Abstract
The sonar systems of bats and dolphins are in many ways superior to man-made sonar and radar systems, and considerable effort has been devoted to understanding the signal-processing strategies underlying these capabilities. A major feature determining the efficiency of sonar systems is the sensitivity to noise and jamming signals. Previous studies indicated that echolocating bats may adjust their signal structure to avoid jamming (‘jamming avoidance response’; JAR). However, these studies relied on behavioural correlations and not controlled experiments. Here, we provide the first experimental evidence for JAR in bats. We presented bats (Tadarida brasiliensis) with ‘playback stimuli’ consisting of recorded echolocation calls at one of six frequencies. The bats exhibited a JAR by shifting their call frequency away from the presented playback frequency. When the approaching bats were challenged by an abrupt change in the playback stimulus, they responded by shifting their call frequencies upwards, away from the playback. Interestingly, even bats initially calling below the playback's frequency shifted their frequencies upwards, ‘jumping’ over the playback frequency. These spectral shifts in the bats' calls occurred often within less than 200 ms, in the first echolocation call emitted after the stimulus switch—suggesting that rapid jamming avoidance is important for the bat.
Keywords: bat echolocation, jamming avoidance response, playback experiments, Tadarida brasiliensis
1. Introduction
Echolocation is a critical sensory system in most bats, and it is used for detecting and assessing prey as well as for orientation and navigation (Griffin 1958; Schnitzler et al. 2003). Most echolocating bats use calling patterns consisting of sequences of short calls (pulses) separated by long periods of silence, during which the bat listens to the returning echoes that provide information about the target (Schnitzler et al. 2003). Many whale and dolphin species also echolocate, using biosonar pulses that differ in design from those of bats (Cranford & Amundin 2004; Nakamura & Tomonari 2004).
Echolocating animals may experience acoustic interference from ambient sources of noise or from the calls of conspecifics (Dusenbery 1992), which may require a jamming avoidance response (JAR), in which the animal adjusts its call structure to minimize interference. It is possible, however, that the signal-processing algorithms of echolocating bats are sufficiently sophisticated that they need not alter their signals. For example, bats may use differences in the direction of arrival of sounds to separate multiple noise and signal sources, similarly to what is done by humans in the ‘cocktail party effect’ (e.g. Bronkhorst & Plomp 1992). Thus, the study of possible JARs provides a window into the signal-processing capabilities of animals that use biosonar.
Early experiments indicated that long-eared bats (Plecotus) are surprisingly resistant to jamming by high intensity white noise (Griffin et al. 1963), but it was unclear whether the bats achieved the reported high performance by changing their calls when the noise was present. In recent years, accumulating indirect evidence has indicated that some bats shift their echolocation call frequencies in the presence of the calls of conspecifics (Habersetzer 1981; Miller & Degn 1981; Obrist 1995; Surlykke & Moss 2000; Ibanez et al. 2004; Ratcliffe et al. 2004; Ulanovsky et al. 2004). These observations have often been interpreted as a JAR. To the best of our knowledge, jamming avoidance has not been studied in echolocating marine mammals.
These previous suggestions for jamming avoidance in bats did not rely on experimental manipulations, but relied rather on the analysis of correlations between call frequency and the absence or presence of conspecifics, or on correlations between call frequency and call amplitude. However, because some bats change the frequencies of their echolocation calls under a variety of circumstances unrelated to conspecific calls (Kalko & Schnitzler 1993), correlation-based inferences do not provide conclusive evidence for a JAR. Moreover, in previous studies (Habersetzer 1981; Miller & Degn 1981; Obrist 1995; Surlykke & Moss 2000; Ibanez et al. 2004; Ratcliffe et al. 2004; Ulanovsky et al. 2004), the spatial positions of the bats were unknown—hence it was unclear whether the directional echolocation beams of the bats (Schnitzler & Grinnell 1977; Hartley & Suthers 1989) were aimed towards each other (which may increase the jamming) or away from each other. The correlational approach meant also that no ‘time zero’ point was available for aligning any observed frequency changes in a bat's calls to the changes in the jamming signals. Thus, demonstrating a JAR that is causally linked to the jamming signals requires experimental presentation of well-controlled acoustic stimuli, designed to provoke a switch in the bat's call frequency at a known time zero.
Here, we report the results of experimental tests of JAR in echolocating bats. In the field, we presented free-flying bats (Tadarida brasiliensis) with playbacks of pre-recorded echolocation calls at one of six different frequencies. Bats consistently minimized spectral overlap with playback signals by shifting the dominant frequencies of their echolocation calls. In a separate experiment, we challenged approaching bats by abruptly switching the frequency of the playback stimulus. Within 200 ms, by the next echolocation call, bats shifted their call frequencies upwards. Our findings provide the first conclusive evidence for a JAR in echolocating animals.
2. Material and methods
(a) Recording site and bats
Experiments involving presentation of playbacks of echolocation calls to freely flying Brazilian free-tailed bats (T. brasiliensis) were conducted using methods approved by the University of Tennessee Animal Care and Use Committee. We performed experiments between 23 May and 9 July 2005 on a cotton farm in the vicinity of Uvalde, South Central Texas, within 10 miles of Frio cave, which has been estimated to contain 10 million T. brasiliensis. Bats were often observed foraging on insects that were found in high densities over these crop fields.
(b) Acoustic playback stimuli and data acquisition
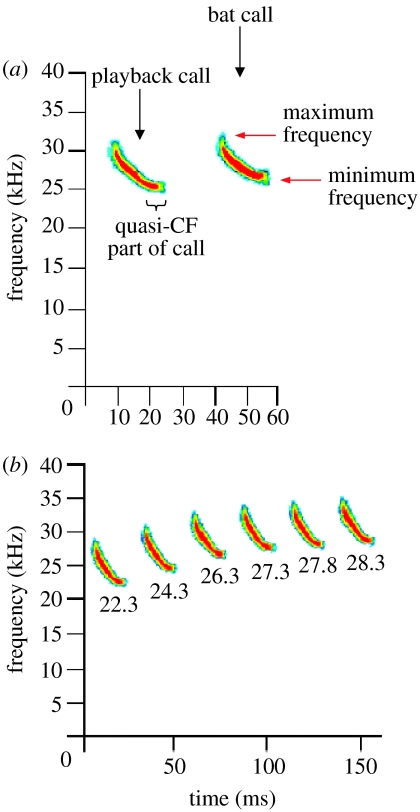
Similar to most insectivorous bats, T. brasiliensis use short frequency-modulated (FM) sweeps for echolocation (figure 1a). Call structure in this species may vary between geographic locations (e.g. Ratcliffe et al. 2004), so to minimize effects due to this variation, playback stimuli were assembled from recordings of bats foraging at the same study site. We constructed the signal using one prototypical call taken from recordings of ‘search-phase’ of bat echolocation (Griffin et al. 1960; figure 1a). Although search calls recorded at the study site often exhibited FM structures very similar to this prototypical call, the call structure varies within and between the individual bats, so further experiments are needed to investigate possible influences of the detailed FM structure on JAR. To create our stimuli, this prototypical call was repeated at 200 ms intervals for 8.8 s, followed by a 1.45 s sequence of ‘approach’ and terminal ‘feeding buzz’ calls (Griffin et al. 1960). This 10.25 s composite signal was repeated to create a 5 min playback sequence. We then created a series of six playback stimuli by shifting the frequency of this playback signal to one of six different frequency positions (we shifted the frequencies of all search, approach, and buzz calls, together). This resulted in playback stimuli with the following six values for the minimum frequencies of the search calls: 22.3; 24.3; 26.3; 27.3; 27.8; and 28.3 kHz (figure 1b). A 5 min control broadcast of silence was also created (no sound was presented during those 5 min).
