Abstract
What is already known about this subject
Prior to the conduct of this study, information on the pharmacokinetics of lamivudine in subjects with renal impairment was limited to patients on haemodialysis. (Br J Clin Pharmacol 1998; 46: 21–7).
No pharmacokinetic data were available on subjects receiving lamivudine who were concurrently receiving peritoneal dialysis.
With increasing numbers of individuals opting for peritoneal dialysis, the need to establish if dose modification was necessary in this setting was important to ensure efficacious and safe drug exposures were being obtained.
What this study adds
This study demonstrated that similar to patients receiving haemodialysis, dose modifications based on reductions in renal function (i.e. decreased CLCR), also applied to subjects on peritoneal dialysis.
Aims
To establish whether peritoneal dialysis (PD) requires dosing modification from the CLCR-corrected lamivudine dose in end-stage renal failure subjects.
Methods
This was an open-label cohort study. A total of 12 subjects undergoing PD, six continuous ambulatory peritoneal dialysis (CAPD) and six automated peritoneal dialysis (APD), for at least 3 months received lamivudine 10 mg (5 mg ml −1 × 2 ml) daily for 8 consecutive days, followed by an intensive pharmacokinetic assessment. Urine and dialysate were collected from 0 to 24 h postdose on day 8 where possible. Pharmacokinetic parameters were calculated using noncompartmental techniques.
Results
The plasma pharmacokinetic results demonstrated that peritoneal dialysis clearance (CLD) of lamivudine was similar between APD and CAPD patients with median (range) of 0.19 l h−1 (0.14–0.25) and 0.1 l h−1 (0.09–0.25), respectively. CLD was approximately 1/15th to 1/30th of plasma clearance, demonstrating that peritoneal dialysis does not contribute significantly to overall lamivudine clearance in this patient population. The AUC(0,24 h) of lamivudine given 10 mg daily to APD and CAPD patients was 3430 ng ml−1 h and 3469 ng ml−1 h, respectively, similar to historical data obtained in patients with normal renal function administered at the normal dose of 100 mg daily (3781 ng ml−1 h). There were no clinically significant changes in any safety assessments that were attributable to lamivudine.
Conclusions
ESRD patients who receive CAPD or APD require no supplemental dosing. These patients should follow the standard dosing reduction for patients infected with HIV or HBV with renal dysfunction.
Keywords: 3TC, ESRD, lamivudine, peritoneal dialysis, pharmacokinetics, Zeffix™
Introduction
Lamivudine (–) 2′,3′-dideoxy, 3′-thiacytidine (also known at 3TC), in combination with other antiretroviral agents is indicated for the treatment of patients with human immunodeficiency virus (HIV) at a recommended dose of 150 mg twice daily and is also indicated for the treatment of chronic hepatitis B (CHB) at a recommended dose of 100 mg once daily. Lamivudine has been shown to be safe and well tolerated in these populations [1–5, 15].
Pharmacokinetics in asymptomatic HIV-infected subjects, hepatits B virus (HBV) infected subjects, and normal healthy volunteers are well established [6–8, 15]. Lamivudine is rapidly absorbed following an oral dose, reaching maximal serum concentrations between 1 and 1.5 h, has good (Gt;80%) absolute bioavailability and exhibits linear kinetics. Renal clearance is the major route of lamivudine elimination with a half-life of 5–7 h. Following oral administration ∼70% of the total dose is excreted unchanged in the urine and only 5–10% undergoes hepatic metabolism to form a trans-sulphoxide metabolite which is then also renally eliminated [9, 10, 15]. Renal dysfunction has been shown to have significant effects on the pharmacokinetics of lamivudine in HIV-infected individuals such that dose modification is recommended at or below 50 ml min−1 creatinine clearance (CLCR) [7] as assessed using the Cockcroft-Gault estimation of renal function [11] (Table 1). This dosing recommendation, based on modifying the daily dose but not the dosing interval, holds true in patients with either HIV infection or HBV infection where the daily dose recommended for efficacy is different, i.e. 150 mg twice daily or 300 mg once daily for HIV and 100 mg once daily for HBV [15].
