Abstract
The transcription coactivator and histone acetyltransferase CAMP response element–binding protein (CBP) has been demonstrated to accumulate in promyelocytic leukemia (PML) bodies. We show that this accumulation is cell type specific. In cells where CBP does not normally accumulate in PML bodies, it can be induced to accumulate in PML bodies through overexpression of either CBP or Pml, but not Sp100. Using fluorescence recovery after photobleaching, we demonstrate that CBP moves rapidly into and out of PML bodies. In contrast, Pml and Sp100 are relatively immobile in the nucleoplasm and within PML nuclear bodies. They possess the characteristics expected of proteins that would play a structural role in the integrity of these subnuclear domains. Our results are consistent with CBP being a dynamic component of PML bodies and that the steady-state level in these structures can be modulated by Pml.
Keywords: nuclear structure, promyelocytic leukemia, PML body, ND10, fluorescence recovery after photobleaching
Introduction
The cell nucleus is highly organized and contains well-defined subdomains. In contrast to cytoplasmic organelles, the different subnuclear compartments are not separated from the surrounding by a lipid bilayer. Instead, subnuclear structures are delineated by an accumulation of specific proteins within a defined volume and retain a typical size, shape, and number under normal growth conditions. It is not known how such compartments are formed, and it remains to be shown whether an underlying structural framework locally concentrates the components or whether the local accumulation results from random aggregations of rapidly diffusing components (Pederson 2000). Specific proteins have been shown to associate and dissociate rapidly between the nucleoplasm and subnuclear compartments (Kruhlak et al. 2000; Phair and Misteli 2000), but how such mobile proteins are concentrated within these compartments is not known.
The promyelocytic leukemia (PML) nuclear body, a nuclear matrix–associated structure of 250–500 nm in diameter, is present in the nucleus of most cell lines (Ascoli and Maul 1991; Stuurman et al. 1992). The first biochemical component of PML nuclear bodies to be identified was the Sp100 nuclear matrix–associated protein, an autoantigen in some patients with primary biliary cirrhosis (Szostecki et al. 1990). This protein may transactivate a variety of promoters (Guldner et al. 1992; Xie et al. 1993). The PML gene product, Pml, is also found in PML nuclear bodies and is the only protein necessary for the formation of PML nuclear bodies (Ishov et al. 1999). In some forms of acute PML, a t(15:17) chromosomal translocation creates a fusion protein of Pml and the retinoic acid receptor α (de The et al. 1990, de The et al. 1991; Kakizuka et al. 1991) which influences PML body integrity. Disruption of the PML nuclear bodies, and not the misregulation of the retinoic acid pathway, is the cause of cell transformation (Kogan et al. 2000). Since some components of the PML nuclear body may not function constitutively, or may function at multiple sites throughout the nucleoplasm, it would be predicted that they would be transient occupants of PML nuclear bodies. However, components that are necessary for the integrity of the domains would be predicted to be residents of PML nuclear bodies.
We have shown that nuclei that have not been exposed to extraction or other disruptive procedures possess a protein-based architecture (Hendzel et al. 1999). Surprisingly, such structures completely devoid of chromatin can be enriched in transcription regulatory factors (Hendzel et al. 1998). The core of the PML nuclear body is an example (Boisvert et al. 2000). CAMP response element–binding protein (CBP), a growth suppressor and histone acetyltransferase (Kwok et al. 1994; Arany et al. 1995; Giordano and Avantaggiati 1999), is highly enriched in PML nuclear bodies (LaMorte et al. 1998; Doucas et al. 1999; Boisvert et al. 2000). Whether PML bodies represent aggregations of randomly diffusing proteins, or alternatively are established by an underlying protein-based architecture, remains to be determined. In this study, we show that some components of the PML body are immobile and may play a structural role, whereas at least one other component, CBP, diffuses into and out of the PML body and accumulates in the PML body under some conditions.
Materials and Methods
Immunodetection of PML Nuclear Bodies
10T1/2, 293, and SK-N-SH cells were cultured directly on glass coverslips under conditions recommended by the American Type Culture Collection. Cells were fixed with 1.0% paraformaldehyde in PBS (pH 7.5) at room temperature for 5 min. Subsequently, cells were permeabilized in PBS containing 0.5% Triton X-100 for 5 min. PML protein was visualized using an anti-PML antibody (5E10; Stuurman et al.. 1992). CBP was labeled using an anti-CBP NH2-terminal antibody (no. sc-369, Santa Cruz Biotechnology, Inc.; or no. 06-297, Upstate Biotechnology). Cells were then labeled using the following secondary antibodies: goat anti–rabbit-Cy3 (Chemicon), goat anti–mouse-Cy5 (Amersham Pharmacia Biotech), and goat anti–human-FITC (Jackson ImmunoResearch Laboratories). After rinsing, the samples were mounted in 1 mg/ml paraphenylenediamine in PBS/90% glycerol, containing DNA-specific staining DAPI at 1 μg/ml. Digital deconvolution confocal imaging was performed using a 14-bit cooled CCD camera (Princeton Instruments) mounted on a Leica DMRE immunofluorescence microscope. VayTek Microtome digital deconvolution software was used to remove out-of-focus contributions, and image stacks were projected into one image plane using Scion Image software. False coloring and superimposition was done with Adobe Photoshop® 5.0.
