Abstract
Sperm adhesion to egg zonae pellucidae initiates sperm acrosome reactions, an exocytotic event that is an early step during fertilization. Previously, it was suggested that zona pellucida-evoked Ca2+ entry into sperm through low voltage-activated Ca2+ channels is an essential step in acrosome reactions, based on the inhibitory effects of Ca2+ channel antagonists. However, analysis of this channel is limited by the inability to apply electrophysiological methods directly to sperm. In this report, optical methods of determining membrane potential and internal Ca2+ levels were used to demonstrate that (i) contact with zonae pellucidae activates a transient Ca2+ response in sperm that has a time course and antagonist sensitivity anticipated of low voltage-activated Ca2+ channels; (ii) these channels are unavailable for opening in uncapacitated sperm because of voltage-dependent, steady state inactivation; (iii) membrane hyperpolarization during sperm capacitation is sufficient to recruit channels into a closed state, from which they are available for opening during fertilization; and (iv) channel conductance state may be a factor in determines the efficacy with which channel antagonists inhibit fertilization. This study provides evidence for the activation of sperm Ca2+ channels during gamete adhesion and offers a mechanism that may account for aspects of the regulation of sperm fertility during capacitation through the control of channel availability. Finally, these results suggest that channel conductance state may be a central feature in the design of channel antagonists that inhibit sperm function.
The acrosome is a secretory vesicle that is present in the apical region of sperm of many animal species, including all mammals. The release of vesicle contents occurs through an exocytotic process known as the acrosome reaction during the early stages of fertilization and must be completed in order for sperm to engage in the later events of fertilization, including gamete fusion. In mammals, acrosome reactions are controlled by both indirect and direct mechanisms. First, sperm must undergo a preliminary activation phase, designated “capacitation,” which regulates the efficiency of exocytosis. Although the essential role of capacitation in fertilization is generally accepted, the molecular mechanisms that underlie this regulatory process are not well understood. Second, exocytosis by capacitated sperm is initiated during sperm-egg contact by ZP3, a component of the egg’s zona pellucida (ZP) extracellular matrix (1–3).
Three lines of evidence suggest that Ca2+ entry through voltage-sensitive channels mediates ZP3 signal transduction. (i) ZP3 produces elevations of sperm intracellular Ca2+ (Ca2+i), as reported by ion-selective fluorescent probes. (ii) ZP3-evoked Ca2+i responses and acrosome reactions are inhibited by Ca2+ channel antagonists. (iii) Under certain circumstances, depolarization of sperm membrane potential (VM) also produces Ca2+i elevations and acrosome reaction, and these are, again, inhibited by Ca2+ channel antagonists (2, 3). These channels may account for the reported male contraceptive effects of 1,4-dihydropyridine-type Ca2+ channel antagonists (4, 5).
Characterization of Ca2+ currents in sperm is complicated by difficulties in applying either molecular genetic methods or patch clamp techniques directly to these cells. An alternative approach has been to examine channels in testicular spermatogenic cells, a developmental precursor stage that synthesize proteins for later use in the translationally quiescent sperm. Low voltage-activated (LVA) T-type channels are detected in rodent spermatogenic cells by using the patch clamp technique (6–12). Moreover, the close correspondence between the effects of channel antagonists on the LVA Ca2+ currents of mouse spermatogenic cells and on the ZP3-evoked Ca2+i response of mouse sperm suggests that these channels mediate Ca2+ influx during acrosome reactions (6). However, LVA currents inactivate rapidly and do not generally produce the protracted and delayed Ca2+i responses that are observed in sperm. This may reflect the fact that earlier studies of ZP3-dependent Ca2+i responses lacked the temporal resolution to detect LVA channels. An understanding of the role of LVA currents during fertilization requires direct analysis of their activation in sperm.
Here, we use fluorescent probe methods to determine Ca2+i and VM in single mouse sperm. These studies demonstrate that ZP3 activates a Ca2+ influx into sperm. This influx produces a Ca2+i elevation that has both a time course and a susceptibility to inhibition by channel antagonists that is anticipated of LVA currents. In addition, we demonstrate that the availability of these channels for opening is controlled during sperm capacitation. This latter form of regulation may contribute to the coordination of exocytosis with gamete contact, as well as providing new targets for channel-based antifertility agents.
MATERIALS AND METHODS
Biological Preparations.
Sperm and spermatogenic cells were obtained from CD-1 mice from caudae epididymides and by manual trituration of testicular slices, respectively (6). The fraction of motile sperm was determined by visual inspection within 20 min, and preparations with <75% motile cells were discarded. Sperm were capacitated in vitro during a 120-min incubation (37°C) in medium HBCM, containing (in mM): NaCl (94), KCl (4.78), MgCl2 (2.1), CaCl2 (1.71), KH2PO4 (1.19), NaHCO3 (10), Hepes [10 (pH 7.4 with NaOH)], sodium lactate (26), sodium pyruvate (1.19), glucose (5.56), and BSA (2% wt/vol) (13). Noncapacitating incubations were carried out in a low BSA (0.1% wt/vol)-HBCM medium (13). Phosphate salts were removed during experiments with La3+.
Capacitation was assayed (i) in single sperm, by chlortetracycline (CTC) fluorescence emission (λEX, 395 nm; λEX, 510 nm) (13, 14) and by determining the ability to undergo ZP3-dependent exocytosis (13); and (ii) in populations by monitoring tyrosine phosphorylation of sperm proteins (15, 16). Acrosome reactions were assayed by Coomassie blue staining (17). Cumulus-free, ZP-intact eggs were fertilized during 4-hr incubations in 100-μl drops of HBCM containing 105 sperm/ml, a concentration at which ≈75% of eggs are fertilized within 4 hr and at which the extent of fertilization depends on the concentration of functional sperm. Fertilization was assayed by the presence of a sperm head within egg cytoplasm (18).
