Abstract
We have studied the effect of the 3′ terminal CCA sequence in precursors of tRNAs on catalysis by the RNase P RNA or the holoenzyme from the cyanobacterium Synechocystis sp. PCC 6803 in a completely homologous system. We have found that the absence of the 3′ terminal CCA is not detrimental to activity, which is in sharp contrast to what is known in other bacterial systems. We have found that this is also true in other cyanobacteria. This situation correlates with the anomalous structure of the J15/16 loop in cyanobacteria, which is an important loop in the CCA interaction in Escherichia coli RNase P, and with the fact that cyanobacteria do not code the CCA sequence in the genome but add it posttranscriptionally. Modification of nucleotides 330–332 in the J15/16 loop of Synechocystis RNase P RNA from GGU to CCA has a modest effect on kcat for CCA-containing substrates and has no effect on cleavage-site selection. We have developed a direct physical assay of the interaction between RNase P RNA and its substrate, which was immobilized on a filter, and we have determined that Synechocystis RNase P RNA binds with better affinity the substrate lacking CCA than the substrate containing it. Our results indicate a mode of substrate binding in RNase P from cyanobacteria that is different from binding in other eubacteria and in which the 3′ terminal CCA is not involved.
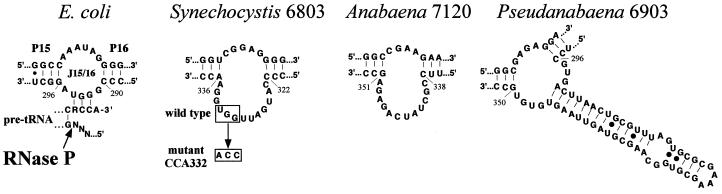
RNase P is a ubiquitous enzyme responsible for generating the 5′ end of pre-tRNAs by a single endonucleolytic cleavage (1, 2). In bacteria, the enzyme is composed of an RNA subunit and a protein subunit. The RNA subunit is the catalytic component and, under appropriate conditions in vitro, it can cleave substrates in the absence of the protein (3). In addition to all the pre-tRNAs, several other natural and artificial substrates are recognized by RNase P (4–7). Several studies have examined how a single enzyme, generally from Escherichia coli and Bacillus subtilis, can recognize so many different substrates and cleave all of them at the correct position (reviewed in refs. 2, 8, and 9). One of the main conclusions from these studies is the crucial role played by the 3′ terminal RCCA sequence of pre-tRNAs, where the underlined nucleotides interact with a GGU sequence in the loop connecting helices P15 and P16 (loop J15/16) (10–12). In addition, the 3′ terminal CCA participates in binding of Mg2+ ions used in catalysis (13). This interaction is important in defining the cleavage site and reaction rate. In cyanobacteria, the J15/16 loop has a structure that deviates from the consensus, and it generally lacks a GGU sequence (Fig. 1) (14, 15). In some instances, there is an extra helix inserted in this loop. We have investigated the role of the 3′ terminal CCA in cyanobacterial pre-tRNAs on cleavage by cyanobacterial RNase P RNA and the holoenzyme. Kinetic analyses indicate that cyanobacteria RNase P has no preference for CCA-containing substrates, as does the E. coli enzyme, under single- or multiple-turnover conditions. The Synechocystis RNase P RNA contains a GGU sequence in the J15/16 loop. We have analyzed how mutagenesis of this sequence affects activity. The relative affinities to Synechocystis RNase P RNA of pre-tRNAs containing or lacking the 3′ terminal CCA have been analyzed by a method based on the immobilization of the pre-tRNAs on a membrane. This procedure facilitates the detection of the RNA–RNA interactions when the binding affinity is too low to be detected by gel retardation. Our results indicate that cyanobacterial RNase P binds and reacts on substrates lacking the 3′ terminal CCA. These results might reflect the fact that in cyanobacteria the 3′ terminal CCA generally is not encoded in the genome but is added posttranscriptionally, and they support the recent suggestion that there is a correlation of the primary structure of tRNA genes with RNase P RNA structure and substrate preference (16).
Figure 1.
Structure of the J15/16 loop in the RNase P RNAs used in this work. The J15/16 loop, connecting helices P15 and P16, is shown from left to right for E. coli, Synechocystis 6803, Anabaena 7120, and Pseudanabaena 6903. The proposed (11) base pairing between the sequence RCCA-3′ in pre-tRNAs and a conserved GGU sequence in J15/16 of E. coli is shown. The GGU sequence mutated in Synechocystis to CCA to generate mutant CCA332 is boxed.
MATERIALS AND METHODS
Preparation of RNAs.
