Abstract
In vivo microscopy has recently revealed the dynamic nature of many cellular organelles. The dynamic properties of several cellular structures are consistent with a role for self-organization in their formation, maintenance, and function; therefore, self-organization might be a general principle in cellular organization.
Keywords: self-organization; cytoskeleton; nucleus; Golgi complex; dynamics
A central question in modern cell biology is how large, macroscopic cellular structures are formed and maintained. It is unknown what determines the different shapes and sizes of cellular organelles, why specific structures form in particular places, and how cellular architecture is affected by function and vice versa.
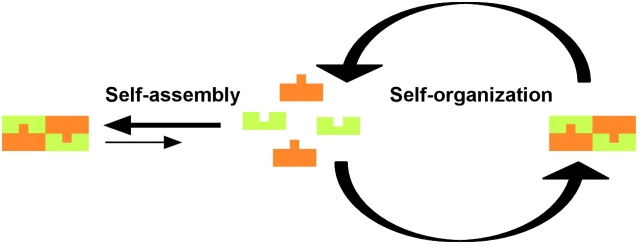
Two fundamentally different mechanisms exist to generate macromolecular structures: self-assembly and self-organization. Whereas self-assembly involves the physical association of molecules into an equilibrium structure (Kushner, 1969), self-organization involves the physical interaction of molecules in a steady-state structure (Fig. 1) (Nicolis and Prigogine, 1977). For example, virus and phage proteins self-assemble to true equilibrium and form stable, static structures. In contrast, most cellular structures (i.e., the cytoskeleton, nuclear subcompartments, or exocytic and endocytic compartments) are open for exchange of energy and matter and are governed by steady-state dynamics.
Figure 1.
Self-assembly versus self-organization. In self-assembly, a set of components assembles into a stable, static structure that reaches a thermodynamic equilibrium. In self-organization, a set of components assembles into a steady-state, dynamic structure.
The concept of self-organization is based on observations of chemical reactions far from equilibrium, and it is well established in chemistry, physics, ecology, and sociobiology (Nicolis and Prigogine, 1977). Self-organization in the context of cell biology can be defined as the capacity of a macromolecular complex or organelle to determine its own structure based on the functional interactions of its components. In a self-organizing system, the interactions of its molecular parts determine its architectural and functional features. The processes that occur within a self-organized structure are not underpinned by a rigid architectural framework; rather, they determine its organization.
For self-organization to act on macroscopic cellular structures, three requirements must be fulfilled: a cellular structure must be dynamic, material must be continuously exchanged, and an overall stable configuration must be generated from dynamic components. Recent advances in live cell imaging have provided, for the first time, insights into the dynamic properties of cellular organelles. Observations from these studies indicate that many cellular structures fulfill the requirements for self-organization. Here I consider the role self-organization might play in the architecture of three major macroscopic cellular structures, namely the cytoskeleton, the cell nucleus, and the Golgi complex. These structures have completely distinct morphological features, diverse functions, and sufficient data regarding their kinetic properties is available to examine whether self-organization affects their architecture. I suggest that self-organization is a more general mechanism for the formation, maintenance, and function of cellular organization than currently anticipated.
The dynamic cell: overall stability from dynamic parts
The interior of a cell is highly dynamic. Proteins, protein complexes, and lipid vesicles move rapidly within the cell. Proteins move with apparent diffusion coefficients of 0.2–20 um2 s−1 in the cytoplasm and in the nucleus, enabling them to travel a distance of several micrometers in a few seconds. Under these conditions, a single protein molecule with an abundance of 50,000 copies per cell encounters a partner molecule of similar abundance every 0.5 s, and any such molecule can reach any place in the cell with equal probability within minutes. Thus, in a living cell, proteins continuously and transiently interact with ever-changing partners (Misteli, 2001).
Many macroscopic cellular structures are also highly dynamic. Although cellular organelles appear static in snapshot observations and can even be biochemically purified, it is now clear that many components of cellular structures are continuously exchanged with their surroundings (Fig. 2). Therefore, apparently stable cellular structures can be generated from highly dynamic components. The generally high mobility of proteins, the high exchange rate, and the generation of stable structures, which result from transient interactions of their components are consistent with a role of self-organization in cellular architecture.
