Abstract
In the face of hybridization, species integrity can only be maintained through post-zygotic isolating barriers (PIBs). PIBs need not only be intrinsic (i.e. hybrid inviability and sterility caused by developmental incompatibilities), but also can be extrinsic due to the hybrid's intermediate phenotype falling between the parental niches. For example, in migratory species, hybrid fitness might be reduced as a result of intermediate migration pathways and reaching suboptimal wintering grounds. Here, we test this idea by comparing the juvenile to adult survival probabilities as well as the wintering grounds of pied flycatchers (Ficedula hypoleuca), collared flycatchers (Ficedula albicollis) and their hybrids using stable isotope ratios of carbon (δ13C) and nitrogen (δ15N) in feathers developed at the wintering site. Our result supports earlier observations of largely segregated wintering grounds of the two parental species. The isotope signature of hybrids clustered with that of pied flycatchers. We argue that this pattern can explain the high annual survival of hybrid flycatchers. Hence, dominant expression of the traits of one of the parental species in hybrids may substantially reduce the ecological costs of hybridization.
Keywords: migration, stable isotopes, hybridization, extrinsic post-zygotic isolation, wintering grounds
1. Introduction
Speciation, i.e. the split of one species into two, is generally viewed as the formation of reproductive barriers between different populations. Reproductive barriers build up as side effects of natural selection or genetic drift in allopatric populations (Mayr 1942) or evolve in response to disruptive selection (e.g. Naisbit et al. 2001). Once populations have diverged genetically, hybridizing individuals experience a cost as a consequence of reduced hybrid viability and/or fertility (i.e. Hewitt 1989; Tegelström & Gelter 1990). These costs result in selection against heterospecific pairing and may lead to additional pre-zygotic or post-zygotic isolation (Dobzhansky 1937). Post-zygotic selection on hybrids may be the result of developmental instability (intrinsic post-zygotic isolation), which has been established in many hybridizing taxa (Coyne & Orr 2004 and references therein). Yet, it may also occur in a different way as an effect of their intermediate phenotype which induces them to fall between the parental niches with reduced fitness as a consequence (extrinsic post-zygotic isolation; Coyne & Orr 2004). For example, intermediate bill size of hybrid Darwin's finches is in most years disadvantageous compared to the resource optimized bill size of the parental species (Grant & Grant 1993, 1996). For migratory passerines, it has been argued that an intermediate migration route acts in a similar way (Helbig 1991a,b; Sutherland 1998; Bensch et al. 1999). Changed migration routes are generally suboptimal (Sutherland 1998) and can therefore enhance post-zygotic reproductive isolation. Bensch et al. (1999) suggested that intermediate suboptimal migration routes may explain the lack of recruitment of hybrids between two willow warbler subspecies (Phylloscopus trochilus trochilus and P. t. acredula). An intermediate migration route, potentially taken by hybrid willow warblers, would take them straight over the central Sahara desert where many may succumb as a result of food and water shortage (Bensch et al. 1999).