RNAs were prepared by in vitro run-off transcription with T7 RNA polymerase from template plasmids as described (17). After transcription, the RNAs were dephosphorylated with calf intestinal phosphatase and labeled at the 5′ end with polynucleotide kinase and [γ-32P]ATP. Alternatively, when uniformly labeled RNA was required, [α-32P]CTP was included in the reaction mix.
Template plasmids for in vitro transcription of E. coli M1 RNA (17), Synechocystis sp. PCC 6803 and Anabaena sp. PCC 7120 RNase P RNAs (14), and pGln (precursor of Synechocystis tRNAGln) (18) already have been described. Pseudanabaena sp. PCC 6903 RNase P was obtained from a template prepared by C. Tous (Sevilla University) on the basis of the published sequence (19).
Plasmid pT7Glu was prepared for the synthesis of Synechocystis 6803 pGlu (precursor of Synechocystis tRNAGlu). The Synechocystis 6803 trnE gene was amplified by PCR from genomic DNA with primers based on the published sequence (20). The forward primer (5′-CGACGGGATCCTAAATACGACTCACTATAGTCTGAAATAACGAACTG-3′) contains a BamHI site and the T7 promoter sequence and overlaps the 5′ end of the pre-tRNA sequence. The reverse primer (5′-GTCCCAAGCTTGGATGGACGCCTGGTACCCCCAAGGGAA-3′) overlaps the 3′ end of pre-tRNA sequence and contains a FokI, a BstNI, and a HindIII site. A PCR product of the expected size was purified, digested with HindIII and BamHI, and ligated into pUC19 treated with the same enzymes. The clone was confirmed by sequencing.
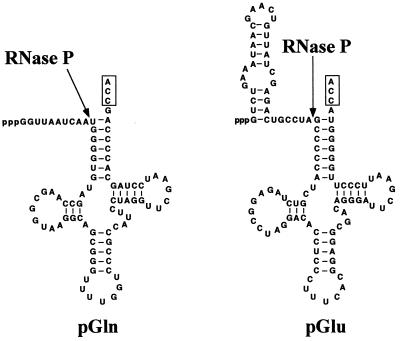
Pre-tRNAs were prepared by T7 RNA polymerase-based run-off transcription by using as templates pT7Gln or pT7Glu digested with either FokI (to generate substrates lacking the 3′ terminal CCA) or BstNI (to generate substrates containing the 3′ terminal CCA). Fig. 2 shows the predicted secondary structures of Synechocystis pGln and pGlu.
Figure 2.
Structure of pre-tRNA substrates used in this work. These substrates could be prepared by in vitro transcription either containing or lacking the 3′ terminal CCA (boxed), depending on the restriction enzyme used to digest the template, as described in the text.
Purification of Proteins.
RNase P protein from E. coli (C5 protein) was purified from cultures of BL21(DE3) carrying plasmid pARE7 as described (17). RNase P protein from Synechocystis was purified from cultures of BL21(DE3) carrying plasmid pARA2 as described (18).
RNase P Activity Assays.
RNase P RNA assays were done in 50 mM Tris⋅HCl, pH 7.5/100 mM MgCl2/1 M KCl. RNase P RNAs were incubated in assay buffer for 5 min at 37°C before addition of substrate. Holoenzyme was reconstituted by direct mixing of RNase P RNA with a 10-fold molar excess of purified RNase P protein in assay buffer and incubated for 5 min at 37°C before addition of substrate. E. coli holoenzyme assays were done in 50 mM Tris⋅HCl, pH 7.5/10 mM MgCl2/400 mM NH4Cl/0.1% Triton X-100 (21). Synechocystis 6803 holoenzyme assays were done in 50 mM Tris⋅HCl, pH 7.5/50 mM MgCl2. These conditions have been found to be optimal for the Synechocystis holoenzyme (unpublished data). Aliquots of the reactions were mixed at different times with loading dye containing urea and EDTA. The amount of processing was estimated by separating reaction products on acrylamide/urea gels and quantification with a Cyclone Phosphor System (Packard). Kinetic constants were estimated from double-reciprocal plots of initial reaction rates at different substrate concentrations as described (22). Substrate concentration was varied in the range of 0.01–100 μM. Enough data were collected for each enzyme-substrate pair to have a reduced error in the estimation of catalytic constants shown in Table 1.
Table 1.
