Abstract
Aims
To compare the pharmacokinetics of mycophenolic acid (MPA) and its metabolite (MPAG) when mycophenolate mofetil (MMF) is administered in combination with sirolimus or ciclosporin (CsA) in renal allograft recipients. Safety and efficacy (biopsy-proven acute rejection (BPAR)) were also assessed.
Methods
Patients (n = 45) were randomized 2 : 1 to receive treatment with sirolimus (n = 30; dosed to maintain trough concentrations of 10–25 ng ml−1 until week 8, and then 8–15 ng ml−1 thereafter) or CsA (n = 15; administered as per centre practice) both in combination with daclizumab, oral MMF and corticosteroids. Pharmacokinetic assessments were performed at day 7, week 4, and months 3 and 6 post-transplant. The primary endpoint was the AUC(0,12 h) for MPA and MPAG. The pharmacokinetics of sirolimus were also assessed.
Results
MPA exposure was 39–50% lower (month 6 mean AUC(0,12 h) (95%CI): 40.4 (33.8, 47.0) vs. 68.5 (54.9, 82.0) µg ml−1 h) and MPAG exposure was 25–52% higher (722 (607, 838) vs. 485 (402, 569) µg ml−1 h at month 6) in the presence of CsA compared with sirolimus across visits. BPAR was 40.0% with sirolimus and 13.3% with CsA. The incidence of hypertension, tremors and hirsutism was higher with CsA than with sirolimus, while the incidence of diarrhoea, hyperlipidaemia and impaired wound closure was higher with sirolimus. No deaths, malignancies or graft losses were reported.
Conclusions
Co-administration of sirolimus with MMF led to greater MPA exposure, but lower MPAG exposure, than co-administration with CsA. As rejection rates were higher in the absence of CsA, further study of calcineurin inhibitor-free regimens is required before general recommendations can be made.
Keywords: ciclosporin, mycophenolate mofetil, pharmacokinetics, renal transplant, sirolimus
Introduction
The advent of potent new immunosuppressive agents over the last decade has led to a dramatic reduction in renal allograft acute rejection rates from 50% to 15–20% [1]. During this period, drug regimens including mycophenolate mofetil (MMF) and calcineurin inhibitors (CNIs) have become standard in immunosuppressive therapy [2]. While CNIs are effective at reducing acute rejection rates, their long-term use is associated with chronic nephrotoxicity that contributes to chronic allograft nephropathy and graft loss [3, 4]. Furthermore, CNIs are associated with other adverse events such as hypertension and hyperlipidaemia that may ultimately affect both graft and patient survival [5–7]. Thus, there has been considerable interest in developing immunosuppressive regimens that eliminate or reduce exposure to CNIs, while maintaining adequate immunosuppression and low acute rejection rates.
One CNI-free regimen of interest is the combination of MMF with sirolimus. While sirolimus binds to the same intracellular binding protein as tacrolimus, it does not inhibit calcineurin [8], and thus, may lack the nephrotoxicity of CNIs [9]. Addition of sirolimus to a ciclosporin-(CSA-)corticosteroid regimen has been shown to reduce significantly the incidence of biopsy-proven acute rejection (BPAR) when compared with azathioprine [10] or placebo [11] in renal allograft recipients. However, initial studies demonstrated that this combination of sirolimus and full-dose CsA exhibited increased nephrotoxicity. This led to further investigation, and to the finding that addition of sirolimus to a CsA-steroid regimen permitted sparing of CsA with improved renal function, while reducing the incidence of acute rejection [12]. Finally, studies have investigated the efficacy of sirolimus as a replacement for CsA in immunosuppressive regimens [9, 13]. The most promising results were reported by Flechner et al.[13] with a regimen consisting of induction with an IL−2 receptor blocker, sirolimus, MMF and steroids.
Preliminary pharmacokinetic data have shown that trough mycophenolic acid (MPA) concentrations were higher in the sirolimus-treated patients compared with CsA-treated patients, even though the sirolimus-treated patients received lower doses of MMF [10, 14]. The present study was primarily designed to provide further analysis of the difference in the pharmacokinetics of MPA and its major glucuronide metabolite (MPAG) in renal transplant recipients when MMF is administered with either sirolimus or CsA.
Methods
Study design and patient population
This was a prospective, randomized, open-label, multicentre study to determine the pharmacokinetics of MPA and MPAG following oral administration of MMF (CellCept®) in combination with daclizumab (Zenapax®), corticosteroids and either sirolimus (Rapamune®) or CsA (Neoral® or bioequivalent formulations). The safety and efficacy of these regimens were also assessed.
Male or female adult (18–75 years) recipients of primary renal allografts who were able to receive oral medication were eligible for the study. Recipients of HLA-identical living-related kidney transplants were excluded from the study. Additional exclusion criteria included a panel reactive antibody (PRA) value >20% within 6 months prior to enrolment, known positivity for HIV-1 or human T-cell lymphotropic virus type-1, presence of hepatitis B surface antigen, a white blood cell count <2.5 × 109 l−1 (IU), a platelet count <100 × 109 l−1 (IU), or haemoglobin <6 g dl−1 at the time of entry into the study, or a history of treated or untreated hyperlipidaemia within the previous year. Patients were also excluded if they had prior malignancies, had previously been treated with daclizumab, were pregnant or nursing, or if they had severe diarrhoea, peptic ulcer disease or other gastrointestinal disorders which might have interfered with the ability to absorb oral medication. Diabetic patients with previously diagnosed diabetic gastroenteropathy were also excluded, as were patients who required concomitant treatment with investigational drugs or prohibited immunosuppressive medications. African-American patients were excluded from participation in the study, as they are generally recognized as a high-risk sub-population for efficacy failure in standard immunosuppressive regimens [15–17]. This exclusion was approved by the ethics review boards based on a safety assessment.
The investigation was conducted in full conformance with the principles of the Declaration of Helsinki and its subsequent amendments. Prior to initiation of the study, the protocol and all subsequent amendments were reviewed and approved by an Institutional Review Board. Written informed consent was obtained from each patient participating in the study after adequate explanation of the aims, methods, anticipated benefits and potential hazards of the study.
Drug regimens
Enrolled patients were randomized prior to transplantation in a 2 : 1 ratio to receive treatment with sirolimus or CsA in combination with daclizumab, oral MMF and corticosteroids. Daclizumab 1 mg kg−1 intravenously was given within 24 h before transplant, followed by four additional doses of 1 mg kg−1 every 2 weeks. MMF, 1 g twice daily orally was started within 24 h pre- or post-transplant. Intraoperative and maintenance corticosteroids were administered to all patients for the duration of the study according to centre practice. Sirolimus was given once daily as an oral solution at 15 mg day−1 on days 1–3 following transplantation and was reduced to 10 mg day−1 (as tablets) beginning on day 4 post-transplant. Sirolimus dosing was adjusted to maintain sirolimus trough concentrations of 10–25 ng ml−1 until 2 months post-transplant, and 8–15 ng ml−1 thereafter. The doses were selected to ensure that adequate sirolimus concentrations were achieved in the immediate post-transplant and early post-transplant period, when patients are most vulnerable to acute rejection. The doses of MMF or sirolimus could be lowered if the investigator considered it necessary to reduce drug-related toxicity, provided that the doses of other agents were high enough to give adequate immunosuppression. Neoral® or bioequivalent CsA was administered according to centre practice.
Prophylactic antibiotics and antiviral medications could be administered as per centre practice. Iron-containing medications or supplements were not to be taken within 3 h after taking MMF, and preferably, MMF was to be taken with food. Patients receiving sirolimus could not take grapefruit juice or ketoconazole.