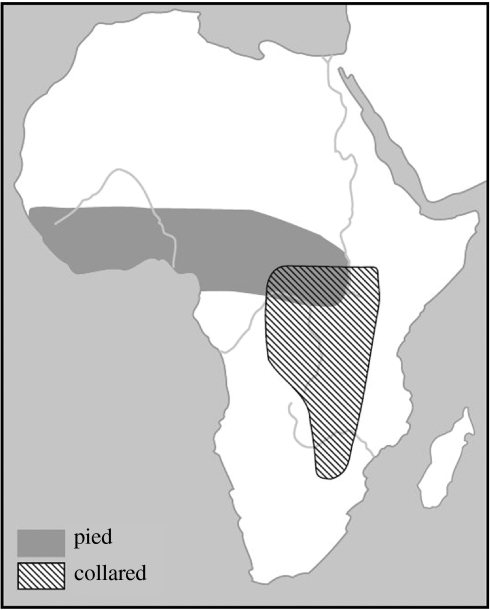
Here, we use pied (Ficedula hypoleuca) and collared (Ficedula albicollis) flycatchers and their hybrids to investigate differentiation in wintering grounds. Pied and collared flycatchers are long-distant migrants with distinct migratory routes and sub-Saharan wintering grounds. In many species, including pied and collared flycatchers, migratory traits like flight direction and distance are at least partially genetically determined. The autumn migration peaks of pied flycatchers are different for adults and first-year migrants, meaning that first-year migrants undertake their journey without parental guidance and probably alone, as in many other nocturnal migrants (Helbig 1991a; Lundberg & Alatalo 1992; Mouritsen & Larsen 1998; Berthold 2003). Pied flycatchers fly a western route, following the Iberian Peninsula and through western Africa (Cramp 1992). Mouritsen & Larsen 1998 showed experimentally that first-year pied flycatchers use the ‘clock-and-compass’ migration model, which appeared to be in accordance with ring recoveries (Mouritsen & Mouritsen 2000). The orientation mechanism in collared flycatcher has not been studied in such detail, but field observations and ring recoveries indicate that they migrate through Italy or further east, passing the Sahara desert on the eastern side (Cramp 1992). Based on ringing recoveries and visual observations, pied flycatchers winter in western to central Africa, while collared flycatchers winter in southeastern Africa (see figure 1). Observations of collared flycatchers in western Africa are generally considered to be vagrants (Cramp 1992; Lundberg & Alatalo 1992). The migratory route of hybrid flycatchers is unknown, but if they were to follow an intermediate route this would entail the crossing of large stretches of unfavourable habitat (i.e. Mediterranean Sea and central Sahara desert) as has been suggested for hybrid willow warblers (Helbig 1991a,b; Bensch et al. 1999).
Figure 1.
Location of the wintering areas of pied and collared flycatchers based on ringing recoveries and visual sightings (after Lundberg & Alatalo 1992).
In order to characterize the wintering locations of the pied, collared and hybrid flycatchers, we used stable isotopes extracted from feathers developed at their wintering sites in Africa, a technique that has been increasingly used over the last decade (Rubenstein & Hobson 2004 and references therein). Stable isotope ratios of the feathers reflect an individual's diet and environmental conditions at the wintering location during feather replacement.
We tested whether hybrid individuals take an intermediate route and thereby suffer from reduced survival as well as whether they reached intermediate wintering grounds between both parental species. The latter potential scenario could result in different carbon and nitrogen isotope ratios, as these areas probably have different precipitation and vegetation types. As alternative hypotheses, we tested whether the wintering grounds of hybrids were determined by sex-linked determination or a parental-species dominance effect. The results were used to infer the most likely migratory pattern of hybrids and to evaluate the implications for post-zygotic isolation.
2. Material and methods
(a) Study species and data collection
Collared and pied flycatchers are insectivorous passerine species with a breeding distribution covering most of Europe. Pied flycatchers breed in western and northern parts of Europe continuing eastwards into Russia. Collared flycatchers breed in Central and Eastern Europe. On the Baltic Islands of Gotland and Öland, both species breed sympatrically and hybridize in low numbers (approx. 3% of breeding pairs; Veen et al. 2001).
Juvenile to adult survival differences were estimated from fledglings recruited back as adults to the breeding population for pied and collared flycatchers as well as hybrids, using 26 years (1980–2005) of data from breeding flycatchers on Gotland (57°10′N, 18°20′E), Sweden (for data collection methods, see Gustafsson 1989; Pärt & Gustafsson 1989). Individuals born in 2004 and 2005 may not yet have recruited and nests from those years were therefore excluded. We collected the median tertial feather of breeding flycatchers. These feathers are fully moulted on the wintering grounds before spring migration (Cramp 1992; Salewski et al. 2004) and since the flycatchers are most probably territorial (Salewski et al. 2002), we assume that the isotope ratios in these feathers reflect their diet and can be used to discern wintering location differentiation between the two species and their hybrids. The feathers were collected at the end of the breeding season, just before pre-migratory moult with permission from the CFN (number M 78-05). Blood samples from birds were collected to ensure correct species identification (see below). Blood samples (3–10 μl) were taken from the brachial vein and stored in 96% ethanol at 4°C.
