Abstract
The L1 adhesion molecule plays an important role in axon guidance and cell migration in the nervous system. L1 is also expressed by many human carcinomas. In addition to cell surface expression, the L1 ectodomain can be released by a metalloproteinase, but the biological function of this process is unknown. Here we demonstrate that membrane-proximal cleavage of L1 can be detected in tumors and in the developing mouse brain. The shedding of L1 involved a disintegrin and metalloproteinase (ADAM)10, as transfection with dominant-negative ADAM10 completely abolishes L1 release. L1-transfected CHO cells (L1-CHO) showed enhanced haptotactic migration on fibronectin and laminin, which was blocked by antibodies to αvβ5 and L1. Migration of L1-CHO cells, but not the basal migration of CHO cells, was blocked by a metalloproteinase inhibitor, indicating a role for L1 shedding in the migration process. CHO and metalloproteinase-inhibited L1-CHO cells were stimulated to migrate by soluble L1-Fc protein. The induction of migration was blocked by αvβ5-specific antibodies and required Arg-Gly-Asp sites in L1. A 150-kD L1 fragment released by plasmin could also stimulate CHO cell migration. We propose that ectodomain-released L1 promotes migration by autocrine/paracrine stimulation via αvβ5. This regulatory loop could be relevant for migratory processes under physiological and pathophysiological conditions.
Keywords: L1; shedding; ADAM10; cell migration; integrins
Introduction
The regulation of cell migration is of paramount importance for many cellular processes. During embryogenesis, cells migrate long distances before reaching their destination. A well-studied example is the formation of the nervous system. The cerebral cortex extends axons long distances to various cortical and subcortical structures. Cell surface receptors that transduce signals from environmental cues direct the guidance of these axons. Environmental cues include diffusible and nondiffusible molecules that can be attractant and/or repellent. Examples include growth factors, semaphorins, netrins, cell adhesion molecules, and extracellular matrix molecules (Tessier-Lavigne and Goodman, 1996). Cell migration remains important in the adult organism in a variety of organ systems. During tumor metastasis, for example, released tumor cells migrate from the primary tumor into the circulatory system, and then invade a new site (Fidler, 1990). Cell adhesion and migration are mediated in many instances by cell surface integrins that link interactions with the substratum to the cytoskeleton inside the cell. Integrins are heterodimeric cell adhesion molecules that were initially found to mediate the interaction of cells to components of the extracellular matrix like laminin, fibronectin, vitronectin, etc. (Hynes, 1992). Integrin binding and clustering initiates not only adhesion, but also activates many intracellular signaling events that regulate diverse cell functions such as cell migration, polarity, survival, or cell growth (for review see Giancotti and Ruoslahti, 1999; Schwartz and Shattil, 2000)
L1 is a 200–220-kD type I membrane glycoprotein of the immunoglobulin family, consisting of 6 Ig-like domains and five fibronectin-type III repeats, followed by a transmembrane region and a highly conserved cytoplasmic tail. In neuronal cells, L1 is involved in several morphogenic events, such as neuron–neuron adhesion, neurite fasciculation, synaptogenesis, neurite outgrowth on Schwann cells and neuronal cell migration (for review see Hortsch, 1996; Schachner, 1997, Brümmendorf et al., 1998). Although initially characterized and most extensively studied in the nervous system, L1 is expressed also by hematopoietic and certain epithelial cells (Kowitz et al., 1992; Ebeling et al., 1996, Pancook et al., 1997; Debiec et al., 1998) and a variety of human tumor cell lines such as neuroblastomas, melanomas, and lung carcinomas (Linnemann et al., 1989; Patel et al., 1991; Reid and Hemperly, 1992; Katayama et al., 1997), suggesting a potential role of the molecule in other adhesion and migration events. L1 supports cellular processes through interaction with extracellular