Clinical assessments
Clinical assessments were performed at baseline and at scheduled visits post-transplantation (days 4 and 7, weeks 2, 4, 6 and 8, and months 3–6).
Laboratory assessments
Laboratory assessments were performed at baseline and at scheduled visits post-transplantation. These included haematology (complete blood count), blood chemistry and fasting lipid profile.
Renal function was assessed by serum creatinine, calculated creatinine clearance (CLCr, calculated using the Cockcroft-Gault formula) [18] and glomerular filtration rate (GFR), measured at a central laboratory (Mayo Clinic) using cold iothalamate meglumine [19].
In order to determine the relationship between serum creatinine and acute rejection, the lowest post-transplant serum creatinine prior to first rejection and the serum creatinine obtained at least 2 weeks after the end of treatment for rejection were recorded.
Sirolimus trough samples were assessed for dose adjustment as per protocol requirements. If sirolimus trough concentrations were found to be outside of the target range, the investigator was to adjust sirolimus dosing as required and to obtain follow-up trough concentrations so as to return trough concentrations to within the target range within 14 days.
Pharmacokinetic assessments
Patients had to meet the following requirements to be eligible for participation in the pharmacokinetic assessments: for the day 4 (trough) and day 7 pharmacokinetic sampling visits, eligible patients included nonsmokers, those who had received a stable uninterrupted dose of MMF twice daily for ≥3 days, had not undergone treatment for rejection since transplantation, and had not received tacrolimus. For the week 4, month 3 and month 6 visits, eligible patients included nonsmokers who had received a stable dose of MMF twice daily for at least 7 days, had not experienced an acute rejection episode/received treatment for acute rejection in the preceding 2 weeks, had not undergone dialysis in the preceding 2 weeks and were maintained within the assigned study group. Pharmacokinetic assessment of both MPA and MPAG were performed on days 4 and 7, week 4 and months 3 and 6 post-transplant, with additional pharmacokinetic assessments for sirolimus from week 4. Blood sampling time points were predose, and at 20, 40 and 75 min, 2, 3, 4, 6, 8 and 12 h after the morning dose.
For analysis of MPA and MPAG, approximately 3 ml of blood was collected for each pharmacokinetic sample in lithium heparinized tubes. Plasma concentrations of MPA and MPAG were determined by Analytico Medinet (the Netherlands) via solid phase extraction and reverse phase high-performance liquid chromatography (HPLC) with ultra-violet detection (F. Hoffman-La Roche Ltd, data on file). The lower limit of quantification for MPA was 0.1 µg ml−1 (accuracy 95.9–102%; precision <12.8%), while the lower limit for MPAG was 4.0 µg ml−1 (accuracy 100–102%; precision <11.7%).
For sirolimus analysis, approximately 3 ml of blood was collected in tubes with EDTA preservative. Sirolimus was extracted from whole blood samples using protein precipitation followed by solid phase extraction and concentrations measured using specific HPLC procedures (Covance, Indianapolis, Indiana, USA) (F. Hoffman-La Roche Ltd, data on file). The lower limit of quantification was 0.25 ng ml−1 (accuracy 89.0–99.5%; precision ≤7.4%).
Treatment of acute rejection and delayed graft function
The diagnosis of allograft rejection was based upon clinical signs and symptoms, serum creatinine, and confirmed by core renal biopsy (Banff criteria 1993–95). Following biopsy confirmation of acute rejection, high-dose corticosteroids (regimen per centre practice) could be administered as the first-line treatment for the rejection episode. Anti-lymphocyte preparations could be administered for steroid-resistant rejection, at the discretion of the investigator. Graft loss was defined as either the institution of chronic dialysis for at least 6 consecutive weeks, transplant nephrectomy, or re-transplantation. Delayed graft function (DGF) was assessed as per study definition (i.e. persistent oliguria, or a decrease in serum creatinine at 24 h post-transplant of <0.5 mg dl−1, or institution of dialysis before adequate graft function was established) or investigator criteria. Patients who required induction therapy with standard antilymphocyte antibody for DGF were to be withdrawn from the study.
Safety assessments
Safety was assessed by monitoring adverse events reported over the course of the study. Pre-existing conditions that worsened during the study were to be reported as adverse events. Opportunistic infections, including cytomegalovirus (CMV), Candida, Aspergillus, Pneumocystis, Cryptococcus, Listeria, herpes zoster and herpes simplex were also assessed.
Statistical analysis
The primary endpoint for this study was the AUC(0,12 h) for MPA and MPAG. The AUC(4,12 h) for MPA and AUC(0,24 h) of sirolimus were also measured. The AUCs were computed using the linear trapezoidal rule. MPAG concentrations were converted to MPA equivalent units by multiplying by the ratio of the molecular weights of MPA (320.3) and MPAG (540.4): MPA equivalent units = (320.3/540.4) × MPAG plasma concentrations. The pharmacokinetic properties of CsA in relation to MMF are well known and were not assessed in this study.
Secondary endpoints included other pharmacokinetic parameters (maximum concentration (Cmax), time to maximum concentration (tmax) and minimum concentration (Cmin), BPAR, patient and graft survival at 6 months post-transplant, renal function at 6 months post-transplant as measured by GFR and calculated CLCr, and treatment failure at 6 months post-transplant (defined as the occurrence of graft loss, death or the use of additional immunosuppressive medication not specified in the assigned treatment group for >7 days for reasons other than rejection).
Pharmacokinetic parameters were derived by ‘noncompartmental’ analysis using WinNonlin® Professional version 4.0 (Pharsight Corporation, Mountain View California, USA). Actual times were used to calculate AUC(0,12 h), AUC(4,12 h) and AUC(0,24 h) for MPA/MPAG, MPA and sirolimus, respectively. All pharmacokinetic parameters were listed and summarized descriptively by time period and treatment group. The AUC(0,12 h) (MPA and MPAG), AUC(4,12 h) (MPA), AUC(0,24 h) (sirolimus), Cmax and Cmin were dose normalized to 1000 mg or 10 mg for MMF and sirolimus, respectively, in the pharmacokinetic analysis. In addition, exploratory analyses (one-way anova) were performed for the dose-adjusted and log-transformed parameter AUC(0,12 h) of MPA when administered with CsA compared with sirolimus, and to calculate 90% confidence intervals for these estimates. The primary patient population for the efficacy analyses was the intent-to-treat (ITT) population, i.e. all patients who were randomized and had received at least one dose of the trial medication, whether on study or prematurely withdrawn, and had at least one follow-up data point. This group also constituted the safety analysis population. The pharmacokinetic analyses were performed using the pharmacokinetic population; all patients in the ITT/safety analysis population who met the pharmacokinetic eligibility criteria were included. However, patients with any missing data that would have influenced the pharmacokinetic analysis were excluded from the analysis.
Results
Patient characteristics and demographics
A total of 45 patients were enrolled into the study, 30 in the sirolimus group and 15 in the CsA group. The demographics and baseline characteristics of the enrolled patients are shown in Table 1. Both groups were balanced with respect to demographics and baseline characteristics except for gender, with the sirolimus group having a higher proportion of females. No patients were excluded from the intent-to-treat or safety analysis populations.
Table 1.