ZP were obtained by manual dissection from germinal vesicle-intact, follicular oocytes. ZP2 and ZP3 were purified by SDS/PAGE (19). Soluble ZP extracts were prepared by heating (80°C, 60 min).
Electrophysiological Methods.
Spermatogenic cells were attached to Cell-Tak-coated plastic culture dishes and were perfused with a solution containing (in mM): NaCl (100), KCl (5), CaCl2 (10), MgCl2 (1), tetraethylammonium–Cl (26), sodium lactate (6), Hepes [10 (pH 7.4 with NaOH)], and glucose (3.3). Ca2+ currents were recorded by using hard glass (Gardner 7052; 2–10 megaohms of pipette resistance), Sylgard-coated pipettes by means of the whole cell-configuration of the patch clamp method (20). The pipette internal solution contained (in mM): cesium glutamate (120), MgCl2 (5), tetraethylammonium–Cl (20), Mg2ATP (3), CsEGTA (10), Hepes [10 (pH 7.0 with CsOH)], and glucose (5). Currents were obtained by using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA), corrected for leakage and capacitance currents, filtered at 3 kHz, digitized every 250 msec, and analyzed by using Biopatch (Biologic, Grenoble, France) as described (6, 8, 9). Voltage protocols and drug treatment regimens are described in Results.
Fluorescent Probe Methods.
Sperm (107 per ml) were labeled with dyes (Molecular Probes) by incubation (30 min, 37°C) in a 0.01% BSA-supplemented medium containing either 3 μM of the acetoxymethyl-ester conjugates of mag-fura 2 or fura 2 or 1 μM di8-ANEPPS 4-[2-[6-(dioctylamino)-2 naphthalenyl] ethenyl]-1-(3-sulfopropyl) (Molecular Probes). Sperm were immobilized on Cell-Tak-coated glass coverslips and were maintained at 37°C on a microscope stage in medium HBCM. Excitation illumination from a DG4 Optical Switch (Sutter Instruments, Novato, CA) was selected by using the following filter combinations: for fura 2 or mag-fura 2, 340 nm and 380 nm; for di-8-ANEPPS, 440 nm and 530 nm. Emission (510 nm) from fura 2 or mag-fura 2 was restricted to single sperm with a field iris and was directed to a photomultiplier tube. Di8-ANEPPS emission (610 nm) from groups of sperm was digitized with a GenIVSys-intensified Dage 72 CCD camera (Dage-MTI) and was analyzed in single cells by using Axon Instruments workbench software (7).
Ca2+i values were estimated by using the algorithm of Grynkiewicz et al. (21), using Kd values for Ca2+:dye complexes of 224 nM for fura 2 (21) and 44 μM for mag-fura 2 (22). Di8-ANEPPS signals were calibrated in the range −100–0 mV, with standard curves obtained from fluorescent emission of voltage-clamped round spermatids. The relationship between VM and dye emission is approximated by the expression VM = 1.002 + (0.00099 * [F440/F530]), in which [F440/F530] is the ratio of fluorescent emission after excitation at the indicated energies. A similar relationship was described for these styryl probes (23). BSA alters the spectral characteristics of di8-ANEPPS. This protein is essential for mouse sperm capacitation (13, 24) but may be omitted from spermatogenic cell cultures. BSA was therefore removed from the bath by superfusion before the determination of VM in immobilized sperm.
RESULTS
ZP3-Dependent Ca2+i Transients in Sperm.
Previously, it was demonstrated that the major component of the Ca2+i response to ZP3 requires minutes to develop (6, 25) and is too slow to reflect ionic influx through LVA channels (26). However, the fluorescence detector systems used in those studies were limited by a temporal resolution of >15 sec. To determine whether this delayed Ca2+i response phase is preceded by more rapid events, we examined the early stages of ZP3 signal transduction in single sperm by using a detector system with a temporal resolution, as estimated by Nyquist’s criterion, of 2–4 msec.
Ca2+i was monitored with mag-fura 2 and with fura 2, which are low- and high-affinity, Ca2+-selective fluorescent probes, respectively. Ca2+i of capacitated sperm was below the level of accurate detection with mag-fura 2 but was determined to be 0.154 ± 0.023 μM (n = 31cells) by using fura 2. Addition of ZP glycoprotein extracts (40 μg/ml; 4 ng of protein/ZP) produced transient Ca2+i elevations that were resolved by mag-fura 2 in 73% (19/26) of cells (Fig. 1 A and B). Similar responses were noted in 65% (24/37) of cells treated with purified ZP3 from an equivalent number of ZP (10 μg/ml; 1 ng ZP3/ZP; Fig. 1B). The presence of nonresponsive cells is consistent with observations that only 40–80% of mouse sperm respond to ZP3 (13, 18, 27) and may reflect inefficient capacitation in vitro. Acrosome reactions occurred within 15 min in 93% (40/43) of cells exhibiting ZP/ZP3-evoked transients but occurred in only 15% (3/20) of cells in which transients were not detected.
Figure 1.