Figure 1.
Spectrograms (frequency versus time) of search-phase bat calls and playback calls. (a) Spectrogram of one search-phase playback call with a minimum frequency of 24.3 kHz (left) and one recorded Tadarida brasiliensis search call with a minimum frequency of 25.8 kHz (right). The spectrogram was computed using a 1024 point fast Fourier transform with 93.75% overlap. Colour scale: linear, with red corresponding to high values and blue to low values. Red arrows: minimum and maximum frequencies of the signal. In all the subsequent analyses, the minimum frequency was used to represent the call frequency, unless stated otherwise. Also shown is the quasi-constant frequency (quasi-CF or QCF) part of the playback call. Dividing the frequency range between the minimum and the maximum frequency into four frequency quartiles, the lowest frequency quartile contained 43.9% of the call duration, whereas the highest frequency quartile contained only 9.5% of the call duration. (b) Spectrograms of all the six playback search calls used in this study; numbers below each call represent the minimum frequency, in kHz. In the static experiment all six frequencies were used, whereas in the dynamic experiment only four frequencies (22.3, 24.3, 26.3 and 28.3 kHz) were used. All the six playback search calls had the same bandwidth, 6.6 kHz, and the same call duration, 14.8 ms.
Several clarifications are needed regarding the playback stimuli. First, unless stated explicitly otherwise, all references to the ‘frequency’ of a call pertain to its minimum frequency (figure 1a, lower red arrow). Second, the playback frequencies used in this study (minimum frequencies of search calls between 22.3 and 28.3 kHz) were selected because preliminary experiments indicated that these frequencies span the range of search call frequencies used by these bats when presented with playback stimuli. This is also supported by the results of the static experiments (see figure 2a, y-range of black dots). Third, bats of some species, including T. brasiliensis, are attracted to feeding buzzes produced by conspecifics (Balcombe & Fenton 1988; E.H. Gillam & G. F. McCracken 2004, personal observations), so the purpose of presenting the approach and feeding buzz calls was to attract more bats into the range of our recording equipment. However, for all of our analyses, we used only data collected during the time periods when search-phase playback calls were presented, and we only measured search-phase calls produced by the bats.
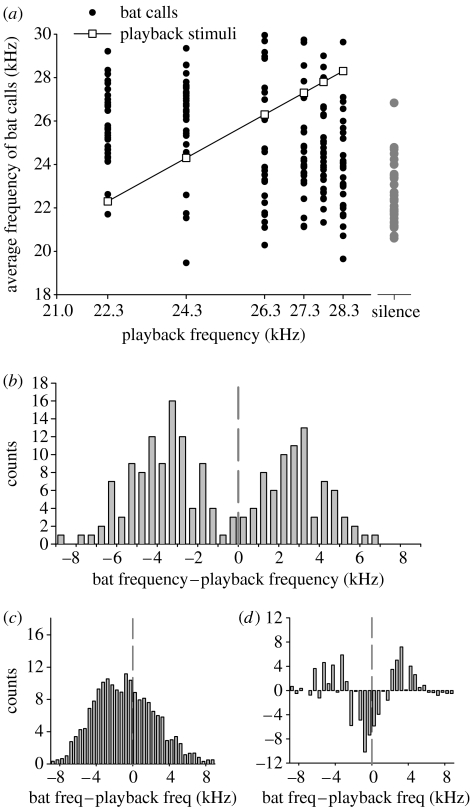
Figure 2.
Static stimulus experiment. (a) Frequency of bat calls versus frequency of playback stimuli. Each dot represents the average frequency of one sequence of search calls, recorded from one bat; black dots: 30 sequences×six playback frequencies (n=180); grey dots: silence control (n=30). Open squares: the six frequency values used for the search-phase playback stimuli. Note that most black dots were spaced above or below the open squares, indicating that the bats tended to avoid the playback frequencies. (b) Distribution of frequency-difference values (average bat frequency−playback frequency), pooled over all the six playback frequencies (n=180); bin size, 0.5 kHz; vertical dashed line indicates zero. (c) Distribution of reshuffled frequency-difference data from the Monte-Carlo simulation (see text). Note that the reshuffled data are unimodally distributed, whereas the original data are bimodally distributed with a trough near zero, indicating a jamming avoidance response. (d) Difference between the original histogram in (b) and the Monte-Carlo simulation in (c).
Each night, we began playbacks at between approximately 20.30 and 20.45, when the first bat was sighted in the area, and continued for 2–3 h, corresponding to the times of peak bat activity. We presented acoustic stimuli through an omnidirectional ultrasonic speaker (Avisoft Magnat 60401, Avisoft Bioacoustics, Berlin, Germany; frequency response ±5 dB between 15 and 43 kHz) mounted 2.5 m above the ground on a tripod. Two condenser microphones (Avisoft CM16; frequency response ±3 dB between 10 and 100 kHz) were placed in opposite directions 10 m from the speaker. Microphones were positioned at a height of 2 m and oriented at 45° above the horizontal and towards the speaker. Stimuli were generated by a Dell Inspiron laptop through a high-speed sound card (DAQCard-6062E, National Instruments, Austin, TX) and an Avisoft 70101 ultrasonic amplifier. High-speed data acquisition was carried out using Avisoft's Ultrasound Gate 416 and Avisoft RECORDER, using the same laptop that was used for stimulus presentation. Recordings were done with 16 bit resolution and a 166 kHz sampling rate. Recorded files were 5 min long and included both the playback signals and the calls of free-flying bats in the area.
(c) Static stimulus experiment
We initially tested for a JAR by broadcasting the six playback stimuli and the silence control in a randomized order and recording the calls of free-flying bats in the vicinity. We changed the playback order on successive nights, and presented each 5 min signal five times per night, on average, and at least 15 times over the course of the entire study. From the data files, we selected recorded call sequences according to the following criteria: (i) only one bat was present near our recording equipment, as evidenced by the stable inter-pulse intervals of recorded search-phase calls (Speakman & Racey 1991; Ulanovsky et al. 2004), (ii) we only used sequences separated by more than 1 min of silence, in order to minimize the chances of analysing multiple recordings of the same bat, (iii) the recorded call sequences had high signal-to-noise ratio, and (iv) the sequences consisted primarily of search-phase echolocation calls. Using these criteria, we selected the 30 highest quality call sequences for each of the seven playback conditions (six frequencies+silence), resulting in a total of 210 sequences. We did not select sequences based on whether any frequency changes were observed in the bat's behaviour. From each sequence, we then selected the highest quality search calls, 7–10 calls per sequence, and used Avisoft SasLab Pro to measure the call parameters (as described below), for a total of n=2070 search calls. We then computed the average pulse parameters for each sequence and used these average values for subsequent analyses of the static stimulus experiment.