Kinetic constants for steady-state cleavage of pGln and pGlnCCA by E. coli M1 RNA, Synechocystis RNase P RNA wild type, and Synechocystis RNase P RNA CCA332
| Enzyme | Substrate | Km, M × 106 | kcat, min−1 | kcat/Km, min−1⋅M−1 × 109 |
|---|---|---|---|---|
| E. coli | pGln | 3.0 ± 0.27 | 0.039 ± 0.0020 | 13.0 |
| pGlnCCA | 0.13 ± 0.05 | 0.003 ± 0.0002 | 23.1 | |
| Synechocystis wild type | pGln | 2.9 ± 0.25 | 0.008 ± 0.002 | 2.8 |
| pGlnCCA | 350.6 ± 2.10 | 0.812 ± 0.012 | 2.3 | |
| Synechocystis CCA332 | pGln | 2.7 ± 0.45 | 0.004 ± 0.002 | 1.5 |
| pGlnCCA | 126.5 ± 1.60 | 0.060 ± 0.005 | 0.5 |
Km and kcat were determined as described in Materials and Methods.
Site-Directed Mutagenesis.
Mutations G330C, G331C, and U332A were introduced simultaneously in the Synechocystis rnpB gene by site-directed mutagenesis with the oligonucleotide 5′-CCTGCCCCATGATTCCAGGAACCGCTTGAGGAATTTGG-3′. The U.S.E. Mutagenesis Kit from Pharmacia was used for this purpose.
Analysis of RNA—RNA Affinity.
Gel retardation of pre-tRNA with RNase P RNAs was done as described (23).
Filter-bound interaction analysis was performed by transferring equal amounts of the pre-tRNAs to be analyzed to a membrane (Hybond N+, Amersham) with a slot–blot apparatus. The RNAs were fixed to the membrane by baking for 2 h at 80°C, and then the filter was blocked by soaking for 2 h at room temperature in 20 ml of 20 mM Hepes⋅KOH, pH 8.0/100 MgCl2/1 M KCl/2 mg/ml yeast RNA (R-6625; Sigma), which was prepared as described (24). After the blocking step, the filter was soaked for 20 min in a minimal volume of the same solution, but without yeast RNA, containing 10,000 cpm/ml of 32P-labeled RNase P RNA. The filter was washed twice for 20 min with the same solution without yeast RNA, and the bound radioactivity was quantified with a Cyclone Phosphor System. The intensity of the signal was proportional to the amount of pre-tRNA bound to the filter in the range of 2–200 pmol. We found that no binding to pre-tRNA is detected when the RNase P RNA is immobilized on the filter instead of the pre-tRNA.
RESULTS
Effect of 3′ Terminal CCA in Pre-tRNA on RNase P Cleavage Kinetics.
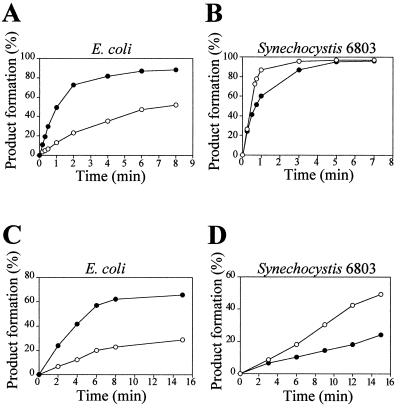
The protein and RNA subunits of RNase P, as well as a pre-tRNA substrate, from the cyanobacterium Synechocystis 6803 have been cloned and characterized (15, 18). Therefore, a completely homologous system to investigate the enzyme—substrate interaction during RNase P catalysis in cyanobacteria is available. We have looked at the effect that the 3′ terminal CCA sequence in the substrate has on cleavage rate and substrate binding. First, we analyzed cleavage rate under single-turnover conditions (pre-steady-state conditions) to determine the effect of the CCA sequence on the rate of the chemical cleavage reaction. In this way the reaction rate was not affected by slow product release that has been shown to limit the overall reaction rate under multiple-turnover conditions in E. coli. Fig. 3 shows that pGln was cleaved slightly faster than pGlnCCA (precursor of Synechocystis tRNAGln containing the 3′ terminal CCA) by Synechocystis RNase P RNA or holoenzyme, whereas E. coli RNase P RNA or holoenzyme had a clear preference for pGlnCCA. The preference of the E. coli enzyme for CCA-containing substrates is well documented (23, 25, 26). Synechocystis RNase P RNA behaved in an opposite way. This result was not a peculiarity of the Synechocystis enzyme or of the pGln substrate. RNase P RNAs from the cyanobacteria Anabaena 7120 and Pseudanabaena 6903 showed a similar behavior (Fig. 4A). Also, when a different substrate was used, Synechocystis pGlu, a similar result was obtained (Fig. 4C). E. coli RNase P RNA had a clear preference for the CCA-containing substrate, whereas the Synechocystis RNase P RNA cleaved the CCA-lacking substrate slightly better.