DNA Constructs and Transient Transfection
The CBP protein (a gift from Dr. X.J. Yang, McGill University, Montreal, Quebec, Canada) has been cloned in the BamHI sites of pEGFP-C1 (CLONTECH Laboratories, Inc.). Sp100 (a gift from Dr. Maul, The Wistar Institute, Philadelphia, PA) was also cloned in pEGFP-C1 (CLONTECH Laboratories, Inc.). The plasmid pCMX–green fluorescent protein (GFP)–PML (a gift from Dr. Evans, The Salk Institute for Biological Studies, La Jolla, CA) has been described elsewhere (Kakizuka et al. 1991). For transient transfection, 2 μg of DNA was diluted in 100 μl of OptiMEM (GIBCO BRL), and 5 μl of Lipofectamine 2000 (GIBCO BRL) was also diluted in 100 μl of OptiMEM. After 5 min at room temperature, the two solutions were mixed and incubated for 20 min to allow complex formation. The mixture was then directly added to the 2 ml of antibiotic-free medium on the cells plated on glass coverslips. The medium was changed 5 h after addition of the transfection mixture, and the cells were allowed to grow for 24 h before fixation.
Interferon Induction
Human 293 cells were grown on coverslips and exposed to human α-interferon (Schering) by addition to the medium at a concentration of 1,000 U/ml (Lavau et al. 1995). After 72 h, the cells were fixed and labeled for immunofluorescence microscopy.
Live Cell Imaging
Coverslips were placed on glass slides containing several drops of medium surrounded by vacuum grease. The vacuum grease allows an airtight seal to form. Cells are capable of growing in these conditions for >24 h at 22°C. Individual cells were located by direct viewing through the microscope eyepieces. For FRAP, the laser scanning microscope (LSM 510; ZEISS) was set to laser scanning mode, and the initial imaging conditions were determined. A 25 × 0.8 NA lens was used for these experiments, and pixel sampling was set between 90 and 120 nm per pixel. The argon laser spectral line at a wavelength of 488 nm was set to an intensity of ≤1.25% of its total power (15 mW) for image collection. A region of interest, such as half of the cell nucleus, was defined, and the software provided with the microscope provided the means to photobleach only this region, using 100 iterations at 25% laser intensity. 12-bit images were collected before, immediately following, and at defined intervals after bleaching. Since significant changes in green fluorescent protein (GFP) signal equilibrium were not observed after 15 min, our total experiment time was set to 900 s to ensure that we observed complete recovery of photobleaching.
Results
CBP Presence in PML Nuclear Bodies Depends on the Cell Line
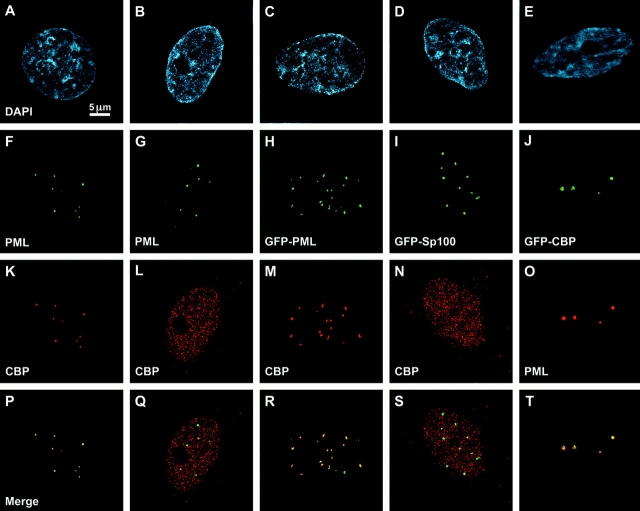
The transcription coactivator CBP is a component of all PML nuclear bodies in HEp-2 (LaMorte et al. 1998), SK-N-SH, COS-1, and CHO cells as detected by immunofluorescence microscopy (Boisvert et al. 2000; Fig. 1A, Fig. F, Fig. K; data not shown). However, we have found that not all cell lines exhibit this property. For example, HeLa, 293, HS-68, and Hep-G2 (Fig. 1 Q; data not shown) cells do not accumulate CBP in PML nuclear bodies. To determine the subnuclear distribution of overexpression of the GFP–CBP fusion protein, 293 cells were transiently transfected with an expression construct coding for this protein. This fusion protein has histone acetyltransferase activity, since the levels of histone H3 and H4 acetylation detected by immunofluorescence rise in proportion to the levels of GFP–CBP overexpression (not shown). This indicates that the GFP tag does not interfere with at least some of CBP's protein–protein interactions. The fusion protein is targeted to PML nuclear bodies as well as being distributed throughout the nucleoplasm (Fig. 1 J). These cells do not normally concentrate CBP in PML nuclear bodies. Furthermore, expression of GFP–Pml (Fig. 1C, Fig. H, Fig. M, and Fig. R) also leads to the accumulation of the endogenous CBP in PML nuclear bodies of these cells (293) (Fig. 1 R). However, overexpression of Sp100 does not bring the endogenous CBP into PML nuclear bodies (Fig. 1D, Fig. I, Fig. N). This shows that the presence of CBP within PML nuclear bodies depends on Pml but not Sp100. Interestingly, expression of Sp100 and Pml increases the number of PML nuclear bodies (Fig. 1H and Fig. I), whereas expression of CBP does not. This indicates that Pml and Sp100, but not CBP, participate in the formation of this nuclear structure.