(d) Dynamic stimulus experiment
To determine whether changes in call frequency were in direct response to the playback signal, we conducted a second experiment in which we abruptly switched the stimulus as an individual bat approached the speaker. We used five out of the seven playback stimuli (22.3, 24.3, 26.3, 28.3 kHz and silence) and performed all of the possible 20 switches between these five conditions. The presence of a single bat was assessed in real time based on the stability of the inter-call intervals, as above, and was later verified offline. The pre-switch playback stimulus was broadcast until an individual bat approached the recording area. We then switched the playback stimulus when the calls of the bat increased in amplitude to a level similar to that of the playback signal, indicating that the bat was approaching our recording system. The switch in playback frequencies resulted in a small temporal gap (less than 1.5 s) between the end of the pre-switch signal and the start of the post-switch signal, and when analysing the data we used the starting time of the post-switch signal as the alignment point, t=0. We continued recording until the echolocation calls of the bat were no longer visible on the oscillograms.
For analysis, we selected the 10 highest quality call sequences for each of the 20 switches, using the same selection criteria as above, and the additional criterion that the sequence contained at least 10 calls pre-switch and 10 post-switch. This resulted in a total of 200 sequences. We then extracted two subsets out of those 200 sequences: (i) the ‘main dataset’, defined as a subset of sequences where before the switch (t<0) the pre-switch playback frequency differed by more than 3 kHz from the bat's frequency (=the average pre-switch bat frequency), and where at the switch (t=0), the new playback frequency differed by less than 1.75 kHz from the bat's average pre-switch frequency. These criteria resulted in 39 sequences in the main dataset (1078 total calls) for which we expected a JAR to occur after the switch (t>0) owing to the small frequency separation between the playback and the bat calls at t=0, and (ii) the ‘control dataset’, defined as a subset of sequences where both before (t<0) and at the switch moment (t=0), the playback frequency differed by more than 3 kHz from the average pre-switch bat frequency. These criteria resulted in 24 sequences in the control dataset (673 total calls), for which we did not expect a JAR to occur after the switch (t>0), owing to the larger frequency separation between the playback and the bat calls at t=0. Selection of a value of greater than 3 kHz for delineating these subsets of the data was informed by the results of the static and dynamic stimulus experiments, as described below (see figures 2b and 3c).
Figure 3.
Dynamic stimulus experiment: examples. (a–b) Examples of recorded sequences of bat search calls, where the bats adjusted their call frequency in response to the playback. Top panels: call frequency versus time; red lines indicate the playback frequency. Note that in both examples, the bats shifted their call frequency upwards, away from the playback frequency. Bottom panels: call amplitudes versus time, showing the gradual increase in recorded amplitude as the bat approached the microphone, and then the gradual decrease as the bat flew away; amplitudes were normalized by the maximal amplitude. (a) The playback stimulus switched at t=0 from a frequency of 22.3–28.3 kHz (red lines); the time gap between the two stimuli, caused by the need to manually run a new playback stimulus, was 0.53 s in this example; t=0 (vertical dashed line) corresponds to the start of the post-switch stimulus. (b) The playback stimulus switched at t=0 from silence to a frequency of 24.3 kHz. Note that the increased frequency variability seen here for t>0 was not the general case, since a similar number of sequences in the main dataset exhibited higher variance for t<0 (17/39 sequences) as for t>0 (22/39 sequences; sign test p>0.50). (c) Population graph showing for each sequence (dots) the bat's frequency shift at t>0 compared with t<0 (y-axis) versus the frequency difference between the bat calls and the playback stimulus (x-axis). We included in this plot all sequences for which the y-value was defined, i.e. in which no silence stimuli occurred before or after the switch (n=164/200 sequences). Grey lines, 25th and 75th percentiles of the y-values of the dots, computed in 2 kHz bins along the x-axis. Note the upward shift in the frequencies of bat calls that occurred for small frequency differences between the bat calls and the playback (x-axis between approx. ±2 kHz), but not for large frequency differences.
For the population analyses of the frequency shifts, we computed for each individual sequence the differences between the frequency of each bat call and the corresponding average pre-switch bat frequency. These differences are, by definition, 0 kHz before the switch (t<0), so that any post-switch frequency shift will be expressed as a deviation from 0 kHz. We then pooled all the 39 sequences of the main dataset, or 24 sequences of the control dataset, and grouped these data into 1 s time bins. For each time bin, we then computed the following three average frequency values: (i) average for all the 39 sequences of the main dataset or 24 sequences of the control dataset, (ii) average only for the sequences in which at t=0, the bat was calling at a frequency above the post-switch playback signal (fplayback<fbat), and (iii) average only for the sequences in which at t=0, the bat was calling at a frequency below the post-switch playback signal (fplayback>fbat). We plotted the data only for time bins that included greater than or equal to 25 calls per bin in all these three averages.
The inter-call interval of search-phase calls in the dynamic stimulus experiment had an average of 227±55 ms (mean±s.d.). Averages were calculated over intervals shorter than 350 ms to remove potential bias due to missed calls. Averaging over intervals shorter than 500 ms resulted in an average inter-call interval of 266±89 ms.
(e) Measurement of pulse parameters
After the conclusion of the experiments, and following selection of all sequences for analysis, we extracted data from each selected file by digitally high-pass filtering the recording using a finite impulse response filter with 5 kHz cut-off, and computed the spectrogram (frequency×time representation) using a 1024 point fast Fourier transform (93.75% overlap). For the 166 kHz sampling rate we used, this gave a 162 Hz frequency resolution.
We excluded all the playback calls, which were easily identified based on their inter-call interval and spectro-temporal shape, both of which were highly reproducible due to our usage of a single replicated call with a fixed interval. From the spectrogram of the search-phase calls of the bats, we measured the following: (i) minimum frequency, and (ii) maximum frequency, defined as the lowest and the highest frequencies above the background noise, respectively (figure 1a; both of these measurements also corresponded well with the −15 dB points below the maximal peak of the power spectrum, data not shown), (iii) call bandwidth, defined as the maximum frequency−minimum frequency, and (iv) inter-call interval, defined as the time between the onsets of consecutive calls. Unless otherwise stated, we used the minimum frequency in our analyses because (i) calls at lower frequencies are less subject to atmospheric attenuation and signal degradation than are calls at higher frequencies (Lawrence & Simmons 1982), and (ii) the quasi-constant frequency (QCF) region near the lowest frequency of the call allows for more precise measurement of minimum frequency than is possible for the higher frequency portions of the call. From the oscillogram, we measured the call amplitudes for the 39 sequences in our main dataset. Finally, we returned to the spectrograms of the original recordings and measured the numerical values of the minimum frequencies of the playback calls (22.3, 24.3, 26.3, 27.3, 27.8 and 28.3 kHz; figure 1b), using the same methods and same settings that were used for measuring the bat calls (1024 point fast Fourier transform, 93.75% overlap).
(f) Estimation of the Doppler shift in the dynamic stimulus experiment
To estimate the effect of the Doppler shift caused by the bat approaching or flying away from our microphones, we used the following values: (i) average flight speed during foraging, v=6 m s−1 (Hayward & Davis 1964; minimal reported flight speeds are 5 m s−1, Vaughan 1966), (ii) average frequency of all bat calls in the main dataset of the dynamic stimulus experiments, f=25.22 kHz, and (iii) speed of sound, c=331.4 m s−1. These values were substituted into the formula of the relative Doppler shift between an approaching bat (t≪0) and a bat flying away (t≫0): 2×v×f/c, yielding a difference value of 0.91 kHz for an approaching bat versus a bat flying away from the microphone.