Figure 3.
Effect of 3′ terminal CCA in pGln on pre-steady-state cleavage kinetics by RNase P RNA or holoenzyme from E. coli and Synechocystis 6803. Ten picomolar 32P-labeled pGln (○) or pGlnCCA (●) was incubated with 20 nM E. coli M1 RNA (A), 40 nM Synechocystis RNase P RNA (B), holoenzyme reconstituted with 0.5 nM E. coli M1 RNA and 5 nM C5 protein (C), or holoenzyme reconstituted with 2 nM Synechocystis RNase P RNA and 20 nM Synechocystis RNase P protein (D) and aliquots withdrawn at different times. Buffer conditions are as described in Materials and Methods. An average of three independent experiments is shown.
Figure 4.
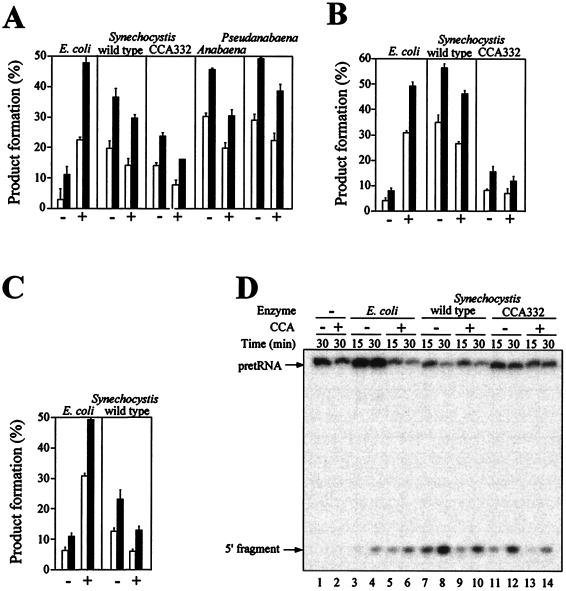
Effect of 3′ terminal CCA in pGln and pGlu on pre-steady-state cleavage kinetics by cyanobacterial RNase P RNAs or holoenzymes. (A–C) Ten picomolar pre-tRNA (−) or pre-tRNACCA (+) was incubated with the indicated RNase P RNAs or reconstituted holoenzymes for 15 min (open bars) or 30 min (solid bars), and the amount of processing was determined. For the RNA-alone reactions, the concentrations used were 0.5 nM of E. coli M1 RNA and 5 nM of all the other RNase P RNAs. The E. coli holoenzyme was reconstituted with 0.1 nM M1 RNA and 1 nM C5 protein. The Synechocystis wild-type and CCA332 mutant holoenzymes were reconstituted with 2 nM RNase P RNA and 20 nM Synechocystis RNase P protein. The average ± SD of three independent experiments is shown. (A) pGln and pGlnCCA cleavage by RNase P RNA. (B) pGln and pGlnCCA cleavage by RNase P holoenzyme. (C) pGlu and pGluCCA cleavage by RNase P RNA. (D) Autoradiogram of a representative assay gel. Ten picomolar 5′ end-labeled pGln (−) or pGlnCCA (+) was incubated for 15 min (lanes 3, 5, 7, 9, 11, and 13) or 30 min (lanes 1, 2, 4, 6, 8, 10, 12, and 14) in the absence of enzyme (lanes 1 and 2) or with 0.5 nM E. coli M1 RNA (lanes 3–6), 5 nM Synechocystis RNase P RNA wild type (lanes 7–10), or 5 nM Synechocystis RNase P RNA CCA332 (lanes 11–14).
Synechocystis is one of the few cyanobacteria that contain a GGU sequence in J15/16. But as has been discussed previously (14), J15/16 has a structure in Synechocystis that differs from that in E. coli. To investigate the role of J15/16 of Synechocystis on enzymatic activity, we have changed the GGU sequence to CCA by site-directed mutagenesis to generate mutant CCA332. Under single-turnover conditions, RNase P RNA CCA332 had the same substrate preference than wild type (Fig. 4A). Holoenzyme reconstituted with RNA CCA332 also behaves similarly (Fig. 4B). RNA CCA332 has a reaction rate about 50% of the wild type in the RNA-alone reaction and about 20% lower of the wild type in the holoenzyme reaction. The holoenzyme activity of RNA CC332 could be increased up to 50% of wild type by increasing the protein concentration (not shown). Therefore, it seemed that RNA CCA332 was partially defective in its interaction with the protein, and, thereby, it required higher protein concentrations than wild type to reach maximum activity. The presence or absence of CCA in the substrate had no effect on the site of cleavage. The modification of J15/16 in mutant CCA332 also had no effect on the site of cleavage. Fig. 4D shows that in all cases the 5′ fragment had the same size, which corresponded to cleavage at the +1 position, as determined by primer extension (18) (A.V., unpublished data).