ligands and transduction of a variety of signaling events through associated proteins (Kamiguchi and Lemmon, 1997; Brümmendorf et al., 1998; Doherty et al., 2000). L1 can undergo homophilic L1-L1 binding involving several Ig domains (De Angelis et al., 1999), and can interact via Ig domain 1 with the proteoglycan neurocan (Oleszewski et al., 1999). The Arg-Gly-Asp (RGD)* sites in Ig domain 6 of L1 support heterophilic binding to integrins including α5β1, αvβ1, and αvβ3, as well as the platelet integrin αIIbβ3 (Ruppert et al., 1995; Ebeling et al., 1996; Montgomery et al., 1996; Felding-Habermann et al., 1997; Oleszewski et al., 1999). Recently, an RGD-independent binding site for α9β1 was identified in the third fibronectin (FN)III domain (Silletti et al., 2000). In addition to the cell surface localization, L1 can be released as a soluble molecule from mouse cerebellar cells in culture (Sadoul et al., 1988) and from mouse and human tumor cells (Montgomery et al., 1996; Beer et al., 1999; Gutwein et al., 2000). We have shown before that L1 release involves membrane-proximal cleavage by an unknown metalloproteinase, most likely of the a disintegrin and metalloproteinase (ADAM) family (Gutwein et al., 2000). An increasing number of soluble proteins are now recognized as being derived from integral plasma membrane proteins (for review see Schlöndorff and Blobel, 1999; Turner and Hooper, 1999; Blobel, 2000). These proteins are diverse in structure and function, and comprise molecules such as TNF-α, FasL, IL-6 receptor, L-selectin, pro–TGF-α, and amyloid precursor protein (APP) (for review see Turner and Hooper, 1999). Members of the ADAM family (membrane proteins composed of a disintegrin and metalloproteinase domain) were shown to be important in ectodomain release (for review see Black and White, 1998; Schlöndorff and Blobel, 1999). ADAM17/TACE mediates the membrane release of TNF-α, L-selectin, and TGF-α (Peschon et al., 1998). ADAM10/Kuz has been described as α-secretase for the cleavage of APP (Lammich et al., 1999), and ADAM9 is involved in the phorbolester-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor (HB-EGF) (Izumi et al., 1998, Gechtman et al., 1999). Recent reports have established an important link between ectodomain cleavage by metalloproteinases and the regulation of migratory processes. The function of the axonal chemoattractant Netrin-1 is regulated by cleavage of its receptor, deleted in colorectal cancer (DCC). Blocking of cleavage by a metalloproteinase inhibitor potentiated axon outgrowth (Galko and Tessier-Lavigne, 2000). The contact-mediated axon repulsion by ephrin-A2 involves cleavage by Kuz, which is triggered by binding of the A2 ligand Eph receptor (Hattori et al., 2000). This proteolytic process allows efficient axon detachment and withdrawal. Finally, a recent study has shown that ectodomain shedding of EGF receptor ligands is required for keratinocyte migration in cutaneous wound healing (Tokumaru et al., 2000). It was hypothesized that the inhibition of EGF receptor ligand shedding abrogated the auto/paracrine stimulation of soluble EGF receptor ligands, which is critical for keratinocyte migration.
In the light of these published results, we have examined the possible biological role of L1 ectodomain shedding. Given the known involvement of L1 in cell migratory processes, we have specifically addressed the question whether the ectodomain release of L1 might have a decisive part in such events. In the present report, we provide evidence that membrane-proximal cleavage of L1 can be detected in tumor cell lines and in the developing mouse brain. In androgen receptor (AR) breast tumor cells, the shedding of L1 involves ADAM10, as transfection with dominant-negative ADAM10 completely abolishes L1 release. We also demonstrate that cleavage of L1 stimulates cell migration on fibronectin and laminin by autocrine binding of soluble L1 to αvβ5 integrins. These observations have important implications for understanding how L1 can function to promote cell migration in the nervous system, as well as how L1 expression in tumors could contribute to dissemination and metastasis.