Demographics and baseline characteristics (intent-to-treat population)
| Sirolimus (n = 30) | Ciclosporin (n = 15) | |
|---|---|---|
| Recipient variables | ||
| Sex | ||
| Female n (%) | 14 (47) | 3 (20) |
| Male n (%) | 16 (53) | 12 (80) |
| Race | ||
| Caucasian n (%) | 25 (83) | 13 (87) |
| Hispanic n (%) | 3 (10) | 1 (7) |
| Asian n (%) | 1 (3) | 1 (7) |
| Other n (%) | 1 (3) | – |
| Age (years) median (range) | 49.0 (21–70) | 47.0 (28–64) |
| Weight (kg) median (range) | 78.2 (58.0–150.6) | 85.0 (41.8–125.0) |
| Primary reason for transplant | ||
| Cystic/polycystic kidney disease n (%) | 3 (10) | 6 (40) |
| Diabetes mellitus n (%) | 4 (13) | 2 (13) |
| Aetiology uncertain n (%) | 1 (3) | 2 (13) |
| Glomerulonephritis n (%) | 5 (17) | – |
| Hypertension n (%) | 6 (20) | 2 (13) |
| Pyelonephritis/interstitial nephritis n (%) | 2 (7) | – |
| Other n (%) | 9 (30) | 3 (20) |
| Donor variables | ||
| Age median (range) | 39.0 (8–59) | 42.0 (19–56) |
| Type of donor: | ||
| Deceased n (%) | 16 (53) | 7 (47) |
| Living related n (%) | 12 (40) | 7 (47) |
| Living unrelated n (%) | 2 (7) | 1 (7) |
| Other variables | ||
| Cold ischaemic time (h) | ||
| 0–30 n (%) | 28 (93) | 15 (100) |
| >30 n (%) | 2 (7) | – |
By 6 months post-transplant, a total of 12 patients had withdrawn from the study; with incidence of withdrawal being higher in the sirolimus group (37% (n = 11) vs. 7% (n = 1)). One patient in the CsA group refused treatment with the assigned regimen and withdrew on day 1. Of the 11 patients who withdrew from the sirolimus group, one withdrew consent on day 16 and five withdrew due to insufficient therapeutic response (BPAR) (one each on days 9, 34, 71, 100 and 153). Five patients withdrew due to adverse events related to MMF or sirolimus (two on day 56 and one each on days 11, 66 and 127), as outlined in Table 2. Follow-up data regarding allograft biopsies, treatment of rejection, malignancies, graft loss and death were available for 43 of the patients. Two patients (one in each treatment group) were not followed to 6 months post-transplantation, both for refusal of treatment (one patient relocated out of state, and the other did not want to participate in the multiple assessments).
Table 2.
Adverse events leading to withdrawal from treatment in the sirolimus group
| Patient | Adverse events | Intensity | Day of onset | Relationship to treatment |
|---|---|---|---|---|
| 1 | Wound dehiscence | Severe | 53 | Possibly related to sirolimus |
| 2 | Leukopenia | Moderate | 51 | Probably related to MMF |
| 3 | Human polyomavirus infection | Moderate | 121 | Possibly related to sirolimus and remotely related to MMF |
| 4 | Diarrhoea | Moderate | 35 | Probably related to MMF |
| 5 | Interstitial nephritis | Severe | 28 | Probably related to sirolimus |
| 5 | Renal tubular necrosis | Severe | 31 | Probably related to sirolimus |
Study medication
Thirty-nine patients (87%) received all five doses of daclizumab. Corticosteroid administration was similar at all time points and was tapered to an average daily dose of 8 mg by month 6. The two groups received similar doses of MMF with mean daily doses being 1.6 g and 1.8 g in the sirolimus and CsA groups, respectively, at both 3 and 6 months. The average daily dose of sirolimus at baseline (15.0 mg) and day 4 (10.2 mg) was in accordance with the protocol-specified doses. The average daily dose was reduced to 4.6 mg by month 3 and 3.3 mg by month 6. Average sirolimus trough concentrations remained within the protocol defined ranges throughout the study (see Methods). The average daily dose of CsA at baseline was 487.5 mg, rising to 571.4 mg at week 2, and then decreasing gradually throughout the remainder of the study to a value of 336.6 mg and 299.9 mg at months 3 and 6, respectively. From day 4 onwards, average CsA trough concentrations remained relatively stable ranging from 224 to 347 ng ml−1 across visits. Two patients received a CsA formulation (GenGraf®) that was bioequivalent to Neoral®.
Pharmacokinetic analysis
The pharmacokinetic population consisted of 42 out of the 45 patients enrolled in the study (28 [93%] sirolimus, 14 [93%] CsA). Not all patients completed each visit.
MPA
The pharmacokinetics of MPA are summarized in Table 3. MPA concentrations, on average, increased over time post-transplant reaching maximal concentrations (dose normalized Cmax) ranging from 12.4–20.6 µg ml−1 in the sirolimus group and 7.08–16.3 µg ml−1 in the CsA group. Median time to peak concentrations was between 0.67–1.21 h and 1.25–1.31 h, respectively. Mean dose normalized Cmax and Cmin were lowest at day 7, with a trend towards increasing MPA concentrations throughout the study period. Overall, the Cmax, Cmin and AUC(0,12 h) for MPA were decreased and tmax increased in the presence of CsA compared with sirolimus.
Table 3.
Pharmacokinetic parameters of MPA (dose-normalized values)
| MPA (mean (95% CI)) | ||
|---|---|---|
| Sirolimus | Ciclosporin | |
| Day 7 | ||
| tmax (h)a | 0.68 (0.33–3.83) | 1.25 (0.33–2.03) |
| n = 23 | n = 12 | |
| Cmax (µg ml−1) | 12.4 (10.7, 14.0) | 7.08 (5.41, 8.75) |
| n = 23 | n = 12 | |
| Cmin (µg ml−1) | 2.73 (1.81, 3.66) | 0.90 (0.73, 1.08) |
| n = 22 | n = 12 | |
| AUC(0,12 h) (µg ml−1 h) | 52.4 (42.4, 62.3) | 25.2 (20.3, 30.0) |
| n = 23 | n = 12 | |
| AUC(0,4 h) (µg ml−1 h) | 25.4 (22.0, 28.9) | 15.1 (12.2, 18.0) |
| n = 23 | n = 12 | |
| AUC(4,12 h) (µg ml−1 h) | 26.9 (19.4, 34.5) | 10.1 (7.06, 13.1) |
| n = 23 | n = 12 | |
| Week 4 | ||
| tmax (h)a | 1.21 (0.33–3.08) | 1.31 (0.63–3.02) |
| n = 24 | n = 14 | |
| Cmax (µg ml−1) | 15.3 (12.2, 18.4) | 8.17 (6.37, 9.97) |
| n = 24 | n = 14 | |
| Cmin (µg ml−1) | 2.86 (2.07, 3.66) | 1.00 (0.69, 1.31) |
| n = 23 | n = 13 | |
| AUC(0,12 h) (µg ml−1 h) | 50.2 (42.1, 58.4) | 28.2 (24.6, 31.8) |
| n = 23 | n = 14 | |
| AUC(0,4 h) (µg ml−1 h) | 28.8 (24.3, 33.3) | 16.8 (13.9, 19.7) |
| n = 23 | n = 12 | |
| AUC(4,12 h) (µg ml−1 h) | 22.8 (17.4, 28.2) | 11.4 (8.82, 14.0) |
| n = 23 | n = 14 | |
| Month 3 | ||
| tmax (h)a | 0.68 (0.67–3.00) | 1.25 (0.67–3.05) |
| n = 19 | n = 14 | |
| Cmax (µg ml−1) | 18.3 (13.8, 22.7) | 11.8 (7.93, 15.6) |
| n = 19 | n = 14 | |
| Cmin (µg ml−1) | 4.16 (3.10, 5.22) | 1.11 (0.89, 1.32) |
| n = 19 | n = 14 | |
| AUC(0,12 h) (µg ml−1 h) | 68.9 (54.3, 83.6) | 34.4 (29.3, 39.6) |
| n = 19 | n = 14 | |
| AUC(0,4 h) (µg ml−1 h) | 36.8 (28.9, 44.8) | 21.5 (16.7, 26.3) |
| n = 19 | n = 14 | |
| AUC(4,12 h) (µg ml−1 h) | 32.1 (23.6, 40.6) | 12.9 (10.8, 15.0) |
| n = 19 | n = 14 | |
| Month 6 | ||
| tmax (h)a | 0.67 (0.33–3.00) | 1.25 (0.42–3.00) |
| n = 18 | n = 14 | |
| Cmax (µg ml−1) | 20.6 (14.4, 26.9) | 16.3 (11.8, 20.8) |
| n = 18 | n = 14 | |
| Cmin (µg ml−1) | 3.93 (2.69, 5.17) | 1.46 (0.99, 1.93) |
| n = 17 | n = 14 | |
| AUC(0,12 h) (µg ml−1 h) | 68.5 (54.9, 82.0) | 40.4 (33.8, 47.0) |
| n = 17 | n = 14 | |
| AUC(0,4 h) (µg ml−1 h) | 37.4 (29.0, 45.9) | 27.0 (22.2, 31.8) |
| n = 17 | n = 14 | |
| AUC(4,12 h) (µg ml−1 h) | 30.3 (23.8, 36.8) | 13.4 (11.0, 15.8) |
| n = 17 | n = 14 | |
CI – Confidence interval;
tmax is reported as median value and observed range.