The results obtained under single-turnover conditions were confirmed fully under multiple-turnover assay conditions. Kinetic constants of Synechocystis wild-type and mutant CCA332 RNase P RNAs were determined under multiple-turnover conditions (steady state) for substrate containing and lacking the 3′ terminal CCA (Table 1). For comparison, E. coli M1 RNA also was analyzed. The effects of the 3′ terminal CCA on Km and kcat of M1 RNA were similar to what has been described previously for a number of substrates. Km was 23-fold lower for the CCA-containing substrate. kcat also was lower (13-fold) because of the lower product release rate for the CCA-containing substrate. Synechocystis RNase P RNA had a Km for pGln similar to that for the E. coli M1 RNA, but the Km for pGlnCCA was more than 100-fold higher. The presence of CCA had a detrimental effect on Km, which was the opposite of what happens in E. coli. Conversely, kcat was 120-fold higher for pGlnCCA than for pGln. The mutation in J15/16 in RNase P RNA CCA332 had no significant effect on Km; it showed just a 3-fold reduction of the Km for pGlnCCA. The kcat for pGlnCCA was reduced 13-fold.
RNase P RNA—Pre-tRNA Interaction.
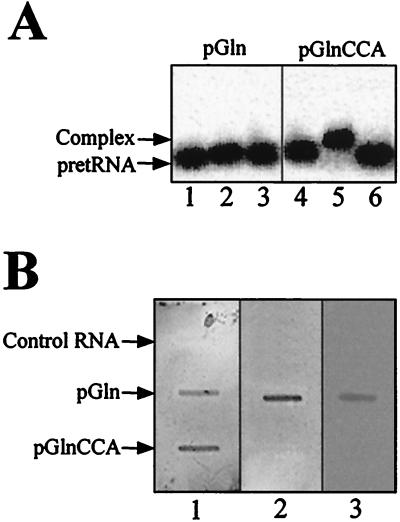
The results described in the previous section indicated that the 3′ terminal CCA in pre-tRNA had an effect on RNase P activity in cyanobacteria that was different from what was described previously in other bacterial RNase P RNAs. The Km was increased drastically when the 3′ terminal CCA was present in the substrate. Therefore, we decided to study whether this effect was due to a defect in enzyme–substrate affinity. It is well established that E. coli M1 RNA has higher affinity for CCA-containing substrates. This has been shown mainly by gel-retardation assays (23). We showed that the same also was true with Synechocystis pGln. pGlnCCA was retarded by M1 RNA but not pGln (Fig. 5A). No retarded complex could be observed with Synechocystis RNase P RNA and either pGln or pGlnCCA with RNA concentrations as high as 1 μM, indicating that for both substrates the affinity was too low to be detected by this assay. Therefore, we have developed a different assay, in which the pre-tRNA was bound to a membrane and then incubated with RNase P RNA. With this assay we could detect the interaction between pre-tRNA and Synechocystis RNase P RNA (Fig. 5B). We have validated the assay with E. coli M1 RNA. This RNA behaved in this assay in a way consistent with the gel-retardation assay. A stronger signal was detected with pGlnCCA than with pGln, which indicated that the relative intensity of the signals was related to the relative affinity between both RNAs. Synechocystis RNase P RNA, however, gave a clear signal with pGln and a barely detectable signal with pGlnCCA. The intensity of the radioactive signal was proportional to the amount of pre-tRNA used (not shown) and was reproducible. Therefore, it was possible to compare the affinities of RNase P RNAs to different substrates by quantification of the radioactivity bound. Our results (from triplicate experiments similar to the one shown in Fig. 5B) indicated that the signal was about 20-fold stronger with pGln than with pGlnCCA for Synechocystis RNase P RNA, but it is about 6-fold stronger with pGlnCCA than with pGln for E. coli M1 RNA. RNA CCA332 behaved in this assay in a way similar to Synechocystis wild-type RNase P RNA.
Figure 5.
