Abstract
Epimorphin was recently described as a mesenchymal factor modulating morphogenesis of murine mammary ducts, skin, liver, and lung in vitro. In this study epimorphin was analyzed in a human, pancreatic adenocarcinoma cell line (A818-6) which develops single layer epithelial hollow spheres resembling normal pancreatic ductal structures in vitro. Soluble 34- and 31-kD isoforms of epimorphin were found in the culture supernatant of A818-6 cells. In lysates of A818-6 cells we detected the 34-and 31-kD isoforms and the dimers, and in lysates of fibroblasts the 150-kD tetramers of epimorphin additionally. A neutralizing monoclonal antibody against epimorphin (MC-1) efficiently blocked the development of hollow sphere structures from A818-6 cells. Coculture of A818-6 cells with fibroblasts stimulated the development of hollow sphere structures in general and increased differentiation in 5–6-d-old hollow spheres. A818-6 hollow sphere development in the presence of fibroblasts was also blocked by MC-1. In this novel system for human duct-like differentiation of pancreatic epithelial cells, we provide evidence for an autocrine and paracrine function of epimorphin as a major mediator for morphogenesis.
Keywords: epimorphin, pancreas, epithelial differentiation, spheres, duct structures
Introduction
Distinct stages in the development of epithelial organs, and in particular of the pancreas, depend on the presence of a mesenchymal support (Sanvito et al. 1994; LeBras et al. 1998). Mesenchymal cells produce and secrete morphogenic factors and mediators that influence epithelial cell growth and morphology. These include cell surface molecules, such as nerve growth factor receptor and ganglioside GD-3 (Sariola et al. 1988, Sariola et al. 1991). It has been suggested that growth factors such as hepatocyte growth factor (HGF/scatter factor), keratinocyte growth factor (KGF), basic fibroblast growth factor (bFGF), and new differentiation factor/heregulin (HRG) act more as growth stimulators than morphogenic factors, and epimorphin has been reported to act as a key morphoregulator for mouse mammary epithelial cells (Hirai et al. 1998). In addition to mammary epithelial cells, epimorphin has been reported to be an extracellular factor involved in the morphogenesis of various cell types, such as endothelial cells, hepatocytes, and lung epithelial cells (Oka and Hirai 1996; Hirai et al. 1998; Watanabe et al. 1998). In Western blot analyses, human epimorphin was detected by an anti–mouse antibody (MC-1) in MRC-5 fibroblast cells or in skin samples from human fetuses (Hirai et al. 1993; Akiyama et al. 1999). As well as MC-1, several polyclonal antibodies against epimorphin have been described (Hirai 1993; Hirai et al. 1998). Epimorphin is expressed in three isoforms. Isoforms I and II display 34-kD proteins and isoform III is a 31-kD protein lacking a putative membrane-spanning region. These isoforms are generated by alternative splicing. The 150- and 70-kD protein bands detected by antibodies against epimorphin in Western blot analyses are comprised of dimers and tetramers of the smaller isoforms (Hirai et al. 1998). MC-1 and affinity-purified polyclonal antibodies against epimorphin were further capable of inhibiting the biological function of epimorphin when added to the cell culture medium (Hirai 1994; Hirai et al. 1998).
Recently, several three-dimensional (3D) model systems have been developed in vitro to mimic the structural and functional characteristics of normal epithelia in a manner that closely approximates in vivo conditions (Boxberger et al. 1993, Boxberger et al. 1997). The 3D ductal architecture has been shown to be of critical importance for the development and maintenance of barrier function, not only in ducts of the human pancreas (Pabst 1987; Arendt 1991; Azghani et al. 1993). In this study we introduce a novel cell culture system consisting of a highly differentiated, adherence-independent growth of the pancreatic adenocarcinoma cell line A818-6. These cells develop single layer epithelial structures (hollow spheres) autonomously when the cells are prevented from adherence. The focus of this study was to unravel the role of epimorphin in this particular 3D model system for the analysis of differentiation in human pancreatic duct cells.
Materials and Methods
Cells
The original cells from which the pancreatic cell line A818 was generated were isolated from the ascites of a 75-yr-old female patient with a differentiated adenocarcinoma in the head of the pancreas. By limited dilution, the subclone A818-6 was obtained together with other subclones. All other cell lines used have been described previously (Kalthoff et al. 1993) or were obtained from the American Type Culture Collection (BxPC-3 CRL-1687; Panc-1 CRL-1469; 293T CRL 1658). Except for 293T, all cell lines were cultured in RPMI 1640 supplemented with 10% FCS, 2 mM glutamine, 1 mM sodium pyruvate, 100 U penicillin/ml, and 100 μg streptomycin/ml. The 293T cells were cultured in DME supplemented with 10% FCS, 2 mM glutamine, 1 mM sodium pyruvate, and 1× nonessential amino acids. Cells were trypsinized with 0.25% trypsin and 0.01% EDTA (both from Life Technologies). Hexadimethrine bromide (polybrene/HDMB) was obtained from Sigma-Aldrich. For standard experiments, cells were seeded in plastic 6-well plates at a density of 1–5 × 105 cells. For 3D growth, cells were seeded in precoated 6-well plates at the same density. The coating solution contained agarose (Life Technologies) at 31 mg/ml mixed with RPMI 1640 (without supplements, preheated to 37°C) at a ratio of 1:3. 1 ml was added to each well. This coating prevented cell adhesion to the tissue culture dishes. After the phase of sphere induction (6–8 d on agarose-coated culture dishes), hollow spheres were transferred back into standard plastic tissue culture dishes. This procedure initiated the phase of hollow sphere maturation (days 8–12). For all subsequent experiments hollow spheres aged ≥2 wk were used. Before immunoprecipitation experiments, A818-6 cells were cultured under serum-free conditions for 24 h. Foreskin-derived fibroblasts (Kif-5) were used for coculture experiments. This fibroblast cell line was established previously in our laboratory. Fibroblasts were grown in fibroblast growth medium (PromoCell) containing all supplements, 10% FCS, 2 mM glutamine, 1 mM sodium pyruvate, 100 U penicillin/ml, and 100 μg streptomycin/ml. Before immunoprecipitation experiments, fibroblasts were cultured under serum-free conditions for 24 h. All cell lines were routinely checked for mycoplasma contamination by the PCR-based mycoplasma detection kit from TaKaRa (BioWhittaker Europe).
Antibodies, Recombinant Proteins, and Plasmids
All secondary antibodies were obtained from Dianova. The antibody KiS5, and all materials used in the telomerase assays were a gift from Prof. Parwaresch (Institute of Hematopathology, Christian-Albrechts University). The antibody against c-met was from NovoCastra (Loxo GmbH). The antibodies against β-catenin, cyclin B, p27/Kip, and β-actin were from Transduction Laboratories. The antibody against carbonic anhydrase II (CAII) was a gift from Dr. Nishimori (Kochi Medical School, Kochi, Japan). The antibody against human IL-13 was obtained from BD PharMingen. Recombinant HGF, bFGF, EGF, TGF-α, TGF-β, and a neutralizing antibody against HGF were obtained from R&D Systems. Antibodies against epimorphin and their detailed characterization have been described previously (Hirai 1993, Hirai 1994; Hirai et al. 1998). phEGFPc-1 containing the humanized enhanced green fluorescent protein (EGFP) gene was purchased from CLONTECH Laboratories, Inc. The plasmid pCMV–vesicular stomatitis virus glycoprotein (VSV-G) was a gift from Dr. Paul Robins (University of Pittsburgh, Pittsburgh, PA). The antibody 214-D4 against MUC-1 was obtained from Dr. John Hilkens (University of Amsterdam, Amsterdam, Netherlands).
Immunocytochemistry/Immunofluorescence
Cells for immunostaining were harvested by trypsinization or scraping and cytocentrifugation onto microscopic slides. Completely developed hollow spheres were centrifuged onto protein-precoated microscope slides from Marienfeld. Cells were fixed on the microscope slides by incubation with acetone for 5 min. Staining was carried out by incubating for 1 h with the primary antibody, followed by three washes with PBS. The incubation time for the secondary antibody was 30 min. Detection was carried out with a DAB kit (Vector Laboratories).