(b) Species identification and molecular genetic methods
Species were identified in the field using biometrics (Svensson 1984), behavioural characteristics and vocalization. Feather samples were collected from all individuals with features that deviated from the normal species range and from pairs whose broods contained infertile eggs or low hatching success, since female hybrids (the heterogametic sex) have reduced fertility (Gelter et al. 1992). Species identity of all birds was genetically determined as part of a large-scale study on genetic introgression between the two species (G.-P. Sætre & A. Qvarnström 2006, unpublished data). These results were used to exclude backcrosses (made possible through fertile hybrid males) and eliminate identification errors. For the genetic analysis, the DNA was extracted and purified using standard phenol–chloroform extraction. Tag-array-based mini-sequencing assays were applied to genotype single nucleotide polymorphisms (SNPs) based on the methods described in Sætre et al. (2003), with the difference that in this study 45 SNPs instead of 20 SNPs were used (G.-P. Sætre & A. Qvarnström 2006, unpublished data). Individuals were assigned as either pied, collared or F1 hybrids using the model-based cluster method described by Pritchard et al. (2000) using the same approach and setting as in Sætre et al. (2003).
The maternal species of F1 hybrids was determined using the amplification of a species-specific 32 bp indel in the mitochondrial DNA (Sætre & Moum 2000).
(c) Stable isotope analysis
The ratios of the stable isotopes 13C/12C and 15N/14N in chloroform-washed feather samples were determined at the Centre for Limnology of the Netherlands Institute of Ecology. Carbon stable isotope ratios (parts per thousand, ‰, difference from the 13C/12C ratio in Vienna PeeDee limestone; henceforth referred to as δ13C) and nitrogen stable isotope ratios (‰ difference from the 15N/14N ratio in atmospheric N2; henceforth referred to as δ15N) were determined in a HEKAtech EuroEA elemental analyser coupled online through a Finnigan con-flo interface to a Finnigan Delta S isotope ratio mass spectrometer. Average reproducibility based on replicate measurements was less than 0.2‰ for δ13C and less than 0.1‰ for δ15N.
(d) Statistical analysis
Survival probabilities were compared between collared flycatchers, pied flycatchers and the two hybrid types (depending on their parental species pairing) using a generalized linear model (GLM) with a logit-link function and number of fledged juveniles within a nest as the denominator. The variable including the categories, collared flycatcher, pied flycatcher and both hybrid types, will henceforth be referred to as ‘species’ for ease of use. Differences in isotope values between the two species and both types of hybrid depending on their parental species paring were analysed separately for δ13C and δ15N using a main effect ANOVA. For all the models tested, we did not find a significant deviation from normality, or an inequality of variances in the data. Besides species, sex, year and lay date (day of first egg) were also included as explanatory variables in the model. Non-significant variables were excluded from the analysis in a backwards elimination process. Pairwise differences between the species categories were analysed using a Tukey's HSD test.
To test whether hybrids fit an intermediate isotope ratio distribution, we compared the combined weighted mean of pied and collared flycatchers to the hybrids using a one-sample t-test for both δ13C and δ15N. Statistical tests were conducted using Statistica v. 7.0, GenStat v. 8.0 and JMP v. 6.0.
To verify our models selected above, we conducted model selection using the Akaike Information Criteria corrected for sample size (AICc; Burnham & Anderson 2002). AIC scores for all models are presented online (electronic supplementary material, appendix 1).
3. Results
The fledged juvenile to adult survival probabilities differed between the species (GLM: χ32=36.76, p<0.0001). However, only the pied flycatcher had a substantially lower survival probability (mean (s.e.)=0.038 (0.014), n=129) compared with the other species (collared flycatcher: mean (s.e.)=0.107 (0.003), n=3308; father pied flycatcher, mother collared flycatcher: mean (s.e.)=0.107 (0.023), n=47; father collared flycatcher, mother pied flycatcher: mean (s.e.)=0.096 (0.021), n=59). The data were overdispersed (overdispersion parameter=1.193) and the scaling parameter was therefore adjusted accordingly.
A total of 78 feather samples collected in 2004 and 2005 were analysed. The analyses involved 24 pied (12 males and 12 females) and 39 collared flycatchers (20 males and 19 females). Feather samples from 15 hybrids were analysed, of which 10 had a pied (3 males and 7 females) and 5 had a collared flycatcher mother (all males).