Table 1.
Summary of demographic and baseline characteristics (mean (±SD) unless otherwise stated)
| CAPD | APD | |
|---|---|---|
| Age (years)† | 48 (21–60) | 38 (30–54) |
| Sex | ||
| Female | 0 | 3 |
| Male | 6 | 3 |
| Race | ||
| Asian | 0 | 2 |
| Caucasian | 6 | 4 |
| Weight (kg) | 74.23 ± 12.08 | 76.25 ± 15.78 |
| Smoking status | ||
| Nonsmokers | 3 | 6 |
| Smokers | 3 | 0 |
| Creatinine clearance (CLCR) (ml min−1) | 9.4 ± 2.3 | 8.8 ± 1.8 |
Median (range).
Continuous ambulatory peritoneal dialysis (CAPD), automated peritoneal dialysis (APD) and haemodialysis are established treatments for subjects with end stage renal disease (ESRD). To date, CAPD and APD are becoming increasingly popular methods for treating ESRD patients, with current estimates of more than 130 000 patients receiving treatment worldwide.
A previous study [9] has shown that haemodialysis does remove lamivudine from the systemic circulation; however, due to a high volume of distribution (>100 l) and the intermittent nature of this technique (3 times weekly), there is no clinically significant reduction in exposure (geometric mean reduction in area under the curve (AUC) was 27%) warranting further dose modification. However, no such study has been done in ESRD patients receiving CAPD or APD.
This study was designed to investigate the pharmacokinetics of lamividine after an 8 day dosing regimen in ESRD patients to substantiate previous findings, and to assess the effect of peritoneal dialysis including CAPD and APD on the clearance of lamivudine to establish whether peritoneal dialysis requires further dosing modification from the creatinine clearance adjusted lamivudine dose in ESRD patients receiving CAPD or APD. The current recommended adjusted daily dose for HBV (10 mg once daily) was chosen, but the results apply to either patient population as they indicate a dependence on renal function for lamivudine clearance.
Methods
The protocol was approved by Manchester Local Research Ethics Committees. Informed consent was taken from each subject.
Subject population
All subjects were between 18 and 65 years old without significant hepatic impairment. Subjects were included if they had severe renal dysfunction (also termed end-stage renal failure) as defined by the subject's record and all subjects were stable, having received CAPD or APD for chronic renal failure for at least 3 months. Subjects were excluded if they had a diagnosis of peritonitis, malignancy, or oedema, smoked more than 15 cigarettes day−1 and were unable to stop from 12 h before to 2 h after the dose on the pharmacokinetic study day (day 8), tested positive for hepatitis B virus surface antigen, hepatitis C virus or human immunodeficiency virus, had a history of drug or alcohol abuse or regularly drank more than 4 units of alcohol day−1 (1 unit = 1/2 pint of beer/1 glass of wine/1 measure of spirits) or weighed more than 100 kg.
Study design
This was an open label study and was performed at the Department of Renal Medicine, Manchester Royal Infirmary, Central Manchester and Manchester Children's University Hospital, Manchester, UK.
Subjects were administered a daily dose of lamivudine 10 mg (given as 2 ml of the 5 mg ml−1 lamivudine solution) for 8 days from day 1 to day 8. On day 8, CAPD subjects had the 12 h overnight dwell time dialysate removed and a new volume installed at approximately 09.00 h. Subjects were then given a dose of 10 mg lamivudine. Subsequent dialysis procedures were three successive 4 h dialysis dwells and then a 12 h dwell overnight. APD subjects attended the Clinic after their overnight cycler-assisted dwell, i.e. they had refreshed their dialysate volume following the 9 h overnight assisted dialysis. At approximately 09.00 h, subjects received their lamivudine dose of 10 mg. Subsequent procedures involved no further dialysis dwell change until the evening, i.e. until the next assisted dialysis overnight. These subjects stayed in the Unit for the next evening.