Figure 2.
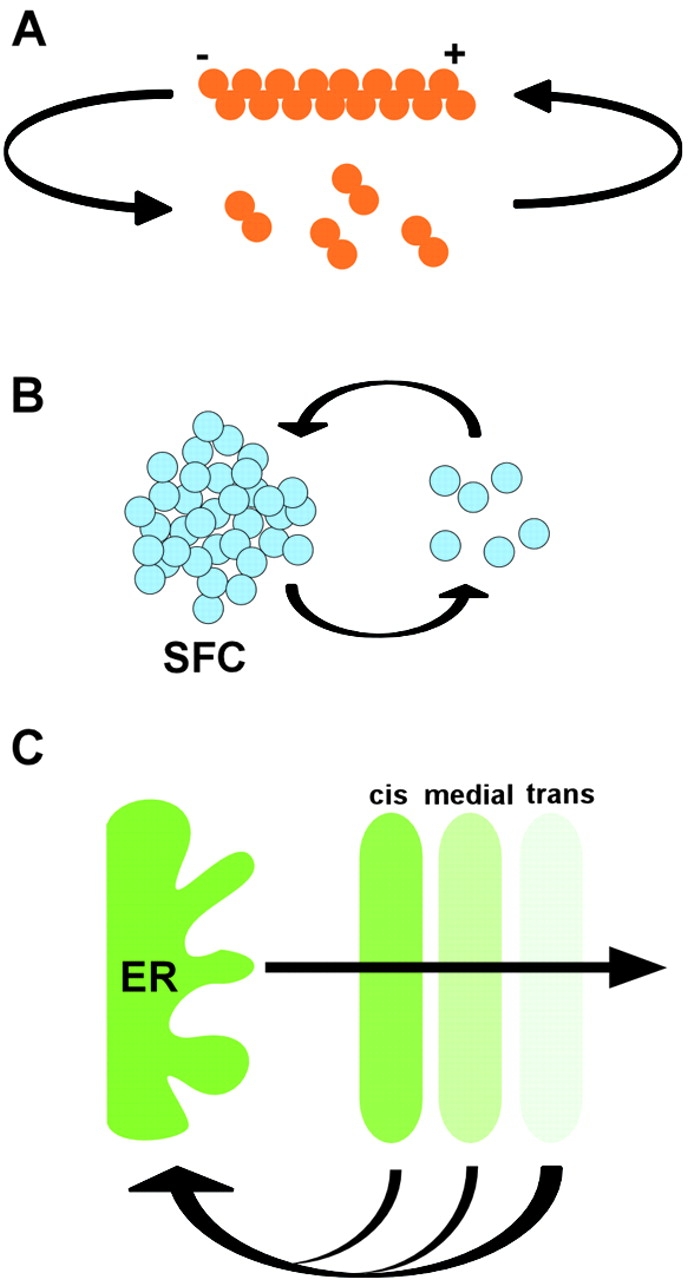
Dynamic components generate stable structures. (A) Tubulin subunits are continuously exchanged from microtubule filaments. (B) Pre-mRNA splicing factors continuously cycle between the SFC and the nucleoplasm. (C) Membrane continuously flows throughout the Golgi complex.
The cytoskeleton: a clear case of self-organization
The concept of self-organization is well established in the study of the actin and microtubule cytoskeletons (Mitchison, 1992). Microtubule and actin polymers determine cell shape and polarity, provide an internal structural framework, and form the spindle apparatus during cell division. Each one of these functions requires a different architecture of the cytoskeletal network, and all of these distinct architectural organizations are generated from a limited set of components. The key to how the same basic cytoskeletal building blocks can assume structurally and functionally different assemblies lies in their highly dynamic nature and their ability to self-organize.