Analysis of RNase P RNA interaction with pGln. (A) Gel retardation. Autoradiogram of 5% acrylamide gels were run as described in Materials and Methods. Samples contained 100 nM 32P-labeled pGln (lanes 1–3) or pGlnCCA (lanes 4–6) and either alone (lanes 1–4), with 500 nM E. coli M1 RNA (lanes 2 and 5), or with 500 nM Synechocystis RNase P RNA (lanes 3 and 6). (B) RNase P RNA interaction with filter-bound substrate. pGlnCCA, pGln, or Synechocystis RNase P RNA (control) was blotted on three similar, nylon membranes, and each membrane was incubated, respectively, with 32P-labeled E. coli M1 RNA (1), Synechocystis RNase P RNA wild type (2), or Synechocystis RNase P RNA CCA332 (3), as described in Materials and Methods.
DISCUSSION
The results described in this paper point to differences in the substrate-recognition mechanism by cyanobacterial RNase P as compared with other bacterial RNase P analyzed so far. The 3′ terminal CCA in the substrate, which is an important determinant for substrate binding in E. coli, has an opposite effect in cyanobacteria. E. coli RNase P RNA or holoenzyme have a clear preference for CCA-containing substrates. In contrast, cyanobacterial RNase P exhibits a preference for CCA-lacking substrates. The differential substrate preference is due to effects both on Km and kcat (Table 1). These differences result in an increased overall catalytic efficiency (kcat/Km) for pGlnCCA vs. pGln with E. coli RNase P RNA of almost 2-fold, whereas there is a better catalytic efficiency for pGln vs. pGlnCCA with the Synechocystis RNase P RNA. Detailed kinetic analysis has shown that the lower kcat of E. coli with CCA-containing substrates is due to slow product release (27, 28). A better affinity for the substrate, as reflected in a lower Km, translates into a greater difficulty of releasing the mature tRNA product. In contrast, Synechocystis RNase P RNA has a much lower Km for the CCA-lacking substrate. That the substrate preference is apparent under single-turnover conditions, where there is no effect of product release, indicates that the rate of the chemical step (k+2) also is affected by the presence of CCA in the substrate. More detailed kinetic analysis of the cyanobacterial system is required to confirm this point.
The results of the filter-bound RNA–RNA interaction assay are in agreement with the kinetic data. The relative intensities of the bands are congruent with the known RNA–RNA affinities (in E. coli) or as expected from the Km values (Synechocystis): E. coli M1 RNA gives a stronger signal with the CCA-containing substrate whereas Synechocystis RNase P RNA does the opposite. The differences in intensities are 6-fold for E. coli and 20-fold for Synechocystis, which is somewhat proportional to the differences in Km (20-fold for E. coli and 120-fold for Synechocystis). A high difference in Km is correlated with a high difference in band intensities in the filter assay.
The filter-bound RNA–RNA interaction technique generally could be useful for comparing relative RNA–RNA affinities when the affinity is too low to be detected by other traditional methods, such as gel retardation, and it can be easily scaled up to analyze the binding of many samples with a 32P-labeled RNA to facilitate the comparison of mutants, structural variants, etc., as well as for the selection of stronger-binding RNAs.
Why does the Synechocystis enzyme have a much lower affinity for the CCA-containing substrate? The J15/16 loop in E. coli is very close to the acceptor stem of the pre-tRNA (29, 30), and it interacts with the CCA sequence. It is possible that in Synechocystis there is steric interference when the CCA-containing substrate is bound because of the larger size of the J15/16 loop. Assuming a similar, overall, three-dimensional structure of Synechocystis RNase P RNA and a similar geometry of binding of the substrate to the E. coli system, the larger size of J15/16 would reduce the space available to fit the substrate and, therefore, make it more difficult to interact with CCA-containing pre-tRNAs. A prediction of this hypothesis is that a reduction of the size of the J15/16 loop in Synechocystis would reduce the preference for CCA-lacking substrates.
The conclusions we have reached with Synechocystis and pGln can be generalized to other substrates, such as pGlu, and to other cyanobacteria, such as Anabaena 7120 and Pseudanabaena 6903. There are published reports that the RNase P RNA from the cyanobacteria Prochlorothrix hollandica and Prochlorococcus marinus are strongly dependent on the presence of CCA in the substrate for activity (31, 32), in contradiction with our results. In those studies, a plant chloroplast substrate was used whereas we have used a completely homologous system. Therefore, it would be interesting to confirm with a cyanobacterial pre-tRNA whether P. hollandica and P. marinus are true exceptions to our observation that cyanobacteria have a preference for CCA-lacking substrates.