ZP3 produces Ca2+i transients that are detected with mag-fura2. (A) Dye (λEX, 360 nm; λEM, 510 nm) distributes throughout sperm and permits ratiometric image acquisition from all regions. (B) Ca2+i responses to ZP3 treatment in the absence (○) and the presence (●) of PN200–110. Cells were treated with buffer (b) or with 1 μM PN200–110 (pn) for 10 min before addition of ZP3 (10 μg/ml; bar). The rising phase of the ZP3-dependent Ca2+i response in the absence of channel antagonists is approximated by the expression Ca2+i = et/τ, where t and τ represent the time and the time constant, respectively (—). In this cell, τ = 12.7 msec. The initial portion of the relaxation trace is approximated by the expression Ca2+i = ae−t/τ, where a designates the maximal response (- - -) and where a = 9.11 μM and τ = 40.9 msec. (C) Peak Ca2+i responses to ZP (40 μg/ml) or to ZP3 (10 μg/ml) are inhibited by La3+ (○) and by Cd2+ (●). Data points represent the means (±SD) of observations on 4–7 different cells and are approximated by the expression R = (100 ∗ IC50)/(IC50 + D). R is observed response; D is antagonist concentration; and IC50 is the drug concentration producing 50% inhibition. Derived IC50 values were La3+, 171 ± 63 μM; and Cd2+, 652 ± 134 μM. Results using ZP3 and ZP are indistinguishable and are pooled for presentation. (D) ZP3-dependent Ca2+i transients are inhibited by LVA current antagonists. Pimozide was added at 15 min of a 120-min incubation in a capacitating medium whereas PN200–110 and Ni2+ were added at 90 min. After incubation for 120 min, sperm were treated with ZP3. Data represent peak Ca2+i values (mean ± SD). The fraction of cells in each treatment group that exhibit transients is indicated. Total cells were: buffer, 12; fetuin (40 μg/ml), 14; ZP (40 μg/ml), 26; ZP2 (40 μg/ml), 14; ZP3 (10 μg/ml), 37; +PN200–110 (0.1 μM), 9; +PN200–110 (1 μM), 11; +pimozide (0.1 μM), 12; +pimozide (1 μM), 16; +Ni2+ (5 μM), 8; +Ni2+ (50 μM), 7.
Sperm were treated in control experiments with proteins (BSA, fetuin, mouse ZP2) that did not promote acrosome reactions. Such treatments failed to initiate Ca2+i transients (0/37 cells), although Ca2+i levels rose linearly to 0.209 ± 0.027 μM during the 10-min treatment period (Fig. 1B). Thus, Ca2+i transients are a specific response to ZP3 stimulation.
The ZP3-dependent Ca2+i transient had the following salient characteristics. (i) It occurred after a time lag (mean = 9 ± 4 sec, n = 43) that reflects, in part, ZP3 diffusion to sperm. (ii) Ca2+i increased from 10 to 90% of peak values in 37 ± 11 msec, and the time course was approximated by a single exponential expression (τ = 11.4 ± 2.3 msec). The rise time reflects Ca2+ influx into and diffusion from the small internal volume of sperm (10−14 liters) (30), where significant changes in Ca2+i may be achieved by a small number of activated channels. (iii) Peak Ca2+i values of 9.2 ± 1.3 μM were resolved. (iv) Ca2+i levels returned to basal levels within 179 ± 23 msec, with the initial portion of the relaxation curve approximated by a single exponential expression (τ = 47.2 ± 6.8 msec). (v) A single transient was observed in 85% (34/40) of responsive sperm, with the remaining cells exhibiting 2–4 spikes of similar magnitude and time course during a 1- to 4-sec interval. The activation time course of this transient is similar to that of the LVA Ca2+ current (6, 8, 9, 11, 12, 26).
To determine whether the ZP3-dependent Ca2+i transient requires influx of extracellular Ca2+, experiments were carried out in media supplemented with either Cd2+ or La3+. These cations block Ca2+entry through a variety of transport mechanisms, including voltage-sensitive channels. La3+ and Cd2+ inhibited peak Ca2+i responses during transients with IC50 values of 171 ± 63 and 652 ± 134 μM, respectively (Fig. 1C).
The relationship of the sperm Ca2+i transient that is detected by optical methods and the spermatogenic cell LVA current detected by patch clamp methods was examined in experiments using channel antagonists. Peak Ca2+i levels during ZP3-dependent transients were inhibited in a concentration-dependent manner by PN200–110, a 1,4-dihydropyridine; by pimozide, a diphenylbutylpiperidine; and by Ni2+ (Fig. 1 B and D). The inhibitory efficacy of these agents is similar to that reported previously for the inhibition of both the LVA Ca2+ current of somatic cells (28–32) and spermatogenic cells (6, 9, 11), as well as for the inhibition of ZP-initiated acrosome reactions (6). These observations demonstrate that ZP3 promotes Ca2+i transients that are coupled to induction of acrosome reactions and that have the anticipated time course and inhibitor sensitivity of LVA Ca2+ currents.
VM Regulation During Sperm Capacitation.
LVA Ca2+ currents exhibit voltage-dependent, steady-state inactivation (26, 32). In spermatogenic cells, the threshold and half-maximal voltages for inactivation were −80 and −72 mV, respectively, and currents were not readily activated from holding potentials more positive than −60 mV (6). To assess the availability of LVA channels for activation during ZP3 stimulation, it is necessary to determine sperm VM.