Pied and collared flycatchers, as well as their hybrids, show a clear difference in the δ13C distribution (ANOVA: ‘species’: F3,74=16.93, p<0.001; all other explanatory variables: p>0.45), whereas only a near significant year effect was found for δ15N (ANOVA: year: F1,76=3.26, p=0.075; all other explanatory variables: p>0.50; figure 2a). Pied and collared flycatchers differed in δ13C, whereas hybrids had δ13C values similar to those of pied flycatchers (Tukey's HSD test, p<0.01; figure 2b). The hybrids differed significantly from the combined weighted mean of pied and collared flycatchers for δ13C (t14=−3.58, p=0.003), but not for δ15N (t14=−0.97, p>0.30).
Figure 2.
δ13C and δ15N isotope ratios for (a) pure pied (PF) and collared (CF) flycatchers and (b) hybrid individuals with maternal species in brackets.
The isotope ratio results above are supported by analyses using model selection theory (see electronic supplementary material, appendix 1).
4. Discussion
Differences in stable isotope ratios in the feathers of collared and pied flycatchers, moulted at the wintering site, were consistent with the presumed largely segregated wintering grounds of the two species. The feather isotope data of the hybrids suggest that the hybrids, regardless of parental species pairing, fall within the range of pied flycatchers.
That the average isotope ratios for nitrogen are indistinguishable for pied and collared flycatchers is in accordance with the recent findings in willow warbler feathers collected in West and Central–East Africa (Bensch et al. 2006). Bensch et al. also found West African willow warbler feathers to have lower carbon isotope ratios than those from Central–East Africa. This exactly mirrors the isotopic findings in pied and collared flycatchers, which are supposedly wintering in West and Central–East Africa, respectively. Variation in carbon is often attributed to the relative abundance of plants using C3 or C4 photosynthetic pathways (Smith & Epstein 1971; Rubenstein & Hobson 2004 and references herein). When using global C3/C4 plant distributions (Still et al. 2003), the pattern found for the willow warblers and flycatchers is opposite to the predicted pattern. However, qualitatively identical patterns found by Bensch et al. 2006 not only validate our findings, but also show that there is need for a better mechanistic understanding of isotope distribution (e.g. Kelly 2000).
Since variation in isotope ratios in tissues is both influenced by environmental and dietary differences, it is important to assess which of these factors is determining the observed stable isotope patterns among the flycatchers in this study. In other words, could the flycatchers winter at the same geographical location but differ in diet, or are they wintering at different locations? There are various studies indicating that dietary choice of pied and collared flycatchers are very similar. Prey items taken by the two flycatcher species during the breeding period largely overlap (Bures 1995). Moreover, nestlings of both species can be successfully raised by foster parents of the alternative species (Qvarnström et al. 2005). On the wintering grounds, the pied flycatcher is an opportunistic forager with a broad diet, which is a common feature of migratory species compared to residents (Salewski et al. 2002). These observations suggest that the differences in feather isotope signatures reflect geographical differences in wintering sites and are not an effect of differences in diet within a single geographical range. This, together with the previously described differences in wintering range between pied and collared flycatchers (Cramp 1992; Lundberg & Alatalo 1992), which matches the isotopic findings in willow warbler feathers collected in these same ranges (Bensch et al. 2006) makes us confident in our conclusion that the two species have segregated wintering distributions. We acknowledge that one of the drawbacks of the now widely used isotope approach is that if no differences in isotope values are found, it does not necessarily mean that the geographical areas are the same. In the future, research such as that conducted by Bensch and his colleagues (2006), resulting in detailed isotope-based maps, will make the linkage between isotope values and geographical area more accurate.
With respect to the hybrid's determination of the wintering grounds, three obvious hypotheses could be put forward. Firstly, determination of the wintering grounds could be additive, which results in ‘intermediate’ wintering grounds of hybrids. This hypothesis can most likely be excluded, as the survival probabilities of the two hybrid types were as high as that of collared flycatchers and higher compared with pied flycatchers. Further, δ13C of hybrids significantly differed from the predicted intermediate isotope ratio of both parental species. However, the latter test assumes a clear-cut difference in habitat types at the contact zone of the parental species. Such assumptions can be tested and further insights gained, once a more accurate linkage between isotope ratios and geographical location becomes available.