Figure 1.
Colocalization by deconvolution immunofluorescence microscopy of CBP (NH2 terminus antibody; Santa Cruz Biotechnology, Inc.) and Pml (5E10 antibody) proteins in SK-N-SH cells (A, F, K, and P) but not in 293 cells (B, G, L, and Q). Overexpression of GFP–Pml in 293 cells (C and H) allows proper targeting into PML bodies (C) and concentrates CBP in PML bodies (M and R). Overexpression of GFP–Sp100 in 293 cells (D and I) does not concentrate CBP in PML bodies (N and S). Overexpression of GFP–CBP in 293 cells (E and J) targets this protein to PML bodies (O and T). Bar, 5 μm.
The observation that CBP is a component of PML nuclear bodies in some cell lines, or that it can be forced to accumulate in PML nuclear bodies with even low levels of overexpression of Pml or CBP itself, indicates that CBP may transit through PML nuclear bodies and accumulate there if the conditions are favorable. Therefore, the steady-state levels of CBP in PML nuclear bodies could be shifted by slight changes in concentration of factors with which CBP interacts.
Effect of Interferon on CBP Accumulation in PML Bodies
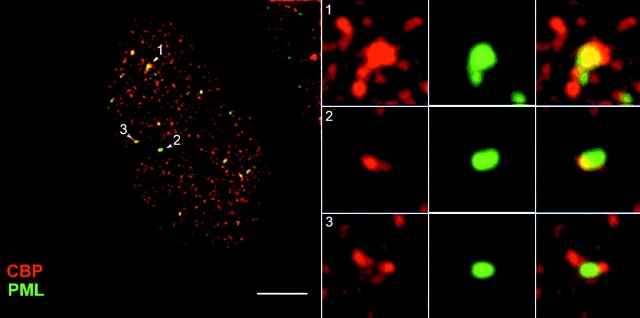
Overexpression of Pml protein leads to an accumulation of CBP in PML bodies in cells that do not otherwise show this localization. We wished to determine whether induction of Pml by α-interferon might also lead to an accumulation of CBP in PML bodies. As expected, after induction of 293 cells with α-interferon, the number of PML bodies increased by three- to fourfold and the total immunofluorescence signal increased approximately threefold (not shown), consistent with previous studies (Lavau et al. 1995). After 72 h of exposure to interferon, the CBP distribution changed significantly. Before induction of 293 cells, CBP is distributed in hundreds of small foci throughout the nucleoplasm. There is almost no overlap of these foci with PML bodies. However, after treatment larger foci appear, and many of these colocalize with PML bodies (Fig. 2, objects 1 and 2 in left panel). Other accumulations of CBP, however, are near but do not align precisely with PML bodies (Fig. 2, objects 2 and 3 in right panel). We do not know the basis for the accumulation of CBP into the larger foci that are not PML bodies upon interferon induction, but the accumulation of CBP in PML bodies mimics the accumulation seen when Pml levels are increased through transient overexpression.
Figure 2.
Localization of Pml and CBP by deconvolution immunofluorescence microscopy after exposure of these 293 cells to α-interferon (1,000 U/ml) for 72 h. Bar: (left panel) 5.5 μm; (right, panels 1–3) 1.38 μm.
