Abstract
How intracellular cytoskeletal and signaling proteins connect and communicate with the extracellular matrix (ECM) is a fundamental question in cell biology. Recent biochemical, cell biological, and genetic studies have revealed important roles of cytoplasmic integrin-linked kinase (ILK) and its interactive proteins in these processes. Cell adhesion to ECM is an important process that controls cell shape change, migration, proliferation, survival, and differentiation. Upon adhesion to ECM, integrins and a selective group of cytoskeletal and signaling proteins are recruited to cell matrix contact sites where they link the actin cytoskeleton to the ECM and mediate signal transduction between the intracellular and extracellular compartments. In this review, we discuss the molecular activities and cellular functions of ILK, a protein that is emerging as a key component of the cell–ECM adhesion structures.
Keywords: integrins; integrin-linked kinase; cell adhesion; cytoskeleton; signal transduction
Integrin-linked kinase–binding proteins
Integrin-linked kinase (ILK)* was identified and cloned 6 yr ago based on its interaction with the β1 integrin cytoplasmic domain (Hannigan et al., 1996). It comprises three structurally distinct regions (Fig. 1). Four ANK repeats lie at the NH2 terminus of ILK. COOH-terminal to the ANK domain is a pleckstrin homology (PH)-like motif. Further downstream and partially overlapping with the PH-like motif is the COOH-terminal domain, which exhibits significant homology to other protein kinase catalytic domains. One of the key functions of ILK is to mediate protein–protein interactions. To date, nine different ILK-binding proteins have been identified (Table I).
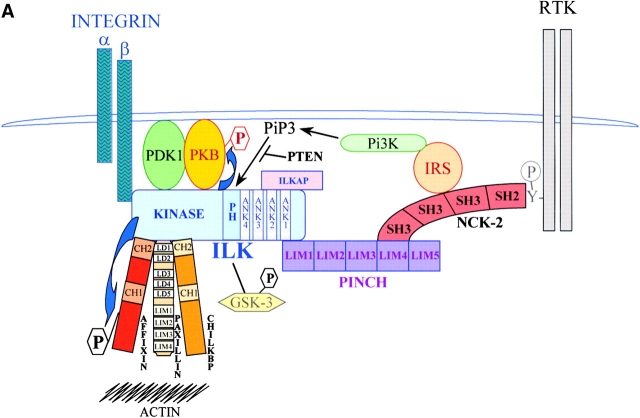
Figure 1.
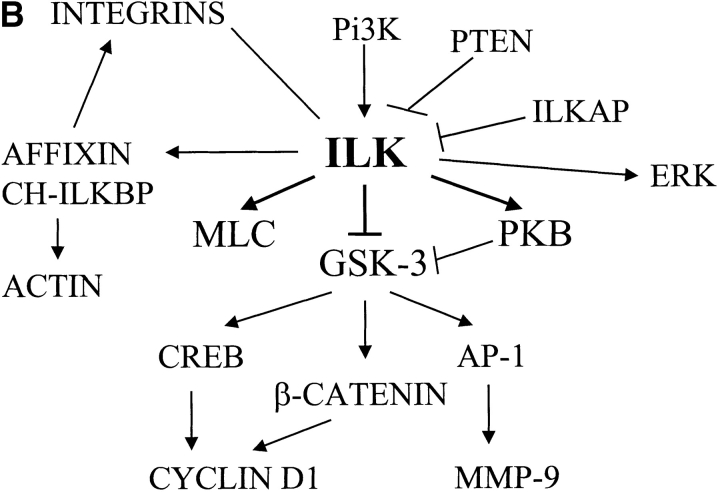
ILK interactions and ILK-mediated signaling pathways. (A) This figure depicts the interaction of ILK with various proteins many of which, including ILK, are found at sites of integrin connections to the actin cytoskeleton. ILK can anchor to integrins by interacting with the cytoplasmic domain of certain β integrin subunits. The NH2-terminal ankyrin repeats mediate interactions with ILKAP, a PP2C phosphatase, which negatively regulates ILK signaling, and PINCH, a LIM domain–only protein, which potentially couples ILK to growth factor receptors and PI 3-kinase by binding to another adaptor protein, Nck-2. PINCH is also required for the localization of ILK to cell adhesion sites and can be found in a stable complex with ILK and another ILK-binding protein, CH-ILKBP. The latter binds to ILK at its COOH terminus, which comprises the kinase domain. A closely related protein, affixin, binds to ILK in the same region, as does paxillin, via its LD1 domain. CH-ILKBP, paxillin, and probably other ILK-binding proteins can bind directly or indirectly to actin filaments, thus coupling integrins to the actin cytoskeleton via ILK. Signaling proteins, PKB, PDK-1, and GSK-3 can also interact with ILK, and ILK can phosphorylate PKB and GSK-3. Affixin is also a substrate of ILK, and the kinase activity of ILK appears to be involved in enhancing the ability of the affixin CH2 domain to inhibit cell spreading. (B) Signaling pathways activated by ILK. PTEN and ILKAP negatively regulate ILK activation. Activated ILK can directly phosphorylate PKB/Akt and GSK-3. Phosphorylation of PKB/Akt by ILK contributes to its activation, leading to suppression of apoptosis and anoikis. Phosphorylation of GSK-3 results in its inhibition, leading to stabilization of β-catenin and stimulation of AP-1 activity. β-catenin and CREB activation via ILK and GSK-3 contribute to the upregulation of cyclin D1, whereas AP-1 activation leads to stimulation of matrix metalloproteinase-9 expression. ILK is also involved in regulating Erk, since overexpression of ILK prevents Erk inactivation during myogenic differentiation. ILK can also directly phosphorylate MLC in a calcium-independent manner, thus potentially regulating smooth muscle contraction and possibly cell motility in nonmuscle cells.