(g) Statistical tests
For the Monte-Carlo simulations of the static stimulus experiment, we randomly reshuffled the playback frequency associated with each bat-call frequency and calculated a new set of frequency differences. This random reshuffling was repeated 1000 times. We constructed histograms of the real and the simulated data using 0.5 kHz bins between −9 and 9 kHz. For the simulated data, we divided the counts by the number of permutations used (n=1000) in order to create an identical sample size for both distributions (n=180). We then performed a χ2-test to compare the real and the simulated distributions (the test's results were similar with other bin sizes). The simulations were done using Matlab (Mathworks, Natick, MA, USA). For this and all other statistical tests, we used a p<0.05 significance level.
3. Results
(a) Static stimulus experiment
A scatter plot of the average frequency in each sequence of bat calls, versus the corresponding playback frequency, indicated that the bat calls were usually displaced above or below the frequency of the playback stimuli (figure 2a). To quantify this observation, we performed three analyses. First, we pooled data from the two lowest-frequency playbacks (22.3 and 24.3 kHz) into a ‘low’ group, and data from the two highest-frequency playbacks (27.8 and 28.3 kHz) into a ‘high’ group (figure 2a, two left most versus two right most columns of black dots). Average call frequency differed between the low and high groups, with bats exhibiting higher frequency calls in the presence of lower frequency playbacks (two-tailed t-test: t=4.37, d.f.=118, p<0.0005).
Second, we subtracted the frequency of the playback from the frequency of the bat's calls and constructed a histogram of these differences (figure 2b). This histogram showed a bimodal distribution of the frequency differences, with a trough near zero and peaks on either side of zero. This pattern indicates that most bats did not call at or near the frequency of the playback, suggesting a JAR. Monte-Carlo simulations of randomly reshuffled frequency differences (figure 2c; see §2) showed a unimodal distribution that was significantly different from the bimodal distribution of our data (χ2-test: χ352=69.57, p<0.0005). This suggests that the trough near zero (figure 2b) is real, and provides evidence for a JAR in the presence of conspecific calls.
Finally, the call frequencies used by bats in the presence of the ‘silence’ control (figure 2a, grey dots) were significantly lower than the frequencies used by bats in the presence of any of the six playback stimuli (black dots; one-sided t-test: t>4.27, d.f.=58, p<0.0001, individually for five out of the six comparisons, with the 28.3 kHz playback yielding t=2.61, p=0.0057; all six t-tests remained significant after application of a Bonferroni correction for multiple comparisons, which yields a significance threshold of 0.0083). This suggests that in the presence of playback calls, the bats tended to shift their call frequencies upwards rather than downwards. Another asymmetry in the bats' behaviour is seen in figure 2d, which shows the difference between the real and the Monte-Carlo-simulated data; although bats employed both positive and negative frequency shifts, they seemed to avoid particularly the frequencies below the playback stimulus, i.e. a larger portion of the frequency differences forming the trough was to the left from 0 than to the right from 0 (figure 2d, sign test for the number of sequences between −3 and 0 kHz versus their number between 0 and +3 kHz: p<0.02). We will return to these asymmetries later.
(b) Dynamic stimulus experiment
Sequences of bat call frequencies collected in the dynamic stimulus experiment (figure 3a–b, top panels) illustrate that the bats shifted their call frequencies upwards in response to the stimulus switch at t=0. In figure 3b, the initial rapid shift upwards was larger than 3 kHz. Note also the gradual increase in the amplitude of the calls as the bat approached the microphone and then the gradual decrease as it flew away (figure 3a–b, bottom panels).
The average frequency difference between the post- and pre-switch bat calls plotted versus the frequency difference between the pre-switch bat call and the post-switch playback stimulus (figure 3c) suggested the following: if at t=0, there was a small frequency difference between the playback and the bat frequency (x-axis less than ±1.75 kHz), the bats shifted their call frequencies, and these shifts are mostly upwards (y>0); however, if at t=0 there was a larger frequency difference between the playback and the bat frequency (x-axis larger than ±3.0 kHz), the bats did not shift frequencies. This was the motivation for dividing our sequences into a main dataset, with x-axis between ±1.75 kHz, and a control dataset, with x-axis larger than ±3.0 kHz, as described above (see §2).
Population analysis of the main dataset (figure 4a) demonstrated that bats made rapid changes to the frequencies of their calls when the playback stimulus was switched at t=0 to within a small frequency difference (less than 1.75 kHz) from the bat's frequency. Such changes were not observed in the control dataset, where the frequency shift of the playback stimulus was to within greater than 3.0 kHz from the bat's frequency (figure 4a, Inset). Very similar results were obtained in the subset of sequences in which the stimulus was switched from silence to a playback frequency that was close to the bat's frequency (data not shown). In other words, the response of the bats was frequency-specific, occurring only when the post-switch playback frequency was close to the bat's frequency—suggesting a JAR.
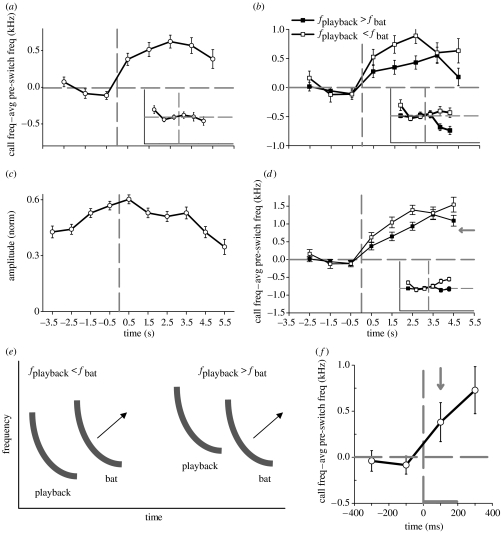
Figure 4.