The J15/16 loop is important in E. coli not only for substrate binding but also for catalysis. Single base substitutions on this loop have strong effects on the kinetic constants and on the cleavage-site selection (11, 28, 33, 34). Although a significant change (i.e., a 3-nt deletion) in the J15/16 loop was introduced in the Synechocystis RNase P, the effect of the change was minimal on Km and it was significant on kcat only with the CCA-containing substrate. The change had no significant effect on the substrate preference of the enzyme, and it did not affect cleavage-site selection with either substrate. This, again, suggests that the role of J15/16 in catalysis by cyanobacterial RNase P is not as relevant as with E. coli or other bacterial RNase P. The 3′ terminal CCA interaction with the J15/16 loop is used in E. coli as part of a measuring device to determine the cleavage site (34–37). Presumably, in cyanobacteria, as in eukaryotes, where the CCA also is unimportant, alternative contacts are used to determine the cleavage site (2). Our results would explain the large variability in sequence and structure of J15/16 in cyanobacteria compared with other bacteria (14). When there are no functional constraints on RNA structure, it will evolve more freely and explore in a neutral way a larger range of possible structures. tRNA genes in cyanobacteria do not code for the 3′ terminal CCA, and, therefore, it is possible that cyanobacterial RNase P function has evolved in a context in which the CCA sequence is not present. In some cases, an extra helix has been inserted in this region (14, 31). The insertion, deletion, or substitution of structures during the evolution of RNase P RNA is widespread. They reflect either specific adaptations in the different RNase P RNAs or random changes in functionally neutral sites. A good example is presented by cyanobacteria again: in heterocyst-forming strains, helix P12 is much larger than usual and contains a short, tandemly repeated sequence (14). These repeats seem to have been inserted recently and independently during the evolution of RNase P RNA in these strains. Interestingly, they can be deleted without significant effect on the kinetics of cleavage (14) or on the binding of the protein subunit (unpublished data).
In summary, our results indicate that cyanobacterial RNase P, in contrast to other bacteria, does not require the 3′ terminal CCA in the substrate for efficient activity. CCA is detrimental to activity and reduces the affinity between enzyme and substrate. This result is in agreement with the fact that CCA is not encoded in cyanobacterial tRNA genes but is added posttranscriptionally, which supports the idea that RNase P evolves to adapt to the primary sequence of tRNAs as proposed recently (16).
Acknowledgments
We thank Cristina Tous for the Pseudanabaena 6903 RNase P RNA template plasmid, Kyle Tanner for comments and style corrections, and Jesús de la Cruz for critical reading. This work was supported by grants from the Human Frontier Science Organization (RG291/1997) and Dirección General de Enseñanza Superior (PB97–0732) and, in part, by Junta de Andalucía. A.P. was supported by a fellowship from the Spanish Ministry of Education and Culture.
ABBREVIATIONS
- pre-tRNA
precursor of tRNA
- pGlu
precursor of Synechocystis tRNAGlu
- pGln
precursor of Synechocystis tRNAGln
- pGlnCCA
precursor of Synechocystis tRNAGln containing the 3′ terminal CCA
References
- 1.Frank D N, Pace N R. Annu Rev Biochem. 1998;67:153–180. doi: 10.1146/annurev.biochem.67.1.153. [DOI] [PubMed] [Google Scholar]
- 2.Altman S, Kirsebom L A. In: The RNA World. Gesteland R, Cech T, Atkins J, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1999. pp. 351–380. [Google Scholar]
- 3.Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. Cell. 1983;35:849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann R K, Heinrich J, Schlegl J, Schuster H. Proc Natl Acad Sci USA. 1995;92:5822–5826. doi: 10.1073/pnas.92.13.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alifano P, Rivellini F, Piscitelli C, Arraiano C M, Bruni C B, Carlomagno M S. Genes Dev. 1994;8:3021–3031. doi: 10.1101/gad.8.24.3021. [DOI] [PubMed] [Google Scholar]