VM was determined from the fluorescence emission of di8-ANEPPS (23, 33). The population-averaged VM, derived from values for single cells as reported by this probe, was −25.7 ± 6.6 mV after 5 min of incubation in capacitating medium (range = −45 to −11 mV; n = 75) and −63.4 ± 17.1 mV by 90–120 min (range at 120 min, −92 to −32 mV; Fig. 2A, ▪). These estimates from immobilized sperm agree with previous data from motile populations (34–36) suggesting that VM is not perturbed by immobilization. In contrast, sperm failed to hyperpolarize during incubation in a 0.1% BSA medium that does not promote efficient capacitation: VM was −23.6 ± 4.7 mV after a 5-min incubation and decreased to only −35.3 ± 6.8 mV after 120 min (Fig. 2A, □; n = 64).
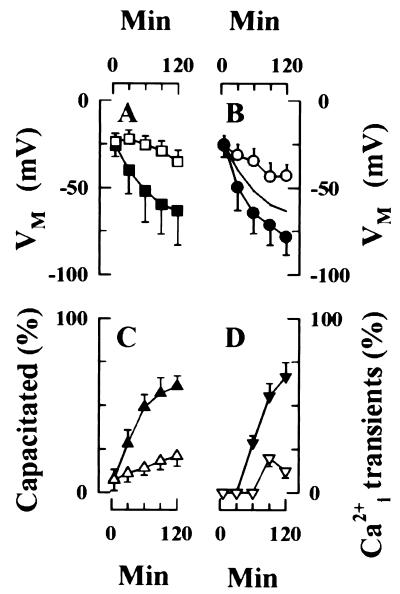
Figure 2.
Capacitation results in membrane hyperpolarization. (A) Hyperpolarization of sperm populations during capacitation. VM values of immobilized sperm were calculated from di8-ANEPPS emission of single cells during a 120-min incubation under conditions that do (■, 75 cells) or do not support capacitation (□, 61 cells). Data are the mean (±SD) of 5–8 different experiments and entail determinations from the indicated number of cells. (B) Membrane hyperpolarization is associated with capacitation. Capacitation state of individual sperm was determined by ability of sperm to initiate exocytosis during a subsequent challenge with ZP. Data from 75 cells were summed to assemble population VM values (—; redrawn from A), as well as from the subpopulations that are (●) or are not (○) capacitated by 120 min. Strong hyperpolarization is restricted to the subpopulation of sperm that capacitate. (C) The time course of capacitation was determined by CTC fluorescence emission. Motile sperm were incubated in a capacitating medium (▴) or in a medium that does not capacitate mouse sperm efficiently (▵). Data are the mean ± SD of triplicate experiments, with 200 sperm assayed/time point in each experiment. (D) Sperm develop the ability to generate ZP/ZP3-dependent Ca2+i transients during capacitation. Sperm were incubated for the indicated times either in a capacitating medium (▾) or in a noncapacitating medium (▿), as described in C and were stimulated with either ZP (40 μg/ml) or ZP3 (10 μg/ml), and Ca2+i responses were recorded. Data represents mean (±SD) of observations on 3–8 cells, with ZP- and ZP3-stimulated responses pooled for presentation.
The relationship between hyperpolarization and capacitation was examined in greater detail by monitoring VM during a 120-min incubation in a capacitating medium. Sperm were subsequently challenged with ZP extracts or with purified ZP3. These preparations induce acrosome reactions only in capacitated sperm (13). In these experiments, 57% (43/75) of sperm acrosome reacted in response to ZP/ZP3 stimulation, and VM of these cells was −78.7 ± 10.2 mV (range = −52 to −92 mV; n = 43) at 120 min (Fig. 2B, ●). In contrast, VM of the group that failed to acrosome react was −43.2 ± 6.8 mV (range = −32 to −57 mV; n = 32; Fig. 2B, ○).
These experiments used immobilized sperm, permitting the reconstruction of time-dependent alterations in VM in individual cells during incubation. Sperm were grouped according to whether they underwent acrosome reactions when challenged with ZP3 after 120 min of incubation (Fig 2B, ●) or failed to do so (○). Fig. 2B shows a time course for changes in VM during incubation. Sperm from both populations had a narrow and overlapping distribution of VM values after 5 min of incubation in capacitating medium (Fig 2B, ●, −25.4 ± 7.1 mV; ○, −25.9 ± 5.9 mV). However, sperm segregated into two populations with different VM ranges during further incubation: Cells that capacitated by 120 min in this assay system hyperpolarized by >50 mV (Fig 2B, ●) whereas cells that failed to capacitate hyperpolarized by <20 mV (Fig. 2B).
The progress of sperm capacitation also was determined by using CTC fluorescence emission. Fig. 2C shows that 61% of sperm capacitated during a 120-min incubation in medium HBCM (▴). The time course of capacitation, as assessed in this independent assay, parallels membrane hyperpolarization (Fig. 2 A and B). In contrast, only 21% of sperm exhibited the CTC fluorescence labeling pattern associated with capacitation during incubation in a low-BSA medium (Fig. 2C, ▵). This limited response parallels the weak hyperpolarization of sperm VM that was noted during incubation in the low-BSA medium (Fig. 2A, □).
In addition, we determined the effects of incubation under capacitating conditions on ZP/ZP3-dependent Ca2+i transients. In these experiments, sperm were incubated for 5–120 min in a capacitating medium and subsequently were stimulated with either ZP extracts or with purified ZP3 (Fig. 2D, ▾). Ca2+i transients were not observed in sperm during the first 30 min of incubation but were apparent after prolonged incubation. A maximal effect, in which >60% of sperm exhibited transient responses, was observed by 90 min of incubation (t1/2 ≈70 min). In contrast, Ca2+i transients were observed in <20% of sperm that were incubated for similar time periods in a medium that does not support capacitation (Fig. 2D, ▿). These experiments demonstrate that ZP3-evoked Ca2+i transients only occur in capacitated sperm populations. Moreover, this response and membrane hyperpolarization developed during incubation with similar time courses.