After the last morning dose of lamivudine on day 8, all subjects underwent serial pharmacokinetic blood sampling from predose to 48 h post-dose. Whole blood (5 ml) was drawn via an intravenous cannula and placed in a nonheparinized plain tube at the following times: predose, 30, 45 min, 1, 1.5, 2, 3, 4, 6, 8, 10, *12–16, *18–24, 30, 36, 48 h post dose (*to allow flexibility to take a mid-point of the dialysis period). Urine was collected between 0 and 24 h postdose when subjects were not anuric. Prior to the dosing, subjects were asked to void their bladder. Dialysate was collected into a plastic container for times postdose 0–4, 4–8, 8–12, 12–24 h (CAPD) and 0–12, 12–24 h (APD).
Blood samples were left at room temperature for at least 30 min prior to being centrifuged at 1500 g (4°C) for 10 min. Serum aliquots were stored at −20°C until analysis. Urine and dialysate samples were collected into a plastic container without preservative. Aliquots (10 ml) of urine and dialysate samples were stored at −20°C until analysis. All samples were sent to the Department of Bioanalysis, Glaxo SmithKline (Research Triangle Park, NC, USA) for analysis.
Assay method
Concentrations of lamivudine in human serum were determined by LC/MS/MS [12]. Linear calibration ranged from 2.5 to 2500 ng ml−1. The detection limit of this assay method was 2 ng ml−1, with lower and upper limits of quantification of 10 ng ml−1 and 1000 ng ml−1, respectively, using 0.5 ml serum. Concentrations of lamivudine in urine and dialysate were determined by LC/MS [13], and LC/MS/MS [14], respectively. Linear calibration of lamivudine ranged from 0.5 to 500 µg ml−1 in urine and 2.5–600 ng ml−1 in dialysate, respectively. A direct injection technique was used for both urine and dialysate samples which provides a lower limit of quantification of 0.5 µg ml−1. Quality control samples (QC), prepared at three different analyte concentrations and stored with study samples, were analyzed with each batch of samples against separately prepared calibration standards. For the analysis to be acceptable, no more than one-third of the QC results were to deviate from the nominal concentration by more than 15%, and at least 50% of the results from each QC concentration should be within 15% of the nominal concentration. The applicable analytical runs met all predefined run acceptance criteria.
Safety
Clinical adverse events (AEs) were monitored continuously throughout the study and at follow-up. Laboratory safety tests (haematology and clinical chemistry), 12 lead ECG and blood pressure/pulse rate measurements were performed at entry to the study and at the post-study visit.
Pharmacokinetic analysis
The maximum drug concentration in serum (Cmax) and the time to Cmax (tmax) were obtained directly from the concentration-time data. The terminal rate constant (λz) was determined by linear least squares regression using logarithmically transformed points in the terminal phase. The number of points to be included in the terminal phase was determined by inspection of a log-linear plot of lamivudine concentration against time. The pharmacokinetic parameters were calculated using standard noncompartmental techniques using WinNonlin version 1.0 (SCI software; Cary, North Carolina).
Dialysis clearance was calculated as follows:
where CLD = dialysis clearance, t = dwell time. (t1 = 0 (predose), t2 = 24 h postdose), A(d) = amount of drug in dialysate and AUC = area under the curve.
Statistical analysis
The population for pharmacokinetic analyses included all subjects who completed the study. All subjects who received study drugs were included in the safety analyses. The sample size of 12 subjects receiving peritoneal dialysis (six CAPD and six APD) was not based on a formal power assessment. Standard summary statistics for demographic data and pharmacokinetic parameter results are presented as mean, SD, and median and range for each group. Additionally, a post hoc analysis was performed across studies to a reference comparator group of volunteers with normal renal function [1]. Loge transformations of AUC (0,t),Cmin, clearance and half-life (t1/2) of lamivuidine were analyzed by analysis of variance fitting renal function in the model. Ratios and associated 90% confidence intervals for the differences between CAPD and a reference group and, between APD and the reference group were constructed. These estimates were then back-transformed to express point and interval estimates on a ratio scale.