Table I. ILK-binding proteins.
| Binding protein | ILK-binding site | Target on ILK | Detectiona |
|---|---|---|---|
| Transmembrane receptors | |||
| (1) β1 integrins | β1 cytoplasmic domain | Kinase domain | 2-hybrid, co-IP (Hannigan et al., 1996) |
| (2) β3 integrins | Not determined | Not determined | Co-IP (Hannigan et al., 1996) |
| Adaptor proteins | |||
| (1) PINCH | LIM1 | ANK domain | Co-IP (Hannigan et al., 1996), ELISA (Tu et al., 1999) |
| (2) CH-ILKBP | CH2 | Kinase domain | 2-hybrid, co-IP, pull-down (Tu et al., 2001) |
| (3) Affixin | CH2 | Kinase domain | 2-hybrid, co-IP (Yamaji et al., 2001) |
| (4) Paxillin | LD1 | Kinase domain | Co-IP, pull-down (Nikolopoulos and Turner, 2001) |
| Catalytic proteins | |||
| (1) ILKAP | Not determined | ANK-PH domains | 2-hybrid, co-IP (Leung-Hagesteijn et al., 2001) |
| (2) PKB/Akt | Not determined | Kinase domain | Co-IP (Persad et al., 2001a) |
| (3) PDK-1 | Not determined | Not determined | Co-IP (Persad et al., 2001a) |
2-hybrid, yeast two-hybrid binding assays; co-IP, coimmunoprecipitation; pull-down, GST fusion protein pull-down assay.
The interaction between ILK and PINCH, a focal adhesion protein comprised primarily of five LIM domains (Wu, 1999), has been studied in detail. ILK forms a complex with PINCH through direct binding of the ILK NH2-terminal ANK domain to the second zinc finger located within the LIM1 domain of PINCH (Li et al., 1999; Tu et al., 1999). Structural studies have revealed that many residues in this zinc finger undergo large chemical shift changes upon ILK binding (Velyvis et al., 2001), suggesting that the interaction likely involves a conformational change of the LIM1 domain.
The COOH-terminal domain of ILK interacts with the β1 integrin cytoplasmic domain (Hannigan et al., 1996) and at least three different cytoplasmic adaptor proteins. CH-ILKBP, which contains two calponin homology (CH) domains, was identified and cloned in a yeast two-hybrid screen of a human cDNA library using the ILK COOH-terminal domain as bait (Tu et al., 2001). The CH2 domain of CH-ILKBP mediates the interaction with ILK (Tu et al., 2001). Two proteins (actopaxin [Nikolopoulos and Turner, 2000] and α-parvin [Olski et al., 2001]) that are closely related structurally to human CH-ILKBP have been identified independently and cloned from rat and mouse cDNA libraries, respectively. Interestingly, actopaxin was identified in a search for proteins that bind to the LD1 motif of paxillin (Nikolopoulos and Turner, 2000), and α-parvin was identified based on its sequence homology with the actin-binding domain of α-actinin (Olski et al., 2001). Although murine actopaxin and α-parvin were identified based on binding activities toward proteins other than ILK, the high degree of sequence similarity of CH-ILKBP, actopaxin, and α-parvin at both the protein level (98% identical) and the cDNA level (90% identical) suggest that the human, rat, and mouse proteins are orthologues and therefore are likely to share the ILK-binding activity. In an independent study, Yamaji et al. (2001) identified and cloned another human protein, affixin, that also binds to the ILK COOH-terminal domain. CH-ILKBP and affixin are encoded by two different genes, but they share significant sequence similarity, particularly in the CH2 domains that mediate the ILK binding, suggesting that they likely recognize a common site on ILK. Affixin is the human orthologue of mouse β-parvin, another recently described actin-binding protein (Olski et al., 2001). These recent studies define a new family of ILK-binding proteins that include CH-ILKBP–actopaxin–α-parvin and affixin–β-parvin. In addition to interacting with the CH2 domains of CH-ILKBP–actopaxin–α-parvin and affixin–β-parvin, the ILK COOH-terminal domain can also be recognized by the paxillin LD1 motif (Nikolopoulos and Turner, 2001).