Indirect immunofluorescence staining was performed in 5-ml plastic tubes from Nunc. Monolayer cells were scraped off the plates, transferred to tubes, and centrifuged. Hollow spheres were directly transferred into the tubes. All experiments were performed with permeabilized cells. Cells were prefixed and permeabilized for 3 min with 70% methanol. All cells were washed with ice-cold PBS containing 0.05% sodium azide and stained with the primary antibody for 1 h. All primary antibodies were used in concentrations ranging from 5 to 20 μg/ml. After washing three times with ice-cold PBS, the incubation with secondary antibody (Cy3-conjugated goat anti–mouse IgG + IgM) was carried out for 30 min. A final antibody-fixation step was carried out by adding 4% paraformaldehyde to the stained cells. Primary antibodies were diluted in PBS/1% BSA. The secondary antibodies were used at concentrations recommended by the supplier and were diluted in PBS containing 1% human serum. Visualization was carried out with an Axioskop2 fluorescence microscope for epifluorescence or with a LSM 510 laser-scanning microscope for confocal microscopy, both from ZEISS.
Transfection of Established Cell Lines and Generation of Transient VSV-G–pseudotyped Retroviral Supernatants
24 h before transfection, 106 293T cells were seeded into Primaria plates (Falcon 3803). 10 μg of each retroviral plasmid vector (EGFP/neo), 7 μg of the packaging plasmid pKAT 1.gag/pol.ATG, and 7 μg of pCMV-VSV-G were then cotransfected into 293T cells by calcium phosphate coprecipitation. Fresh medium was added 1 d later and viral supernatants were harvested 24 and 48 h later, filtered through 0.45-mm low protein binding Acrodisc™ filters (no. 4184; Gelman Sciences), and stored at −80°C.
Transduction of Kif-5 Fibroblasts
105 Kif-5 fibroblasts were seeded into 6-cm2 dishes containing complete medium and 8 μg/ml polybrene. 24 h later, EGFP/neo retroviral supernatants generated from the 293T producer cell line were used to infect this cell line at a multiplicity of infection of 10. For long term evaluation of gene expression and provector copy analysis, hEGFP/neo transduced fibroblasts were selected in G418 for 2 wk using 700 μg/ml.
Electron Microscopy
For transmission electron microscopy, confluent cells were harvested by scraping, and cells or hollow spheres in suspension were concentrated by centrifugation at 100 g and fixed in ice-cold 2% glutaraldehyde/2% formaldehyde solution at pH 7.4 with 0.1 M cacodylate buffer. Fixed cells and spheres were postfixed in 1% osmium tetroxide and embedded in Epon. Silver thin sections were contrasted with uranyl and lead and viewed under an electron microscope (EM 10; ZEISS).
Cytokine and Growth Factor Treatment of A818-6 Cells
HGF (100 ng/ml), EGF (10 ng/ml), TGF-α (10 ng/ml), TGF-β (10 ng/ml), and bFGF (1 μg/ml) were added to either freshly seeded A818-6 cells or completely developed hollow spheres at the indicated concentrations. In a second experiment, an HGF-neutralizing antibody and an epimorphin-neutralizing antibody (MC-1) were used at a concentration of 50 μg/ml to neutralize exogenously added HGF or to block intrinsic HGF/epimorphin produced by the cells. Additionally, MC-1 (100 μg/ml) was added to cocultures of freshly seeded A818-6 cells and Kif-5 fibroblasts. An anti–interleukin (IL)-13 antibody (rat IgG) was used as control for MC-1 experiments in concentrations of 50 and 100 μg/ml, respectively. All cytokines and corresponding antibodies were added on days 2, 5, and 7 after seeding. The cultures were checked microscopically daily.
Proliferation Assays with A818-6 Cells
A818-6 monolayer cells were grown to 60–70% confluence, harvested, and prepared for immunocytochemistry as described above. All further steps were carried out following the instruction manual for Vectastain kits using the KiS5 antibody against the Ki67 antigen as the primary antibody. The nuclei were counterstained with hemalaun and the number of positive cells was evaluated using an Olympus BH-2 microscope.
The telomerase assay was performed as described previously (Klapper et al. 1998). Protein was extracted from A818-6 hollow spheres or from A818-6 monolayer cells. A total amount of 25 ng protein was taken and five independent measurements were performed for each phenotype.
Western Blot Analysis and Immunoprecipitation
For Western blot analyses, protein extracts from A818-6 hollow sphere cells, monolayer cells, and fibroblasts were isolated with standard RIPA buffer (0.1% SDS, 1% NP-40, 0.5% sodium deoxycholate). 20 μg of total protein was loaded per lane. The separation was carried out under denaturing conditions in 12.5–15% PAGE gels. Blotting was performed for 1.5 h at 400 mA in a blotting chamber (Bio-Rad Laboratories) onto polyvinylidene difluoride membranes (Immobilon P; Millipore). All washes were performed with PBST buffer (Life Technologies) containing 0.1% Tween 20 (Bio-Rad Laboratories). All antibodies were diluted in PBST containing 5% (wt/vol) skim milk. The transfer efficiency and the correct protein size were checked by using a RainbowTM protein marker from Amersham Pharmacia Biotech. All blots were normalized with an antibody against β-actin (42 kD) and detection was carried out with the ECL labeling kit from Amersham Pharmacia Biotech according to the manufacturer's instructions. The resulting bands were visualized on x-ray films (Eastman Kodak Co.).
Immunoprecipitation was performed with supernatants from A818-6 hollow spheres or monolayer cultures, fibroblasts, and cocultures from hollow spheres and fibroblasts. 1–3 μg of primary antibodies was added to up to 2 ml of supernatant and rotated at 4°C overnight. Protein G–Sepharose (Amersham Pharmacia Biotech) was equilibrated overnight for the corresponding cell culture medium and then added to the primary antibody solution, followed by rotation for 30 min at 4°C. Sepharose beads were collected by centrifugation at 14,000 rpm for 2 min and washed four times with TNE buffer (0.5 M Tris, pH 8, 0.15 M NaCl, 0.1% NP-40, 0.125 M EDTA). The pellet was then taken up in 1× Laemmli buffer, boiled for 4 min at 95°C, and loaded onto a 15% SDS-PAGE gel for separation. Detection was carried out as described for Western blot analyses.
Coculture of A818-6 Cells with Fibroblasts
Fibroblast cocultures with A818-6 cells were carried out with dermal-derived fibroblasts (KIF-5). In the first experiment, fibroblasts were mixed at a ratio of 1:1 with A818-6 cells after trypsinization. The mixture was seeded into agarose-coated culture dishes. As a control, A818-6 cells were seeded without fibroblasts. In a second experiment, fibroblasts were seeded under standard cell culture conditions and grown in monolayer cultures. After reaching confluence, freshly trypsinized A818-6 cells were seeded onto these fibroblast monolayer cultures and incubated for 48 h. In a third set-up, premature hollow spheres (5–6-d-old) were seeded onto a fibroblast monolayer culture. 2 d before coculturing fibroblasts with A818-6 cells, the fibroblasts were adapted to RPMI 1640 medium by mixing both media at a ratio of 1:1. On the day of the experiment, both fibroblasts and A818-6 cells were incubated in RPMI 1640 medium. To obtain fibroblast-free protein from fibroblast-stimulated A818-6 hollow spheres, the coculture was transferred to noncoated tissue culture plates. Fibroblasts adhered to these plates but hollow spheres did not. Results were evaluated using an Axiovert microscope (ZEISS).
Results
Hollow Sphere Development
Under standard cell culture conditions, A818-6 cells grew as a monolayer culture (Fig. 1 a) with a doubling time of 69 h. When adherence of these cells to culture substratum was prevented by coating tissue culture plates with solid agarose, hollow sphere development was observed within a time frame of 6–12 d after the initial seeding. The development of hollow spheres can be subdivided into two phases: (a) initiation and (b) maturation. The phase of initiation proceeded from the day of seeding to days 6–8 of the development. Signet ring–like cells with an expanded vacuole (Fig. 1 b, white arrows) appeared. 6–8 d after seeding, hollow spheres were the predominant component of the culture. Other remaining structures were identified as compact spheroids and signet ring–like cells. The maturation phase commenced after hollow spheres were retransferred into noncoated standard cell culture flasks. The hollow spheres became more homogenous in size and shape over the next 4 d (Fig. 1 c). In comparison to the early stages, only a few apoptotic cells were visible inside the hollow spheres. In contrast to other remaining cell clusters, hollow spheres did not attach to the surface of tissue culture grade flasks. Reattachment of the hollow spheres to the surface of the dish was only seen after mechanical disruption and was associated with regrowth as a monolayer culture (data not shown).
Figure 1.