FRAP Analysis of GFP–CBP Movement
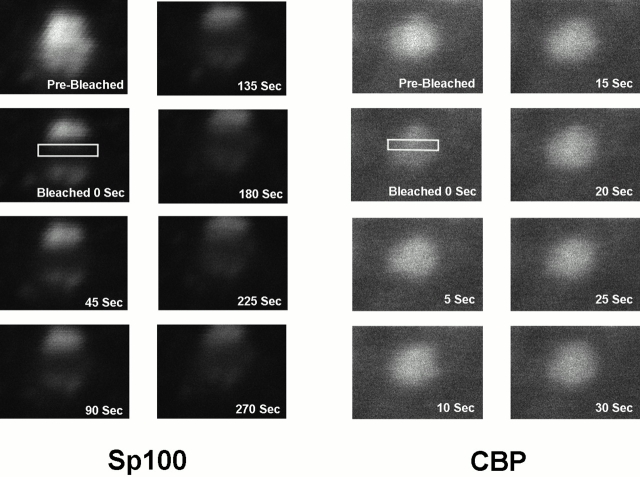
To determine whether CBP is a mobile molecule in the nucleus and moves in or out of PML nuclear bodies, we measured its mobility using the FRAP technique. The approach uses a high intensity laser from a laser scanning microscope to irreversibly bleach a region in the nucleus of a cell expressing a GFP fusion protein (boxes in Fig. 3 represent the bleached region). After photobleaching, the cell is imaged at different times to follow the redistribution of the unbleached GFP fusion proteins. Because the bleaching process irreversibly eliminates fluorescence of the GFP without affecting the rest of the protein, it is possible to visualize the normal movement of the unbleached protein in the cell. The recovery of the fluorescence is a measure for the mobility of the protein.
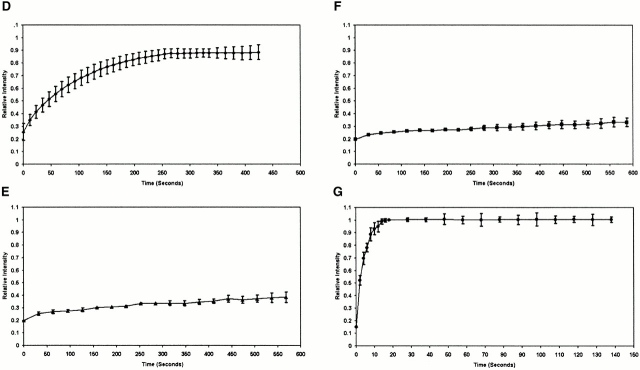
Figure 3.
FRAP of half-nucleus of 293 cells expressing GFP–Pml (A), GFP–Sp100 (B), or GFP–CBP (C). Cells were imaged before the bleaching (Pre-Bleached), immediately after the bleaching (Bleached, time = 0 s), and during fluorescence recovery at the indicated times. A box indicates the area bleached, corresponding to half the nucleus. The fluorescence intensity in the bleached and the unbleached regions was measured and expressed as a relative intensity where a value of one is equal intensity in both halves. The relative intensity over time is shown. Fluorescence recovery curves for CBP (D), Pml (E), and Sp100 (F) show the kinetics of redistribution of the different fluorescent proteins after bleaching. As a control, recovery of GFP alone was measured (G).
A region corresponding to approximately half of the cell nucleus was photobleached (Fig. 3 C). After 40 and 80 s, a wave of recovery of fluorescence can be seen to move across the nucleus from the unbleached to the bleached side and where the redistributed fluorescence is ∼50% of that before photobleaching. Plots of the gray values up to 200 s also show this directional wave, though the effect is not apparent in the images after 80 s due to the limited gray value resolution in the images (Fig. 3 C). Transient expression of GFP–CBP shows two different populations, one that is concentrated in PML nuclear bodies and one that is dispersed throughout the nucleus (Fig. 1 J and Fig. 3 C). The GFP–CBP observed outside of PML nuclear bodies, dispersed throughout the nucleoplasm, moves >50 times slower than GFP alone (Fig. 3E and Fig. G). This difference in mobility is likely not a consequence of the size of the protein, but rather due to interaction with other nuclear components since some large structures (500 nm in diameter) can move very rapidly through the nucleoplasm (Seksek et al. 1997; Kruhlak et al. 2000). Fluorescence recovery of these proteins is significantly faster than GFP–histone H2B, which is immobile over very long periods of time (4 h) (Phair and Misteli 2000; our unpublished observations). A time period of 7.5 min was necessary for the GFP–CBP signal to reach equilibrium with the bleached half of the nucleus compared with only 14 s for the GFP protein alone. The half-recovery time was calculated to be 92 and 1.8 s, respectively, for GFP–CBP and GFP alone. There is no apparent difference in rates of recovery between CBP in PML nuclear bodies and the CBP population that is dispersed throughout the nucleoplasm (Fig. 3 C). This means that high-affinity binding sites for CBP exist in PML nuclear bodies and throughout the nucleoplasm. In control experiments in which the entire nuclear fluorescence was bleached, recovery was not observed over long time periods (30 min), indicating that de novo synthesis, import of a cytoplasmic pool, or refolding of the GFP molecule did not contribute significantly to fluorescence recovery (data not shown). Moreover, cells can be bleached several times and will still show similar kinetics of recovery, indicating that there is no immediate damage induced by scanning the cell using high intensity 488-nm-wavelength light. Photobleaching does not affect the cell's ability to enter mitosis and does not affect the mobility of organelles or subnuclear domains as visualized by differential interference optics. We have also shown that paraformaldehyde-fixed cells show no recovery after photobleaching.