Some, although probably not all, of the interactions described above occur simultaneously in cells. It has been demonstrated recently that ILK binds to PINCH and CH-ILKBP simultaneously through two separate domains (the NH2-terminal ANK domain and the COOH-terminal kinase domain), resulting in the formation of a multicomponent PINCH–ILK–CH-ILKBP complex in cells (Tu et al., 2001). On the other hand, paxillin was not detected as part of the multicomponent ILK complex, despite its ability to interact with both ILK and CH-ILKBP through the LD1 motif.
Localization of ILK to cell matrix contact sites
The interactions of mammalian cells with extracellular matrix (ECM) are mediated by molecularly and morphologically distinct structures, among which the best characterized are focal adhesions (Burridge and Chrzanowska-Wodnicka, 1996) and fibrillar adhesions (or ECM contacts) (Zamir et al., 1999). ILK localizes to both focal adhesions (Hannigan et al., 1996; Li et al., 1999; Mulrooney et al., 2000; Nikolopoulos and Turner, 2001) and fibrillar adhesions (Guo et al., 2001), which is consistent with a role of ILK in cell adhesion, spreading, and fibronectin matrix assembly (Hannigan et al., 1996; Wu et al., 1998; Tu et al., 2001). The localization of ILK to these adhesion sites is mediated by at least two interactions. Mutations in the ILK NH2-terminal domain that disrupt the PINCH binding impair the ability of ILK to localize to these sites, indicating that the PINCH binding is required in this process (Li et al., 1999). However, PINCH binding is not sufficient, since deletion of the ILK COOH-terminal domain also abolishes the localization of ILK to these sites (Li et al., 1999). Because the ILK COOH-terminal domain mediates interactions with multiple proteins, including integrins, CH-ILKBP–actopaxin–α-parvin, affixin–β-parvin, and paxillin, the requirement of the ILK COOH-terminal domain likely reflects the need of one or more of these interactions. Mutations in an ILK COOH-terminal sequence that is 15–33% identical to the paxillin-binding sequences in vinculin, FAK, and actopaxin, disrupt the focal adhesion localization of ILK, supporting a role of paxillin in this process (Nikolopoulos and Turner, 2001). However, because paxillin is deficient in fibrillar adhesions (Zamir et al., 1999) other ILK COOH-terminal–binding proteins are likely required in the targeting of ILK to the cell matrix contact sites.
A crucial role of ILK in anchoring the actin filaments to cell matrix contact sites
In genetic model systems such as Drosophila and Caenorhabditis elegans, null mutations of ILK cause defects similar to loss of integrin function (Zervas et al., 2001) (Mackinnon, A.C., and B.D. Williams. 2000. 40th American Society for Cell Biology Annual Meeting. 2664 [Abstr.]). Closer examination of ilk-null mutants in Drosophila has revealed that actin filaments are detached from the membranes at the muscle attachment sites (Zervas et al., 2001). Several lines of evidence suggest that CH-ILKBP–actopaxin–α-parvin functions as one of the key bridge molecules, linking ILK to the actin filaments. First, CH-ILKBP–actopaxin–α-parvin can interact with not only ILK but also actin (Nikolopoulos and Turner, 2000; Olski et al., 2001; Tu et al., 2001). Second, overexpression of an ILK-binding CH2 fragment of CH-ILKBP–actopaxin–α-parvin in cells inhibits the actin stress fiber formation and cell shape change (Nikolopoulos and Turner, 2000; Tu et al., 2001). Third, mutants of the C. elegans homologue of CH-ILKBP–actopaxin–α-parvin like those of C. elegans ILK display defects in the assembly of muscle dense bodies that attach actin filaments to sarcolemma (Lin, X., and B.S. Williams. 2000. 40th American Society for Cell Biology Annual Meeting. 2666 [Abstr.]).