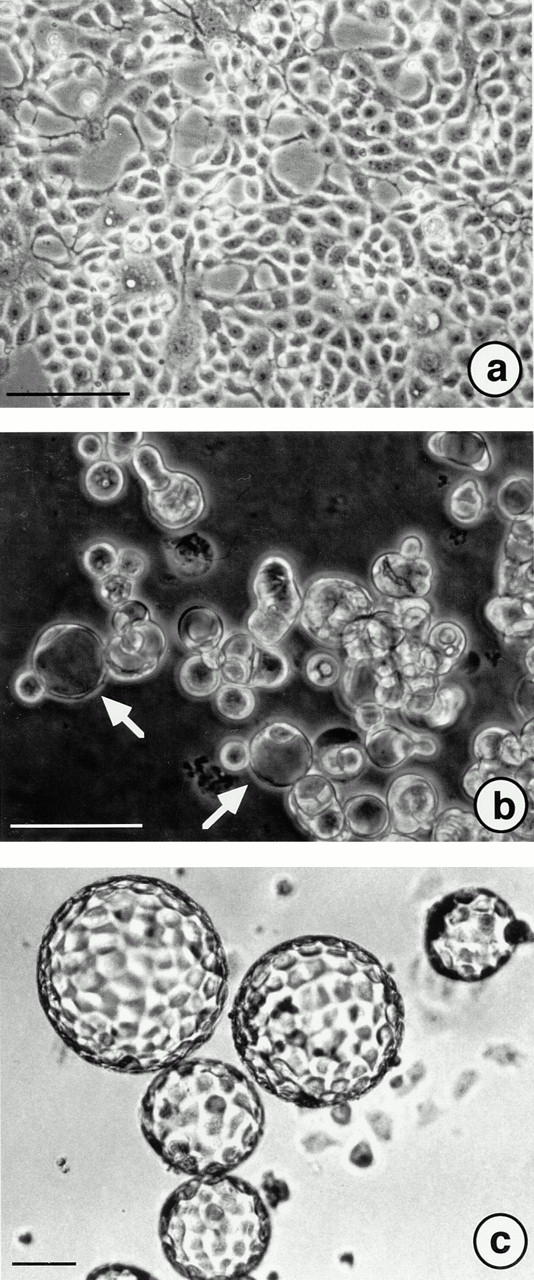
A818-6 hollow sphere development as documented by phase–contrast microscopy. (a) A818-6 cells grown as a monolayer culture under standard cell culture conditions. (b) Signet ring–like cells (arrows) displaying a preform of hollow sphere development observed 2 d after seeding the cells on solid agarose. (c) Mature hollow spheres after 14 d in culture. Bars, 10 μm.
In electron microscopy analysis, the initial step in hollow sphere development was the formation of cell clusters with multiple cell–cell contacts between neighboring cells (Fig. 2 a). These cell clusters no longer had any cell-substratum adhesion, but were held together by multiple adherence junctions between A818-6 cells. Some of these cells contained large vacuoles and a central or lateral space within the cluster formed and dilated progressively. In the next phase of development, mature hollow spheres were found in abundance, now consisting of a single cell layer (Fig. 2 b) which was sealed towards the outside of the sphere by clearly defined tight junctions between neighboring cells. No tight junctions sealing the internal surface of hollow sphere–forming cells were detected, but lateral cell–cell contacts corresponded ultrastructurally to adherence junctions. At the same time interval, the plasmalemma facing the outside of the sphere had developed an often dense and highly developed surface structure of microvilli (Fig. 2 c). During later phases of hollow sphere development, occasional cell death by apoptosis was found. Cells undergoing apoptosis were initially found in situ within the single cell layer of hollow spheres still in contact with unaffected neighboring cells and were then apparently sequestered to the lumen of the sphere, as indicated by apoptotic or partially digested cells in the sphere's lumen (Fig. 2 d).
Figure 2.
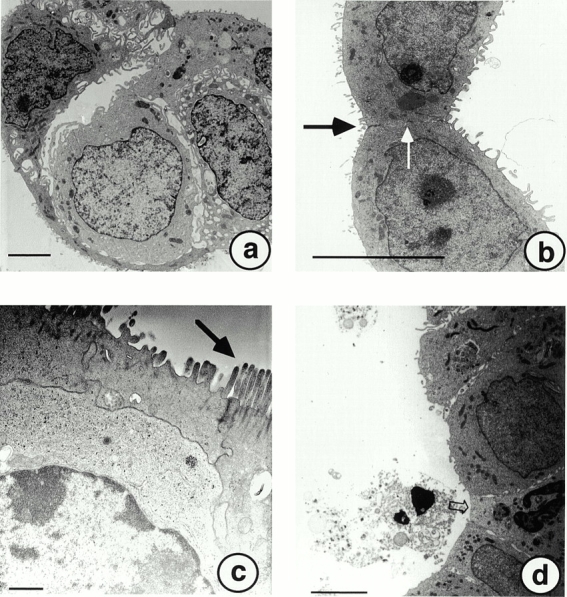
Ultrastructural analyses at different stages of A818-6 hollow sphere development. (a) After 2 d in culture, clusters of A818-6 cells formed with multiple cell–cell contacts between neighboring cells. (b) Mature hollow spheres (10–12-d-old) developed tight junctions (black arrow) and adherence junctions constituting the polarized epithelial phenotype (white arrow). (c) Highly developed microvilli were found on the apical surface of A818-6 hollow sphere cells that were older than 10 d (black arrow). Cells with condensed chromatin but intact junctions to neighboring cells undergoing apoptosis were observed throughout the entire hollow sphere development. These cells were sequestered to the lumen of A818-6 hollow spheres (d). Bars: (a, b, and d) 10 μm; (c) 2 μm.
Expression of Pancreatic Duct Cell Differentiation Marker Antigens
As bicarbonate secretion of ductal epithelial cells is an important physiological feature, CAII is a reliable marker for this type of cell. We consequently detected CAII in hollow sphere cells by indirect immunofluorescence staining (Fig. 3 a), as well as in the corresponding monolayer cells (data not shown). In normal ductal epithelial cells of the pancreas, highly glycosylated proteins of the mucin family are facing the lumen of the duct. A818-6 hollow sphere cells also expressed mucin-1 (MUC-1) antigen at the outer surface, which was therefore identified as the apical membrane, which resembles the lumen-orientated membrane in pancreatic ducts in vitro (Fig. 3 b). In differentiated ductal epithelial cells, cell adhesions are formed at the basolateral cell surface. The cytosolic binding partner of E-cadherin, β-catenin, exhibited a mainly lateral staining in A818-6 cells under confocal laser scanning microscopy (Fig. 3 c). This localization of β-catenin correlated with the ultrastructural observations of intact adherence junctions between hollow sphere cells at the lateral cell membrane (Fig. 2 a). Furthermore, we found a weaker, but also laterally localized, staining for α-catenin, γ-catenin, and E-cadherin (data not shown). Normal epithelial cells produce proteins of the extracellular matrix such as collagen type IV or laminin. A818-6 hollow sphere cells were found to produce small amounts of laminin at their basolateral membranes (Fig. 3d and Fig. e) but were negative for collagen IV as assessed by immunofluorescence staining.
Figure 3.
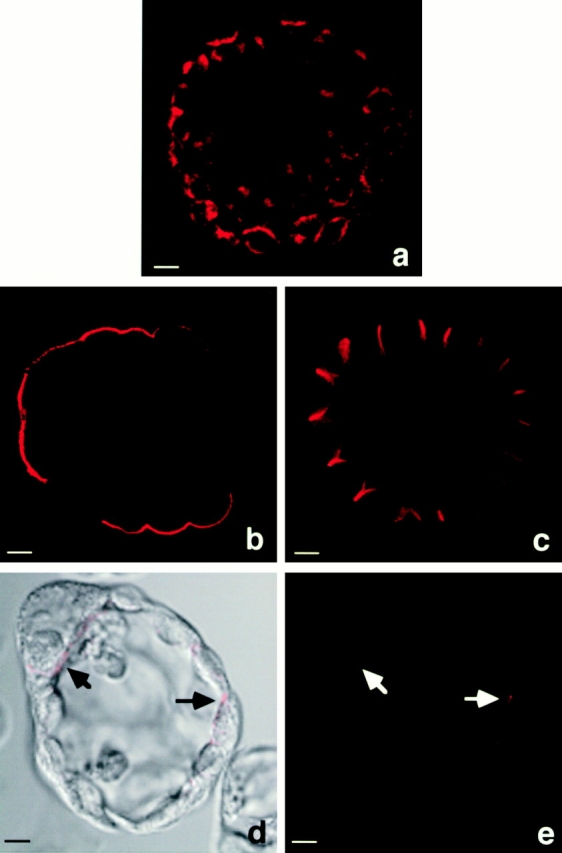
Immunofluorescence staining of hollow sphere cells for duct cell differentiation marker antigens. (a) Cytosolic localization of CAII is shown as a duct cell marker. Mucin-1 staining depicts the apical membrane side of A818-6 hollow spheres (b); β-catenin staining outlines the basolateral membranes of A818-6 hollow sphere cells (c). Analyses were performed using confocal laser scanning microscopy. Laminin staining (d and e, black and white arrows) depicts the basal potion of the basolateral membranes. Bars, 10 μm.