Secondly, the determination of wintering grounds could be sex linked, in which case hybrids should have isotope ratios overlapping those of both parental species. In the case of Z-linkage, the wintering range would be paternally determined in daughters (ZW). Alternatively, wintering grounds could be maternally determined through the W chromosome (resulting in maternal inheritance in daughters) or cytoplasmic DNA inheritance (resulting in complete maternal inheritance). Neither of these hypotheses is supported by our data, but the sample sizes are small making firm conclusion difficult.
Thirdly, dominance of the determination of wintering grounds of one species would create the clustering of hybrids with one parental species, which is consistent with the observed pattern. The most parsimonious explanation for our data is that the pied flycatcher's migration route is dominant and hence all hybrids follow this route. This would explain both the lack of a reduced survival among hybrids, since these hybrids do not follow an intermediate and potentially suboptimal migration route (Helbig 1991b; Bensch et al. 1999) and the clustering of hybrids with pied flycatchers based on isotope ratios.
Sutherland (1998) states that ‘the change (of migration routes) will be most rapid when the genetic system is simple, there is assortative mating or the new route is dominant…’ In this study, we find indications for dominance in migratory traits when two populations hybridize, but an important question is whether dominance can evolve within populations and whether it has done so. Recently, there has been much discussion on the evolutionary potential of migratory traits (Berthold 2003; Pulido & Berthold 2003; Pulido & Widmer 2005) and an increasing body of evidence suggests that maintaining genetic variation and hence the potential for a response to selection of these traits is adaptive (e.g. Berthold et al. 1992; Sutherland 1998; Bearhop et al. 2005). Dominance effects in migratory traits therefore need to be taken into consideration, since they can have marked effects on the evolution of changes in migratory traits.
More recently, an interest in the potential effects of changes in migratory behaviour on reproductive isolation has arisen. Bearhop et al. (2005) argue that in blackcaps, a switch of wintering location can lead to temporal segregation of sympatric breeding populations and eventually lead to reproductive isolation and sympatric speciation. At the moment, the effect of an accelerated change of migration route on speciation is still poorly understood and remains a challenge to be solved.
To our knowledge, this is the first study to find indications of a dominance effect on the location of wintering grounds between two closely related species. By following one of the parental species' migration routes and using their wintering grounds, hybrids will not bear the potential costs of an intermediate migration route. Thereby, the dominance effect reduces the extrinsic post-zygotic isolation between the two flycatcher species, since hybrid individuals do not suffer from intermediate maladaptive traits. Further, species integrity is negatively affected by the reduced reproductive isolation between the two species. Not only can the observed dominance effect in migratory traits affect reproductive isolation, but also it can have implication for the speed with which migratory traits can change in response to a changing environment.
Acknowledgments
We would like to thank Harry Korthals for the stable isotope ratio determinations; Ben Sheldon, Franjo Weissing, Francisco Pulido, Thomas Alerstam, Lars Gustafsson and two anonymous referees for helpful comments and discussions during preparation of the manuscript; Reija Dufva, Glenn-Peter Sætre and Chris Wiley for help in the laboratory and Dick Visser for making figure 1. Financial support was obtained from the Netherlands Organization for Scientific Research (grant NWO-ALW 812.04.001) (T.V.), the Swedish Research Council Formas (M.B.H.), Academy of Finland, project no. 202388 (J.T.F.), European Commission (Marie Curie Intra-European Fellowship, MEIF-CT-2003-500554, J.T.F.) and the Swedish Research Council (A.Q.). This is publication 3952 from The Netherlands Research Council (KNAW-N100).