The actin and microtubule cytoskeletons are intrinsically unstable, and they undergo continuous turnover of subunits by addition at their plus end and depolymerization at their minus end (Fig. 2 A). The continuous dynamic exchange of the subunits and their interactions with associated proteins are essential for the polymerization of structures of different patterns. The self-organizing properties of microtubule networks have been elegantly demonstrated in vitro by simply combining tubulin, microtubule motors, and ATP (Nedelec et al., 1997). Depending on the relative concentrations of motors and tubulin, structurally different patterns (i.e., random networks, vortices, or asters) are formed. The outcome of the polymerization process is solely determined by the concentration of reactants and the kinetics of their interactions (Surrey et al., 2001). As would be expected for a self-organizing system, the transitions between distinct assembly patterns are not gradual but are sharp, and multiple sets of assembly conditions can result in the same assembly pattern (Surrey et al., 2001).
The physiological relevance of self-organization is evident from the study of cell crawling. Crawling of fibroblasts occurs by continuous, apparently random, remodeling of the actin cytoskeleton. It has been unclear how crawling cells can move towards a target by a persistent random walk. Recent computer modeling of actin dynamics demonstrates that actin filaments close to the membrane are more likely to grow into the crawling direction than in other directions, thereby favoring forward movement. These results demonstrate that the autocatalytic polymerization kinetics of actin are sufficient to promote persistent crawling (Sambeth and Baumaertner, 2001).
The limited set of components in the cytoskeleton has allowed the use of pioneering computational modeling approaches to determine the parameter combinations that result in the collective properties of cytoskeletal networks (Sambeth and Baumaertner, 2001; Surrey et al., 2001). These studies have permitted the direct and quantitative testing of the role of self-organization in the formation of cytoskeletal networks, thus elevating the concept of self-organization from a largely theoretical consideration to a cell biological reality. Although the behavior of other macroscopic cellular structures is more complex, cellular compartments such as the nucleus and the Golgi complex show hallmarks of self-organizing systems and may, therefore, follow similar organizational principles.
Self-organization in the cell nucleus
The mammalian cell nucleus contains a number of distinct subcompartments, the most prominent being the nucleolus, the splicing factor compartments (SFC),*and the family of small nuclear bodies including Cajal and promyelocytic leukemia bodies. However, it has not been clear how these compartments are formed and maintained (Lewis and Tollervey, 2000). Analogous with cytoskeletal elements, nuclear compartments are not defined by membranes, and recent analysis of the dynamics of nuclear compartments demonstrates that their resident proteins are continuously exchanged with the nucleoplasm (Misteli, 2001).
A role for self-organization has been suspected in the biogenesis of the nucleolus (Lewis and Tollervey, 2000; Misteli, 2001). Although the nucleolus is a permanent subnuclear structure, nucleolar proteins are continuously exchanged between the nucleolus and the nucleoplasm, generating an overall stable compartment from dynamic components. The morphological appearance of the nucleolus is directly linked to its functional status. When ribosomal gene transcription is inhibited experimentally, the nucleolus disassembles (Oakes et al., 1993). Conversely, introduction of extra copies of ribosomal genes into cells generates micronucleoli (Karpen et al., 1988; Oakes et al., 1998). Even more telling is the behavior of the nucleolus during cell division (Olson et al., 2000). During M phase ribosomal genes are repressed, the nucleolus disassembles, and nucleolar proteins are dispersed throughout the dividing cell. During telophase, rDNA transcription resumes and the nucleolar structure reforms. In vivo microscopy experiments suggest that the mitotic reassembly of the nucleolus might occur by self-organization. rRNA processing factors are highly mobile in the reforming nucleus, and as they diffuse throughout the nucleus they search the nuclear volume for binding sites on nascent rRNAs. As synthesis of nascent transcripts resumes, the processing enzymes associate and gradually accumulate at ribosomal transcription sites, thus reforming the nucleolus as more nascent rRNAs are made (Dundr et al., 2000).