To determine whether hyperpolarization is necessary for exocytosis, sperm were incubated under capacitating conditions, and VM was adjusted to −46.7 ± 7.2 mV (range = −29 to −54 mV; n = 41) with 30 mM K+o. As shown in Table 1, hyperpolarization was required for the induction of acrosome reactions by ZP.
Table 1.
Effect of K+o on the ZP-dependent acrosome reaction
| Percent of acrosome-reacted sperm
|
||
|---|---|---|
| 30 mM K+o | 3 mM K+o | |
| +Buffer | 15 (10/68) | 12 (5/43) |
| +Ionomycin | 71 (76/107) | 84 (70/83) |
| +ZP | 20 (17/83) | 57 (43/75) |
Sperm were incubated in medium HBCM for 120 min; medium K+o was adjusted to the indicated values; sperm were treated with equal volumes of buffer, of ionomycin (5 μM final concentration), or of ZP (40 μg/ml final protein concentration); and the fraction of acrosome reacted cells was determined after an additional 30-min incubation. Data represent the percent of acrosome-reacted cells as well as the number of acrosome reacted and total sperm examined.
Effects of Sperm VM on Ca Channel Antagonist Action.
Thus, VM attains values more negative than −75 mV during capacitation, as measured fluorometrically. This value would be sufficient to relieve steady-state, voltage-dependent inactivation of LVA Ca2+ currents (6, 26). This suggests that LVA channels shift during capacitation from an inactivated state to a closed state that can be subsequently activated during ZP3-evoked depolarization. This hypothesis was tested by using pimozide, an agent that previously was shown to inhibit the inactivated state of spermatogenic cell LVA currents with high affinity but to block the closed state of this channel with lower affinity (9). Sperm were treated with pimozide (0.001–50 μM) either after 15 or 90 min of incubation. Two aspects of sperm function that depend on LVA channel activity were measured: ZP-dependent acrosome reactions and fertilization.
Exocytosis and fertilization were inhibited with IC50 values of 0.53 ± 0.02 and 0.38 ± 0.03 μM, respectively, when pimozide was introduced after 15 min of incubation in capacitating medium, (Fig. 3 A and B). This efficacy is similar to that for the inhibition of the inactivated state of the LVA current in spermatogenic cells (IC50 = 0.72 ± 0.13 μM; Fig. 3C) (9). However, when pimozide was added at 90 min of incubation, it inhibited exocytosis and fertilization with IC50 values of 28.7 ± 1.6 and 14.1 ± 3.6 μM, respectively (Fig. 3 A and B). These values are similar to that with which it inhibits the closed state of the spermatogenic cell LVA current (IC50 = 14.0 ± 2.1 μM; Fig. 3C).
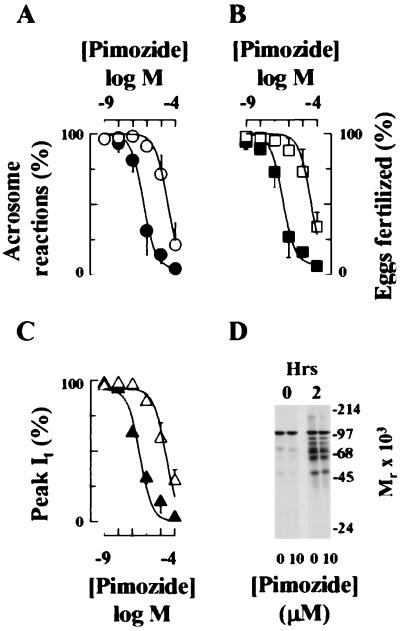
Figure 3.
Effects of capacitation state on the inhibition of ZP3-dependent exocytosis and fertilization by Ca2+ channel antagonists. (A and B) Pimozide was added to sperm either at 15 min (●, ■) or at 90 min (○, □) of a 120-min incubation in a capacitating medium. Sperm subsequently were assayed for the ability to undergo ZP-dependent exocytosis (A, ● and ○) or fertilize eggs in vitro (B, ■ and □). Data are the results (mean ± SD) of 2–3 separate experiments in which each experiment determined the acrosome reaction status of 200 sperm or the fertilization status of 31–56 eggs. (C) Inhibition of LVA current of a pachytene spermatocyte by pimozide. Germ cells were treated with pimozide at −80 mV (▵) or to −45 mV (▴), when channels are predominantly in the closed and inactivated states, respectively. VM subsequently was returned to holding potential (−90 mV), and Ca2+ currents were measured during steps to a −20-mV test potential. Drug was present during prepulse and test pulse intervals. (A–C) Data were corrected for background levels of exocytosis, fertilization, or leak currents and then were approximated by the expression R = (100 * IC50)/(IC50 + D). These terms are defined in Fig. 1. Values in the absence of pimozide were background exocytosis, 23 ± 8%; exocytosis in ZP-treated sperm, 74 ± 9%; fertilization, 71 ± 8%. IC50 values are provided in the text. (D) Pimozide does not inhibit capacitation. Capacitation of sperm populations was assayed by protein tyrosine phosphorylation after a 120-min incubation in capacitating medium. Lanes: 1, uncapacitated sperm, 0 min; 2, uncapacitated sperm, + pimozide, 0 min; 3, sperm incubated for 120 min in capacitating medium; 4, sperm incubated 120 min in capacitating medium containing pimozide.