Results
A total of 12 subjects were enrolled in this study, six of each in the APD and CAPD dialysis groups. All 12 subjects completed the study. No major protocol violations were reported during this study with the exception of one subject in the APD group who had received APD therapy for <1 month prior to the screening visit and was admitted to the study because the patient was considered stabilized on her APD therapy (per protocol, all subjects were stable, having received CAPD or APD for chronic renal failure for at least 3 months).
Demographic and baseline characteristics, including creatinine clearance are shown in Table 1. Creatinine clearance was very similar between the two groups (9.4 ± 2.3 ml min−1 for CAPD patients and 8.8 ± 1.8 ml min−1 for APD patients, respectively). With regards to renal history for each subject, in the CAPD group, duration of current chronic dialysis therapy ranged from approximately 1 year and 5 months to 4 years and 11 months. In the APD group, with the exception of one subject (protocol violation), duration of current chronic dialysis therapy ranged from approximately 7 months to 8 years and 9 months. Overall, the majority of subjects (n = 7) had undergone at least one renal transplant, including three subjects in the CAPD group and four subjects in the APD group. One subject in each group had undergone two renal transplants.
Pharmacokinetic results
Lamivudine pharmacokinetic parameters are summarized in Table 2. The peritoneal dialysis clearance (CLD) and AUC(0,24 h) of lamivudine were similar between APD and CAPD patients. Peritoneal dialysis clearance was approximately one fifteenth to one thirtieth of oral clearance for both APD and CAPD groups. Post hoc analysis of the pharmacokinetic parameters was conducted and ratio estimates and associated 90% confidence intervals for the differences between CAPD and subjects with normal renal function and between APD and subjects with normal renal function are presented in Table 3. Daily exposure, (AUC(0,24 h)), was similar between subjects receiving APD and administered the recommended 10 mg daily dose of lamivudine, and subjects with normal renal function administered the standard HBV therapeutic dose of lamivudine at 100 mg daily (ratio (90% CI) 0.81 (0.63, 1.05)). Similar AUC(0,24 h) findings were also demonstrated between subjects receiving CAPD and subjects with normal renal function (ratio (90% CI) 0.77 (0.59, 0.99)). The geometric least square mean, minimum drug concentration (Cmin) values on day 8 were, 91 ng ml−1 and 87 ng ml−1 for subjects receiving either APD or CAPD, respectively. Comparison testing between APD and normal renal function and CAPD and individuals with normal renal function demonstrated a three fold increase in Cmin in APD and CAPD dialyzed individuals, respectively, over their normal renal function comparators (APD ratio (90% CI) 3.01 (2.17, 4.16); CAPD ratio 2.89 (2.09, 4.0). The half-life (h) of lamivudine in ESRD subjects receiving CAPD or APD increased by greater than six-fold (APD ratio (90% CI) 6.12 (4.84, 7.75); CAPD ratio (90%CI) 7.35 (5.81, 9.31)) as compared with subjects with normal renal function. Median lamivudine pharmacokinetic profiles of APD and CAPD subjects are shown in Figure 1. Figure 2 compares lamivudine pharmacokinetic profiles of ESRD patients receiving ADP and CAPD with those of HBV infected subjects with normal renal function.
Table 2.