Proliferation of A818-6 Cells
Proliferation of hollow sphere and monolayer cells was monitored by immunoperoxidase staining for the proliferation-associated antigen Ki67 with the antibody KiS5. The Ki67 antigen is detectable in all phases of the cell cycle except the G0 phase. Therefore, cells that are negative for Ki67 are arrested in the G0 phase of the cell cycle. During hollow sphere formation we observed a dramatic decrease in proliferation (Fig. 4 A). Monolayer cultures contained 95% proliferating, Ki67-positive cells, whereas 31.5% of hollow sphere cells were positive for Ki67. These results were supported by findings on cell cycle–associated proteins by Western blot analysis. The cell cycle inhibitor p27/Kip was expressed in larger amounts in hollow sphere cells than in monolayer cells. The converse result was seen upon comparison of the expression of the cell cycle promoter cyclin B between monolayer cells and hollow sphere cells (Fig. 4 B). Other cell cycle–associated proteins, such as cdk4, cdc2, and cyclin D1, were found in equal amounts in hollow sphere and monolayer cells (data not shown). A telomerase assay was carried out as described previously (Klapper et al. 1998) using a modified telomeric repeat amplification protocol assay. Recent publications have shown a correlation between the status of differentiation of cells and the telomerase activity (Reichman et al. 1997). Consequently, measurement of telomerase activity can be used as a further indicator for differentiation in A818-6 hollow sphere cells. In comparison to the corresponding monolayer, the relative telomerase activity was found to be approximately sixfold lower in hollow spheres (Fig. 4 D).
Figure 4.
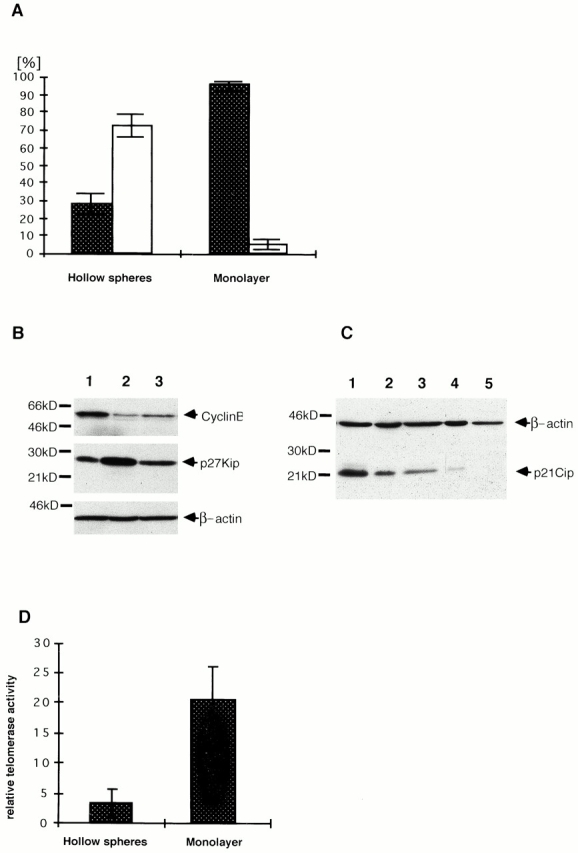
Comparison of proliferation rates between A818-6 monolayer and hollow sphere cells. (A) A818-6 monolayer cells and A818-6 hollow sphere cells were stained for the Ki67 antigen. The total amount of positive cells for Ki67 is shown in percent by dark columns. The amount of negative cells is represented by white bars (A818-6 hollow spheres: mean value = 31.5% positive cells; mean value = 68.5% negative cells; A818-6 monolayer: mean value = 95% positive cells; mean value = 5% negative cells). (B) Western blot analyses for cell cycle–associated proteins p27/Kip and cyclin B were carried out on lysates of A818-6 monolayer cells (lane 1) and hollow sphere cells (lane 2) and in cocultures of A818-6 cells with fibroblasts (lane 3). (C) Western blot analyses for p21Cip after TGF-β treatment of A818-6 cells. Lane 1, TGF-β–treated A818-6 monolayer cells; lane 2, TGF-β–treated A818-6 hollow sphere cells; lane 3, untreated A818-6 monolayer cells; lane 4, untreated hollow sphere cells; lane 5, negative control. (D) Measurement of the relative telomerase activity of A818-6 monolayer cells and hollow sphere cells was carried out by telomeric repeat amplification protocol assay. Lysates of A818-6 monolayer cells and A818-6 hollow sphere cells were measured against the telomerase activity of lysates of the cell line L428 used as a standard. A818-6 monolayer cells exhibited approximately sixfold higher telomerase activity than A818-6 hollow sphere cells.
Hollow Sphere Development in the Presence of Fibroblasts
Fibroblast cocultures with A818-6 cells were set up using foreskin-derived KIF-5 fibroblasts. When A818-6 cells were seeded onto a confluent fibroblast monolayer culture, invasive growth and the development of plaques of A818-6 tumor cells were seen (Fig. 5 a). When premature A818-6 hollow spheres (Fig. 5 b) were seeded onto a confluent fibroblast monolayer culture, these hollow spheres increased in size by increasing the number of cells per hollow sphere and developed a homogenous shape within 48 h (Fig. 5 c). In a second experiment, A818-6 monolayer cells were mixed with fibroblasts and subsequently seeded onto agarose-coated dishes. This procedure was also found to enhance A818-6 hollow sphere development. Hollow sphere development was seen 2 d after initial coculture of A818-6 cells with KIF-5 fibroblasts; it was normally observed after 6–8 d in cultures without fibroblasts (Fig. 5 d). The same experiment was repeated with EGFP-transduced fibroblasts and revealed a localization of fibroblasts in the lumen of hollow spheres closely associated to the basal side of A818-6 cells (Fig. 6, a–d). Fibroblast-conditioned medium was not able to replace the direct presence of fibroblasts in the same manner (data not shown). Analysis of the cell cycle–associated proteins cyclin B and p27/Kip after coculture revealed a downregulation of p27/Kip and a weak upregulation of cyclin B in A818-6 hollow spheres (Fig. 4 B, lane 3). This is in accordance with an observed stimulatory effect of fibroblasts on A818-6 hollow sphere development (Fig. 5b and Fig. c).
Figure 5.
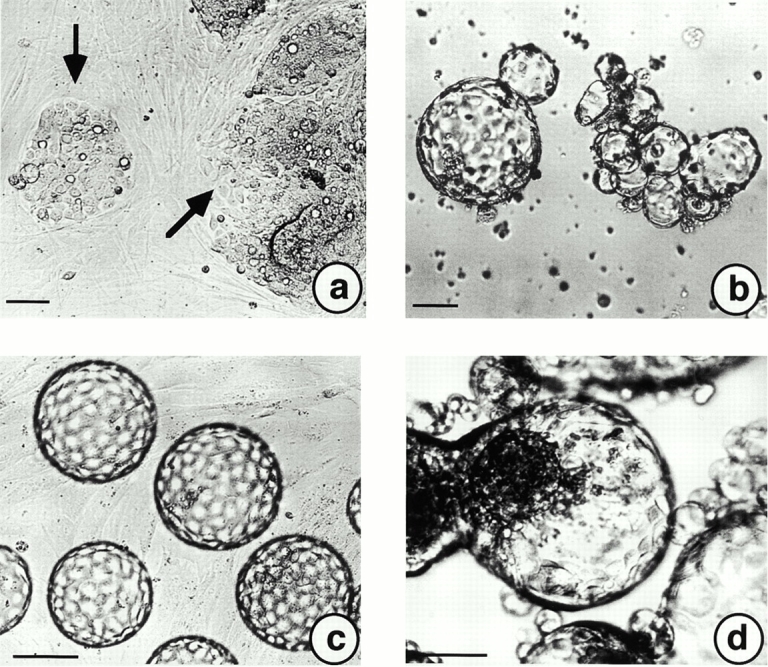
Influences of foreskin-derived fibroblasts (Kif-5) on A818-6 hollow sphere development in coculture experiments. (a) A818-6 monolayer cells were trypsinized and seeded onto a preformed, confluent fibroblast monolayer. A818-6 monolayer plaques (black arrows) are shown after 2 d in coculture. (b) In a second experimental set-up, premature (5–6-d-old) hollow spheres were seeded onto a confluent fibroblast monolayer. (c) Premature hollow spheres grew to a homogenous size and regular shape within 2 d. Coseeding of trypsinized A818-6 cells together with fibroblasts onto solid agarose at a ratio of 1:1 increased the speed of A818-6 hollow sphere development dramatically. Premature hollow spheres were observed after only 2 d in coculture (d). This day of development is normally characterized by signet ring–like cells (see Fig. 1 b). Bars, 50 μm.