CBP Moves In and Out of PML Nuclear Bodies
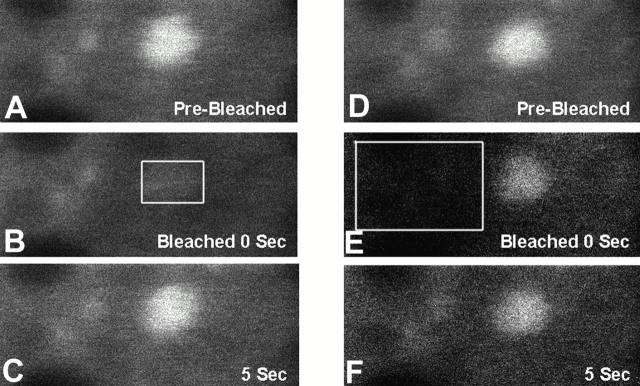
The rate of fluorescence recovery of CBP in PML nuclear bodies is equivalent to that found in the nucleoplasm. To determine whether the direction of CBP movement is only into PML nuclear bodies or is bidirectional into and out of these domains, we performed both FRAP and fluorescence loss in photobleaching (FLIP) experiments. To determine the rate of movement from the nucleoplasm into PML nuclear bodies, we bleached an entire PML nuclear body in a cell expressing GFP–CBP (Fig. 4, A–C). Complete fluorescence recovery of the PML nuclear body was observed after 5 s. This indicates that CBP can move rapidly from the nucleoplasm into PML nuclear bodies. To determine whether CBP can leave the PML nuclear body, we bleached a region just outside the domain to see whether we could drain some fluorescence from it (FLIP) (Fig. 4D and Fig. E). Indeed, we observed a loss of fluorescence from the PML nuclear body followed by a quick reequilibration (5 s) of the fluorescence. The integrated intensity of signal from the PML body shown in Fig. 4 E decreased from 180 to 131 (gray values) after bleaching but rebounded to 160 within 5 s. Our interpretation is that fluorescent molecules that moved from the PML body to the bleached region outside were rapidly replaced (5 s) by fluorescent molecules moving into the PML body from unbleached but nearby regions of the nucleoplasm. Therefore, we conclude that the movement of GFP–CBP between the nucleoplasm and the PML nuclear bodies is bidirectional. The movement cannot be described as freely mobile since it is significantly slower than that seen for a freely diffusing molecule such as GFP (Fig. 3 G). These experiments indicate that CBP in PML nuclear bodies is not an insoluble aggregation of molecules which form by random clustering of diffusing molecules. The bidirectional movement further demonstrates that CBP molecules are not recruited to these domains, stored, and then degraded (Maul 1998).
Figure 4.
Determination of the direction of movement of GFP–CBP. A whole PML nuclear body was bleached (B, box) in a 293 cell expressing GFP–CBP. 5 s after bleaching, an image showing the fluorescence recovery was recorded (5 s; C). A region just outside the PML nuclear body was then bleached (E, box), indicating that fluorescence can be drained from the PML nuclear body (E), which is followed by a rapid reequilibration (5 s; F) of the fluorescence. Bleached box in Fig. 4 B is 400 nm in length.