Are there other linkages between ILK and the actin cytoskeleton? The answer is almost certainly yes. One potentially important connection could be provided by PINCH. In C. elegans, PINCH like ILK is required for the assembly of muscle-dense bodies (Hobert et al., 1999). Paxillin can interact with ILK and actin-binding proteins, including viculin and CH-ILKBP–actopaxin–α-parvin, and therefore could provide another linkage to the actin filaments. An additional connection could be provided by affixin–β-parvin. Overexpression of an affixin fragment containing the ILK-binding CH2 domain inhibits initial spreading of the cells (Yamaji et al., 2001). However, despite extensive efforts a direct interaction between affixin–β-parvin and actin has not been detected in vitro (Yamaji et al., 2001). Finally, ILK can interact with the β1 integrin cytoplasmic domain and thereby could be potentially linked to the actin filaments through other actin- and β1 integrin–binding proteins such as talin. It is worth noting that an interaction between Drosophila ILK and the cytoplasmic domain of integrin βPS was not detected in yeast two-hybrid analyses (Zervas et al., 2001). Consistent with this, Drosophila ILK is localized normally to muscle attachment sites in the absence of the βPS integrin (Zervas et al., 2001). However, in C. elegans integrin is required for the proper localization of ILK (Mackinnon, A.C., and B.D. Williams. 2000. 40th American Society for Cell Biology Annual Meeting. 2664 [Abstr.]), suggesting a connection between integrin and ILK in this organism.
The multiple interactions between ILK and the actin cytoskeleton have at least two important implications. First, it could allow cells to modulate the physical strength of the connection at the cell matrix contact sites. Second, it could facilitate signal transduction and regulation through the cell matrix contact sites. In addition to binding to ILK through the LIM1 domain, PINCH can interact with Nck-2 (also known as Nckβ or Grb4), an adaptor protein containing three Src homology (SH)3 domains and one SH2 domain, through the LIM4 domain (Tu et al., 1998) (Fig. 1). In turn, Nck-2 could potentially help to bring other components of the growth factor and small GTPase signaling pathways into proximity of the adhesion sites through interactions mediated by the SH2 and SH3 domains.
Signaling role of ILK
ILK has a low basal kinase activity, which is stimulated transiently by cell–ECM interactions and by certain growth factors (Dedhar, 2000). The activity is stimulated in a phosphatidylinositol (PI) 3-kinase–dependent manner and likely involves binding of the phosphoinositide phospholipid product of PI 3-kinase, PI 3,4,5-triphosphate, to the PH-like domain of ILK (Dedhar, 2000). ILK activity is regulated negatively by two phosphatases: PTEN, a tumor suppressor lipid phosphatase, which dephosphorylates PI 3,4,5-triphosphate to PI 4,5-bisphosphate, and a PP2C protein phosphatase, ILKAP (Morimoto et al., 2000; Persad et al., 2000; 2001b; Leung-Hagesteijn et al., 2001). ILK activity is activated constitutively in PTEN-null tumor cells (Persad et al., 2000, 2001b). Both PI 3,4,5-triphosphate binding and (auto)phosphorylation appear to be crucial for ILK activation, since mutations in the PH domain (Arg 211) or in the activation loop serine (ser) 343 render the kinase inactive and unable to phosphorylate protein kinase B (PKB)/Akt (Persad et al., 2001a).