Figure 6.
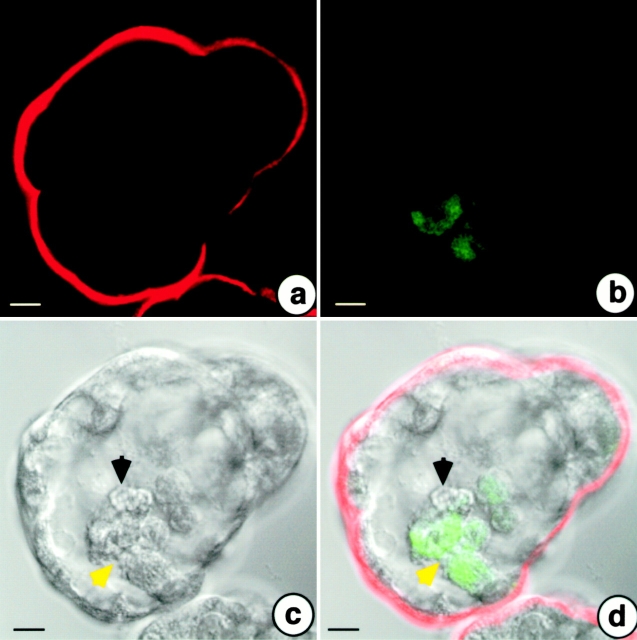
Confocal laser scanning analysis of coculture experiments of A818-6 cells with Kif-5 fibroblasts transduced with the gene coding for the EGFP. After 6 d of hollow sphere development, A818-6 cells were stained for the mucin-1 antigen. Mucin-1 displayed a staining of apical membranes of A818-6 hollow sphere cells (a). Fibroblasts are depicted by EGFP-derived fluorescence (b). (c) A differential interference contrast image of the analyzed hollow sphere with fibroblasts closely associated with the basal side of hollow sphere cells (c and d, yellow arrow) accompanied by a putative sequestered, nonfluorescing single A818-6 cell (c and d, black arrow). Bars, 10 μm.
Response to Cytokine Treatment
HGF, EGF, TGF-α, TGF-β, and bFGF were added to either freshly seeded A818-6 cells or completely developed hollow spheres at concentrations indicated in Materials and Methods. HGF, EGF, and TGF-α showed no effect on either hollow sphere development or mature hollow spheres. bFGF was found to enhance A818-6 hollow sphere development and was able to stimulate the growth of mature hollow spheres. TGF-β induced p21/Cip in A818-6 monolayer and hollow sphere cells (Fig. 4 C) which was accompanied by a very drastic decrease in proliferation, leading to a delay in A818-6 hollow sphere development. Although p21/Cip was induced by TGF-β, no morphological effect on mature hollow spheres was observed.
Inhibition of A818-6 Hollow Sphere Development by MC-1
A rat monoclonal antibody, MC-1, was found to react with native epimorphin (Hirai 1993; Hirai et al. 1993) and inhibited branching morphogenesis in mammary duct cells (Hirai et al. 1998). Incubation of freshly seeded A818-6 with 50 μg/ml of MC-1 under 3D culture conditions resulted in the inhibition of A818-6 hollow sphere development (Fig. 7, a and b). A rat IgG antibody against human IL-13 (50 μg/ml) served as a control and showed no effect on A818-6 hollow sphere development (Fig. 7 c). So far, it has been generally assumed that fibroblasts are essential for the mediation of an epimorphin effect by a 150-kD form associated with the membrane of fibroblasts. However, in our experiments we found an epimorphin effect which was independent of the presence of fibroblasts in A818-6 cultures. Therefore, epimorphin is suggested to act as a soluble factor in an autocrine manner in the A818-6 system. Neither a neutralizing antibody against HGF/SF nor recombinant HGF/SF added to freshly seeded cells had an influence on hollow sphere formation, even though HGF/SF and its receptor c-met were detected in A818-6 monolayer and hollow sphere cells by immunocytochemistry with intense staining for the receptor and faint staining for the ligand (data not shown). As described above, the presence of fibroblasts enhanced A818-6 hollow sphere development (Fig. 5 d). MC-1 at a concentration of 100 μg/ml was able to prevent the fibroblast-mediated stimulation and complete hollow sphere development (Fig. 7d and Fig. e). Large compact aggregates of A818-6 cells and fibroblasts developed in contrast to A818-6 cells alone. In this coculture experiment the inhibition of hollow sphere development by MC-1 was not permanent. When MC-1 application was stopped after day 7, hollow spheres developed from the compact spheroids over the following days (data not shown). A rat IgG antibody against human IL-13 (100 μg/ml) served as a control and showed no effect on A818-6 hollow sphere development (Fig. 7 f).
Figure 7.
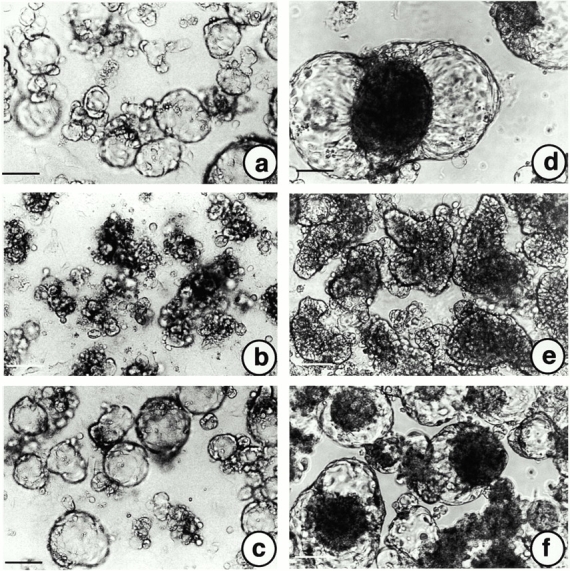
Inhibiting influence of a monoclonal antibody against epimorphin, MC-1, on A818-6 hollow sphere development. (a) A818-6 cells cultured on solid agarose for 8 d without MC-1. (b) A818-6 cells were cultured on solid agarose for 8 d in the presence of MC-1 (50 μg/ml). (c) A818-6 cells cultured on solid agarose for 8 d in the presence of an antibody to human IL-13 used as an isotype control. (d) Coculture of A818-6 cells with fibroblasts but without MC-1. (e) Coculture of A818-6 cells with fibroblasts and MC-1 (100 μg/ml). (f) Coculture of A818-6 cells with fibroblasts and an antibody against human IL-13 used as an isotype control. The antibodies were added on days 2, 5, and 7 after seeding. Bars, 50 μm.