FRAP Analysis of GFP–PML and GFP-Sp100 Movement
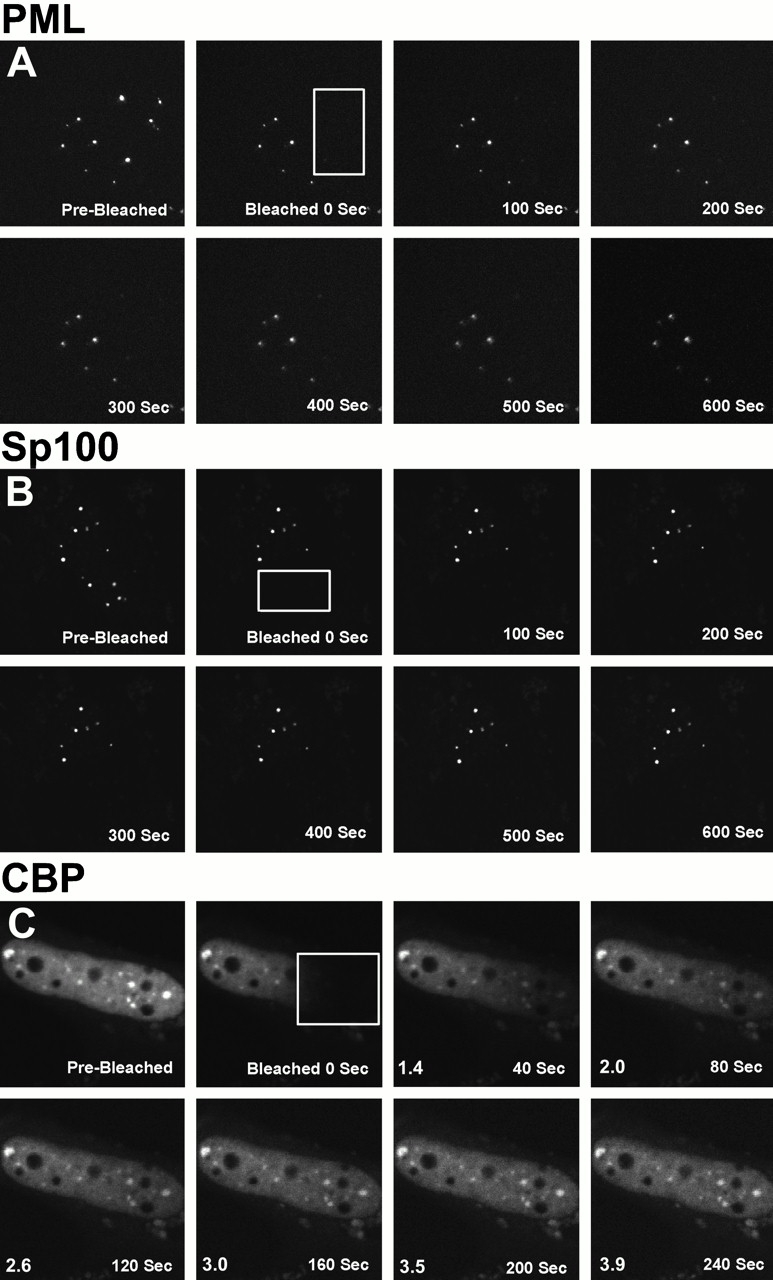
In contrast to the rate of movement of GFP–CBP in live cells, GFP–Pml (Fig. 3A and Fig. E) and GFP-Sp100 (Fig. 3B and Fig. F) fluorescence recovery occurred over much longer times with very little recovery even after 10 min. The recovery times are extremely long, indicating that these molecules are immobile. This indicates that Pml and Sp100 are not local concentrations of protein that form through stochastic aggregation events from freely diffusing molecules. Instead, the dynamic properties of Sp100 and Pml are consistent with structural proteins that contribute to the integrity of the PML nuclear body, which we propose is a specialized component of the protein-based nuclear architecture.
Sp100 and PML Are Immobile inside the PML Nuclear Bodies
We wished to determine whether Sp100 and Pml could be distinguished from CBP on the basis of mobility within the PML nuclear body itself. To determine whether GFP-Sp100 is moving within the PML nuclear body, we performed FRAP at high resolution, bleaching a line passing through the middle of a single PML nuclear body (Fig. 5 A). We found that the fluorescence within the PML nuclear body only recovers after relatively long periods (4 min). The same result was obtained for Pml within PML nuclear bodies (data not shown). In contrast, GFP–CBP (Fig. 5 B) fluorescence recovers rapidly from such a treatment, making it difficult to observe the bleached line inside the PML nuclear body in the first image recorded after the bleaching step. After 5 s, the fluorescence of GFP–CBP has been completely redistributed throughout the PML nuclear bodies. The rate of redistribution is greater than that observed when a half nucleus is bleached because the distance the proteins have to travel is comparatively much less. Again, the lack of movement of Sp100 and Pml within the PML nuclear bodies lead us to conclude that they play a structural role in these domains and that the steady-state accumulations of Sp100 and Pml are very stable.
Figure 5.
FRAP of subregions of PML bodies. A line of photobleaching through the middle of one PML nuclear body (boxes) was created in 293 cells expressing GFP-Sp100 (A) and GFP–CBP (B). After the bleaching, images were recorded over time. Bleached boxes are ∼300 nm in length.