To investigate the effect of sirolimus vs. CsA on MPA exposure, the relative exposure of the AUC(0,12 h) of MPA and its 90% confidence interval (90% CI) were estimated for the CsA relative to the sirolimus group for each visit. The ratios (90% CI) were 50% (40, 62%), 59 (48, 72%), 53 (42, 67%) and 61% (50, 75%) for day 7, week 4, and months 3 and 6, respectively. Thus, the AUC(0,12 h) of MPA in the presence of CsA was consistently lower than that in the presence of sirolimus (Figure 1a). Figure 2 shows the plasma concentration profile of MPA over 0–12 h at month 6 in a typical patient from each group. No correlation was noted between MPA exposure and incidence of rejection within groups. Furthermore, no correlation was observed between sirolimus exposure and incidence of rejection, but this may have been due to the small number of events and lack of availability of exposure data in those patients making any definitive correlation unfeasible.
Figure 1.
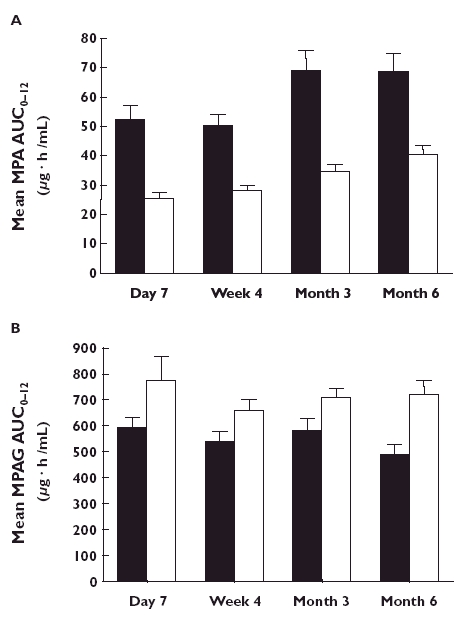
Mean (± SD) AUC(0,12 h) values at each assessment point in patients receiving regimens containing MMF and sirolimus, (▪); or MMF and ciclosporin A. (□); a) Mean values for MPA and b) mean values for MPAG
Figure 2.
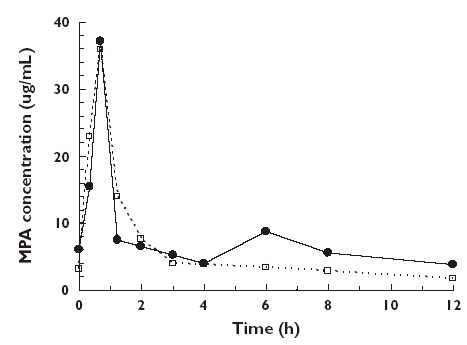
Representative individual MPA plasma concentration–time profiles at month 6; one patient on MMF and sirolimus (SRL), (•); and one on MMF and ciclosporin A (CsA), (□)
In addition, to investigate the effect of CsA on MPA enterohepatic recirculation, the mean AUC(4,12 h) of MPA for each group was estimated for each visit (Table 3). Within each group, the AUC(4,12 h) for MPA was consistent over time. However, the AUC(4,12 h) for MPA was markedly reduced in the presence of CsA compared with sirolimus (ranging from 51 to 63% reduction). The mean AUC(0,4 h) of MPA was also higher in the sirolimus group than the CsA group at each time point (Table 3). The ratio of AUC(0,4 h) : AUC(0,12 h) remained relatively constant over time within each regimen.
MPAG
The pharmacokinetics of MPAG following administration of MMF in combination with sirolimus or CsA are presented in Table 4. The median tmax was 2 h vs. 2–3 h in the sirolimus and the CsA groups, respectively. In the presence of CsA, the AUC(0,12 h) of MPAG was increased by 27% at day 7, 28% at week 4, 25% at month 3 and 52% at month 6 compared with the sirolimus-treated group (Figure 1b). In addition, Cmin and Cmax were higher in the presence of CsA.
Table 4.
Pharmacokinetic parameters of MPAG (dose-normalized values)
| MPAG (MPA equivalent units, mean (95%CI)) Sirolimus | Ciclosporin | |
|---|---|---|
| Day 7 | ||
| tmax (h)a | 2.0 (1.25–12.00) | 3.0 (1.25–3.00) |
| n = 23 | n = 12 | |
| Cmax (µg ml−1) | 65.0 (57.1, 72.8) | 82.8 (63.2, 102) |
| n = 23 | n = 12 | |
| Cmin (µg ml−1) | 36.9 (29.9, 43.9) | 50.5 (34.1, 67.1) |
| n = 22 | n = 12 | |
| AUC(0,12 h) (µg ml−1 h) | 592 (511, 674) | 775 (569, 980) |
| n = 22 | n = 12 | |
| Week 4 | ||
| tmax (h)a | 2.0 (0.67–4.00) | 3.0 (2.00–6.00) |
| n = 24 | n = 14 | |
| Cmax (µg ml−1) | 71.2 (47.7, 94.7) | 73.3 (63.8, 82.8) |
| n = 24 | n = 14 | |
| Cmin (µg ml−1) | 34.7 (28.1, 41.3) | 38.9 (31.5, 46.4) |
| n = 23 | n = 13 | |
| AUC(0,12 h) (µg ml−1 h) | 540 (463, 617) | 658 (574, 742) |
| n = 23 | n = 14 | |
| Month 3 | ||
| tmax (h)a | 2.0 (0.67–4.00) | 3.0 (1.5–4.00) |
| n = 19 | n = 14 | |
| Cmax (µg ml−1) | 68.7 (57.7, 79.7) | 75.5 (69.0, 82.0) |
| n = 19 | n = 14 | |
| Cmin (µg ml−1) | 33.4 (27.6, 39.4) | 43.1 (35.1, 51.0) |
| n = 19 | n = 14 | |
| AUC(0,12 h) (µg ml−1 h) | 577 (474, 680) | 705 (630, 781) |
| n = 19 | n = 14 | |
| Month 6 | ||
| tmax (h)a | 2.0 (0.67–6.00) | 2.0 (0.00–6.00) |
| n = 18 | n = 14 | |
| Cmax (µg ml−1) | 61.3 (52.1, 70.6) | 80.6 (70.0, 91.5) |
| n = 18 | n = 14 | |
| Cmin (µg ml−1) | 28.1 (10.8, 35.3) | 41.9 (32.8, 51.1) |
| n = 17 | n = 14 | |
| AUC(0,12 h) (µg ml−1 h) | 485 (402, 569) | 722 (607, 838) |
| n = 17 | n = 14 | |
CI – Confidence interval;
tmax is reported as median value and observed range.