Detection of Soluble Epimorphin
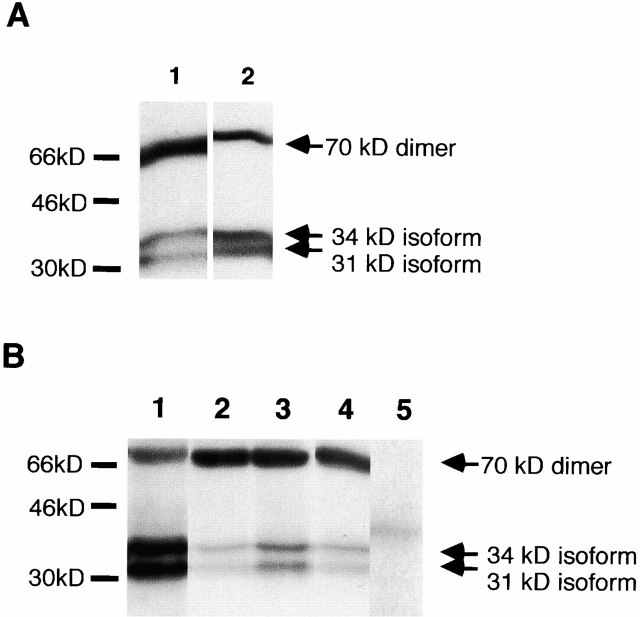
The supernatants of A818-6 cells and fibroblasts were analyzed for putative soluble forms of epimorphin. Immunoprecipitation, followed by Western blot analysis, was carried out with the monoclonal MC-1 antibody and an affinity-purified polyclonal antibody against epimorphin. The identical immunoreactivity of MC-1 and the affinity-purified polyclonal antibody was demonstrated first. MC-1 was used to probe precipitates made with the affinity-purified antibody and vice versa using supernatants from fibroblasts as a source. Both antibodies detected antigens of similar mobility (Fig. 8 A). We subsequently found that both antibodies were able to detect the monomeric 34- and 31-kD isoforms of epimorphin and the dimeric form of ∼70 kD in the supernatants of A818-6 hollow spheres, Kif-5 fibroblasts and cocultures of fibroblasts, and hollow spheres. No tetrameric complex of 150 kD was detected in any of the supernatants (data not shown). Epimorphin was not found in the pure cell culture medium or detected in the precipitate of an anti–human IL-13 (rat IgG) antibody used as a control (Fig. 8 B).
Figure 8.
Detection of epimorphin in the supernatants of A818-6 hollow spheres and Kif-5 fibroblasts by immunoprecipitation with the monoclonal antibody MC-1 and an affinity-purified polyclonal antibody against epimorphin. (A) Western blot analysis after immunoprecipitation from fibroblast (Kif-5) supernatants to confirm the detection of the same antigen (human epimorphin) by both antibodies used under these conditions. In lane 1 the precipitate obtained with the monoclonal antibody MC-1 was probed with the polyclonal rabbit serum against epimorphin and affinity-purified against recombinant epimorphin. In lane 2, the precipitate obtained with the affinity-purified antibody was probed with the monoclonal rat IgG antibody for epimorphin MC-1. Epimorphin isoforms and dimers are indicated by arrows. (B) Western blot analysis after immunoprecipitation from A818-6 hollow sphere supernatants, Kif-5 fibroblast supernatants, and supernatants obtained from cocultures (premature hollow spheres and fibroblasts). Precipitates obtained with both antibodies (MC-1 and the affinity-purified polyclonal antibody against epimorphin) were resolved by SDS-PAGE (15%) and transferred to a nitrocellulose membrane. In lane 1, epimorphin was precipitated from hollow sphere supernatant by MC-1 and detected by MC-1. In lane 2, epimorphin was precipitated from hollow sphere supernatant and detected by the affinity-purified polyclonal antibody. Epimorphin was also precipitated and detected by the affinity-purified polyclonal antibody from coculture supernatants (lane 3) and fibroblast supernatants (lane 4). In lane 5, an anti–human IL-13 (rat-IgG) antibody was used for precipitation from hollow sphere supernatants. This precipitate was probed with MC-1 on the succeeding Western blot (negative control). Epimorphin isoforms and dimers of the monomers are indicated by arrows.
Analysis of the Expression of Epimorphin in Cell Lysates of Pancreatic Carcinoma Cell Lines
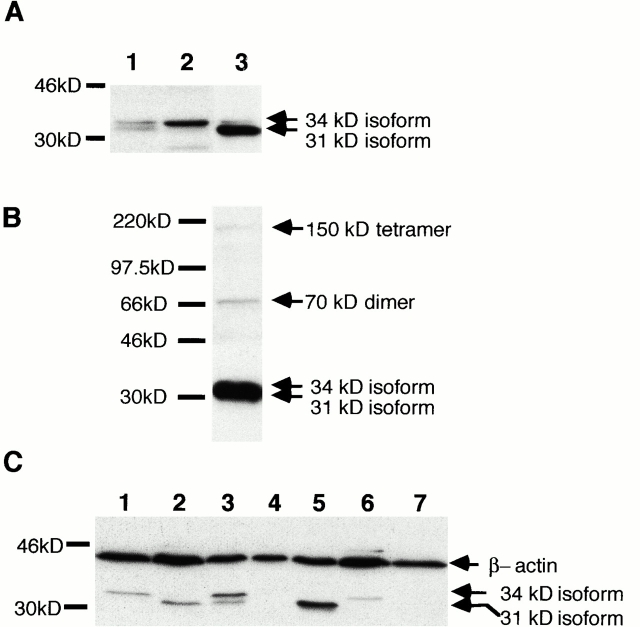
All forms of epimorphin described previously by others were detected with an affinity-purified antibody against epimorphin, as assessed by Western blot analysis (Fig. 9A and Fig. B). The 150-kD tetramer of epimorphin was only detected in the lysates of fibroblasts (Fig. 9 B), whereas it was not detectable by Western blot analysis in lysates of A818-6 monolayer cells and hollow sphere cells. No difference in the expression pattern of 70-kD dimers was observed upon comparison of fibroblasts, A818-6 monolayer cells, and A818-6 hollow sphere cells. Differences were observed in the expression pattern of monomeric 34- and 31-kD isoforms of epimorphin. All monomeric isoforms were expressed in A818-6 monolayer cells whereas 3D hollow spheres exclusively expressed the larger 34-kD isoforms. Fibroblasts expressed larger amounts of the smaller, 31-kD isoform, than of the 34-kD isoforms (Fig. 9 A). Subsequently, we compared cell lines that did not develop lumenal structures with cell lines which grew with a lumenal phenotype under our cell culture conditions. All cell lines capable of lumen formation expressed the larger 34-kD isoforms of epimorphin occasionally together with the 31-kD isoform. Cell lines not capable of lumen formation expressed only the smaller 31-kD isoform or failed to express any (Fig. 9 C). The cell lines Capan-2 and BxPC-3 that expressed the 34-kD isoforms responded upon fibroblast coculture with stimulation of the development of lumenal structures. Cell lines expressing only the 31-kD isoform or none (Capan-1, A818-4, Panc-1) showed no induction of lumen formation in fibroblast coculture (data not shown).
Figure 9.
Detection of epimorphin in protein lysates of pancreatic carcinoma cell lines and fibroblasts. (A) Western blot analyses of epimorphin in protein lysates of A818-6 monolayer cells (lane 1), hollow sphere cells (lane 2), and Kif-5 fibroblasts (lane 3). Monolayer cells expressed the 34- and 31-kD isoforms, whereas hollow sphere cells lacked the 31-kD isoform. Fibroblasts expressed larger amounts of the 31-kD isoforms of epimorphin than of the 34-kD isoforms (lane 3). (B) The 150-kD tetrameric complex, 70-kD dimer, and monomers of epimorphin detected in the lysate of Kif-5 fibroblasts. (C) Western blot analysis of epimorphin in protein lysates of the pancreatic carcinoma cell lines Capan-1 (lane 2), Capan-2 (lane 3), A818-4 (lane 4), Panc-1 (lane 5), BxPC-3 (lane 6), and A818-6 hollow sphere cells (lane 1) as control; it was also taken as negative control by omitting the primary antibody (lane 7). All cell lines which were capable of lumen formation (A818-6, Capan-2, BxPC-3) expressed the 34-kD isoforms of epimorphin occasionally together with the 31-kD isoform. Cell lines which did not develop lumenal structures (Capan-1, A818-4, Panc-1) expressed no epimorphin or only the 31-kD isoform. Epimorphin isoforms are indicated by arrows.