Discussion
We have shown previously that a protein-based nuclear architecture exists in the eukaryotic nucleus under native, nondisruptive conditions (Hendzel et al. 1999). The PML nuclear body represents a specialization of the protein-based architecture and exists as an entity that is independent of either DNA or RNA (Boisvert et al. 2000). Several different proteins have the potential to localize to varying degrees within these subnuclear domains, including Pml and Sp100, which are consistently found there. CBP, on the other hand, is concentrated in PML nuclear bodies only under certain conditions. For example, there are several cell lines where immunofluorescence microscopy cannot detect an accumulation of CBP in these domains. However, even the lowest levels of overexpression of Pml (where the size and number of PML bodies are unaffected) or CBP above the endogenous levels in these cells can lead to an accumulation of CBP in these bodies. A similar effect is observed when endogenous Pml levels are increased through α-interferon induction. Thus, it appears that one function of Pml is to target CBP to PML nuclear bodies. One model for the formation of such protein enrichments in the nucleoplasm is that rapidly diffusing molecules can associate with and dissociate from binding sites within subnuclear domains (Phair and Misteli 2000). This model may accurately apply to the formation of some subnuclear domains but apparently does not apply to all of the components of PML nuclear bodies. At least two components, Pml itself and Sp100, are relatively immobile proteins in the nucleoplasm. They represent nuclear proteins that are tightly bound and do not move significantly even within individual PML bodies. In contrast, CBP moves relatively rapidly into and out of these domains, behaving more similarly to the alternate splicing factor in relation to nuclear speckles (Kruhlak et al. 2000; Phair and Misteli 2000). Though CBP's movement is relatively rapid compared with Pml, it is not freely mobile because it moves significantly slower than GFP, a protein that does not bind specifically to any subnuclear complexes. Therefore, there appear to be sites throughout the nucleoplasm with which CBP can interact but discrete domains where the equilibrium is such that local accumulation is maintained. The number of these domains as well as their size and shape can be modulated by stresses such as heat shock, interferons, and viral infections. An alternative explanation for the apparent slower movement of CBP is that it is part of a large complex that has a lower diffusion constant. We think that this explanation is unlikely because we have observed very large structures (up to 500 nm in diameter) that can move very rapidly through the nucleoplasm (Kruhlak et al. 2000).
We propose that Pml acts as an anchor for concentrating factors such as CBP, thus providing a protein-based rather than DNA-based affinity site able to concentrate CBP in local nuclear volumes. The local accumulation of CBP, a transcriptional coactivator and histone acetyltransferase, may create a domain on the periphery of PML nuclear bodies that is enriched in acetylated and transcriptionally active chromatin. Indeed, we have observed that the chromatin surrounding PML nuclear bodies is highly acetylated, and nascent RNA is associated with the periphery of these domains (Boisvert et al. 2000). We propose that CBP accumulates in regions where a greater concentration is required. Disruption of nuclear bodies, and thereby disruption of the levels of CBP and other PML body components in certain subnuclear volumes, may influence the level of activity of the surrounding genes. Hence, biochemical alteration of the domain, by altering composition or physical integrity, may lead to aberrant gene regulation and a transformed phenotype (Zhong et al. 2000).
Acknowledgments
We thank Ying Ren and Priscilla Hill for technical assistance, and Dr. W. Michael Schoel for assistance with the digital fluorescence microscopy. We are grateful to Dr. R. van Driel for the 5E10 antibody against Pml. F.-M. Boisvert and M.J. Kruhlak were recipients of studentships from the Alberta Heritage Foundation for Medical Research.
The work was funded by an operating grant from the Cancer Research Society, Inc.
Footnotes
Abbreviations used in this paper: CBP, CAMP response element–binding protein; GFP, green fluorescent protein; PML, promyelocytic leukemia.