Lamivudine pharmacokinetic parameters in ESRD patients receiving peritoneal dialysis, CAPD or APD, compared with healthy subjects. Data indicate median (range); n = 6 for each group (CAPD and APD)
| Pharmacokinetic parameter | CAPD (Lamivudine 10 mg) | APD (Lamivudine 10 mg) | Normal renal function† (Lamivudine 100 mg) |
|---|---|---|---|
| Cmax (ng ml−1) | 232 (185–317) | 271 (247–309) | 1002 (860–1167) |
| AUC(0,24 h) (ng ml−1 h) | 3430 (2500–4904) | 3469 (3237–4656) | 3781 (3505–4079) |
| t1/2 (h) | 46.3 (35.7–56.3) | 37.6 (30.1–46.2) | 8.3 (6.4–10.6) |
| CL/F (l h−1) | 2.9 (2.0–4.0) | 2.9 (2.2–3.1) | 29.6 (27.6–31.8) |
| CLD (l h−1) | 0.19 (0.14–0.25) | 0.1 (0.09–0.25) | – |
| Cmin (ng ml−1) | 93 (62–165) | 95 (69–117) | 33 (18–47) |
| Cmin/IC50‡ | ∼20 | ∼20 | ∼5–10 |
Data from [15]
IC50 for HBV: 4–7 ng ml−1.
Table 3.
Post hoc interstudy analysis of lamivudine pharmacokinetic differences between CAPD or APD and a reference comparator group of volunteers with normal renal function [1]
| Geometric least square mean | Ratio (90% CI) | ||||
|---|---|---|---|---|---|
| Pharmacokinetic parameter | CAPD | APD | Normal renal function | CAPD | APD |
| Cmax (ng ml−1) | 236.8 | 272.8 | 1144 | 0.21 (0.15, 0.29) | 0.24 (0.17, 0.34) |
| AUC(0,24 h) (ng ml−1 h) | 3383 | 3596 | 4415 | 0.77 (0.59, 0.99) | 0.24 (0.17, 0.34) |
| t1/2 (h) | 45.3 | 37.7 | 6.2 | 7.4 (5.8, 9.3) | 6.1 (4.8, 7.8) |
| CL/F (l h−1) | 3.0 | 3.0 | 23.0 | 0.13 (0.1, 0.17) | 0.12 (0.1, 0.16) |
| Cmin (ng ml−1) | 87.8 | 91.4 | 30.4 | 2.9 (2.09, 4) | 3.0 (2.2, 4.2) |
Figure 1.
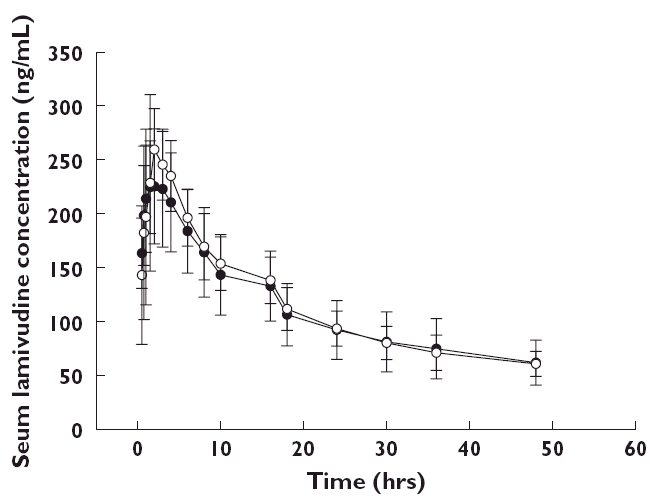
Mean (±SD) serum lamivudine concentration–time profiles for ESRD patients receiving peritoneal dialysis, CAPD, (•, n = 6); or APD, (○, n = 6)
Figure 2.