Despite having a somewhat unusual kinase catalytic domain (Dedhar et al., 1999; Lynch et al., 1999; Dedhar, 2000), ILK has been shown recently to directly phosphorylate proteins such as PKB (PKB/Akt) on ser 473 (Persad et al., 2001a), glycogen synthase kinase 3 (GSK-3) on ser 9 (Persad et al., 2001b), myosin light chain (MLC) on ser 18/thr 19 (Deng et al., 2001), and ILK-binding protein affixin (Yamaji et al., 2001). In addition, ILK can phosphorylate the cytoplasmic domain of β1 integrin subunit in vitro (Hannigan et al., 1996), although whether this occurs in intact cells is not clear. On the other hand, Lynch et al. (1999) have suggested that ILK regulates the phosphorylation of PKB/Akt on ser 473 indirectly. This suggestion was based on their inability to detect kinase activity in ILK immunoprecipitates and on the reversal of a dominant negative form of ILK to wild type by mutation of ser 343 to aspartic acid. However, more recent work by Persad et al. (2001a) has shown that a similar mutation in another dominant negative form of ILK does not cause this reversal and that ser 343 is critical for ILK kinase activity. Thus, the issue of whether ILK has kinase activity remains controversial, although the recent demonstration of ILK as a kinase (Deng et al., 2001; Persad et al., 2001a) seems to tip the balance in favor of ILK being a bona fide serine/threonine protein kinase. However, it is also likely that certain functions of ILK may not require its kinase activity. This is apparent from the suggestion that the regulation of integrin–actin cytoskeleton interaction by ILK in Drosophila may not require kinase activity (Zervas et al., 2001), although it should be pointed out that the mutation in the ILK kinase domain on which this conclusion was based results in the retention of substantial amount of residual ILK activity (Persad et al., 2001a; Yamaji et al., 2001).
The role of ILK in the stimulation of various signaling pathways (Fig. 1 B) has been demonstrated by analyzing ILK activity in cells stimulated by ECM or growth factors and in cells overexpressing ILK. Overexpression of ILK in epithelial cells resulted in an increased phosphorylation of PKB/Akt and GSK-3 (Lynch et al., 1999; Dedhar, 2000), activating the former and inhibiting the latter. The hallmarks of overexpressing ILK in normal epithelial cells is the loss of cell–cell adhesion, due to the downregulation of E-cadherin expression, and nuclear translocation of β-catenin (Dedhar, 2000; Somasiri et al., 2001). The downregulation of E-cadherin expression may involve ILK-mediated activation of the E-cadherin repressor Snail (Tan et al., 2001), and nuclear β-catenin activation may involve ILK-mediated inhibition of GSK-3 activity, resulting in β-catenin stabilization (Persad et al., 2001b). The inhibition of GSK-3 by ILK also results in the activation of the transcription factor AP-1 (Dedhar, 2000), which along with β-catenin/Tcf and the transcription factor CREB may also be responsible for ILK-mediated upregulation of cyclin D1 expression (D'Amico et al., 2000). ILK-induced AP-1 activity also results in the stimulation of expression of matrix metalloproteinase-9 (Troussard et al., 2000). It should be pointed out that these readily demonstrated signaling functions of ILK in mammalian cells appear not to be conserved in Drosophila, since inactivating mutation of Drosophila ILK does not appear to affect PKB/Akt, GSK-3, or β-catenin pathways (Zervas et al., 2001).
One of the consequences of constitutive ILK activation, or overexpression, in mammalian cells is suppression of apoptosis and anoikis (Attwell et al., 2000; Persad et al., 2000). Both of these effects involve ILK-mediated activation of PKB/Akt and suppression of activation of caspase 3. The central role of ILK in these pathways can be inferred from experiments in which inhibiting ILK with a dominant negative form of ILK or by a pharmacological inhibitor of ILK induced apoptosis in cells in which PKB/Akt is activated constitutively (Persad et al., 2000). It is interesting that mammary gland tumors arise in ILK transgenic mice, and the tumor tissues exhibit many of the hallmarks of ILK overexpression in tissue culture cells, namely phosphorylation of PKB/Akt and GSK-3 but also downregulation of expression of E-cadherin and phosphorylation and activation of extracellular signal–regulated kinase (Erk) (West et al., 2001). ILK also appears to regulate muscle differentiation by activating Erk, which suppresses transcription factors required for myogenic differentiation (Huang et al., 2000). The signaling pathway involved in the ILK-mediated Erk activation remains to be determined.
Therefore, it is apparent that ILK can control the activities of key signaling pathways, leading to the stimulation of downstream effector kinases and transcription factors, which either activate or repress the expression of genes encoding proteins involved in the regulation of cell survival, the cell cycle, cell adhesion, and ECM modification (Fig. 1 B). In addition, ILK may also be involved in the regulation of cell migration, cell motility, and contractility by directly phosphorylating proteins such as MLC (Deng et al., 2001) and affixin (Yamaji et al., 2001). The phosphorylation of the latter may also affect early stages of cell spreading by modulating the interaction of affixin with actin and ILK (Yamaji et al., 2001). Although not demonstrated yet, it is likely that ILK can also phosphorylate CH-ILKBP, since affixin and CH-LIKBP share significant sequence similarity and appear to bind to ILK via similar domains. ILK may also modulate adhesion and spreading by directly or indirectly regulating the phosphorylation of the β1 integrin cytoplasmic domain, since serine phosphorylation of this subunit has been implicated in integrin-mediated regulation of cell adhesion, spreading, and formation of focal adhesion plaques (Mulrooney et al., 2000).