Sirolimus
The average dose of sirolimus administered on the morning of each visit was 7 ± 3, 5 ± 3, and 3 ± 2 mg for week 4, and months 3 and 6, respectively. Following co-administration with MMF, concentrations of sirolimus increased over time post-transplant, reaching an average dose-normalized Cmax between 62.4 and 85.7 ng ml−1 over the three visits, with a median tmax of between 1.25 and 2.00 h (Table 5). The dose normalized AUC(0,24 h) values for sirolimus ranged from 835 to 1100 ng ml−1 h over the three visits.
Table 5.
Pharmacokinetic parameters of sirolimus (dose-normalized values)
| Visit | Values mean (95%CI) |
|---|---|
| Week 4 | |
| tmax (h)a (n = 15) | 1.25 (0.67–6.00) |
| Cmax (µg ml−1) (n = 15) | 62.4 (45.2, 79.7) |
| Cmin (µg ml−1) (n = 14) | 25.0 (18.9, 31.2) |
| AUC(0,12 h) (µg ml−1 h) (n = 14) | 835 (632, 1037) |
| Month 3 | |
| tmax (h)a (n = 13) | 2.00 (1.25–6.00) |
| Cmax (µg ml−1) (n = 13) | 85.7 (66.9, 105) |
| Cmin (µg ml−1) (n = 13) | 32.5 (22.8, 42.2) |
| AUC(0,12 h) (µg ml−1 h) (n = 13) | 1078 (870, 1285) |
| Month 6 | |
| tmax (h)a (n = 18) | 2.00 (0.67–4.00) |
| Cmax (µg ml−1) (n = 18) | 85.2 (62.5, 108) |
| Cmin (µg ml−1) (n = 17) | 31.8 (23.0, 40.8) |
| AUC(0,12 h) (µg ml−1 h) (n = 17) | 1100 (820, 1381) |
CI – Confidence interval;
tmax is reported as median value and observed range.
Despite the loading doses, target trough concentrations were only achieved by 64.3% of patients at week 4 (average 17.3 ± 11.1 ng ml−1), 69.2% of patients at month 3 (average, 11.9 ± 4.6 ng ml−1) and 59% of patients at month 6 (average, 8.7 ± 2.8 ng ml−1).
Efficacy
By 6 months post-transplant, the incidence of BPAR or treatment failure was higher in the group receiving sirolimus compared with the group receiving CsA (40.0% vs. 13.3% (including borderline)) and 36.7% vs. 0.0% (excluding borderline). In the sirolimus group, 11 patients experienced BPAR (borderline n = 1; grade I rejection, n = 6; grade IIa rejection, n = 3; grade IIb rejection, n = 1), and three patients experienced treatment failure. In contrast, only two patients in the CsA group experienced BPAR (both borderline) and no patients experienced treatment failure. All episodes of BPAR and treatment failure occurred within the first 4 months post-transplant, with early acute rejection (within the first 2 months) only occurring in the group receiving sirolimus (n = 5). Except for the patient with borderline rejection, all other patients with acute rejection in the sirolimus group received treatment for rejection, while only one of the two patients in the CsA group received treatment. A total of four patients (all receiving sirolimus) received anti-lymphocyte therapy as treatment for rejection (either without first attempting steroids (n = 1), simultaneously with steroids (n = 1), simultaneously with steroids for the first rejection episode and without steroids for the second episode (n = 1), or not given on the first day postrejection [steroids alone], then simultaneously with steroids on the second day post-rejection (n = 1)).
Delayed graft function (DGF) was recorded for nine patients (n = 7 (23.3%) in the sirolimus group; n = 2 (13.3%) in the CsA group) with three patients needing dialysis (n = 2 and n = 1, respectively). One patient (CsA group) required induction therapy and was included in the rejection assessment. None of the patients with DGF died, lost their graft, or experienced chronic rejection during the study, and only two patients with DGF (both in the sirolimus group) experienced BPAR (at days 7 and 95). One patient had a renal biopsy on day 2 post-transplant demonstrating mild interstitial fibrosis, but this most likely reflected the condition of the donor organ.
Patients in the sirolimus group had slightly better renal function at 6 months post-transplant according to serum creatinine values (1.2 ± 0.4 vs. 1.5 ± 0.3 mg dl−1, respectively) and calculated creatinine clearances (82.7 ± 24.4 vs. 77.8 ± 15.1 ml min−1, respectively) compared with the CsA group. Measured GFR values for the two treatment groups were more comparable (54.6 ± 16.4 vs. 55.2 ± 21.3 ml min−1 1.73 m−2, respectively). More patients had their renal function evaluated at 6 months using serum creatinine or calculated creatinine clearances (33 patients) than measured GFR (25 patients).
In the group receiving sirolimus, pre- and post-rejection serum creatinine values were available for 10 patients. Five patients experienced a decrease in serum creatinine concentrations post-rejection, and five patients had an increase in serum creatinine concentrations. The mean serum creatinine value was slightly lower following rejection (1.5 vs. 1.7 mg dl−1). Comparison with the CsA group was not possible, as only one patient had matched pre- and post-rejection data (with this patient experiencing a slightly higher serum creatinine concentration after rejection).
There were no graft losses during the first 6 months after transplantation.
Safety
All adverse events with an overall incidence of >10% which occurred up to 6 months post-transplant are listed in Table 6. All 45 patients experienced >1 adverse event by 6 months post-transplant with most events being mild-to-moderate in intensity and considered unrelated to daclizumab (98%), MMF (88%), sirolimus (74%) or CsA (81%). The most common adverse events considered related to MMF were leukopenia and diarrhoea, and these were more common in the sirolimus group. Adverse events known to be associated with CsA use, such as hypertension and tremors, were more common in that group. Similarly, adverse events known to be associated with sirolimus, such as anaemia, thrombocytopenia, hyperlipidaemia, hypercholesterolaemia, and pneumonia were more common in patients receiving sirolimus. The proportion of patients who failed to achieve primary closure of their surgical wound at 1 month post-transplant was higher in the sirolimus group (37% vs. 13%). One patient in this group required lymphocele intervention within the first 6 months post-transplant. There was no correlation between these events and the patients' BMI.
Table 6.