The behavior of nuclear SFCs is also consistent with self-organization. Pre-mRNA splicing factors are concentrated in irregularly shaped, intranuclear membraneless structures termed SFCs. Splicing factors reside only transiently in SFCs, and the proteins shuttle continuously between SFCs and sites of transcription which are dispersed throughout the nucleoplasm. Thus, the relative rate of association and dissociation with SFCs determines the occupancy of SFCs and their morphological appearance (Fig. 2 B). Upon inhibition of transcription, SFCs lose their normally amorphous appearance and reorganize into round bodies in which splicing factors accumulate (Sinclair and Brasch, 1978). This morphological change can be explained by the self-organizing properties of the compartments. Upon inhibition of transcription, splicing factors, which normally bind to nascent transcripts outside of SFCs, find no binding targets and reassociate with the SFC more frequently, leading to the morphological rounding-up of the compartment. Evidence for a role of self-organization of some of the small nuclear bodies comes from the observation that expression of the Cajal body resident protein, p80-coilin, is sufficient to generate complete Cajal bodies in p80-coilin knockout cells (Tucker et al., 2001).
The molecular basis for self-organization of nuclear structures is unknown. A striking feature of many proteins found in subnuclear compartments is the presence of self-interaction domains. At least one of the prominent proteins in each of the major nuclear bodies contains an oligomerization domain, and some of these self-interacting proteins have been shown to be required for the formation of the respective nuclear bodies (Lorson et al., 1998; Chen et al., 1999; Ishov et al., 1999; Hebert and Matera, 2000). Similarly, the SR protein splicing factors, which are highly enriched in SFCs, contain an arginine–serine rich protein–protein interaction domain, and many nucleolar proteins are highly charged which may facilitate their self-interaction.
Nuclear structures might not form at random locations, and gene sequences might provide nucleation sites for their formation. Ribosomal genes provide a template for the formation of the nucleolus. Similarly, Cajal bodies frequently form near histone and U2 snRNA genes (Frey and Matera, 1995; Smith et al., 1995; Schul et al., 1998), and the Oct1/PTF/transcription domain is formed in preferential association with sequences of chromosome 6 (Pombo et al., 1998). In contrast, no potential templates for any of the other nuclear bodies are known, and it is unclear whether they form at random positions within the nucleus.
Self-organization might also play a role in chromatin organization (Wolffe and Hansen, 2001). It is becoming increasingly clear that chromatin is highly dynamic and in flux. Many architectural proteins have short residence times on chromatin and are continuously exchanged with the nucleoplasm. Similarly, transcriptional activators only interact transiently with their target sequences. Furthermore, the chromatin fiber itself is subject to structural alternations by remodeling activities. It is likely that chromatin and its associated proteins are in continuous transition, thus allowing the formation of protein configurations on chromatin by the combinatorial interactions of proteins that follow the principles of self-organization (Misteli, 2001).
The Golgi complex: self-organization of a membrane compartment
A case for self-organization can also be made for membrane compartments, particularly those of the exocytic transport pathway. Proteins destined for secretion or targeting to the plasma membrane are transported into the ER. From there, they are transported through the Golgi complex and the trans-Golgi network to the plasma membrane. The compartments of the exocytic pathway have long been considered permanent, static structures. However, tracking of resident and cargo molecules through the exocytic pathway using in vivo microscopy reveals that the compartments continuously and rapidly exchange material (Lippincott-Schwartz et al., 2000). The Golgi complex, which is at the center of the exocytic pathway, receives membrane from the ER and discharges membrane to the trans-Golgi network and back to the ER (Fig. 2 C). As is the case for membraneless compartments, an overall stable structure is generated from dynamic components.
The morphological appearance of the Golgi complex is greatly dependent on the balanced flux of material through the compartment (Glick, 2000). Inhibition of ER to Golgi transport results in the disintegration of the Golgi complex (Zaal et al., 1999). Similarly, inhibition of membrane budding from the trans-Golgi network results in the enlargement of the TGN and a size reduction of the Golgi complex (Griffiths et al., 1989). The physiological relevance of the balance of influx and outflux becomes clear during mitosis, when the Golgi complex disassembles into a large number of vesicles. It has been proposed that the reason for the disassembly is the continued budding of membranes with concomitant inhibition of their fusion (Warren, 1993; Lowe et al., 1998). This tips the balance to efflux and results in the disassembly of the organelle. The Golgi fragments reassemble during telophase and the polarized interphase stacks reform. Remarkably, the reformation process can be recapitulated in vitro, yielding polarized stacks of Golgi membranes and demonstrating the inherent self-organizing potential of this organelle (Rabouille et al., 1995).