Conclusion
The combination of genetic, biochemical, and cell biological studies demonstrates that ILK plays a central role in connecting integrins to actin filaments, and it also plays crucial roles in the regulation of cell survival, cell proliferation, and cell–cell adhesion. In addition, in its ability to phosphorylate myosin in smooth muscle cells ILK may also have an important role in regulating smooth muscle contraction and cell motility in nonmuscle cells. Thus, ILK provides a molecular scaffold for the assembly of proteins such as PINCH, CH-ILKBP, affixin, and paxillin, which bridge the ECM and growth factors via integrins and growth factor receptors to the actin cytoskeleton (Fig. 1 A). ILK also couples integrins and growth factor receptors to downstream signaling components (Fig. 1 B). It should be pointed out that these two functions of ILK need not be mutually exclusive, since it is entirely possible that the scaffold function of ILK is required for its signaling function. This is indeed the case for PINCH, which has the potential, via Nck-2, to couple ILK to growth factor receptors. In addition, the recent demonstration that PKB/Akt and PI 3-kinase–dependent kinase (PDK)-1, a key regulator of PKB/Akt, can complex with ILK also suggests a role for ILK in the compartmentalization of signaling components. Furthermore, since many proteins appear to bind to ILK within the kinase catalytic domain it is possible that the activity of ILK and its signaling properties are also regulated by such interactions. The challenge for the future is going to be the dissection of the roles of the various ILK interactions in cell anchorage, migration, and signaling.
Finally, dysregulation of ILK activity and/or expression appears to play important roles in certain disease states. Overexpression, or constitutive activation of ILK, due to inactivating mutations in tumor suppressors such as PTEN or APC lead to oncogenic transformation of cell lines and tumor formation in transgenic mouse models (Dedhar, 2000; West et al., 2001). In addition, ILK expression and activity are upregulated in Ewings sarcomas, advanced prostate cancers (Graff et al., 2001), and colonic poyposis and colorectal cancers (Marotta et al., 2001). ILK has also been demonstrated recently to be upregulated in the kidney glomeruli in patients with diabetic nephropathy (Guo et al., 2001), from children with congenital nephrotic syndrome, and in kidney podocytes from two murine models of proteinuria (Kretzler et al., 2001).
Given the central role of ILK in connecting integrins to the actin cytoskeleton and regulation of cell growth, dysregulation of ILK is likely to have much wider implications in many developmental and somatic diseases, underscoring the need to understand fully the mechanism of its regulation and activation.
Acknowledgments
This work was supported by National Institutes of Health grant DK54639, American Cancer Society research project grant 98-220-01-CSM to C. Wu, and grants from the National Cancer Institute of Canada to S. Dedhar.
Footnotes
Abbreviations used in this paper: CH, calponin homology; ECM, extracellular matrix; Erk, extracellular signal–regulated kinase; GSK, glycogen synthase kinase; ILK, integrin-linked kinase; MLC, myosin light chain; PDK-1, PI 3-kinase–dependent kinase; PH, pleckstrin homology; PI, phosphatidylinositol; PKB, protein kinase B; ser, serine; SH, Src homology.