Adverse events occurring up to 6 months post-transplant (overall incidence >10%), safety population
| Adverse event n (%) | Sirolimus (n = 30) | Ciclosporin (n = 30) |
|---|---|---|
| Peripheral oedema | 17 (56.7) | 7 (46.7) |
| Anaemia | 16 (53.3) | 1 (6.7) |
| Diarrhoea | 16 (53.3) | – |
| Constipation | 14 (46.7) | 9 (60.0) |
| Hyperlipidaemia | 14 (46.7) | 6 (40.0) |
| Incision site complication | 12 (40.0) | 3 (20.0) |
| Hypertension | 11 (36.7) | 8 (53.3) |
| Leukopenia | 11 (36.7) | 2 (13.3) |
| Headache | 10 (33.3) | 3 (20.0) |
| Tremor | 3 (10.0) | 7 (46.7) |
| Insomnia | 7 (23.3) | 2 (13.3) |
| Pruritis | 7 (23.3) | 1 (6.7) |
| Pyrexia | 7 (23.3) | 1 (6.7) |
| Hypophosphataemia | 6 (20.0) | 2 (13.3) |
| Acne | 6 (20.0) | 1 (6.7) |
| Hypokalaemia | 6 (20.0) | 1 (6.7) |
| Pharyngolaryngeal pain | 6 (20.0) | 1 (6.7) |
| Impaired healing | 6 (20.0) | – |
| Hyperglycaemia | 4 (13.3) | 3 (20.0) |
| Dysuria | 4 (13.3) | 3 (20.0) |
| Dyspepsia | 4 (13.3) | 3 (20.0) |
| Fatigue | 4 (13.3) | 1 (6.7) |
| Oedema | 4 (13.3) | 1 (6.7) |
| Dyspnea | 3 (10.0) | 4 (26.7) |
| Vomiting | 3 (10.0) | 3 (20.0) |
| Fluid overload | 3 (10.0) | 2 (13.3) |
Six patients experienced ≥1 opportunistic infection, with an overall incidence of 17% (n = 5) in the sirolimus group and 7% (n = 1) in the CsA group. The most frequent opportunistic infection was candidiasis (4/30 patients in the sirolimus group (13.3%), either mucocutaneous (n = 3) or respiratory (n = 2) in nature. The next most frequent opportunistic infections were CMV (2/30 (6.7%) vs. 1/15 (6.7%), sirolimus vs. CsA, respectively) and herpes simplex (clinical diagnosis and pathology confirmed; 1/30 (3.3%) vs. 0/15 (0%)).
A total of 23 patients (57% vs. 40%, sirolimus vs. CsA, respectively) experienced ≥1 serious adverse event during the study. The most common serious events by body system were infections and infestations (particularly cytomegalovirus and candida infections), which occurred in 23.3% and 6.7% of sirolimus and CsA recipients, and renal and urinary disorders, which occurred in 13.3% of patients in each treatment group. The most common individual serious events (occurring in at least two patients) were anaemia (n = 2 (7%)), diarrhoea (n = 2 (7%)) and pyrexia (n = 2 (7%)), which all occurred in the group receiving sirolimus. The majority of serious adverse events were considered to be unrelated to daclizumab (100%), MMF (82%), sirolimus (61%) and CsA (83%). Serious adverse events considered to be related to MMF were diarrhoea in two patients and odynophagia, polyomavirus infection, anaemia and pyrexia (each in one patient), all of which occurred in patients in the sirolimus group. Events considered to be related to sirolimus were diarrhoea in two patients and odynophagia, polyomavirus infection, wound infection, interstitial nephritis and urethral obstruction (each in one patient). The one serious adverse event thought to be related to CsA was therapeutic drug toxicity, manifested by nephrotoxicity.
Five patients receiving sirolimus discontinued treatment due to adverse events (Table 2). Two patients withdrew from MMF (one due to leukopenia and one due to diarrhoea). Two other patients withdrew from sirolimus (one due to wound dehiscence, and one due to interstitial nephritis and renal tubular necrosis), and one patient withdrew from MMF and sirolimus (due to polyomavirus infection).
The most frequent marked laboratory abnormalities were hypophosphataemia, erythropaenia and lymphopaenia, and these were consistently higher in the group receiving sirolimus. The incidence of marked thrombocytopenia was also higher in the group receiving sirolimus (23% vs. 0%). However, the incidence of laboratory events associated with CsA use (nephrotoxicity and hyperuricaemia) was comparable between the two treatment groups (n = 1 for both groups). No changes occurred in any other laboratory parameter.
No malignancies were reported up to 6 months post-transplant, and there were no deaths during the study.
Discussion
The present study has shown that, compared with CsA, the concomitant use of sirolimus with MMF results in increased exposure to MPA and decreased exposure to MPAG in renal transplant patients.
Exposure to MPA in renal transplant recipients was reduced by 39–50% in the presence of CsA compared with sirolimus, while exposure to MPAG was increased in patients receiving CsA. The reduced exposure to MPA in the presence of CsA agrees with recently published data [20–22]. In 30 renal transplant patients, exposure to MMF and CsA resulted in a reduction of 30–50% in MPA exposure compared with patients receiving sirolimus, even though patients received similar amounts of MMF [20]. Similarly, in a 12-month study by Cattaneo et al. (n = 21) [22], which was published after the completion of the current study, with a relatively low MMF dose (500 mg twice daily), the mean MPA AUC(0,12 h) was reduced in the CsA group by a similar degree to that observed in the current study (sirolimus group to CsA group ratio of 1.5) [22]. A possible explanation is that a drug–drug interaction could occur between MMF and CsA [23–27]. Indeed, it is hypothesized that CsA inhibits or interferes with the enterohepatic recycling of MPA by inhibiting excretion of MPAG into the bile. This reduces the amount of MPAG available for conversion back to MPA in the gut, and subsequent MPA reabsorption. CsA is a known inhibitor of the bile salt pump (BSep) [28] and the multidrug resistance protein 2 pump (Mrp2) [29–31], either of which could be responsible for transport of MPAG from hepatocytes into the bile. The net effect of inhibition of either mechanism would be an increased concentration of MPAG, and a decreased concentration of MPA, in the blood. Interestingly, this hypothesis is validated by a comparison made between the ratio of AUC(4,12 h) to AUC(0,12 h) for both groups, in which the ratios were consistently higher for the sirolimus compared with the cyclosporin group. This highlights the reduction of MPA enterohepatic recycling during 4–12 h post-transplant period in patients receiving CsA. The AUC(0,4 h) and AUC(0,4 h) to AUC(0,12 h) ratio indicated that the early post-dose effect of CsA on the overall MPA exposure was slightly higher than that of sirolimus. However, as expected, the magnitude of the partial MPA AUC also increased over time after transplantation similar to that of the overall MPA AUC. As a consequence of the reduced enterohepatic recycling, it was noted that patients receiving CsA had exposures of MPA that were below those recommended (30 µg ml−1 h) [32] for the first 4 weeks. Based on these data, it may be reasonable to suggest that patients receiving CsA start on a dose of 3 g day−1 of MMF for the first month and include routine monitoring of MPA exposures (AUC(0,12 h)) within this period, to ensure adequate protection against acute rejection and reduce the incidence of adverse event to a minimum. However, this regimen would have to be studied further in future, well-designed, clinical trials.
The target concentrations of sirolimus used within the present study were based upon previous data obtained from kidney transplant recipients [13, 14]. While not all patients reached target, the resulting sirolimus trough concentrations were similar to those that have previously been published in kidney transplant recipients receiving CsA, sirolimus and corticosteroids alone (8–13 ng ml−1) [33]. In contrast to patients receiving CsA, patients receiving sirolimus reached recommended concentration of MPA. Furthermore calculation of the ratios of MPA trough concentrations to AUC(0,12 h) for both groups showed that values were consistently higher in the sirolimus group (data not shown), indicating that, for equivalent exposures, higher MPA trough concentrations should be expected in patients treated with sirolimus.