The Golgi complex is a polarized organelle. Particular Golgi residents are found preferentially at the cis-, medial-, or trans-portion of the Golgi complex. The dynamic flow of membrane and the transient interactions of the cargo through the exocytic pathway might provide a means to maintain protein gradients by self-organizing protein–protein interactions. Proteins entering the stack from the ER are moved along the pathway until they find appropriate interaction partners, thus slowing their progress; they accumulate and, in turn, provide additional binding sites for incoming interaction partners. In such a “distillation process,” local concentrations are built up leading to distinct cis-, medial- or trans-Golgi subcompartments (Rothman, 1981; Rothman and Wieland, 1996). The flow of proteins through different compartments is essential for this mechanism, as it allows their interaction with various partners. The imperfect efficiency of this filtration process is illustrated by the fact that “resident” proteins are typically not found exclusively in one compartment, but appear to spill into neighboring compartments. Polarity within the Golgi stack might be established in a similar manner at the end of mitosis, when Golgi fragments fuse and their contents interact to literally sort themselves out.
The architecture of the Golgi complex is extreme in its complexity compared with other endomembrane structures. However, the same principles of organization may apply to other exocytic and endocytic compartments, as all of them are characterized by a high membrane flux.
Why self-organization?
Macroscopic cellular structures are characterized by two apparently contradictory properties. On one hand, they must be architecturally stable, on the other hand they must be flexible and prepared for change. Self-organization ensures structural stability without loss of plasticity. Fluctuations in the interaction properties of its components do not have deleterious effects on the structure as a whole. However, global and persistent changes rapidly result in morphological changes. The basis for the responsiveness of self-organized structures is the transient nature of the interactions among their components. The dynamic interplay of components generates frequent windows of opportunity during which proteins can change their interaction partners or be modified. The effective availability of components is controlled by posttranslational modifications via signal transduction pathways.
Why does a cell not simply build stable, static structures? To change stable structures, dedicated machineries must exist to break them down and rebuild them again when needed. Self-organizing, dynamic structures can easily be modified by simple modification of their subunits. The observed transitions of microtubule networks into spindles, and the complete, rapid disassembly and reassembly of SFCs and the Golgi complex, occur suddenly and typically with no significant intermediates. The absence of gradual intermediates in the reorganization of cellular structures is consistent with self-organization, as self-organizing systems are frequently in a state of criticality; that is, a point at which system properties can change suddenly.
Self-organization is an elegant, efficient way to organize complex structures. The properties that determine the organization are the intrinsic properties of the structure's components. In protein polymers, the protein–protein interaction properties determine the architecture; in membrane structures, the flow of membrane determines the architecture. Self-organized structures do not require complex mechanisms to establish, maintain, and regulate their architecture. Thus, self-organization is a simple but effective way to optimally organize cellular structures.
Concluding remarks
Many macroscopic cellular structures are highly dynamic, and their architecture is determined by the transient interactions of their components. These properties are consistent with a role of self-organization in their biogenesis. It remains to be seen which cellular structures are formed by processes of self-organization. For example, whereas the dynamic behavior of focal adhesions or lipid rafts in the plasma membrane are compatible with self-organization, it is not clear whether self-organization also plays a role in the organization of complexes such as ribosomes, spliceosomes, the transcription machineries, or the nuclear pore complex. It will be important to determine which, if any, cellular structures require templates for their formation, how the templates themselves are organized, and how the presence or absence of templates affects organelle inheritance.