References
- Arany Z., Newsome D., Oldread E., Livingston D.M., Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature. 1995;374:81–84. doi: 10.1038/374081a0. [DOI] [PubMed] [Google Scholar]
- Ascoli C.A., Maul G.G. Identification of a novel nuclear domain. J. Cell Biol. 1991;112:785–795. doi: 10.1083/jcb.112.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert F.-M., Hendzel M.J., Bazett-Jones D.P. Promyelocytic leukemia (PML) nuclear bodies are protein structures that do not accumulate RNA. J. Cell Biol. 2000;148:282–292. doi: 10.1083/jcb.148.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de The H., Chomienne C., Lanotte M., Degos L., Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 1990;347:558–561. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]
- de The H., Lavau C., Marchio A., Chomienne C., Degos L., Dejean A. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991;66:675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- Doucas V., Tini M., Egan D., Evans R.M. Modulation of CREB binding protein function by the promyelocytic (PML) oncoprotein suggests a role for nuclear bodies in hormone signaling. Proc. Natl. Acad. Sci. USA. 1999;96:2627–2632. doi: 10.1073/pnas.96.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A., Avantaggiati M.L. p300 and CBPpartners for life and death. J. Cell. Physiol. 1999;181:218–230. doi: 10.1002/(SICI)1097-4652(199911)181:2<218::AID-JCP4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Guldner H.H., Szostecki C., Grotzinger T., Will H. IFN enhances expression of Sp100, an autoantigen in primary biliary cirrhosis. J. Immunol. 1992;149:4067–4073. [PubMed] [Google Scholar]
- Hendzel M.J., Kruhlak M.J., Bazett-Jones D.P. Organization of highly acetylated chromatin around sites of heterogeneous nuclear RNA accumulation. Mol. Biol. Cell. 1998;9:2491–2507. doi: 10.1091/mbc.9.9.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendzel M.J., Boisvert F.-M., Bazett-Jones D.P. Direct visualization of a protein nuclear architecture. Mol. Biol. Cell. 1999;10:2051–2062. doi: 10.1091/mbc.10.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishov A.M., Sotnikov A.G., Negorev D., Vladimirova O.V., Neff N., Kamitani T., Yeh E.T., Strauss J.F.R., Maul G.G. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 1999;147:221–234. doi: 10.1083/jcb.147.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizuka A., Miller W.H.J., Umesono K., Warell R.P.J., Frankel S.K., Murty V.V., Dmitrovsky E., Evans R.M. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell. 1991;66:663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- Kogan S.C., Hong S.H., Shultz D.B., Privalsky M.L., Bishop J.M. Leukemia initiated by PMLRARalphathe PML domain plays a critical role while retinoic acid-mediated transactivation is dispensable. Blood. 2000;95:1541–1550. [PubMed] [Google Scholar]
- Kruhlak M.J., Levert M.A., Fischle W., Verdin E., Bazett-Jones D.P., Hendzel M.J. The mobility of the GFP:ASF splicing factor in live cells. J. Cell Biol. 2000;150:41–51. doi: 10.1083/jcb.150.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok R.P., Lundblad J.R., Chrivia J.C., Richards J.P., Bachinger H.P., Brennan R.G., Roberts S.G., Green M.R., Goodman R.H. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- LaMorte V.J., Dyck J.A., Ochs R.L., Evans R.M. Localization of nascent RNA and CREB binding protein with the PML-containing nuclear body. Proc. Natl. Acad. Sci. USA. 1998;95:4991–4996. doi: 10.1073/pnas.95.9.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavau C., Marchio A., Fagioli M., Jansen J., Falini B., Lebon P., Grosveld F., Pandolfi P.P., Pelicci P.G., Dejean A. The acute promyelocytic leukaemia-associated PML gene is induced by interferon. Oncogene. 1995;11:871–876. [PubMed] [Google Scholar]
- Maul G.G. Nuclear domain 10, the site of DNA virus transcription and replication. Bioessays. 1998;20:660–667. doi: 10.1002/(SICI)1521-1878(199808)20:8<660::AID-BIES9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Pederson T. Half a century of “the nuclear matrix.”. Mol. Biol. Cell. 2000;11:799–805. doi: 10.1091/mbc.11.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phair R.D., Misteli T. High mobility of proteins in the mammalian cell nucleus. Nature. 2000;404:604–609. doi: 10.1038/35007077. [DOI] [PubMed] [Google Scholar]
- Seksek O., Biwersi J., Verkman A.S. Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus. J. Cell Biol. 1997;138:131–142. doi: 10.1083/jcb.138.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuurman N., de Graaf A., Floore A., Josso A., Humbel B., de Jong L., van Driel R. A monoclonal antibody recognizing nuclear matrix-associated nuclear bodies. J. Cell Sci. 1992;101:773–784. doi: 10.1242/jcs.101.4.773. [DOI] [PubMed] [Google Scholar]
- Szostecki C., Guldner H.H., Netter H.J., Will H. Isolation and characterization of cDNA encoding a human nuclear antigen predominantly recognized by autoantibodies from patients with primary biliary cirrhosis. J. Immunol. 1990;145:4338–4347. [PubMed] [Google Scholar]
- Xie K., Lambie E.J., Snyder M. Nuclear dot antigens may specify transcriptional domains in the nucleus. Mol. Cell. Biol. 1993;13:6170–6179. doi: 10.1128/mcb.13.10.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S., Salomoni P., Pandolfi P.P. The transcriptional role of PML and the nuclear body. Nat. Cell Biol. 2000;2:85–90. doi: 10.1038/35010583. [DOI] [PubMed] [Google Scholar]