Dynamic stimulus experiment: population analysis. (a) Average frequency difference values (bat call frequency minus average pre-switch bat frequency) versus time. Main plot: main dataset, in which we expect to see a jamming avoidance response (n=39 sequences, see text). Inset: control dataset, in which we do not expect to see a jamming avoidance response (n=24 sequences). Averages were obtained by aligning the sequences on the start of the post-switch stimulus (t=0), pooling all the sequences and grouping them into 1 s time bins. Error bars denote mean±s.e.m., here and in all subsequent plots. Vertical dashed line, t=0; horizontal dashed line, frequency difference=0. Note that in the main dataset the frequency increased at t>0; such increase was not observed in the control dataset. (b) Average frequency difference versus time, using the same data as in (a), decomposed based on whether the post-switch playback frequency was above the average pre-switch bat frequency (closed symbols, n=25 sequences) or below it (open symbols, n=14). Both curves showed a clear upward frequency shift at t>0. (c) Average amplitude of recorded bat calls, computed for the main dataset (n=39 sequences). Prior to averaging, we normalized the call amplitudes in each sequence by the maximum amplitude of that sequence. (d) Data from (b) corrected for the Doppler shift (see text). In both the datasets, none of the pre-switch data points were significantly different from zero (two-sided t-tests for all pre-switch data points: p>0.1), whereas all the post-switch data points did significantly differ from zero (two-sided t-tests for all post-switch data points: p<0.001). Grey arrow: average frequency difference between the playback frequency and the bat frequency, for the group of sequences where fplayback>fbat. Note that for t>0 the bats in this group shifted their frequency towards and, for t≫0, beyond the playback frequency (i.e. closed symbols data are above the grey arrow). (e) Schematic of a spectrogram summarizing the bats' responses in the dynamic stimulus experiment. When the playback frequency is below the bat's frequency (fplayback<fbat, left), the bat shifts its frequency upwards, away from the playback; when the playback frequency is above the bat frequency (fplayback>fbat, right), the bat also shifts its call frequency upward, towards and beyond the playback frequency. From this, we hypothesize that the bat attempts to keep the QCF part of the playback calls outside the bandwidth of the bat's own calls. (f) Average frequency difference values (bat call frequency−average pre-switch bat frequency) versus time, plotted as in (a) but on a finer time-scale, with a bin size of 200 ms. Arrow: first time bin that showed a significant upwards frequency shift by the bats; this bin was centred at t=100 ms and spanned the time from 0 to 200 ms (denoted by the horizontal grey bar). This demonstrates that jamming avoidance occurred rapidly, within less than 200 ms (the end-time of that bin).
To examine the effect of having a positive versus negative initial frequency difference between the playback and the bat calls, we decomposed the dataset into two groups of sequences (figure 4b), based on whether the average pre-switch bat frequency was above the post-switch playback frequency (open squares) or below it (closed squares). The bats that used frequencies above the playback (open squares) shifted their call frequencies upwards, away from the playback, as expected from a JAR. However, many bats that used frequencies below the playback (closed squares) also shifted their frequency upwards—towards the playback frequency. Comparison of the average frequency at t>0 versus t<0 showed that 100% of the sequences in the fplayback<fbat group exhibited an upward frequency shift (14/14 sequences, sign test: p<0.0005) and 72% of the sequences in the fplayback>fbat group also exhibited an upward frequency shift (18/25, sign test: p<0.05). These upward frequency shifts were maintained almost as long as we could reliably record the bats as they flew away from the speaker (on average, up to t=3.5 s). No upward frequency shifts were observed in the control dataset and a downward shift was observed for the control group with fplayback>fbat (figure 4b, Inset).
Two possible explanations can be invoked for the counter-intuitive frequency shift of bat calls towards the playback frequency. First, this may be an artefact caused by the Doppler shift due to the bats' motion. Second, the bats may have been shifting their frequencies towards and beyond the playback frequencies, perhaps in order to ‘jump’ over the playback frequency: figure 5 (electronic supplementary material) shows an example of a recorded bat sequence where this seems to be the case, with the bat slowly shifting its call frequencies upwards, eventually reaching frequencies higher than the playback.
To determine the magnitude of the Doppler shift due to the bats' motion, we first plotted the average amplitudes of recorded bat calls (figure 4c). These amplitudes increased as the bat approached our recording system (t∼0), remained high as the bat flew near our system, and then decreased as the bat flew away (t≥3.5 s). Since we performed the frequency switch of the playback as the bat was approaching the microphones (figure 4c, t=0 is on the rising phase of the amplitude curve), this meant that at times t≪0 there was a positive Doppler shift from the approaching bat—so the actual pre-switch frequencies were lower than what we recorded. Conversely, owing to the negative Doppler shift for a bat flying away, the post-switch frequencies were higher than those recorded. Using the estimate of a Doppler shift of 0.91 kHz for a bat approaching the microphone (t≪0) versus a bat flying away (t≫0; see §2), we re-plotted the data from figure 4b with a linear rise in the Doppler shift from a 0 kHz shift at t=0 to a 0.91 kHz shift at t=4.5 s (figure 4d). A linear change in the Doppler shift was used because we did not know the direction of the bat's flight immediately after t=0. Therefore, this estimate may be inaccurate at t∼0, but at t≫0 it provides a reasonable approximation of the Doppler shift. The main point conveyed by figure 4d is that the Doppler-corrected frequency shift (figure 4d) was even larger than our initial measurements (figure 4b).
Next, to determine whether the bats indeed shifted their frequency beyond the playback frequency for sequences with fplayback>fbat (figure 4d main plot, closed squares), we computed the average value of fplayback−fbat for these sequences using pre-switch fbat and post-switch fplayback. This frequency difference was 0.82 kHz. We then plotted this difference value in figure 4d (grey arrow). Since the bat frequencies after the switch were above the grey arrow (figure 4d closed squares, t=3.5 and 4.5 s bins), this demonstrated that the bats shifted their frequency not only towards, but also beyond the playback frequency. For the fplayback>fbat group, we also directly examined individual call sequences for evidence of upward shifts, calculating the percentage of sequences in which the bat's call frequency was above the playback frequency presented to the bat. For time bins 0.5, 1.5, 2.5, 3.5 and 4.5 s, these percentages were 29, 42, 48, 65 and 63%, respectively. This demonstrates that after 5 s there was an increase of greater than twofold in the number of bats calling above the playback signal, with the majority of sequences surpassing the playback frequency by the last two time bins.
Figure 4e shows a schematic summarizing this behaviour of the bats. When the bats used call frequencies above the playback (figure 4e, left), they shifted their call frequencies upwards, away from the playback. When the bats used call frequencies below the playback (figure 4e, right), they also tended to shift their call frequencies upwards, towards and beyond the playback—jumping over the playback frequency.
Finally, to address how quickly the bats reacted to the stimulus switch, we re-examined the dataset from figure 4a using smaller, 200 ms, time bins rather than 1 s bins (figure 4f). This higher temporal resolution demonstrates that a significant upward frequency shift was apparent already in the first time bin after the switch (figure 4f, arrow; one-sided t-test for this bin: t=1.80, d.f.=30, p<0.05). This bin was centred at t=100 ms and spanned the times from t=0 to 200 ms (grey horizontal bar). Therefore, on average, the bats shifted their call frequencies upwards within less than 200 ms. Since the bats' average inter-call interval during search-phase was 227±55 ms (mean±s.d.), this means that many bats shifted their call frequencies upwards already in their first call after the stimulus switch.
(c) Changes in call parameters other than the minimum frequency
Components of calls other than the minimum frequency also changed in response to the playback stimuli. A plot of the changes in the maximum frequency of the bat calls, Fmax, shows that at t>0, the bats rapidly shifted their Fmax upwards, with an average shift of +3 kHz at t=3.5 s (figure 6a, electronic supplementary material). The bandwidth of the bat calls also increased at t>0, as shown in figure 6b (electronic supplementary material) (t-test of −0.5 s time bin versus 3.5 s time bin in figure 6c (electronic supplementary material): t=3.92, p<0.0002). In the static experiment, the bandwidth also increased in the presence of playbacks compared with the silence condition (bandwidth=6.74±2.30 kHz, mean±s.d., compared with 4.15±1.93 kHz for silence; t-test: t=5.86, p<0.0001). The increase in bandwidth, combined with the upward frequency shift, suggests that the bats were decreasing the frequency overlap between their calls and the playback stimuli. A slight, but significant decrease in the frequency overlap, from 71 to 65% overlap, was indeed observed between the t=−0.5 s time bin and the t=4.5 s time bin (figure 6c (electronic supplementary material), one-sided t-test: t=1.35, p<0.05).