Overall, these data indicate that a better understanding of the relationships between the pharmacokinetics and pharmacodynamics of sirolimus and MMF are needed when they are used in combination.
The acute rejection rate for patients receiving sirolimus in this multicentre study (40%) was greater than that observed by Kreis et al. (28%) or Flechner et al. (7%) [13, 14]. The reasons for the higher rate of rejection in the sirolimus arm of the current study are unclear as the study was well matched to the other studies with respect to treatment regimens and the demographics of both recipients and donors. However, the present study had fewer patients than these previous studies (n = 78 for Kreis et al.[14], n = 61 for Flechner et al.[13]), although the number of patients in the sirolimus arms were similar (n = 30, 30 and 40 for the current study, Flechner et al.[13] and Kreis et al.[14] respectively). Furthermore, our study was also a multicentre study, as opposed to a single centre study, and so could have included more variables that might have affected patient response. The present study also used lower steroid doses in the immediate postoperative period (approximately 400 mg steroids intraoperatively, 60 mg by day 4 and up to 37 mg by day 7) compared with Kreis et al. (500 mg of methylprednisolone intraoperatively followed by 200 mg of either prednisolone or prednisone tapered to 30 mg by day 7), or Flechner et al. (500 mg of methylprednisolone for 3 days, then prednisone 120 mg tapered to 30 mg by day 8). Either of these factors may have contributed to the higher incidence of acute rejection in this study. In this study, we were not able to show a definitive correlation between sirolimus exposure and rejection. This was due to the low number of acute rejections and lack of availability of sirolimus pharmacokinetics in the majority of the patients which did not allow for a detailed analysis to be performed. In the trial by Kreis et al.[14], there were no significant differences in the mean trough concentrations of sirolimus between rejectors and nonrejectors in the first 2 months after transplantation.
Patients receiving CsA experienced a higher incidence of events known to be related to CsA use, including hypertension, tremors and hirsutism. The long-term use of CsA is also associated with progressive renal toxicity, which can ultimately lead to chronic rejection and graft loss [3]. Although there was little evidence of renal toxicity in the present study, and no episodes of chronic rejection or graft loss, the length of the study (6 months) may have been too short to draw any conclusions regarding the effect of CsA vs. sirolimus on long-term renal function. In addition, the study was probably too small to detect such differences. Compared with patients on CsA, those who received sirolimus experienced a higher incidence of failure to achieve primary closure of the surgical wound by 1 month post-transplant, lymphocele requiring intervention by 6 months post-transplant, thrombocytopenia and hyperlipidaemia, events known to be associated with sirolimus use [34, 35]. The effect of sirolimus on wound closure has been previously noted [36, 37], and while no concentration-related effect on wound closure has been observed with sirolimus, it is possible that the high loading dose used in the present study may have exacerbated this effect. In addition, while none of the patients in the CsA arm experienced diarrhoea, 53% of those in the sirolimus arm reported this adverse event. This may be due to a combination of the effects of sirolimus and increased MPA exposure in the sirolimus group. A higher proportion of patients in the sirolimus arm also experienced adverse events which led to study drug discontinuation (17% vs. 0%) or dose modification or interruption (70% vs. 33%).
In summary, the use of sirolimus in combination with MMF, daclizumab and steroids, resulted in increased MPA exposure and decreased MPAG exposures when compared with CsA. While rejection rates in the sirolimus arm were higher in the current study than reported elsewhere, the total enrolment was too small to make any definitive conclusion on efficacy or safety. Nevertheless, the rejection rates, in addition to the adverse event profile, in the sirolimus arm are such that the regimen of sirolimus, MMF, daclizumab and corticosteroids, as administered in this study, could not be generally recommended. There are large, randomized, multicentre studies in progress that are investigating the effectiveness of this combination, and more definitive recommendations await their conclusion.
Acknowledgments
Funding for this study was provided by F. Hoffmann-La Roche Ltd, Basel, Switzerland. We would like to thank Dr Richard Mamelok for critically reading the manuscript and Dr Richard Glover for editorial assistance.
References
- 1.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–83. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman DB, Shapiro R, Lucey MR, Cherikh WS, Bustami RT, Dyke D. Immunosuppression: practice and trends. Am J Transplant. 2004;4(Suppl 9):38–53. doi: 10.1111/j.1600-6135.2004.00397.x. [DOI] [PubMed] [Google Scholar]
- 3.Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–33. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 4.de Mattos AM, Olyaei AJ, Bennett WM. Nephrotoxicity of immunosuppressive drugs. long-term consequences and challenges for the future. Am J Kidney Dis. 2000;35:333–46. doi: 10.1016/s0272-6386(00)70348-9. [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL, Tortorice KL, Heim-Duthoy KL, Awni WM, Rao KV. The adverse impact of cyclosporine on serum lipids in renal transplant recipients. Am J Kidney Dis. 1991;17:700–7. doi: 10.1016/s0272-6386(12)80355-6. [DOI] [PubMed] [Google Scholar]
- 6.Schorn TF, Kliem V, Bojanovski M, Bojanovski D, Repp H, Bunzendahl H, Frei U. Impact of long-term immunosuppression with cyclosporin A on serum lipids in stable renal transplant recipients. Transpl Int. 1991;4:92–5. doi: 10.1007/BF00336404. [DOI] [PubMed] [Google Scholar]
- 7.Mayer AD, Dmitrewski J, Squifflet JP, Besse T, Grabensee B, Klein B, Eigler FW, Heemann U, Pichlmayr R, Behrend M, Vanrenterghem Y, Donck J, van Hooff J, Christiaans M, Morales JM, Andres A, Johnson RW, Short C, Buchholz B, Rehmert N, Land W, Schleibner S, Forsythe JL, Talbot D, Pohanka E. Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation. 1997;64:436–43. doi: 10.1097/00007890-199708150-00012. [DOI] [PubMed] [Google Scholar]
- 8.Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31:335–40. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 9.Groth CG, Backman L, Morales JM, Calne R, Kreis H, Lang P, Touraine JL, Claesson K, Campistol JM, Durand D, Wramner L, Brattstrom C, Charpentier B. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation. 1999;67:1036–42. doi: 10.1097/00007890-199904150-00017. [DOI] [PubMed] [Google Scholar]
- 10.Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet. 2000;356:194–202. doi: 10.1016/s0140-6736(00)02480-6. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald AS. A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation. 2001;71:271–80. doi: 10.1097/00007890-200101270-00019. [DOI] [PubMed] [Google Scholar]