References
- Bearhop S, Fiedler W, Furness R.B, Votier S.C, Waldron S, Newton J, Bowen G.J, Berthold P, Farnsworth K. Assortative mating as a mechanism for rapid evolution of a migratory divide. Science. 2005;310:502–504. doi: 10.1126/science.1115661. doi:10.1126/science.1115661 [DOI] [PubMed] [Google Scholar]
- Bensch S, Andersson T, Åkesson S. Morphological and molecular variation in a migratory divide in willow warblers, Phylloscopus trochilus. Evolution. 1999;56:1925–1935. doi: 10.1111/j.1558-5646.1999.tb04573.x. doi:10.2307/2640451 [DOI] [PubMed] [Google Scholar]
- Bensch S, Bengtsson G, Åkesson S. Patterns of stable isotope signatures in willow warbler Phylloscopus trochilus feathers collected in Africa. J. Avian Biol. 2006;37:323–330. doi:10.1111/j.2006.0908-8857.03628.x [Google Scholar]
- Berthold P, Helbig A.J, Mohr G, Querner U. Rapid microevolution of migratory behavior in a wild bird species. Nature. 1992;360:668–670. doi:10.1038/360668a0 [Google Scholar]
- Berthold P. Genetic basis and evolutionary aspects of bird migration. In: Naquib M, et al., editors. Advances in the study of behaviour. Academic Press, Inc.; London, UK: 2003. pp. 175–229. [Google Scholar]
- Bures S. Comparisons of diet between collared flycatcher (Ficedula albicollis) and pied flycatcher (F. hypoleuca) nestlings in a hybrid zone. Folia Zool. 1995;44:247–253. [Google Scholar]
- Burnham K.P, Anderson D.R. 2nd edn. Springer; New York, NY: 2002. Model selection and multimodel inference. [Google Scholar]
- Coyne J.A, Orr H.A. Sinauer Associates; Sunderland, MA: 2004. Speciation. [Google Scholar]
- Cramp S. Oxford University Press; Oxford, UK: 1992. The birds of the Western Palearctic. vol. 6. [Google Scholar]
- Dobzhansky T. Columbia University Press; New York, NY: 1937. Genetics and the origin of species. [Google Scholar]
- Gelter H.P, Tegelström H, Gustafsson L. Evidence from hatching success and DNA fingerprinting for the fertility of hybrid Pied×Collared Flycatchers Ficedula hypoleuca×albicollis. Ibis. 1992;134:62–68. [Google Scholar]
- Gustafsson L. Collared flycatcher. In: Newton I, editor. Life time reproduction in birds. Academic Press, Inc.; London, UK: 1989. pp. 75–88. [Google Scholar]
- Grant B.R, Grant P.R. Evolution of Darwin's finches caused by a rare climatic event. Proc. R. Soc. B. 1993;251:111–117. [Google Scholar]
- Grant B.R, Grant P.R. High survival of Darwin's finch hybrids: effects of beak morphology and diets. Ecology. 1996;77:500–509. doi:10.2307/2265625 [Google Scholar]
- Helbig A.J. Inheritance of migratory direction in a bird species: a cross-breeding experiment with SE- and SW-migrating blackcaps (Sylivia atricapilla) Behav. Ecol. Sociobiol. 1991a;28:9–12. doi:10.1007/BF00172133 [Google Scholar]
- Helbig A.J. SE- and SW-migrating Blackcap (Sylvia atricapilla) populations in Central Europe: orientation of birds in the contact zone. J. Evol. Biol. 1991b;4:657–670. doi:10.1046/j.1420-9101.1991.4040657.x [Google Scholar]
- Hewitt G.M. The division of species by hybrid zones. In: Otte D, Endler J.A, editors. Speciation and its consequences. Sinauer Associates; Sunderland, MA: 1989. pp. 85–110. [Google Scholar]
- Lundberg A, Alatalo R.V. T & AD Poyser; London, UK: 1992. The Pied flycatcher. [Google Scholar]
- Kelly J.F. Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can. J. Zool. 2000;78:1–27. doi:10.1139/cjz-78-1-1 [Google Scholar]
- Mayr E. Columbia University Press; New York, NY: 1942. Systematics and the origin of species. [Google Scholar]
- Mouritsen H, Larsen O.N. Migrating young pied flycatchers Ficedula hypoleuca do not compensate for geographical displacements. J. Exp. Biol. 1998;201:2927–2934. [Google Scholar]
- Mouritsen H, Mouritsen O. A Mathematical expectation model for bird navigation based on the clock-and-compass strategy. J. Theor. Biol. 2000;207:283–291. doi: 10.1006/jtbi.2000.2171. doi:10.1006/jtbi.2000.2171 [DOI] [PubMed] [Google Scholar]