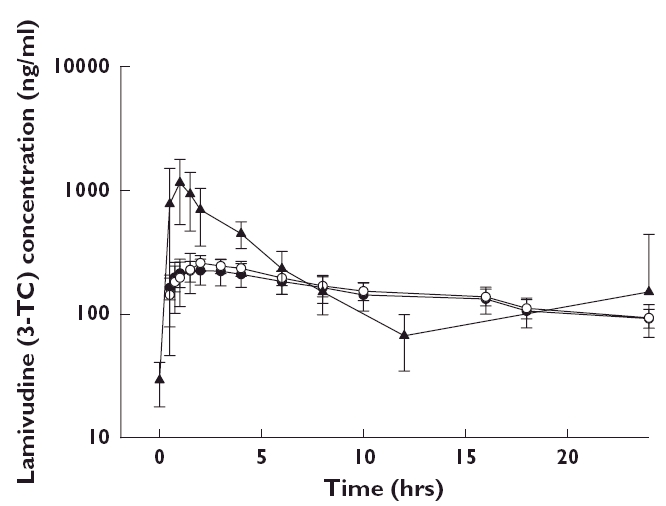
Mean (±SD) serum lamivudine (10 mg daily) concentration–time profiles for ESRD patients receiving peritoneal dialysis, CAPD (•, 3TC 10 mg, n = 6); or APD (○, 3TC 10 mg, n = 6); as compared with HBV infected subjects with normal renal function (▴, 3TC 100 mg) receiving lamivudine 100 mg daily
Since approximately half of the subjects did not have measurable urine concentrations, calculation of urine pharmacokinetic parameters (Ae and CLr) was not performed.
Safety profile
Lamivudine 10 mg daily was well tolerated in ESRD patients receiving CAPD or APD. The most frequent adverse events were eye redness and diarrhoea (n = 2 for each group). No other AE was reported in more than one subject, and no AE was assessed by the investigator as related to the study drug. One nonfatal SAE (gastritis) was reported, which the investigator assessed as not related to the study drug. The gastritis had an onset 17 days after the first dose of study drug (and 10 days after the final dose). None of the laboratory (haematology and clinical chemistry) abnormalities observed during this study were assessed by the investigator as related to study drug.
There were no changes in any safety assessment that were attributable to lamivudine which were of clinical concern.
Discussion
Drugs that are renally eliminated require dose adjustment in patients with renal dysfunction. One complicating factor in making dosing adjustments is the potential for dialysis, either peritoneal (APD or CAPD) or haemodialysis, to alter significantly the elimination of the drug, which would require an additional dose following dialysis. A previous study of lamivudine demonstrated the lack of a significant removal effect of lamivudine following intermittent haemodialysis (4 h, two to three times weekly) [9]. Continuous haemodialysis, however, may necessitate an increase in dose to maintain concentrations above the IC50 and total drug exposure that are needed for antiviral effects as suggested by the author.
The current study examined whether peritoneal dialysis (APD or CAPD) significantly removed lamivudine. We found that lamivudine pharmacokinetics were similar in ESRD patients receiving intermittent dialysis (CAPD or APD). The contribution of CAPD or APD to the clearance and elimination of lamivudine was minimal, as demonstrated by the low clearance values for CAPD and APD compared with plasma clearance. Dose adjustment according to renal function (10 mg daily) normalized total exposure of lamivudine (AUC(0,24 h)) to a standard dose in patients with normal renal function (100 mg daily). Half-life increased by greater than six-fold in the dialyzed groups vs. the normal renal function comparator group which is consistent with previous studies where significant renal impairment resulted in a prolonged lamivudine half-life [7, 9]. Additionally, Cmin values were approximately three fold greater than those reported in subjects with normal renal function [15]. Overall, lamivudine was well tolerated at a dose of 10 mg daily in this subject population.
In conclusion, based on the results from this study, ESRD patients who require CAPD or APD do not need supplemental dosing. These patients should follow the standard dosing reduction for patients infected with HIV or HBV with renal dysfunction.
Acknowledgments
This research was sponsored by GlaxoSmithKline Inc.
The authors wish to thank the subjects who took part in this study, the research team at Manchester Royal Infirmary, Manchester, and employees of GlaxoSmithKline for their contribution.
References
- 1.GlaxoSmithKline Clinical Trial Registry. Registered office: 980 Great West Road, Brentford, Middlesex, TW8 9GS, United Kingdom (updated July 4th, 2005). Available from: http://ctr.gsk.co.uk/Summarylamivudine/II_NUCB2002.pdf.