This reduced efficacy was not the indirect effect of an inhibition of capacitation, as demonstrated by control studies in which capacitation was determined by CTC fluorescence (data not shown) or by sperm protein tyrosine phosphorylation (Fig. 3D). Additional control experiments addressed the effects of the duration of drug treatment on efficacy. Sperm were incubated in medium HBCM for 90 min, at which time capacitation was maximal (Fig. 2C), and then were treated with pimozide for 105 min. In this protocol, pimozide acted as a low-efficacy inhibitor of the acrosome reactions and fertilization, with IC50 values of 11.4 ± 4.2 and 8.9 ± 2.5 μM, respectively. Thus, the higher efficacy of inhibition by pimozide when this agent was added at early times during capacitation is not caused by the duration of drug treatment. These data support the hypothesis that LVA channels are predominantly in the inactivated state in uncapacitated sperm but do not remain predominantly in this state after capacitation. Moreover, conductance state-specific antagonists may be selected that are highly efficacious antagonists of LVA channels in uncapacitated sperm.
DISCUSSION
The two central observations of this study are that (i) transient Ca2+i elevations occur during the initial seconds of sperm stimulation by ZP3; and (ii) membrane hyperpolarization during capacitation regulates the ability of sperm to generate transient Ca2+i elevations. Ca2+i is a mediator of the acrosome reaction that is initiated during contact with eggs. Previously, an essential role of sperm LVA currents in ZP3 signal transduction had been deduced, based on the presence of these currents in spermatogenic cells (6, 10, 11) and on the effects of LVA channel antagonists on acrosome reactions (6). However, it had not been possible to detect LVA currents in sperm because patch clamp recordings are not readily obtained from these cells and because attempts to monitor Ca2+i responses associated with LVA channel activity were limited previously by the low temporal resolution (≥15 sec) of fluorescence detector systems (7).
We used an optical detector with an estimated time resolution of 2–4 msec to resolve a Ca2+i transient during the first seconds of ZP3 stimulation. This transient has the kinetic and pharmacological features anticipated of LVA currents. First, the time courses for activation and inactivation were similar. The time constants for Ca2+i transient activation and inactivation, as determined optically, were 10–15 msec and 40–50 msec, respectively. Similar values are observed for LVA currents of rodent spermatogenic cells and of somatic cells by using whole cell–patch clamp methods (6, 26, 31, 32, 37, 38).
Second, structurally unrelated classes of Ca2+ channel antagonists had similar inhibitory effects on the spermatogenic cell LVA current and on the sperm Ca2+i transient. For example, pimozide and Ni2+ inhibited peak Ca2+i responses to ZP3 with IC50 values of 0.1–1 and 5–50 μM (Fig. 1B), respectively, and these same agents inhibited spermatogenic cell LVA currents with IC50 values of 0.46 and 34 μM, respectively (6, 9). The 1,4-dihydropyridines inhibit LVA currents from many cells, although a wide range of efficacies are reported. In spermatogenic cells (6, 9), as well in several neuronal preparations and in atrial myocytes (28, 29, 39–41), LVA currents are inhibited by these drugs with IC50 values ≤1 μM. The 1,4-dihydropyridine, PN200–110, inhibited both peak Ca2+i responses in sperm and LVA current in spermatogenic cells with similar efficacies (IC50 values of 0.1–1 and 0.04 μM, respectively). Finally, the Ca2+i transient was more sensitive to inhibition by Ni2+ than by Cd2+, a characteristic shared by LVA currents but not by most other classes of voltage-sensitive Ca2+ channels (26, 31, 42, 43). These shared kinetic and pharmacological features strongly suggest that the ZP3-evoked Ca2+i transient reflects the activation of LVA currents during sperm contact with the ZP.
The rapid Ca2+i transients in response to ZP3 stimulation that are documented here were not observed in previous reports. That failure may be attributed to the lower time resolution of the detector systems used in those earlier studies. Instead, we and others had recorded Ca2+i responses to ZP3 that developed with a slower time course and were of lower peak amplitude (6, 25, 44–46). This delayed response is inhibited by LVA current antagonists and likely reflects the activation of a downstream Ca2+i regulatory mechanism that depends on the LVA current. One proposed mechanism includes the release of sequestered Ca2+ from intracellular stores through an IP3 receptor pathway (47) and is compatible with the well established modulation of that channel by cytosolic Ca2+ (48, 49).
A signature feature of LVA currents is a low threshold for voltage-dependent inactivation. These currents inactivate at holding potentials between −80 and −60 mV, and currents cannot readily be activated from more positive holding potentials (6, 26, 50, 51). This inactivation range has two implications.
First, it suggests that sperm must maintain a hyperpolarized VM during the early stages of interaction with the ZP. Previous VM determinations were based on population-averaged signals obtained from sperm suspensions, and the derived values were sufficiently depolarized so as to impose complete voltage-dependent inactivation on LVA currents (7, 34–36). It was observed in those studies that membrane hyperpolarization accompanies capacitation, but the final VM was only ≈−55 mV (36) and hence was not sufficient to relieve inactivation. However, the interpretation of those data was complicated by observations that only a portion of sperm populations capacitate in vitro (1). In the present study, VM of single sperm is estimated and is correlated with capacitation state. These studies confirm that VM of uncapacitated sperm is relatively depolarized. Sperm that capacitate in vitro, as assessed by both signal transduction-based and fluorescent probe assays, exhibit strong hyperpolarization to a value that is sufficient to relieve voltage-dependent inactivation. In contrast, sperm that fail to capacitate during these incubations exhibit a weak hyperpolarization that will not remove inactivation.
