(89)90070-6. doi:10.1016/0018-506X(89)90070-6 [DOI] [PubMed] [Google Scholar]
- Sergent C, Dehaene S. Neural processes underlying conscious perception: experimental findings and a global neuronal workspace framework. J. Physiol. Paris. 2004;98:374–384. doi: 10.1016/j.jphysparis.2005.09.006. doi:10.1016/j.jphysparis.2005.09.006 [DOI] [PubMed] [Google Scholar]
- Smith A.P.R, Stephan K.E, Rugg M.D, Dolan R.J. Task and content modulate amygdala–hippocampal connectivity in emotional retrieval. Neuron. 2006;49:631–638. doi: 10.1016/j.neuron.2005.12.025. doi:10.1016/j.neuron.2005.12.025 [DOI] [PubMed] [Google Scholar]
- Sockman K.W, Gentner T.Q, Ball G.F. Complementary neural systems for the experience-dependent integration of mate-choice cues in European starlings. J. Neurobiol. 2005;62:72–81. doi: 10.1002/neu.20068. doi:10.1002/neu.20068 [DOI] [PubMed] [Google Scholar]
- Urano A, Gorbman A. Effects of pituitary hormonal treatment an responsiveness of anterior preoptic neurons in male leopard frogs, Rana pipiens. J. Comp. Physiol. 1981;141:163–171. [Google Scholar]
- Walkowiak W. Neuroethology of anuran call recognition. In: Fritzsch B, Ryan M.J, Wilczynski W, Hetherington T.E, Walkowiak W, editors. The evolution of the amphibian auditory system. Wiley; New York, NY: 1988. pp. 253–273. [Google Scholar]
- Walkowiak W, Luksch H. Sensory motor interfacing in acoustic behavior of anurans. Am. Zool. 1994;34:685–695. [Google Scholar]
- Walkowiak W, Berlinger M, Schul J, Gerhardt H.C. Significance of forebrain structures in acoustically guided behavior in anurans. Eur. J. Morphol. 1999;37:177–181. doi: 10.1076/ejom.37.2.177.4740. doi:10.1076/ejom.37.2.177.4740 [DOI] [PubMed] [Google Scholar]
- Westhoff G, Roth G. Morphology and projection pattern of medial and dorsal pallial neurons ind the frog Discoglossus pictus and the salamander Plethodon jordani. J. Comp. Neurol. 2002;225:97–121. doi: 10.1002/cne.10136. doi:10.1002/cne.10136 [DOI] [PubMed] [Google Scholar]
- Westhoff G, Roth G, Straka H. Topographic representation of vestibular and somatosensory signals in the anuran thalamus. J. Neurosci. 2004;124:669–683. doi: 10.1016/j.neuroscience.2003.12.022. doi:10.1016/j.neuroscience.2003.12.022 [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Allison J.D. Acoustic modulation of neural activity in the hypothalamus of the leopard frog. Brain Behav. Evol. 1989;33:317–324. doi: 10.1159/000115939. [DOI] [PubMed] [Google Scholar]
- Wilczynski, W. & Endepols, H. 2007 Central auditory pathways in anuran amphibians: the anatomical basis of hearing and sound communication. In Hearing and sound communication in amphibians Springer handbook of auditory research, vol. 28 (ed. A. N. Popper, A. S. Feng & P. N. Narins), Berlin, Germany: Springer.
- Wilczynski W, Northcutt R.G. Connections of the bullfrog striatum: efferent projections. J. Comp. Neurol. 1983a;214:333–343. doi: 10.1002/cne.902140310. doi:10.1002/cne.902140310 [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Northcutt R.G. Connections of the bullfrog striatum: afferent organization. J. Comp. Neurol. 1983b;214:321–332. doi: 10.1002/cne.902140309. doi:10.1002/cne.902140309 [DOI] [PubMed] [Google Scholar]
- Will U, Luhede G, Görner P. The area octavo-lateralis in Xenopus laevis I. The primary afferent projections. Cell Tissue Res. 1985;239:147–161. doi:10.1007/BF00214915 [Google Scholar]
- Yamada Y, Hada Y, Imamura K, Mataga N, Watanabe Y, Yamamoto M. Differential expression of immediate-early genes, c-fos and zif268, in the visual cortex of young rats: effects of a noradrenergic neurotoxin on their expression. Neuroscience. 1999;92:473–484. doi: 10.1016/s0306-4522(99)00003-2. doi:10.1016/S0306-4522(99)00003-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ANCOVA table showing tests of stimulus- and movement-related egr-1 levels in each brain region
Recordings of natural mating calls used as acoustic stimuli and photomicrographs showing boundaries of brain regions
Additional results graphs showing dependence of egr-1 mRNA levels in each brain region on stimulus condition and movement