- Naisbit R.E, Jiggins C.D, Mallet J. Disruptive sexual selection against hybrids contributes to speciation between Heliconius cydno and H. melpomene. Proc. R. Soc. B. 2001;268:1849–1854. doi: 10.1098/rspb.2001.1753. doi:10.1098/rspb.2001.1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pärt T, Gustafsson L. Breeding dispersal in the collared flycatcher (Ficedula albicollis): possible causes and reproductive consequences. J. Anim. Ecol. 1989;58:307–322. [Google Scholar]
- Pulido F, Berthold P. Quantitative genetic analysis of migratory behavior. In: Berthold P, Gwinner E, Sonnenschein E, editors. Avian migration. Springer; Berlin, Germany: 2003. pp. 53–77. [Google Scholar]
- Pulido F, Widmer M. Are long-distance migrants constrained in their evolutionary response to environmental change. Causes of variation in the timing of Autumn migration in blackcap (S. atricapilla) and two garden warbler (Sylvia borin) populations. Ann. NY Acad. Sci. 2005;1046:228–241. doi: 10.1196/annals.1343.020. doi:10.1196/annals.1343.020 [DOI] [PubMed] [Google Scholar]
- Pritchard J.K, Stephens M, Donnelly P.J. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvarnström A, Svedin N, Wiley C, Veen T, Gustafsson L. Cross-fostering reveals seasonal changes in the relative fitness of two competing species of flycatchers. Biol. Lett. 2004;1:68–71. doi: 10.1098/rsbl.2004.0265. doi:10.1098/rsbl.2004.0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein D.R, Hobson K.A. From birds to butterflies: animal movement patterns and stable isotopes. Trend Ecol. Evol. 2004;19:256–263. doi: 10.1016/j.tree.2004.03.017. doi:10.1016/j.tree.2004.03.017 [DOI] [PubMed] [Google Scholar]
- Salewski V, Bairlein F, Leisler B. Different wintering strategies of two Palearctic migrants in West Africa—a consequence or foraging strategies? Ibis. 2002;144:85–93. doi:10.1046/j.0019-1019.2001.00007.x [Google Scholar]
- Salewski V, Altwegg R, Erni B, Falk K.H, Bairlein F, Leisler B. Moult of three Palearctic migrants in their West African winter quarters. J. Ornithol. 2004;145:109–116. doi:10.1007/s10336-004-0020-2 [Google Scholar]
- Sætre G.-P, Moum T.A. A simple molecular method for species identification of pied and collared flycatchers. Hereditas. 2000;132:171–172. doi:10.1111/j.1601-5223.2000.00171.x [Google Scholar]
- Sætre G.-P, Borge T, Lindroos K, Haavie J, Sheldon B.C, Primmer C, Syvänen A.-C. Sex chromosome evolution and speciation in Ficedula flycatchers. Proc. R. Soc. B. 2003;270:53–59. doi: 10.1098/rspb.2002.2204. doi:10.1098/rspb.2002.2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B.N, Epstein S. Two categories of 13C/12C ratios for higher plants. Plant Physiol. 1971;47:380–384. doi: 10.1104/pp.47.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Still C.J, Berry J.A, Collatz G.J, DeFries R.S. Global distribution of C3 and C4 vegetation: carbon cycle implications. Global Biogeochem. Cycle. 2003;17:1006–1029. doi:10.1029/2001GB001807 [Google Scholar]
- Sutherland W.J. Evidence for flexibility and constraint in migration systems. J. Avian Biol. 1998;29:441–446. [Google Scholar]
- Svensson L. Märstatryck; Stockholm, Sweden: 1984. Identification guide to European passerines. [Google Scholar]
- Tegelström H, Gelter H.P. Haldane's rule and sex biased gene flow between two hybridizing flycatcher species (Ficedula albicollis and F. hypoleuca, Aves: Muscicapidae) Evolution. 1990;44:2012–2021. doi: 10.1111/j.1558-5646.1990.tb04307.x. doi:10.2307/2409611 [DOI] [PubMed] [Google Scholar]
- Veen T, Borge T, Griffith S.C, Sætre G.-P, Bures S, Gustafsson L, Sheldon B.C. Hybridization and adaptive mate choice in flycatchers. Nature. 2001;411:45–50. doi: 10.1038/35075000. doi:10.1038/35075000 [DOI] [PubMed] [Google Scholar]