Sperm have only a single secretory vesicle, and exocytosis must be coordinated with egg contact for efficient fertilization (1, 3). The present study supports a mechanism in which the ability of sperm to undergo ZP3-dependent acrosome reactions is regulated by the availability of LVA channels for opening and in which that availability is controlled during capacitation by VM. Specifically, membrane depolarization of uncapacitated sperm, with the attendant voltage-dependent inactivation of LVA currents, provides a means of suppressing premature secretion until the completion of capacitation-associated hyperpolarization, at which time sperm have arrived near the site of fertilization and are primed for ZP3-dependent activation. Previous studies indicate that this hyperpolarization is mediated by an increased contribution of K+ permeability to sperm VM (36). In this regard, a large number of biochemical modifications have been described within sperm during capacitation (1, 52), yet the role of such events in the acquisition of fertility remains poorly understood. The present study represents a case in which a molecular mechanism can account for certain aspects of capacitation.
The recognition of the voltage-dependent inactivation of sperm LVA currents, and the regulation of conductance states during capacitation, also has implications with regard to the design of channel-based antifertility agents. Antagonists that bind to the inactivated state with high affinity, such as pimozide (9), are likely to be most effective when VM is relatively depolarized, as occurs in uncapacitated sperm and also may occur during sperm storage within the male (53). Pimozide also inhibits sperm function after capacitation, albeit with lower efficacy. This may be attributable to the fact that transitions between conductance states are stochastic and occur to some extent at all VM values (26). In contrast, agents that inhibit conductance by binding to the closed state of germ cell LVA channels with high affinity, such as 1,4-dihydropyridines (9), are expected to inhibit fertilization with high efficacy only after capacitation. The significance of these observations lies not in the specific feasibility of pimozide for such use but, rather, in the demonstration that LVA channel conductance state is regulated as sperm prepare for fertilization and can provide new targets for channel-based antagonists.
Acknowledgments
We thank Drs. Kathleen Dunlap, Michele Jacob, and Bayard Storey for valuable discussions. This work was supported by the Centre National de la Recherche Scientifique (C.A. and M.V.) and by the National Institutes of Health (Grant HD32177 to H.M.F. and Grant HD06274 to G.S.K.).
ABBREVIATIONS
- ZP
zona pellucida
- LVA
low voltage-activated
- CTC
chlortetracycline
- di8-ANEPPS
4-[2-[6-(dioctylamino)-2 naphthalenyl]ethenyl]-1-(3-sulfopropyl)]
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Yanagimachi R. In: Mammalian Fertilization. Knobil E, Neill J D, editors. New York: Raven; 1994. pp. 189–317. [Google Scholar]
- 2.Wassarman P M, Florman H M. In: Handbook of Physiology. Hoffman J F, Jamieson J, editors. Vol. 14. New York: Oxford Univ Press; 1997. pp. 885–938. [Google Scholar]
- 3.Wassarman P M. Cell. 1999;95:176–183. [Google Scholar]
- 4.Benoff S, Cooper G W, Hurley I, Mandel F S, Rosenfeld D L, Scholl G M, Gilbert B R, Hershlag A. Fertil Steril. 1994;62:606–617. [PubMed] [Google Scholar]
- 5.Hershlag A, Cooper G W, Benoff S. Hum Reprod. 1995;10:599–606. doi: 10.1093/oxfordjournals.humrep.a135996. [DOI] [PubMed] [Google Scholar]
- 6.Arnoult C, Cardullo R A, Lemos J R, Florman H M. Proc Natl Acad Sci USA. 1996;93:13004–13009. doi: 10.1073/pnas.93.23.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnoult C, Zeng Y, Florman H M. J Cell Biol. 1996;134:637–645. doi: 10.1083/jcb.134.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnoult C, Lemos J R, Florman H M. EMBO J. 1997;16:1593–1599. doi: 10.1093/emboj/16.7.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnoult C, Villaz M, Florman H M. Mol Pharmacol. 1998;53:1104–1111. [PubMed] [Google Scholar]
- 10.Hagiwara S, Kawa K. J Physiol. 1984;356:135–149. doi: 10.1113/jphysiol.1984.sp015457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lievano A, Santi C M, Serrano J, Trevino C L, Bellve A R, Hernandez-Cruz A, Darszon A. FEBS Lett. 1996;388:150–154. doi: 10.1016/0014-5793(96)00515-7. [DOI] [PubMed] [Google Scholar]
- 12.Santi C M, Darszon A, Hernandez-Cruz A. Am J Physiol. 1996;271:C1583–C1593. doi: 10.1152/ajpcell.1996.271.5.C1583. [DOI] [PubMed] [Google Scholar]
- 13.Ward C R, Storey B T. Dev Biol. 1984;104:287–296. doi: 10.1016/0012-1606(84)90084-8. [DOI] [PubMed] [Google Scholar]