Discussion
Ductal epithelial cells of the human pancreas develop a strictly polarized phenotype to establish their barrier function and to fulfil other physiological roles, such as polarized secretion of fluid, electrolytes, and mucus. The enzyme CAII was described as a typical marker for the differentiation of pancreatic duct epithelial cells (Nishimori et al. 1999) and consequently A818-6 cells were specified for this cell type. The apical membranes of normal ductal cells are characterized by the production of a mucus that mainly consists of highly glycosylated proteins of the mucin family, for which MUC-1 serves as a paradigm (Ho et al. 1993; Cheung et al. 1998). Apical MUC-1 was detected on the outer surface of A818-6 hollow spheres by immunofluorescence analysis, whereas monolayer cells had lost their polar expression pattern of MUC-1. At the ultrastructural level the apical membranes of hollow sphere cells were covered with well-developed microvilli and sealed by tight junctions, the existence of which is further evidence for polarized epithelial development. Additionally, the process of tight junction formation was accompanied by translocation of symplekin from the nucleus to the membrane of A818-6 hollow sphere cells. This recruitment was not observed in monolayer cells (data not shown). Symplekin has been identified as a novel type of protein localized on the plaque associated with the cytoplasmic face of the tight junction–containing zone (zonula occludens) of polar epithelial cells (Keon et al. 1996). Microvilli and tight junctions are characteristic features of normal duct epithelial cells of the pancreas (Gumbiner 1987; Stevenson and Keon 1998). In addition to tight junctions, adherence junctions were formed between hollow sphere cells at their lateral membranes. Loss of E-cadherin (Bracke et al. 1997; Perl et al. 1998) and changes in the expression of catenins (Hague et al. 1997; Sparks et al. 1998) displaying major components of adherence junctions, generally leading to a loss of contact inhibition, can change tissue integrity and have been described as crucial for the development of a variety of malignant processes. The staining pattern for β-catenin in hollow spheres in particular confirmed the identification of lateral adherence junctions at the electron microscopy level and underlines the strictly polar orientation of A818-6 cells in hollow spheres. The extreme reduction in proliferation (monitored by p27/Kip, cyclin B, Ki67 expression, and telomerase assay) during hollow sphere development characterizes A818-6 hollow sphere cells as normal duct epithelial cells of the pancreas, with the majority of cells being arrested in the G0 phase of the cell cycle. Although the cell line A818-6 is a pancreatic adenocarcinoma cell line carrying distinct genetic alterations typically described in pancreatic cancer, i.e., k-ras and p53 mutations (Sirivatanauksorn et al. 1998; Matsubayashi et al. 1999), these cells were able to reverse the malignant phenotype of the monolayer culture upon development of differentiated 3D hollow spheres. A similar observation, where structure dominated over the genotype, has been reported by Ingber et al. 1986. In comparison to A818-6 hollow spheres, lumenal structures from other cell lines, such as Capan-2 and BxPC-3, showed a more irregular organization of cells within the structure. After transferring these structures from agarose-coated dishes to uncoated dishes (phase of maturation) all complexes from Capan-2 and BxPC-3 attached to the surface and grew out as monolayers, whereas premature A818-6 hollow spheres remained unattached. This attachment might be explained by Capan-2 or BxPC-3 cells facing towards the medium with their basolateral membranes. This type of orientation has been described previously when Capan-2 cells were embedded in collagen type I gels (Brinkmann et al. 1995).
The development of a differentiated, 3D phenotype of the cell line A818-6 was stimulated by coculture with mesenchymal cells, pointing to the importance of mesenchymal factors in the differentiation of ductal epithelial cells. Epimorphin has recently emerged as an important morphoregulator derived from mesenchymal cells, also found in epithelial cells. Epimorphin has further been found to be responsible for the branching morphogenesis and lumen formation in breast epithelial cells of the mouse, and is known to have a primary role in the regeneration of the liver (Hirai et al. 1998; Watanabe et al. 1998). Here we show that certain isoforms of epimorphin are important factors for the development of lumenal structures of pancreatic duct epithelial cells. As described for hair follicle growth and lung epithelial tubular formation (Hirai et al. 1992), the antibody MC-1 was found to be a potent inhibitor of A818-6 hollow sphere development. In line with findings for mammary epithelial cell development (Hirai et al. 1998), no effects on hollow sphere development were observed using a neutralizing antibody to HGF. We detected all reported isoforms of epimorphin in fibroblasts, whereas the 150-kD tetramer of epimorphin was not detectable in lysates of A818-6 cells as assessed by Western blot analysis. Interestingly, we found a strict correlation between the lumen-formation capacity of pancreatic duct cells and the expression pattern of the different isoforms of epimorphin in cell lysates. It is now thought that the extracellular and membrane-associated tetrameric complex (150 kD) represents the morphoregulatory form of epimorphin (Hirai et al. 1998; Zhang et al. 1999). We detected all monomeric isoforms (34 and 31 kD) and the dimeric form (70 kD) of epimorphin in the supernatants of A818-6 cells and fibroblasts but no tetrameric form of 150 kD. As immunoprecipitation data for conditioned medium from A818-6 hollow spheres without cocultivated fibroblasts revealed only the monomeric isoforms which are able to either form homodimeric or heterodimeric complexes, morphogenic activity is attributed to these smaller forms. Interestingly, the 31-kD isoform of epimorphin was reported to lack a putative membrane-spanning domain (Hirai et al. 1998). Additionally, dimer formation should only be seen for undegraded monomers of epimorphin, pointing to an ectodomain of epimorphin with functional interaction sites (Miller et al. 1995; Gilboa et al. 1998). Epimorphin and its related molecules form oligomeric products via complex intermolecular and intramolecular interactions in tissues and each product appeared to present different antigenic properties (Hirai 1994). The antiepimorphin monoclonal antibody MC-1 detects an epitope in the middle of the epimorphin molecule (Hirai 1994). Thus, dimerization may hide these epitopes, explaining the lower affinity of MC-1 for the dimeric form of epimorphin. Polyclonal antibodies were prepared from serum of rabbits immunized with recombinant epimorphin produced in bacterial cells; the primary structure of the immunogen (epimorphin) in both cases is identical, but the secondary and tertiary structure may not be. In this study the presence of epimorphin, in particular soluble epimorphin, is demonstrated in human pancreatic adenocarcinoma cells and, moreover, a functional effect of epimorphin on human epithelial cells exhibiting duct-like development has been shown for the first time.
Further studies are required to establish whether soluble epimorphin displays the active form of this competent morphoregulator in other tissues too and whether quantitative differences in the ratio of monomeric to dimeric forms, as they are shown in Fig. 8, are of functional relevance. The in vitro differentiation of the A818-6 hollow sphere system will therefore help to shed light on the interaction between mesenchymal and epithelial cells and the particular role of epimorphin in the differentiation of ductal epithelial cells.
Acknowledgments
We thank Dr. Klaus Heidorn of the Institute of Hematopathology, Christian-Albrechts University, for the performance and validation of the telomerase assay. We further thank Dr. Bradley Howard for producing retroviral supernatants for the transduction of fibroblasts with the EGFP gene.
This work forms part of the Ph.D. thesis of L. Lehnert and is supported by the Deutsche Forschungsgemeinschaft (KA1346/11).
Footnotes
Abbreviations used in this paper: 3D, three-dimensional; bFGF, basic FGF; CAII, carbonic anhydrase II; EGFP, enhanced green fluorescent protein; HGF, hepatocyte growth factor; HRG, heregulin; IL, interleukin; KGF, keratinocyte growth factor; VSV-G, vesicular stomatitis virus glycoprotein.