Measures of non-frequency call parameters showed that the amplitude of the calls did not increase after the stimulus switch in the dynamic experiment (no stepwise increase in amplitude at t=0 in figure 4c), indicating that the bats did not increase their call loudness in response to the playback. However, the inter-call interval was slightly and significantly shorter in the static stimulus experiment under the playback versus the silence conditions (mean±s.d.=247±34 versus 262±17 ms, respectively; two-sided t-test: t=2.12, d.f.=197, p<0.05), suggesting that the bats increased their call rate in the presence of conspecifics. The duration of the calls also was shorter under the playback versus the silence conditions (11.8±1.6 versus 13.7±0.9 ms; two-sided t-test: t=5.81, d.f.=197, p<0.0001). As a result, the duty cycle, defined as the percentage of time when a bat is calling, was not significantly different between the playback and the silence conditions (duty cycle: 4.98±0.97 and 5.33±0.74%, respectively; two-sided t-test: t=1.72, d.f.=197, n.s.). This suggests that the bats did not increase the redundancy of their signals, an increase that has been previously reported as a response to noise in other taxa (e.g. Lengagne et al. 1999).
In summary, the JAR in T. brasiliensis consisted of several changes to the bats' calls, including an upward frequency shift, increase in bandwidth, decrease in duration, slight decrease in spectral overlap between the bat call and the jamming call, and an increase in call rate.
4. Discussion
JAR and its role in electrolocation have been well documented in a number of weakly electric fishes, particularly the knife fish Eigenmannia (Watanabe & Takeda 1963; for reviews see Heiligenberg 1991; Metzner 1999). Here, we provide the first direct experimental evidence for jamming avoidance in echolocating animals. Our ‘static stimulus experiment’, where we presented playbacks of pre-recorded calls shifted to one out of six frequencies, demonstrated that free-flying bats (T. brasiliensis) avoided using frequencies that were close to the presented stimulus frequency, creating a notch in the distribution of used frequencies (figure 2b). A causal link between stimulus and response was demonstrated in the ‘dynamic stimulus experiment’, which involved abruptly switching the playback stimulus as a bat approached our recording equipment. Here, bats clearly exhibited a JAR by shifting their call frequencies upwards (figures 3 and 4). Surprisingly, the bats that originally used frequencies below the playback frequency also shifted upwards, ‘jumping’ over the frequency of the playback stimulus (figure 4d). Finally, we showed that the JAR was very rapid, with the bats shifting their frequencies within less than 200 ms of the stimulus switch (figure 4f).
(a) Comparisons with previous studies in echolocating bats and weakly electric fishes
Several previous studies in echolocating bats have provided evidence that some bat species which produce FM signals (‘FM bats’) shift their call frequency in response to conspecifics (Habersetzer 1981; Miller & Degn 1981; Obrist 1995; Surlykke & Moss 2000; Ibanez et al. 2004; Ratcliffe et al. 2004; Ulanovsky et al. 2004). This includes the species studied here (Ratcliffe et al. 2004) and the related species Tadarida teniotis (Ulanovsky et al. 2004). In species that produce constant frequency (CF) signals, so-called ‘CF bats’, no robust frequency shifts have been found (Jones et al. 1994)—but this may be expected, since auditory neurons in CF bats have an extremely narrow-band tuning to the bat's call frequency (Suga et al. 1987), making spectral jamming less likely. Similarly, no shifts were found in the bat Taphozous perforatus, which is an FM bat that uses unusually narrowband calls (Ulanovsky et al. 2004).
In some of the previous studies of FM bats, the evidence for frequency shifts consisted of examples of recordings in which two or three bats were flying together and maintained particularly large frequency differences between their calls (Habersetzer 1981; Miller & Degn 1981; Surlykke & Moss 2000). Other studies have shown that groups of bats flying in the same area exhibit a larger variation in frequencies compared with ‘virtual groups’ constructed from calls of bats flying alone (Obrist 1995; Ibanez et al. 2004; Ratcliffe et al. 2004; Ulanovsky et al. 2004). The most extensive evidence for frequency shifts involved a recent study of T. teniotis (Ulanovsky et al. 2004), which suggested long-term ‘static’ frequency shifts as well as more rapid dynamic shifts within approximately 1 s time-scale, when two bats were flying together. Interestingly, several of these previous studies have indicated a bias for upward frequency shifts (Obrist 1995; Ibanez et al. 2004; Ulanovsky et al. 2004), similar to the current study.
Although the frequency differences observed in previous studies can be interpreted as a JAR, other interpretations are likely, particularly because echolocating bats are known to shift their call frequencies under a variety of circumstances, such as when approaching a cluttered environment (Kalko & Schnitzler 1993). For example, bats flying in groups may fly at different speeds compared with solitary bats, or at different heights, or at different distances from vegetation—all of which may aid in collision avoidance. Therefore, changes in call design reported in previous studies (Habersetzer 1981; Miller & Degn 1981; Obrist 1995; Surlykke & Moss 2000; Ibanez et al. 2004; Ratcliffe et al. 2004; Ulanovsky et al. 2004) may have been due to these or other behavioural factors, rather than to a JAR to the conspecific calls. Owing to the lack of experimental manipulations, these studies do not provide information about the behavioural significance of any observed frequency shifts. Moreover, several methodological difficulties were inherent to all previous studies, which relied on recording the calls of free-flying bats and then using a post hoc correlation analysis of call parameters. First, the locations of the recorded bats relative to each other and to the recording microphone were unknown, so it was unclear whether the bats were approaching or departing from each other, which may influence whether jamming avoidance was to be expected at all. Second, because experimental manipulations were not used, there was no ‘time zero’ around which to measure any presumed frequency changes, confounding the analysis of any dynamic frequency shifts. In the current study, explicit experimental manipulations allowed us to overcome these methodological limitations. By switching the playback frequency as the bat approached our speaker and then by aligning the analysis to the switch time (time zero), we provide the first demonstration that frequency shifts are causally linked to experimental playback stimuli. These frequency shifts were very rapid, occurring in some bats within less than 200 ms, suggesting that these frequency shifts are not caused by factors such as changes in the bat's height or the level of ultrasonic clutter, which are unlikely to change appreciably within 200 ms—but were, in fact, induced by the playback calls themselves.
The jamming avoidance described in this study differs from the JAR in the electrolocation system of weakly electric fishes in that jamming avoidance in fishes typically develops slowly, sometimes over a few tens of seconds (e.g. Kawasaki 1997), in contrast to the very rapid frequency shifts that occurred in the bats. In other respects, the asymmetric response that we report for the bat T. brasiliensis, which shifted its frequencies mostly upwards, is similar to some species of fish (Apteronotidae) that also exhibit an asymmetric response, always shifting their discharge frequency upwards (Heiligenberg et al. 1996). However, in weakly electric fishes, the picture is known to be more complex, as other species (Eigenmannidae) exhibit a symmetric JAR, shifting their frequency upwards when encountering a lower frequency conspecific signal and shifting downwards when encountering a higher frequency signal (Heiligenberg 1991). Other species of echolocating bats may also exhibit a symmetric JAR similar to that of the weakly electric fish Eigenmannia.