- 14.Saling P M, Storey B T. J Cell Biol. 1979;83:544–555. doi: 10.1083/jcb.83.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visconti P E, Moore G D, Bailey J L, Leclerc P, Connors S A, Pan D, Olds-Clarke P, Kopf G S. Development (Cambridge, UK) 1995;121:1139–1150. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- 16.Visconti P E, Bailey J L, Moore G D, Pan D, Olds-Clarke P, Kopf G S. Development (Cambridge, UK) 1995;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- 17.Miller D J, Gong X, Shur B D. Development (Cambridge, UK) 1993;118:1279–1289. doi: 10.1242/dev.118.4.1279. [DOI] [PubMed] [Google Scholar]
- 18.Florman H M, Storey B T. Dev Biol. 1982;91:121–130. doi: 10.1016/0012-1606(82)90015-x. [DOI] [PubMed] [Google Scholar]
- 19.Bleil J D, Wassarman P M. Cell. 1980;20:873–882. doi: 10.1016/0092-8674(80)90334-7. [DOI] [PubMed] [Google Scholar]
- 20.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 21.Grynkiewicz G, Poenie M, Tsien R Y. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 22.Konishi M, Hollingworth S, Harkins A B, Baylor S M. J Gen Physiol. 1991;97:271–301. doi: 10.1085/jgp.97.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montana V, Farkas D L, Loew L M. Biochemistry. 1989;28:4536–4539. doi: 10.1021/bi00437a003. [DOI] [PubMed] [Google Scholar]
- 24.Go K J, Wolf D P. Biol Reprod. 1985;32:145–152. doi: 10.1095/biolreprod32.1.145. [DOI] [PubMed] [Google Scholar]
- 25.Florman H M, Tombes R M, First N L, Babcock D F. Dev Biol. 1989;135:133–146. doi: 10.1016/0012-1606(89)90164-4. [DOI] [PubMed] [Google Scholar]
- 26.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer; 1992. pp. 1–594. [Google Scholar]
- 27.Bleil J D, Wassarman P M. Dev Biol. 1983;95:317–324. doi: 10.1016/0012-1606(83)90032-5. [DOI] [PubMed] [Google Scholar]
- 28.Akaike N, Kostyuk P G, Osipchuk Y V. J Physiol. 1989;412:181–195. doi: 10.1113/jphysiol.1989.sp017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen C J, McCarthy R T. J Physiol. 1987;387:195–225. doi: 10.1113/jphysiol.1987.sp016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enyeart J J, Biagi B A, Day R N, Sheu S-S, Maurer R A. J Biol Chem. 1990;265:16373–16379. [PubMed] [Google Scholar]
- 31.Herrington J, Lingle C J. J Neurophysiol. 1992;68:213–232. doi: 10.1152/jn.1992.68.1.213. [DOI] [PubMed] [Google Scholar]
- 32.Huguenard J R. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- 33.Bedlack R S, Wei M-D, Loew L M. Neuron. 1992;9:393–403. doi: 10.1016/0896-6273(92)90178-g. [DOI] [PubMed] [Google Scholar]
- 34.Babcock D F, Pfeiffer D R. J Biol Chem. 1987;262:15041–15047. [PubMed] [Google Scholar]
- 35.Espinosa F, Darszon A. FEBS Lett. 1995;372:119–125. doi: 10.1016/0014-5793(95)00962-9. [DOI] [PubMed] [Google Scholar]
- 36.Zeng Y, Clark E N, Florman H M. Dev Biol. 1995;171:554–563. doi: 10.1006/dbio.1995.1304. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez J L, Vassort G. J Gen Physiol. 1992;100:519–545. doi: 10.1085/jgp.100.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coulter D A, Huguenard J R, Prince D A. J Physiol. 1989;414:587–604. doi: 10.1113/jphysiol.1989.sp017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akaike N, Kanaide H, Kuga T, Nakamura M, Sadoshima J-I, Tomoike H. J Physiol. 1989;416:141–160. doi: 10.1113/jphysiol.1989.sp017754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richard E A, Lisman J E. Nature (London) 1992;356:336–338. doi: 10.1038/356336a0. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K, Akaike N. J Pharmacol Exp Ther. 1991;256:169–175. [PubMed] [Google Scholar]
- 42.Bourinet E, Zamponi G W, Stea A, Soong T W, Lewis B A, Jones L P, Yue D T, Snutch T P. J Neurosci. 1996;16:4983–4993. doi: 10.1523/JNEUROSCI.16-16-04983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ertel S I, Ertel E A. Trends Pharmacol Sci. 1997;18:37–42. doi: 10.1016/s0165-6147(96)01021-8. [DOI] [PubMed] [Google Scholar]
- 44.Bailey J L, Storey B T. Mol Reprod Dev. 1994;39:297–308. doi: 10.1002/mrd.1080390307. [DOI] [PubMed] [Google Scholar]
- 45.Florman H M. Dev Biol. 1994;165:152–164. doi: 10.1006/dbio.1994.1242. [DOI] [PubMed] [Google Scholar]
- 46.Storey B T, Hourani C L, Kim J B. Mol Reprod Dev. 1992;32:41–50. doi: 10.1002/mrd.1080320108. [DOI] [PubMed] [Google Scholar]
- 47.Walensky L D, Snyder S H. J Cell Biol. 1995;130:857–869. doi: 10.1083/jcb.130.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bezprozvanny I, Watras J, Ehrlich B E. Nature (London) 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 49.Finch E A, Turner T J, Goldin S M. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- 50.Tsien R W, Lipscombe D, Madison D V, Bley K R, Fox A P. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- 51.Tsien R W, Lipscombe D, Madison D, Bley K, Fox A. Trends Neurosci. 1995;18:52–54. [PubMed] [Google Scholar]
- 52.Ward C R, Kopf G S. Dev Biol. 1993;158:9–34. doi: 10.1006/dbio.1993.1165. [DOI] [PubMed] [Google Scholar]
- 53.Hinton B T, Palladino M A. Microsc Res Tech. 1995;30:67–81. doi: 10.1002/jemt.1070300106. [DOI] [PubMed] [Google Scholar]