References
- Akiyama M., Amagai M., Smith L.T., Hashimoto K., Shimizu H., Nishikawa T. Epimorphin expression during human foetal hair follicle development. Br. J. Dermatol. 1999;141:447–452. doi: 10.1046/j.1365-2133.1999.03037.x. [DOI] [PubMed] [Google Scholar]
- Arendt T. Penetration of lanthanum through the main pancreatic duct epithelium in cats following exposure to infected human bile. Dig. Dis. Sci. 1991;36:75–81. doi: 10.1007/BF01300091. [DOI] [PubMed] [Google Scholar]
- Azghani A.O., Gray L.D., Johnson A.R. A bacterial protease perturbs the paracellular barrier function of transporting epithelial monolayers in culture. Infect. Immun. 1993;61:2681–2686. doi: 10.1128/iai.61.6.2681-2686.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxberger H.J., Sessler M.J., Maetzel B., Meyer T.F. Highly polarized primary epithelial cells from human nasopharynx grown as spheroid-like vesicles. Eur. J. Cell Biol. 1993;62:140–151. [PubMed] [Google Scholar]
- Boxberger H.J., Meyer T.F., Grausam M.C., Reich K., Becker H.D., Sessler M.J. Isolating and maintaining highly polarized primary epithelial cells from normal human duodenum for growth as spheroid-like vesicles. In Vitro Cell Dev. Biol. Anim. 1997;33:536–545. doi: 10.1007/s11626-997-0096-0. [DOI] [PubMed] [Google Scholar]
- Bracke M.E., Depypere H., Labit C., Van Marck V., Vennekens K., Vermeulen S.J., Maelfait I., Philippe J., Serreyn R., Mareel M.M. Functional downregulation of the E-cadherin/catenin complex leads to loss of contact inhibition of motility and of mitochondrial activity, but not of growth in confluent epithelial cell cultures. Eur. J. Cell Biol. 1997;74:342–349. [PubMed] [Google Scholar]
- Brinkmann V., Foroutan H., Sachs M., Weidner K.M., Birchmeier W. Hepatocyte growth factor/scatter factor induces a variety of tissue-specific morphogenic programs in epithelial cells. J. Cell Biol. 1995;131:1573–1586. doi: 10.1083/jcb.131.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C.Y., Wang X.F., Chan H.C. Stimulation of HCO3 − secretion across cystic fibrosis pancreatic duct cells by extracellular ATP. Biol. Signals Recept. 1998;7:321–327. doi: 10.1159/000014555. [DOI] [PubMed] [Google Scholar]
- Gilboa L., Wells R.G., Lodish H.F., Henis Y.I. Oligomeric structure of type I and type II transforming growth factor beta receptorshomodimers form in the ER and persist at the plasma membrane. J. Cell Biol. 1998;140:767–777. doi: 10.1083/jcb.140.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B. Structure, biochemistry, and assembly of epithelial tight junctions. Am. J. Physiol. 1987;253:C749–C758. doi: 10.1152/ajpcell.1987.253.6.C749. [DOI] [PubMed] [Google Scholar]
- Hague A., Hicks D.J., Bracey T.S., Paraskeva C. Cell-cell contact and specific cytokines inhibit apoptosis of colonic epithelial cellsgrowth factors protect against c-myc-independent apoptosis. Br. J. Cancer. 1997;75:960–968. doi: 10.1038/bjc.1997.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai Y. Molecular cloning of human epimorphinidentification of isoforms and their unique properties. Biochem. Biophys. Res. Commun. 1993;191:1332–1337. doi: 10.1006/bbrc.1993.1363. [DOI] [PubMed] [Google Scholar]
- Hirai Y. Sodium-dodecyl-sulfate-resistant complex formation of epimorphin monomers and interaction of the 150-kDa complex with the cell surface. Eur. J. Biochem. 1994;225:1133–1139. doi: 10.1111/j.1432-1033.1994.1133b.x. [DOI] [PubMed] [Google Scholar]
- Hirai Y., Takebe K., Takashina M., Kobayashi S., Takeichi M. Epimorphina mesenchymal protein essential for epithelial morphogenesis. Cell. 1992;69:471–481. doi: 10.1016/0092-8674(92)90448-l. [DOI] [PubMed] [Google Scholar]
- Hirai Y., Nakagawa S., Takeichi M. Reexamination of the properties of epimorphin and its possible roles. Cell. 1993;73:426–427. doi: 10.1016/0092-8674(93)90129-e. [DOI] [PubMed] [Google Scholar]
- Hirai Y., Lochter A., Galosy S., Koshida S., Niwa S., Bissell M.J. Epimorphin functions as a key morphoregulator for mammary epithelial cells. J. Cell Biol. 1998;140:159–169. doi: 10.1083/jcb.140.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S.B., Niehans G.A., Lyftogt C., Yan P.S., Cherwitz D.L., Gum E.T., Dahiya R., Kim Y.S. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993;53:641–651. [PubMed] [Google Scholar]
- Ingber D.E., Madri J.A., Jamieson J.D. Basement membrane as a spatial organizer of polarized epithelia. Exogenous basement membrane reorients pancreatic epithelial tumor cells in vitro. Am. J. Pathol. 1986;122:129–139. [PMC free article] [PubMed] [Google Scholar]
- Kalthoff H., Schmiegel W., Roeder C., Kasche D., Schmidt A., Lauer G., Thiele H.G., Honold G., Pantel K., Riethmuller G. p53 and K-RAS alterations in pancreatic epithelial cell lesions. Oncogene. 1993;8:289–298. [PubMed] [Google Scholar]
- Keon B.H., Schafer S., Kuhn C., Grund C., Franke W.W. Symplekin, a novel type of tight junction plaque protein. J. Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper W., Heidorn K., Kuhne K., Parwaresch R., Krupp G. Telomerase activity in ‘immortal’ fish. FEBS Lett. 1998;434:409–412. doi: 10.1016/s0014-5793(98)01020-5. [DOI] [PubMed] [Google Scholar]
- LeBras S., Czernichow P., Scharfmann R. A search for tyrosine kinase receptors expressed in the rat embryonic pancreas. Diabetologia. 1998;41:1474–1481. doi: 10.1007/s001250051094. [DOI] [PubMed] [Google Scholar]
- Matsubayashi H., Watanabe H., Yamaguchi T., Ajioka Y., Nishikura K., Iwafuchi M., Yamano M., Kijima H., Saito T. Multiple K-ras mutations in hyperplasia and carcinoma in cases of human pancreatic carcinoma. Jpn. J. Cancer Res. 1999;90:841–848. doi: 10.1111/j.1349-7006.1999.tb00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Knorr R., Ferrone M., Houdei R., Carron C.P., Dustin M.L. Intercellular adhesion molecule-1 dimerization and its consequences for adhesion mediated by lymphocyte function associated-1. J. Exp. Med. 1995;182:1231–1241. doi: 10.1084/jem.182.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimori I., FujikawaAdachi K., Onishi S., Hollingsworth M.A. Carbonic anhydrase in human pancreashypotheses for the pathophysiological roles of CA isozymes. Ann. NY Acad. Sci. 1999;880:5–16. doi: 10.1111/j.1749-6632.1999.tb09505.x. [DOI] [PubMed] [Google Scholar]
- Oka Y., Hirai Y. Inductive influences of epimorphin on endothelial cells in vitro. Exp. Cell Res. 1996;222:189–198. doi: 10.1006/excr.1996.0024. [DOI] [PubMed] [Google Scholar]
- Pabst R. The anatomical basis for the immune function of the gut. Anat. Embryol. 1987;176:135–144. doi: 10.1007/BF00310046. [DOI] [PubMed] [Google Scholar]
- Perl A.K., Wilgenbus P., Dahl U., Semb H., Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- Reichman T.W., Albanell J., Wang X., Moore M.A., Studzinski G.P. Downregulation of telomerase activity in HL60 cells by differentiating agents is accompanied by increased expression of telomerase-associated protein. J. Cell. Biochem. 1997;67:13–23. [PubMed] [Google Scholar]
- Sanvito F., Herrera P.L., Huarte J., Nichols A., Montesano R., Orci L., Vassalli J.D. TGF-beta 1 influences the relative development of the exocrine and endocrine pancreas in vitro. Development. 1994;120:3451–3462. doi: 10.1242/dev.120.12.3451. [DOI] [PubMed] [Google Scholar]
- Sariola H., Aufderheide E., Bernhard H., Henke-Fahle S., Dippold W., Ekblom P. Antibodies to cell surface ganglioside GD3 perturb inductive epithelial-mesenchymal interactions. Cell. 1988;54:235–245. doi: 10.1016/0092-8674(88)90556-9. [DOI] [PubMed] [Google Scholar]
- Sariola H., Saarma M., Sainio K., Arumae U., Palgi J., Vaahtokari A., Thesleff I., Karavanov A. Dependence of kidney morphogenesis on the expression of nerve growth factor receptor. Science. 1991;254:571–573. doi: 10.1126/science.1658930. [DOI] [PubMed] [Google Scholar]
- Sirivatanauksorn V., Sirivatanauksorn Y., Lemoine N.R. Molecular pattern of ductal pancreatic cancer. Langenbecks Arch. Surg. 1998;383:105–115. doi: 10.1007/s004230050101. [DOI] [PubMed] [Google Scholar]
- Sparks A.B., Morin P.J., Vogelstein B., Kinzler K.W. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–1134. [PubMed] [Google Scholar]
- Stevenson B.R., Keon B.H. The tight junctionmorphology to molecules. Annu. Rev. Cell Dev. Biol. 1998;14:89–109. doi: 10.1146/annurev.cellbio.14.1.89. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Hirose M., Wang X.E., Ikejima K., Oide H., Kitamura T., Takei Y., Miyazaki A., Sato N. A novel hepatic stellate (Ito) cell-derived protein, epimorphin, plays a key role in the late stages of liver regeneration. Biochem. Biophys. Res. Commun. 1998;250:486–490. doi: 10.1006/bbrc.1998.9339. [DOI] [PubMed] [Google Scholar]
- Zhang L., Ishikawa O., Takeuchi Y., Yokoyama Y., Miyachi Y. Influences of keratinocyte-fibroblast interaction on the expression of epimorphin by fibroblasts in vitro. J. Dermatol. Sci. 1999;20:191–196. doi: 10.1016/s0923-1811(98)00081-4. [DOI] [PubMed] [Google Scholar]