(b) Hypotheses accounting for jamming avoidance in the bat
Several explanations may account for the upward shifts in the bats' call frequencies. First, the bats may be exhibiting a vocal startle response to the playback stimuli (a ‘surprise response’), rather than be avoiding jamming, and this startle response may be expressed as an upward frequency shift. However, the long duration of the response, lasting several seconds—as long as we could record the calls (figure 3a,b)—suggests that these frequency shifts do not reflect an instinctive, transient, startle response. Second, if the bats are avoiding jamming, they may prefer to shift their frequencies upwards rather than downwards if they have more sensitive hearing at above-average frequencies than at below-average frequencies. However, this explanation is unlikely because published audiograms of T. brasiliensis suggest that the hearing of this bat is most sensitive over a wide frequency range between 10 and 40 kHz (Henson 1970), covering frequencies both above and below the bat's dominant frequency. Third, the observed increase in Fmax (figure 6a, electronic supplementary material) and bandwidth (figure 6b, electronic supplementary material) after the stimulus switch could reflect an attempt by the bats to specifically avoid jamming of their highest call frequencies. Yet, similar increases in these call parameters are often noted when bats are attempting to gain more detailed information about their environment, such as when foraging in the presence of vegetative clutter (Obrist 1995). Brazilian free-tailed bats often forage in the presence of multiple conspecifics (Ratcliffe et al. 2004), so increasing the Fmax and bandwidth would provide a foraging bat with more precise information about the location of nearby conspecifics, which may be helpful for reducing mid-air collisions. Fourth, the bats may be changing their calls in order to minimize the frequency overlap with the playback stimuli, as reflected by the significant decrease in overlap that was observed following the stimulus switch (figure 6c, electronic supplementary material). However, the decrease in overlap was small in size, from 71% overlap just before the switch (at t=−0.5 s) to 65% overlap long after the switch (at t=4.5 s). This small decrease was most likely caused by the increased bandwidth of post-switch calls rather than by frequency shifts and suggests that the bats were not attempting to substantially reduce the frequency overlap.
A fifth hypothesis, which can account for the results of both the static- and the dynamic-stimulus experiments, is that the jamming power of playback calls is not uniform across frequencies—but that the narrowband, so-called ‘quasi-constant frequency’ (QCF) component that occurs near the end of the playback call (figure 1a), produces the most effective jamming. Two factors may add to the jamming potency of the QCF part of the playback call. First, this part of the playback call is relatively long in duration (see figure 1a). Second, it contains lower frequencies, which are least subject to atmospheric attenuation (Lawrence & Simmons 1982). Therefore, we propose that the bat's sonar is most jammed if the lowest (QCF) frequency of the playback call is anywhere within the bandwidth of the bat's own call. This hypothesis explains why in the dynamic stimulus experiment the bats tended to shift their frequencies upwards, above the playback frequency (figure 4d)—because an upward shift puts the QCF part of the playback call below the bandwidth of the bat's own call.
This hypothesis also accounts for several results of the static stimulus experiment. First, the hypothesis explains why, compared with the silence condition, the bats preferentially shifted their frequency upwards when the playback frequencies were presented (figure 2a). Second, the hypothesis suggests that the bats' frequencies should form an asymmetric ‘hole’ mostly below the playback frequency, because the bats calling below the playback would shift their frequencies upwards, above the playback frequency. This asymmetry in the hole was observed in the static stimulus experiment (figure 2d). The hypothesis does not explain however the finding that bats used lower call frequencies when presented with higher playback frequencies, which is the opposite of what we might expect (figure 2a)—although this could reflect a physical limit of the bats' ability to shift their frequency upwards when presented with the highest playback frequencies. Thus, our hypothesis explains the results of the dynamic stimulus experiment (figures 3 and 4) as well as most of the results of the static stimulus experiment (figure 2).
In conclusion, several intriguing questions remain. For example, what happens when two bats approach one another: do they both shift their frequencies upwards? What, if any, are the rules that govern their ‘group behaviour’ under such conditions? One way to address these questions experimentally is to use sequences of playback calls that do not have a fixed frequency as in this study, but rather change their frequencies across successive calls, according to the time course reported here for the real bats. It would also be informative to digitally manipulate the lowest (QCF) and the highest frequency parts of the playback calls in order to test the hypothesis that there are differential effects of various parts of the playback call on the bats' behaviour. These and other experiments could help elucidate the ability of echolocating bats to forage and avoid collisions when flying in high-density groups that often consist of tens or hundreds of bats (Adams & Simmons 2002)—an ability that is yet to be matched by man-made airborne radars.
Acknowledgments
We thank C. Moss, B. Fenton, M. Aytekin and G. Spanjer for their critical reading of the manuscript. This research was supported by an EPA Science to Achieve Results Graduate Research Fellowship, a University of Tennessee SARIF grant, and a research award from the Department of Ecology and Evolutionary Biology at the University of Tennessee.
Supplementary Material
Additional example of a call sequence
(a) Average frequency difference versus time, using the maximal frequency, Fmax, instead of the minimum frequency that was used in all the previous plots. Same sequences were used here as in (figure 4a). (b) Average call bandwidth versus time. Arrow shows the bandwidth of the playback call, 6.6 kHz. The bandwidth increased at t>0 in the main dataset but not in the control dataset (inset). (c) Average frequency overlap between the bat calls and the playback, defined as the percentage of the bat call bandwidth overlapped by that of the playback. The decrease in overlap prior to the stimulus switch, at time bins less than 0, results from the increase in bandwidth at t<0 seen at (c), which in turn was probably a by-product of the increase in call amplitude seen in (figure 4c), whereby more of the call is above background noise. Inset, control dataset; overlap values were much lower in the control dataset since, by definition, the control dataset has much larger frequency separation between the playback and the bat calls
References
- Adams R.A, Simmons J.A. Directionality of drinking passes by bats at water holes: is there cooperation? Acta Chiropt. 2002;4:195–199. [Google Scholar]