Results
A 32-kD cytoplasmic fragment of L1 is retained in the membrane after release of the L1 ectodomain
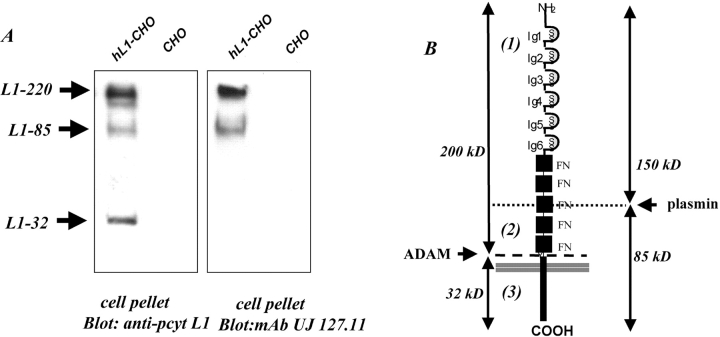
Soluble L1 is present in the culture medium of various L1-positive mouse and human carcinoma cell lines (Beer et al., 1999; Gutwein et al., 2000). The release from the cell surface requires proteolytic cleavage of the ectodomain, which in adherent cells is induced by detachment with EDTA and incubation at 37°C (Gutwein et al., 2000). To identify the COOH-terminal cleavage product and to further investigate the cleavage process, a polyclonal antibody against the cytoplasmic tail of L1 (pcytL1) was used. L1-transfected CHO cells (CHO-L1) were incubated in suspension at 37°C for 1 h, and the cell pellets were lysed and analyzed by Western blotting. As shown in Fig. 1 A, pcytL1 detected protein bands at 220, 85, and 32 kD in CHO-L1 cells, but not in untransfected CHO cells. A mAb against the ectodomain of human L1 (UJ 127.11) reacted with the 220- and the 85-kD fragment, but not with the 32-kD fragment (Fig. 1 A). The latter band was also not detected by pcytL1 in CHO cells transfected with mouse L1 carrying a deletion of the cytoplasmic portion (unpublished data). This suggested that a 32-kD fragment comprising the COOH-terminal portion of L1 remained associated with the membrane after cleavage. The protein bands at 220 kD represents full-length L1. The 85-kD L1 fragment represents the COOH-terminal fragment after proteolytic cleavage of L1 in the third FNIII repeat which contains the dibasic sequence QRKHSKRHIH (Fig. 1 B). Serine proteases like plasmin (Nayeem et al., 1999) can cleave at this site and release a soluble 140–150-kD fragment into the supernatant (see below).
Figure 1.
L1 is shed without cytoplasmic tail. (A) CHO or L1-transfected CHO cells were incubated at 37°C in suspension in medium without FCS for 1 h. The cell pellet was lysed and analyzed by Western blot with mAb UJ127.11 to the ectodomain or pcytL1 to the cytoplasmic domain of L1. (B) Schematic representation of L1 cleavage fragments and their relative localization. The location of antibody recognition sites are indicated. (1), mAb 5G3; (2), mAb UJ 127.11; (3) pcytL1.
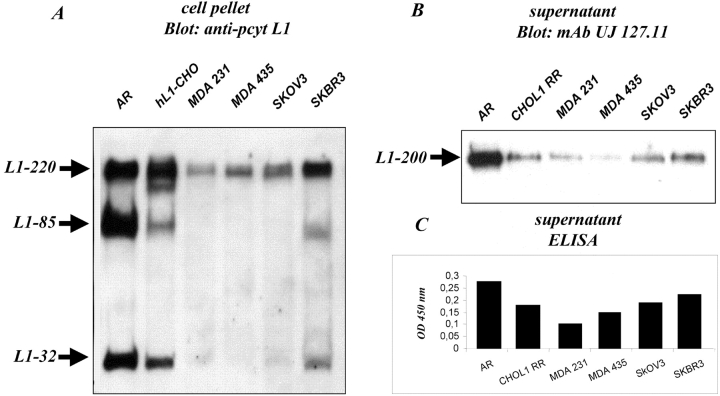
L1-positive tumor cell lines were tested for the presence of the 32-kD cleavage fragment. As shown in Fig. 2 A, the 32-kD fragment was clearly detected in lysates from the breast carcinoma lines AR and SKBR3, but only minor amounts were detected in MDA-231, MDA-435, or SKOV3 cell lysates. As shown by Western blot analysis in Fig. 2 B and by capture ELISA in Fig. 2 C, the level of shed L1 in the supernatants paralleled the presence of L1-32 in the cell lysate. A close correlation between the amount of L1 in the supernatant and the level of L1 expression was noted, suggesting that the release of L1 is an intrinsic property of many human tumor lines.
Figure 2.
Presence of a L1-32 indicates membrane-proximal cleavage. Cells were incubated at 37°C in suspension in medium without FCS for 1 h (A) Cell pellets were lysed and blotted with pcytL1 followed by ECL detection. Note that a parallel blot using the secondary antibody only was negative. (B) Supernatants were analyzed for the presence of shed L1 using blotting with mAb UJ127.11 to the ectodomain of human L1 followed by ECL detection. (C) Supernatants were also probed for the presence of soluble L1 using a capture ELISA.