References
- Attwell, S., C. Roskelley, and S. Dedhar. 2000. The integrin-linked kinase (ILK) suppresses anoikis. Oncogene. 19:3811–3815. [DOI] [PubMed] [Google Scholar]
- Burridge, K., and M. Chrzanowska-Wodnicka. 1996. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 12:463–518. [DOI] [PubMed] [Google Scholar]
- D'Amico, M., J. Hulit, D. Amanatullah, B. Zafonte, C. Alabanese, B. Bouzahzah, M. Fu, L.H. Augenlicht, L. Donehover, K. Takemura, et al. 2000. The integrin-linked kinase regulates cyclin D1 gene through glycogen synthase kinase 3beta and CREB-dependent pathways. J. Biol. Chem. 275: 32649–32657. [DOI] [PubMed] [Google Scholar]
- Dedhar, S. 2000. Cell-substrate interactions and signaling through integrin linked kinase (ILK). Curr. Opin. Cell Biol. 12:250–256. [DOI] [PubMed] [Google Scholar]
- Dedhar, S., B. Williams, and G. Hannigan. 1999. Integrin-linked kinase (ILK): a regulator of integrin and growth factor signaling. Trends Cell Biol. 9:319–323. [DOI] [PubMed] [Google Scholar]
- Deng, J.T., J.E.V. Lierop, C. Sutherland, and M.P. Walsh. 2001. Calcium-independent smooth muscle contraction: a novel role for integrin linked kinase. J. Biol. Chem. 276:16365–16373. [DOI] [PubMed] [Google Scholar]
- Graff, J.R, J.A. Deddens, B.W. Konicek, B.M. Colligan, B.M. Hurst, H.W. Carter, and J.H. Carter. 2001. Integrin-linked kinase expression increases with prostate tumor grade. Clin. Cancer Res. 7:1987–1991. [PubMed] [Google Scholar]
- Guo, L., P.W. Sanders, A. Woods, and C. Wu. 2001. The distribution and regulation of integrin-linked kinase in normal and diabetic kidneys. Am. J. Pathol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan, G.E., C. Leung-Hagesteijn, L. Fitz-Gibbon, M.G. Coppolino, G. Radeva, J. Filmus, J.C. Bell, and S. Dedhar. 1996. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 379:91–96. [DOI] [PubMed] [Google Scholar]
- Hobert, O., D.G. Moerman, K.A. Clark, M.C. Beckerle, and G. Ruvkun. 1999. A conserved LIM protein that affects muscular adherens junction integrity and mechanosensory function in Caenorhabditis elegans. J. Cell Biol. 144:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y., J. Li, Y. Zhang, and C. Wu. 2000. The roles of integrin-linked kinase in the regulation of myogenic differentiation. J. Cell Biol. 150:861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzler, M., V.P. Teixeira, P.G. Unschlud, C.D. Cohen, R. Wanke, I. Edenhofer, P. Mundel, D. Schlondorff, and H. Holthoffer. 2001. Integrin-linked kinase as a candidate downstream effector in proteinuria. FASEB. J. 15:1843–1846. [DOI] [PubMed] [Google Scholar]
- Leung-Hagesteijn, C., A. Mahendra, I. Naruszewicz, and G.E. Hannigan. 2001. Modulation of integrin signal transduction by ILKAP, a protein phosphatase 2C associating with the integrin-linked kinase, ILK1. EMBO J. 20:2160–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F., Y. Zhang, and C. Wu. 1999. Integrin-linked kinase is localized to cell-matrix focal adhesions but not cell-cell adhesion sites and the focal adhesion localization of integrin-linked kinase is regulated by the PINCH-binding ANK repeats. J. Cell Sci. 112:4589–4599. [DOI] [PubMed] [Google Scholar]
- Lynch, D.K., C.A. Ellis, A.W. Edwards, and I.D. Hiles. 1999. Integrin linked kinase regulates phosphorylation of serine 473 of protein kinase B by an indirect mechanism. Oncogene. 18:8024–8032. [DOI] [PubMed] [Google Scholar]
- Marotta, A., C. Tan, V. Gray, S. Malik, S. Gallinger, J. Sanghera, B. Dupuis, D. Owen, S. Dedhar, and B. Salh. 2001. Dysregulation of integrin-linked kinase (ILK) signaling in colonic polyposis. Oncogene. 20:6250–6257. [DOI] [PubMed] [Google Scholar]
- Morimoto, A.M., M.G. Tomlinson, K. Nakatani, J.B. Bolen, R.A. Roth, and R. Herbst. 2000. The MMAC tumor suppressor phosphatase inhibits phospholipase C and integrin linked kinase activity. Oncogene. 19:200–209. [DOI] [PubMed] [Google Scholar]
- Mulrooney, J., K. Foley, S. Vineberg, M. Barreuther, and L. Grabel. 2000. Phosphorylation of the beta1 integrin subunit cytoplasmic domain: toward an understanding of function and mechanism. Exp. Cell Res. 258:332–341. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos, S.N., and C.E. Turner. 2000. Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. J. Cell Biol. 151:1435–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos, S.N., and C.E. Turner. 2001. Integrin-linked kinase (ILK) binding to paxillin LD1 motif regulates ILK localization to focal adhesions. J. Biol. Chem. 276:23499–23505. [DOI] [PubMed] [Google Scholar]