- Balcombe J.P, Fenton M.B. Eavesdropping by bats: the influence of echolocation call design and foraging strategy. Ethology. 1988;79:158–166. [Google Scholar]
- Bronkhorst A.W, Plomp R. Effect of multiple speechlike maskers on binaural speech recognition in normal and impaired hearing. J. Acoust. Soc. Am. 1992;92:3132–3139. doi: 10.1121/1.404209. doi:10.1121/1.404209 [DOI] [PubMed] [Google Scholar]
- Cranford T.W, Amundin M. Biosonar pulse production in odontocetes: the state of our knowledge. In: Thomas J.A, Moss C.F, Vater M, editors. Echolocation in bats and dolphins. The University of Chicago Press; Chicago, IL: 2004. pp. 27–35. [Google Scholar]
- Dusenbery D.B. Freeman; New York, NY: 1992. Sensory ecology. [Google Scholar]
- Griffin D.R. Yale University Press; New Haven, CT: 1958. Listening in the dark. [Google Scholar]
- Griffin D.R, Webster F.A, Michael C.R. The echolocation of flying insects by bats. Anim. Behav. 1960;8:141–154. doi:10.1016/0003-3472(60)90022-1 [Google Scholar]
- Griffin D.R, McCue J.J.G, Grinnell A.D. The resistance of bats to jamming. J. Exp. Zool. 1963;152:229–250. doi:10.1002/jez.1401520303 [Google Scholar]
- Habersetzer J. Adaptive echolocation sounds in the bat Rhinopoma hardwickei. J. Comp. Physiol. 1981;144:559–566. doi:10.1007/BF01326841 [Google Scholar]
- Hartley D.J, Suthers R.A. The sound emission pattern of the echolocating bat, Eptesicus fuscus. J. Acoust. Soc. Am. 1989;85:1348–1351. doi: 10.1121/1.395684. doi:10.1121/1.397466 [DOI] [PubMed] [Google Scholar]
- Hayward B, Davis R. Flight speeds in western bats. J. Mammal. 1964;45:236–242. doi:10.2307/1376986 [Google Scholar]
- Heiligenberg W. MIT Press; Cambridge, MA: 1991. Neural nets in electric fish. [Google Scholar]
- Heiligenberg W, Metzner W, Wong C.J.H, Keller C.H. Motor control of the jamming avoidance response of Apteronotus leptorhynchus: evolutionary changes of a behavior and its neuronal substrates. J. Comp. Physiol. A. 1996;179:653–674. doi: 10.1007/BF00216130. doi:10.1007/BF00216130 [DOI] [PubMed] [Google Scholar]
- Henson W.O., Jr . The ear and audition. In: Wimsatt W.A, editor. Biology of bats. vol. II. Academic Press; New York, NY: 1970. pp. 181–264. [Google Scholar]
- Ibanez C, Juste J, Lopez-Wilchis R, Nunez-Garduno A. Habitat variation and jamming avoidance in the echolocation calls of the sac-winged bat (Balantiopterix plicata) J. Mammal. 2004;85:38–42. doi:10.1644/1545-1542(2004)085<0038:HVAJAI>2.0.CO;2 [Google Scholar]
- Jones G, Sripathi K, Waters D.A, Marimuthu G. Individual variation in the echolocation calls of three sympatric Indian hipposiderid bats, and an experimental attempt to jam bat echolocation. Folia Zool. 1994;43:347–361. [Google Scholar]
- Kalko E.K.V, Schnitzler H.-U. Plasticity in echolocation signals of European pipistrelle bats in search flight: implications for habitat use and prey detection. Behav. Ecol. Sociobiol. 1993;33:415–428. doi:10.1007/BF00170257 [Google Scholar]
- Kawasaki M. Sensory hyperacuity in the jamming avoidance response of weakly electric fish. Curr. Opin. Neurobiol. 1997;7:473–479. doi: 10.1016/s0959-4388(97)80025-6. doi:10.1016/S0959-4388(97)80025-6 [DOI] [PubMed] [Google Scholar]
- Lengagne T, Aubin T, Lauga J, Jouventin P. How do king penguins (Aptenodytes patagonicus) apply the mathematical theory of information to communicate in windy conditions? Proc. R. Soc. B. 1999;266:1623–1628. doi:10.1098/rspb.1999.0824 [Google Scholar]
- Lawrence B.D, Simmons J.A. Measurements of atmospheric attenuation at ultrasonic frequencies and the significance for echolocation by bats. J. Acoust. Soc. Am. 1982;71:585–590. doi: 10.1121/1.387529. doi:10.1121/1.387529 [DOI] [PubMed] [Google Scholar]
- Metzner W. Neural circuitry for communication and jamming avoidance in gymnotiform electric fish. J. Exp. Biol. 1999;202:1365–1375. doi: 10.1242/jeb.202.10.1365. [DOI] [PubMed] [Google Scholar]
- Miller L.A, Degn H.J. The acoustic behavior of four species of vespertilionid bats studied in the field. J. Comp. Physiol. 1981;142:67–74. doi:10.1007/BF00605477 [Google Scholar]
- Nakamura K, Tomonari A. Comparison of click characteristics among odontocete species. In: Thomas J.A, Moss C.F, Vater M, editors. Echolocation in bats and dolphins. The University of Chicago Press; Chicago, IL: 2004. pp. 36–40. [Google Scholar]
- Obrist M.K. Flexible bat echolocation: the influence of individual, habitat and conspecifics on sonar signal design. Behav. Ecol. Sociobiol. 1995;36:207–219. doi:10.1007/s002650050142 [Google Scholar]
- Ratcliffe J.M, et al. Conspecifics influence call design in the Brazilian free-tailed bat, Tadarida brasiliensis. Can. J. Zool. 2004;82:966–971. doi:10.1139/z04-074 [Google Scholar]
- Schnitzler H.-U, Grinnell A.D. Directional sensitivity of echolocation in the horseshoe bat, Rhinolophus ferrumequinum. I. Directionality of sound emission. J. Comp. Physiol. 1977;116:51–61. doi:10.1007/BF00605516 [Google Scholar]
- Schnitzler H.-U, Moss C.F, Denzinger A. From spatial orientation to food acquisition in echolocating bats. Trends Ecol. Evol. 2003;18:386–394. doi:10.1016/S0169-5347(03)00185-X [Google Scholar]
- Speakman J.R, Racey P.A. No cost of echolocation for bats in flight. Nature. 1991;350:421–423. doi: 10.1038/350421a0. doi:10.1038/350421a0 [DOI] [PubMed] [Google Scholar]
- Suga N, Niwa H, Taniguchi I, Margoliash D. The personalized auditory cortex of the mustached bat: adaptation for echolocation. J. Neurophysiol. 1987;58:643–654. doi: 10.1152/jn.1987.58.4.643. [DOI] [PubMed] [Google Scholar]
- Surlykke A, Moss C.F. Echolocation behavior of big brown bats, Eptesicus fuscus, in the field and the laboratory. J. Acoust. Soc. Am. 2000;108:2419–2429. doi: 10.1121/1.1315295. doi:10.1121/1.1315295 [DOI] [PubMed] [Google Scholar]
- Ulanovsky N, Fenton M.B, Tsoar A, Korine C. Dynamics of jamming avoidance in echolocating bats. Proc. R. Soc. B. 2004;271:1467–1475. doi: 10.1098/rspb.2004.2750. doi:10.1098/rspb.2004.2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan T.A. Morphology and flight characteristics of molossid bats. J. Mammal. 1966;47:249–260. doi:10.2307/1378121 [Google Scholar]
- Watanabe A, Takeda K. The change of discharge frequency by AC stimulus in a weakly electric fish. J. Exp. Biol. 1963;40:57–66. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional example of a call sequence
(a) Average frequency difference versus time, using the maximal frequency, Fmax, instead of the minimum frequency that was used in all the previous plots. Same sequences were used here as in (figure 4a). (b) Average call bandwidth versus time. Arrow shows the bandwidth of the playback call, 6.6 kHz. The bandwidth increased at t>0 in the main dataset but not in the control dataset (inset). (c) Average frequency overlap between the bat calls and the playback, defined as the percentage of the bat call bandwidth overlapped by that of the playback. The decrease in overlap prior to the stimulus switch, at time bins less than 0, results from the increase in bandwidth at t<0 seen at (c), which in turn was probably a by-product of the increase in call amplitude seen in (figure 4c), whereby more of the call is above background noise. Inset, control dataset; overlap values were much lower in the control dataset since, by definition, the control dataset has much larger frequency separation between the playback and the bat calls