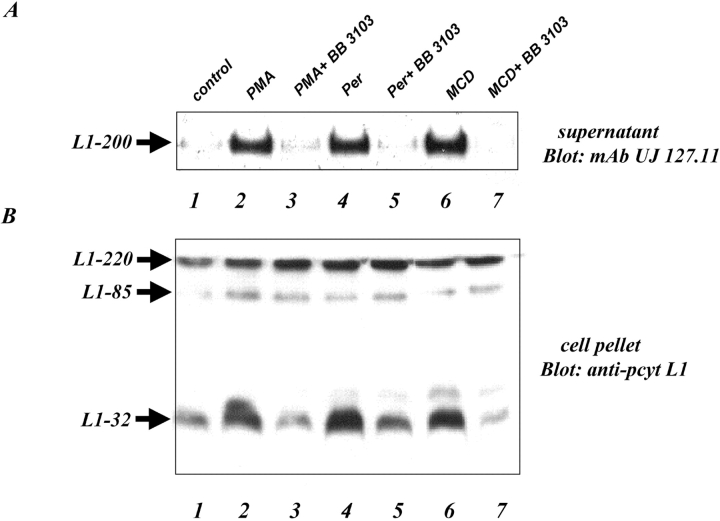
Treatment of attached cells with PMA or pervanadate also induced shedding of L1 that can be blocked by hydroxamate-based metalloproteinase inhibitors (Gutwein et al., 2000). We examined the presence of L1-32 in AR cells after induction of cleavage in the absence or presence of BB-3103 metalloproteinase inhibitor. As shown in Fig. 3 A, dramatically increased levels of soluble L1 were detected in the supernatant after PMA (lane 2) or pervanadate treatment (lane 4) that were reduced to background levels in the presence of BB-3103 (lanes 3 and 5). Surprisingly, enhanced L1 release was observed by treatment of cells with methyl-β-cyclodextrin (MCD) (lane 6), a drug known to interfere with membrane cholesterol levels. Also, the MCD-induced release of L1 was inhibited by BB-3103 (lane 7). As shown in Fig. 3 B, the induction of L1 cleavage by PMA, pervanadate, or MCD clearly augmented the presence of L1-32 from a basal level in untreated cells. Collectively, the data suggested that L1-32 represents the portion of L1 remaining associated with the cells after shedding of the ectodomain.
Figure 3.
Regulated cleavage of L1 is blocked by metalloproteinase inhibitor. Attached AR cells were treated with PMA (100 ng/ml), pervanadate (200 mmolar), or MCD for 1 h at 37°C in the absence or presence of BB-3103 inhibitor. (A) Supernatants were immunoprecipitated with mAb to L1 coupled to Sepharose and analyzed for shed L1 using blotting with mAb UJ127.11 followed by ECL detection. (B) Cell pellets were lysed and blotted with pcytL1 followed by ECL detection.
Ectodomain cleavage of L1 occurs in the developing mouse brain
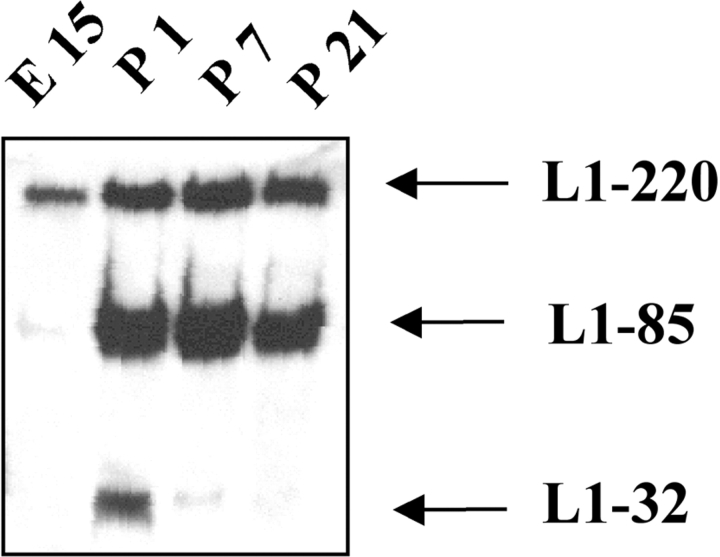
L1 plays an important role in brain development. We examined the possibility that ectodomain cleavage of L1 occurred in vivo. Mouse brains of different ages were lysed, and equal amounts of protein were analyzed by Western blotting using pcytL1. To avoid post-lysis cleavage of L1, the lysates were prepared in the presence of 10 mM EDTA and 2 mM 1,10 phenanthroline, conditions that are known to block metalloproteinase activity. As shown in Fig. 4, L1-32 was most prominent in newborn P1 mice but declined at later stages. The low level of L1-220 and the absence of L1-85 in E15 brain is in agreement with earlier studies using rat brain (Liljelund et al., 1994). Densitometric analysis of band intensities revealed a L1-220/L1-32 ratio of approximately 4:1 in P1 brain that changed at later ages. This suggests that the level of L1-32 cleavage is highest in P1 brain, but the onset of cleavage probably occurred earlier. These data confirm and extend the results of Liljelund, showing that the expression of L1 and the proteolytic cleavage are developmentally regulated.