- 6.Bothwell A L, Garber R L, Altman S. J Biol Chem. 1976;251:7709–7716. [PubMed] [Google Scholar]
- 7.Guerrier-Takada C, van Belkum A, Pleij C W, Altman S. Cell. 1988;53:267–272. doi: 10.1016/0092-8674(88)90388-1. [DOI] [PubMed] [Google Scholar]
- 8.Kirsebom L A. Mol Microbiol. 1995;17:411–420. doi: 10.1111/j.1365-2958.1995.mmi_17030411.x. [DOI] [PubMed] [Google Scholar]
- 9.Kirsebom L A, Vioque A. Mol Biol Rep. 1995;22:99–109. doi: 10.1007/BF00988713. [DOI] [PubMed] [Google Scholar]
- 10.Oh B K, Pace N R. Nucleic Acids Res. 1994;22:4087–4094. doi: 10.1093/nar/22.20.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirsebom L A, Svärd S G. EMBO J. 1994;13:4870–4876. doi: 10.1002/j.1460-2075.1994.tb06814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Easterwood T R, Harvey S C. RNA. 1997;3:577–585. [PMC free article] [PubMed] [Google Scholar]
- 13.Oh B K, Frank D N, Pace N R. Biochemistry. 1998;37:7277–7283. doi: 10.1021/bi973100z. [DOI] [PubMed] [Google Scholar]
- 14.Vioque A. Nucleic Acids Res. 1997;25:3471–3477. doi: 10.1093/nar/25.17.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vioque A. Nucleic Acids Res. 1992;20:6331–6337. doi: 10.1093/nar/20.23.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brännvall M, Mattsson J G, Svärd S G, Kirsebom L A. J Mol Biol. 1998;283:771–783. doi: 10.1006/jmbi.1998.2135. [DOI] [PubMed] [Google Scholar]
- 17.Vioque A, Arnez J, Altman S. J Mol Biol. 1988;202:835–848. doi: 10.1016/0022-2836(88)90562-1. [DOI] [PubMed] [Google Scholar]
- 18.Pascual A, Vioque A. Eur J Biochem. 1996;241:17–24. doi: 10.1111/j.1432-1033.1996.0017t.x. [DOI] [PubMed] [Google Scholar]
- 19.Pascual A, Vioque A. Biochim Biophys Acta. 1994;1218:463–465. doi: 10.1016/0167-4781(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 20.O’Neill G P, Söll D. J Bacteriol. 1990;172:6363–6371. doi: 10.1128/jb.172.11.6363-6371.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talbot S J, Altman S. Biochemistry. 1994;33:1399–1405. doi: 10.1021/bi00172a016. [DOI] [PubMed] [Google Scholar]
- 22.Guerrier-Takada C, Lumelsky N, Altman S. Science. 1989;246:1578–1584. doi: 10.1126/science.2480641. [DOI] [PubMed] [Google Scholar]
- 23.Hardt W D, Schlegl J, Erdmann V A, Hartmann R K. Nucleic Acids Res. 1993;21:3521–3527. doi: 10.1093/nar/21.15.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sägesser R, Martinez E, Tsagris M, Tabler M. Nucleic Acids Res. 1997;25:3816–3822. doi: 10.1093/nar/25.19.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerrier-Takada C, McClain W H, Altman S. Cell. 1984;38:219–224. doi: 10.1016/0092-8674(84)90543-9. [DOI] [PubMed] [Google Scholar]
- 26.Hardt W D, Schlegl J, Erdmann V A, Hartmann R K. J Mol Biol. 1995;247:161–172. doi: 10.1006/jmbi.1994.0130. [DOI] [PubMed] [Google Scholar]
- 27.Tallsjö A, Kirsebom L A. Nucleic Acids Res. 1993;21:51–57. doi: 10.1093/nar/21.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tallsjö A, Kufel J, Kirsebom L A. RNA. 1996;2:299–307. [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J L, Nolan J M, Harris M E, Pace N R. EMBO J. 1998;17:1515–1525. doi: 10.1093/emboj/17.5.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massire C, Jaeger L, Westhof E. J Mol Biol. 1998;279:773–793. doi: 10.1006/jmbi.1998.1797. [DOI] [PubMed] [Google Scholar]
- 31.Fingerhut C, Schön A. FEBS Lett. 1998;428:161–164. doi: 10.1016/s0014-5793(98)00519-5. [DOI] [PubMed] [Google Scholar]
- 32.Hess W R, Fingerhut C, Schon A. FEBS Lett. 1998;431:138–142. doi: 10.1016/s0014-5793(98)00729-7. [DOI] [PubMed] [Google Scholar]
- 33.Kirsebom L A, Svärd S G. J Mol Biol. 1993;231:594–604. doi: 10.1006/jmbi.1993.1312. [DOI] [PubMed] [Google Scholar]
- 34.Kufel J, Kirsebom L A. J Mol Biol. 1996;263:685–698. doi: 10.1006/jmbi.1996.0608. [DOI] [PubMed] [Google Scholar]
- 35.Svärd S G, Kirsebom L A. J Mol Biol. 1992;227:1019–1031. doi: 10.1016/0022-2836(92)90518-o. [DOI] [PubMed] [Google Scholar]
- 36.Svärd S G, Kirsebom L A. Nucleic Acids Res. 1993;21:427–434. doi: 10.1093/nar/21.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kufel J, Kirsebom L A. J Mol Biol. 1994;244:511–521. doi: 10.1006/jmbi.1994.1749. [DOI] [PubMed] [Google Scholar]