- 2.Lessells R, Leen C. Management of hepatitis B in patients co-infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis. 2004;23:366–74. doi: 10.1007/s10096-004-1127-3. [DOI] [PubMed] [Google Scholar]
- 3.Clercq ED. Antiviral drugs in current clinical use. J Clin Virol. 2004;30:115–33. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Doong S-L, Tsai C-H, Shinazi RF, Liotta DC, Cheng YC. Inhibition of the replication of hepatitis B virus in vitro by 2′3′-dideoxy-3′thiacytidine and related analogues. Proc Nat Acad Sci. 1991;88:8495–9. doi: 10.1073/pnas.88.19.8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pluda JM, Cooley TP, Montaner JS, Shay LE, Reinhalter NE, Warthan SN, Ruedy J, Hirst HM, Vicary CA, Quinn JB, Yuen GJ, Wainberg MA, Rubin M, Yarchoan R. A phase I/II study of 2′-deoxy-3′-thiacytidine (lamivudine) in subjects with advanced human immunodeficiency virus infection. J Infect Dis. 1995;171:1438–47. doi: 10.1093/infdis/171.6.1438. [DOI] [PubMed] [Google Scholar]
- 6.Van Leeuwen R, Lange JMA, Hussey EK, Donn KH, Hall ST, Harker AJ, Jonker P, Danner SA. The safety and pharmacokinetics of a reverse transcriptase inhibitor, 3-TC, in subjects with HIV infection: a phase I study. AIDS. 1992;6:1415–7. doi: 10.1097/00002030-199212000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Heald AE, Hsyu PH, Yuen GJ, Robinson P, Mydlow P, Bartlett JA. Pharmacokinetics of lamivudine in human immunodeficiency virus-infected subjects with renal dysfunction. Antimicrob Agents Chemother. 1996;40:31–41. doi: 10.1128/aac.40.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuen GJ, Morris DM, Mydlow PK, Haidar S, Hall ST, Hussey EK. Pharmacokinetics, absolute bioavailability, and absorption characteristics of lamivudine. J Clin Pharmacol. 1995;35:1174–80. doi: 10.1002/j.1552-4604.1995.tb04043.x. [DOI] [PubMed] [Google Scholar]
- 9.Johnson MA, Verpooten GA, Daniel MJ, Plumb R, Moss J, Van Caesbroeck D, De Broe ME. Single dose pharmacokinetics of lamivudine in subjects with impaired renal function and the effect of haemodialysis. Br J Clin Pharmacol. 1998;46:21–7. doi: 10.1046/j.1365-2125.1998.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry CM, Faulds D. Lamivudine, a review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in the management of HIV infection. Drugs. 1997;53:657–80. doi: 10.2165/00003495-199753040-00008. [DOI] [PubMed] [Google Scholar]
- 11.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 12.Harker AJ, Evans GL, Hawley AE, Morris DM. High-performance liquid chromatographic assay for 2′-deoxy-3′-thiacytidine in human serum. J Chromatogr B Biomed Appl. 1994;657:227–32. doi: 10.1016/0378-4347(94)80092-8. [DOI] [PubMed] [Google Scholar]
- 13.Plumb RS, Gray RD, Harker AJ, Taylor S. High-performance chromatographic assay for the sulphoxide metabolite of 2′-deoxy-3′-thiacytidine in human urine. Chromatogr B Biomed Appl. 1996;687:457–61. doi: 10.1016/s0378-4347(96)00247-2. [DOI] [PubMed] [Google Scholar]
- 14.Hsyu PH, Lloyd TL. Automated high-performance chromatographic analysis of (−) -2′-deoxy-3′-thiacytidine in biological fluids using the automated sequential trace enrichment of dialysate systems. Chromatogr B Biomed Appl. 1994;655:253–9. doi: 10.1016/0378-4347(94)00114-6. [DOI] [PubMed] [Google Scholar]
- 15.Johnson MA, Moore KHP, Yuen GJ, Bye A, Pakes GE. Clinical pharmacokinetics of lamivudine. Clin Pharmacokinet. 1999;36:41–66. doi: 10.2165/00003088-199936010-00004. [DOI] [PubMed] [Google Scholar]