The study of the dynamic behavior of cellular structures requires new tools. The behavior of dynamic cellular structures can not be described accurately by conventional equilibrium dynamics or by static observations. To understand the behavior of dynamic systems, the kinetic characteristics of their components must be known. In contrast to the study of molecular mechanisms, it is not sufficient to understand in detail the behavior of single molecules; rather, the rules that govern the collective behavior of systems must be uncovered. The future of cell biology will be to understand the collective behavior of cellular structures at the molecular level using novel tools, such as in vivo microscopy and computational modeling. In moving from analyzing single molecule behavior to studying the cell biological behavior of entire systems, we are bound to encounter many surprises. The possible role of self-organization as a basic principle in cellular architecture might be just the beginning.
Acknowledgments
I thank Graham Warren and Matthias Becker for discussion and critical comments. Due to space limitations, many of the relevant primary publications could not be cited.
Footnotes
Abbreviation used in this paper: SFC, splicing factor compartments.
References
- Chen, T., F.M. Boisvert, D.P. Bazett-Jones, and S. Richard. 1999. A role for the GSG domain in localizing Sam68 to novel nuclear structures in cancer cell lines. Mol. Biol. Cell. 10:3015–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundr, M., T. Misteli, and M.O.J. Olson. 2000. The dynamics of postmitotic reassembly of the nucleolus. J. Cell Biol. 150:433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey, M.R., and A.G. Matera. 1995. Coiled bodies contain U7 small nuclear RNA and associate with specific DNA sequence in interphase human cells. Proc. Natl. Acad. Sci. USA. 92:5915–5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick, B.S. 2000. Organization of the Golgi apparatus. Curr. Opin. Cell Biol. 12:450–456. [DOI] [PubMed] [Google Scholar]
- Griffiths, G., S.D. Fuller, R. Back, M. Hollinshead, S. Pfeiffer, and K. Simons. 1989. The dynamic nature of the Golgi complex. J. Cell Biol. 108:277–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert, M.D., and A.G. Matera. 2000. Self-association of p80 coilin reveals a common theme in nuclaer body formation. Mol. Biol. Cell. 11:4159–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishov, A.M., A.G. Sotnikov, D. Negorev, O.V. Vladimirova, N. Neff, T. Kamitani, E.T. Yeh, J.F. Strauss, and G.G. Maul. 1999. PML is critical for ND10 formation and recruits the PML-interacting protein Daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 147:221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen, G.H., J.E. Schaefer, and C.D. Laird. 1988. A Drosophila rRNA gene located in euchromatin is active in transcription and nucleolus formation. Genes Dev. 2:1745–1763. [DOI] [PubMed] [Google Scholar]
- Kushner, D.J. 1969. Self-assembly of biological structures. Bacteriol. Rev. 33:302–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, J.D., and D. Tollervey. 2000. Like attracts like: getting RNA processing together in the nucleus. Science. 288:1385–1389. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., T. Roberts, and K. Hirschberg. 2000. Secretory protein trafficking and organelle dynamics in living cells. Annu. Rev. Cell Dev. Biol. 16:557–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorson, C.L., J. Strasswimmer, J.D. Yao, J.D. Baleja, E. Hahnen, T. Wirth, T. Le, A.H. Burghes, and E.J. Androphy. 1998. SMN oligomerization defect correlates with spinal muscular atrophy severity. Nat. Genet. 19:63–66. [DOI] [PubMed] [Google Scholar]
- Lowe, M., N. Nakamura, and G. Warren. 1998. Golgi division and membrane traffic. Trends Cell Biol. 8:40–44. [DOI] [PubMed] [Google Scholar]
- Misteli, T. 2001. Protein dynamics: Implications for nuclear architecture and gene expression. Science. 291:843–847. [DOI] [PubMed] [Google Scholar]
- Mitchison, T.J. 1992. Self-organization of polymer-motor systems in the cytoskeleton. Phil. Trans. R. Soc. Lond. B. Biol. Sci. 336:99–106. [DOI] [PubMed] [Google Scholar]
- Nedelec, F., T. Surrey, A.C. Maggs, and S. Leibler. 1997. Self-organization of microtubules and motors. Nature. 389:305–308. [DOI] [PubMed] [Google Scholar]
- Nicolis, G., and I. Prigogine. 1977. Self-organization in Nonequilibrium Systems. John Wiley & Sons, Inc., New York. 512 pp.