- Olski, T.M., A.A. Noegel, and E. Korenbaum. 2001. Parvin, a 42 kDa focal adhesion protein, related to the alpha-actinin superfamily. J. Cell Sci. 114:525–538. [DOI] [PubMed] [Google Scholar]
- Persad, S., S. Attwell, V. Gray, M. Delcommene, A. Troussard, J. Sanghera, and S. Dedhar. 2000. Inhibition of integrin linked kinase (ILK) suppresses activation of PKB/Akt and induces cell cycle arrest and apoptosis in PTEN-null prostate cancer cells. Proc. Natl. Acad. Sci. USA. 97:3207–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persad, S., S. Attwell, V. Gray, N. Mawji, J.T. Deng, D. Leung, J. Yan, J. Sanghera, M.P. Walsh, and S. Dedhar. 2001. a. Regulation of protein kinase B/Akt-serine-473 phosphorylation by integrin linked kinase (ILK): critical roles for kinase activity and amino acids arginine-211 and serine-343. J. Biol. Chem. 276:27462–27469. [DOI] [PubMed] [Google Scholar]
- Persad, S., A. Troussard, T. McPhee, D. Mulholland, and S. Dedhar. 2001. b. Tumor suppressor PTEN inhibits beta-catenin nuclear localization and T cell/lymphoid enhancer factor 1–mediated transcriptional activation. J. Cell Biol. 153:1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasiri, A., A. Howarth, D. Goswami, S. Dedhar, and C. Roskelley. 2001. Overexpression of integrin linked kinase initiates a mesenchymal transformation of mammary epithelial cells. J. Cell Sci. 114:1125–1136. [DOI] [PubMed] [Google Scholar]
- Tan, C., P. Costello, J. Sanghera, D. Dominguez, A. Garcia de Harreros, and S. Dedhar. 2001. Inhibition of integrin linked kinase (ILK) suppresses beta-catenin-Lef/Tcf-dependent transcription and expression of the E-cadherin suppressor, snail in APC −/− human colon carcinoma cells. Oncogene. 20:133–140. [DOI] [PubMed] [Google Scholar]
- Troussard, A., P. Costello, N. Yoganathan, S. Kumagai, C. Roskelley, and S. Dedhar. 2000. The integrin linked kinase induces an invasive phenotype via Ap-1 transcription factor dependent upregulation of matrix metalloproteinase (MMP-9). Oncogene. 19:5444–5453. [DOI] [PubMed] [Google Scholar]
- Tu, Y., F. Li, and C. Wu. 1998. Nck-2, a novel Src homology2/3-containing adaptor protein that interacts with the LIM-only protein PINCH and components of growth factor receptor kinase signaling pathways. Mol. Biol. Cell. 9:3367–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, Y., F. Li, S. Goicoechea, and C. Wu. 1999. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol. Cell. Biol. 19:2425–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, Y., Y. Huang, Z. Zhang, Y. Hua, and C. Wu. 2001. A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J. Cell Biol. 153:585–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velyvis, A., Y. Yang, C. Wu, and J. Qin. 2001. Solution structure of the focal adhesion adaptor PINCH LIM1 domain and characterization of its interaction with integrin linked kinase ankyrin repeat domain. J. Biol. Chem. 276:4932–4939. [DOI] [PubMed] [Google Scholar]
- West, D.E., R.D. Cardiff, S. Dedhar, and W.J. Muller. 2001. Mammary epithelial specific expression of the integrin linked kinase (ILK) in transgenic mice results in the induction of hyperplasias and tumors. Oncogene. In press. [DOI] [PubMed] [Google Scholar]
- Wu, C. 1999. Integrin-linked kinase and PINCH: partners in regulation of cell-extracellular matrix interaction and signal transduction. J. Cell Sci. 112:4485–4489. [DOI] [PubMed] [Google Scholar]
- Wu, C., S.Y. Keightley, C. Leung-Hagesteijn, G. Radeva, M. Coppolino, S. Goicoechea, J.A. McDonald, and S. Dedhar. 1998. Integrin-linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression, and tumorigenicity. J. Biol. Chem. 273:528–536. [DOI] [PubMed] [Google Scholar]
- Yamaji, S., A. Suzuki, Y. Sugiyama, Y. Koide, M. Yoshida, H. Kanamori, H. Mohri, S. Ohno, and Y. Ishigatsubo. 2001. A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell–substrate interaction. J. Cell Biol. 153:1251–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir, E., B.Z. Katz, S. Aota, K.M. Yamada, B. Geiger, and Z. Kam. 1999. Molecular diversity of cell-matrix adhesions. J. Cell Sci. 112:1655–1669. [DOI] [PubMed] [Google Scholar]
- Zervas, C.G., S.L. Gregory, and N.H. Brown. 2001. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J. Cell Biol. 152:1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]