- 12.Kahan BD, Julian BA, Pescovitz MD, Vanrenterghem Y, Neylan J. Sirolimus reduces the incidence of acute rejection episodes despite lower cyclosporine doses in Caucasian recipients of mismatched primary renal allografts: a phase II trial. Rapamune Study Group. Transplantation. 1999;68:1526–32. doi: 10.1097/00007890-199911270-00016. [DOI] [PubMed] [Google Scholar]
- 13.Flechner SM, Goldfarb D, Modlin C, Feng J, Krishnamurthi V, Mastroianni B, Savas K, Cook DJ, Novick AC. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus vs. cyclosporine. Transplantation. 2002;74:1070–6. doi: 10.1097/00007890-200210270-00002. [DOI] [PubMed] [Google Scholar]
- 14.Kreis H, Cisterne JM, Land W, Wramner L, Squifflet JP, Abramowicz D, Campistol JM, Morales JM, Grinyo JM, Mourad G, Berthoux FC, Brattstrom C, Lebranchu Y, Vialtel P. Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation. 2000;69:1252–60. doi: 10.1097/00007890-200004150-00009. [DOI] [PubMed] [Google Scholar]
- 15.Dunn J, Vathsala A, Golden D, Kerman R, Lawen J, Van Buren CT, Lewis R, Kahan BD. Impact of race on the outcome of renal transplantation under cyclosporine-prednisone. Transplant Proc. 1989;21:3946–8. [PubMed] [Google Scholar]
- 16.Opelz G, Pfarr E, Engelmann A, Keppel E. Kidney graft survival rates in black cyclosporine-treated recipients. Collaborative Transplant Study. Transplant Proc. 1989;21:3918–20. [PubMed] [Google Scholar]
- 17.Katznelson S, Gjertson DW, Cecka JM. The effect of race and ethnicity on kidney allograft outcome. Clin Transpl. :379–94. [PubMed] [Google Scholar]
- 18.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 19.Guesry P, Kaufman L, Orloff S, Nelson JA, Swann S, Holliday M. Measurement of glomerular filtration rate by fluorescent excitation of non-radioactive meglumine iothalamate. Clin Nephrol. 1975;3:134–8. [PubMed] [Google Scholar]
- 20.Buchler M, Lebranchu Y, Beneton M, Le Meur Y, Heng AE, Westeel PF, le Guellec C, Libert F, Hary L, Marquet P, Paintaud G. Higher exposure to mycophenolic acid with sirolimus than with cyclosporine cotreatment. Clin Pharmacol Ther. 2005;78:34–42. doi: 10.1016/j.clpt.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 21.El Haggan W, Ficheux M, Debruyne D, Rognant N, Lobbedez T, Allard C, Coquerel A, Ryckelynck JP, Hurault de Ligny B. Pharmacokinetics of mycophenolic acid in kidney transplant patients receiving sirolimus vs. cyclosporine. Transplant Proc. 2005;37:864–6. doi: 10.1016/j.transproceed.2004.12.217. [DOI] [PubMed] [Google Scholar]
- 22.Cattaneo D, Merlini S, Zenoni S, Baldelli S, Gotti E, Remuzzi G, Perico N. Influence of co-medication with sirolimus or cyclosporine on mycophenolic acid pharmacokinetics in kidney transplantation. Am J Transplant. 2005;5:2937–44. doi: 10.1111/j.1600-6143.2005.01107.x. [DOI] [PubMed] [Google Scholar]
- 23.Gregoor PJ, de Sevaux RG, Hene RJ, Hesse CJ, Hilbrands LB, Vos P, van Gelder T, Hoitsma AJ, Weimar W. Effect of cyclosporine on mycophenolic acid trough levels in kidney transplant recipients. Transplantation. 1999;68:1603–6. doi: 10.1097/00007890-199911270-00028. [DOI] [PubMed] [Google Scholar]
- 24.Smak Gregoor PJ, van Gelder T, Hesse CJ, van der Mast BJ, van Besouw NM, Weimar W. Mycophenolic acid plasma concentrations in kidney allograft recipients with or without cyclosporin: a cross-sectional study. Nephrol Dial Transplant. 1999;14:706–8. doi: 10.1093/ndt/14.3.706. [DOI] [PubMed] [Google Scholar]
- 25.Pou L, Brunet M, Cantarell C, Vidal E, Oppenheimer F, Monforte V, Vilardell J, Roman A, Martorell J, Capdevila L. Mycophenolic acid plasma concentrations: influence of comedication. Ther Drug Monit. 2001;23:35–8. doi: 10.1097/00007691-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Shaw LM, Korecka M, DeNofrio D, Brayman KL. Pharmacokinetic, pharmacodynamic, and outcome investigations as the basis for mycophenolic acid therapeutic drug monitoring in renal and heart transplant patients. Clin Biochem. 2001;34:17–22. doi: 10.1016/s0009-9120(00)00184-3. [DOI] [PubMed] [Google Scholar]
- 27.Aw MM, Brown NW, Itsuka T, Gonde CE, Adams JE, Heaton ND, Tredger JM, Mieli-Vergani G, Dhawan A. Mycophenolic acid pharmacokinetics in pediatric liver transplant recipients. Liver Transpl. 2003;9:383–8. doi: 10.1053/jlts.2003.50022. [DOI] [PubMed] [Google Scholar]
- 28.Stieger B, Fattinger K, Madon J, Kullak-Ublick GA, Meier PJ. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology. 2000;118:422–30. doi: 10.1016/s0016-5085(00)70224-1. [DOI] [PubMed] [Google Scholar]
- 29.Morrow CS, Smitherman PK, Townsend AJ. Role of multidrug-resistance protein 2 in glutathione S-transferase P1-1-mediated resistance to 4-nitroquinoline 1-oxide toxicities in HepG2 cells. Mol Carcinog. 2000;29:170–8. doi: 10.1002/1098-2744(200011)29:3<170::aid-mc6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 30.Bramow S, Ott P, Thomsen Nielsen F, Bangert K, Tygstrup N, Dalhoff K. Cholestasis and regulation of genes related to drug metabolism and biliary transport in rat liver following treatment with cyclosporine A and sirolimus (Rapamycin) Pharmacol Toxicol. 2001;89:133–9. doi: 10.1034/j.1600-0773.2001.d01-147.x. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi M, Saitoh H, Tadano K, Takahashi Y, Hirano T. Cyclosporin A, but not tacrolimus, inhibits the biliary excretion of mycophenolic acid glucuronide possibly mediated by multidrug resistance-associated protein 2 in rats. J Pharmacol Exp Ther. 2004;309:1029–35. doi: 10.1124/jpet.103.063073. [DOI] [PubMed] [Google Scholar]
- 32.van Gelder T, Le Meur Y, Shaw LM, Oellerich M, DeNofrio D, Holt C, Holt DW, Kaplan B, Kuypers D, Meiser B, Toenschoff B, Mamelok R. Therapeutic drug monitoring of mycophenolate mofetil in transplantation. Is Ther Drug Monit. 28:145–54. doi: 10.1097/01.ftd.0000199358.80013.bd. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan B, Meier-Kriesche HU, Napoli KL, Kahan BD. The effects of relative timing of sirolimus and cyclosporine microemulsion formulation coadministration on the pharmacokinetics of each agent. Clin Pharmacol Ther. 1998;63:48–53. doi: 10.1016/S0009-9236(98)90120-5. [DOI] [PubMed] [Google Scholar]
- 34.Brattstrom C, Wilczek H, Tyden G, Bottiger Y, Sawe J, Groth CG. Hyperlipidemia in renal transplant recipients treated with sirolimus (rapamycin) Transplantation. 1998;65:1272–4. doi: 10.1097/00007890-199805150-00023. [DOI] [PubMed] [Google Scholar]
- 35.Hong JC, Kahan BD. Sirolimus-induced thrombocytopenia and leukopenia in renal transplant recipients. risk factors, incidence, progression, and management. Transplantation. 2000;69:2085–90. doi: 10.1097/00007890-200005270-00019. [DOI] [PubMed] [Google Scholar]
- 36.Valente JF, Hricik D, Weigel K, Seaman D, Knauss T, Siegel CT, Bodziak K, Schulak JA. Comparison of sirolimus vs. mycophenolate mofetil on surgical complications and wound healing in adult kidney transplantation. Am J Transplant. 2003;3:1128–34. doi: 10.1034/j.1600-6143.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 37.Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, Kremers WK, Stegall MD. Wound-healing complications after kidney transplantation. a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation. 2004;77:1555–61. doi: 10.1097/01.tp.0000123082.31092.53. [DOI] [PubMed] [Google Scholar]