- Oakes, M., J.P. Aris, J.S. Brockenbrough, H. Wai, L. Vu, and M. Nomura. 1998. Mutational analysis of the structure and localization of the nucleolus in the yeast Saccharomyces cerevisiae. J. Cell Biol. 143:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes, M., Y. Nogi, M.W. Clark, and M. Nomura. 1993. Structural alterations of the nucleolus in mutants of Saccharomyces cerevisiae defective in RNA polymerase I. Mol. Cell. Biol. 13:2441–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, M.O.J., M. Dundr, and A. Szebeni. 2000. The nucleolus: An old factory with unexpected capabilities. Trends Cell Biol. 10:189–196. [DOI] [PubMed] [Google Scholar]
- Pombo, A., P. Cuello, W. Schul, J.-B. Yoon, R.G. Roeder, P.R. Cook, and S. Murphy. 1998. Regional and temporal specialization in the nucleus: a transcriptionally-active nuclear domain rich in PTE, Oct1 and PIKA antigens associates with specific chromosomes early in the cell cycle. EMBO J. 17:1768–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille, C., T. Levine, M. Peters, and G. Warren. 1995. An NSF-like ATPase, p97, and NSF mediate cisternal regrowth from mitotic Golgi fragments. Cell. 82:905–914. [DOI] [PubMed] [Google Scholar]
- Rothman, J.E. 1981. The Golgi apparatus: two organelles in tandem. Science. 213:1212–1219. [DOI] [PubMed] [Google Scholar]
- Rothman, J.E., and F.T. Wieland. 1996. Protein sorting by transport vesicles. Science. 272:227–234. [DOI] [PubMed] [Google Scholar]
- Sambeth, R., and A. Baumaertner. 2001. Autocatalytic polymerization generates persistent random walk of crawling cells. Phys. Rev. Lett. 86:5196–5199. [DOI] [PubMed] [Google Scholar]
- Schul, W., R. van Driel, and L. de Jong. 1998. Coiled bodies and U2 snRNA genes adjacent to coiled bodies are enriched in factors required for snRNA transcription. Mol. Biol. Cell. 9:1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair, G.D., and K. Brasch. 1978. The reversible action of a-amanitin on nuclear structure and molecular composition. Exp. Cell Res. 111:1–14. [DOI] [PubMed] [Google Scholar]
- Smith, K.P., K. Carter, C. Johnson, and J.B. Lawrence. 1995. U2 and U1 snRNA gene loci associate with coiled bodies. J. Cell. Biochem. 59:473–485. [DOI] [PubMed] [Google Scholar]
- Surrey, T., F. Nedelec, S. Leibler, and E. Karsenti. 2001. Physical properties determining self-organization of motors and microtubules. Science. 292:1167–1171. [DOI] [PubMed] [Google Scholar]
- Tucker, K.E., M.T. Berciano, E.Y. Jacobs, D.F. LePage, K.B. Shpargel, J.J. Rossire, E.K.L. Chan, M. Lafarga, R.A. Conlon, and A.G. Matera. 2001. Residual Cajal bodies in coilin knckout mice fail to recruit Sm snRNPs and SMN, the spinal muscular atrophy gene product. J. Cell Biol. 154:293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, G. 1993. Membrane partitioning during cell division. Annu. Rev. Biochem. 62:323–348. [DOI] [PubMed] [Google Scholar]
- Wolffe, A., and J.C. Hansen. 2001. Nuclear visions: functional flexibility from structural instability. Cell. 104:631–634. [DOI] [PubMed] [Google Scholar]
- Zaal, K.J.M., C.L. Smith, R.S. Polishchuk, N. Altan, N.B. Cole, J. Ellenberg, K. Hirschberg, J.F. Presley, T.H. Roberts, E. Siggia, R.D. Phair, and J. Lippincott-Schwartz. 1999. Golgi membranes are absorbed into and reemerge from the ER during mitosis. Cell. 99:589–601. [DOI] [PubMed] [Google Scholar]