Figure 4.
L1-32 can be detected in the developing mouse brain. Mouse brains of different ages were homogenized in lysis buffer, and equal amounts of protein were analyzed by SDS-PAGE and Western blotting using pcytL1 followed by peroxidase-conjugated secondary antibodies and ECL detection.
Expression of L1 augments cell migration
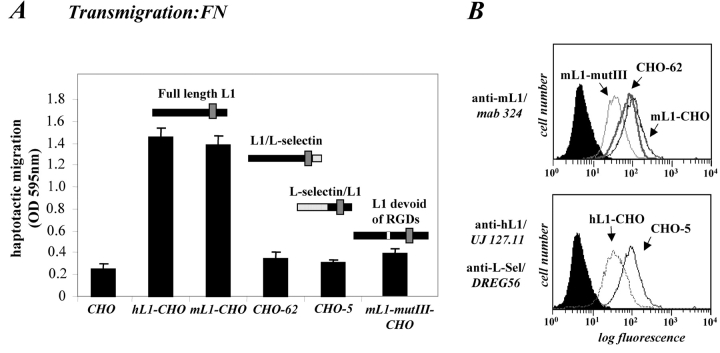
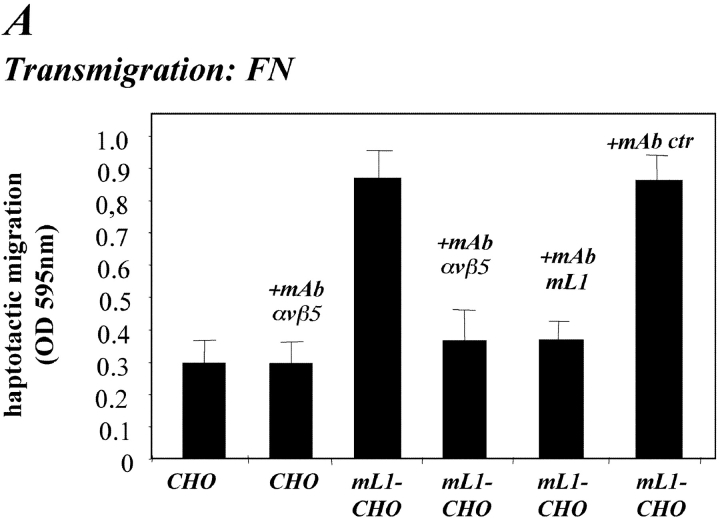
Having established that ectodomain cleavage of L1 occurs in human tumor cells and during mouse brain development, we set out to explore the physiological role of the shedding process. In the brain, L1 plays an important role in the migration of neural cells. Therefore, we examined the role of L1 and its ectodomain cleavage process in cell migration. As shown in Fig. 5 A, we observed that CHO cells stably transfected with mouse or human L1 (mL1-CHO or hL1-CHO cells) showed a three- to fivefold increase in haptotactic migration on fibronectin compared with CHO cells. In contrast, CHO cells expressing L1 in which both RGD sites were mutated to RGE (mL1-mutIII-CHO) or those expressing mutant forms of L1 containing either the cytoplasmic and transmembrane portion of L-selectin (L1/L-selectin) or the ectodomain of L-selectin (L-selectin/L1) did not show increased migration (Fig. 5 A). The transfected CHO cells expressed L1 or the chimeric forms of L1 at comparable level as detected by FACS analysis (Fig. 5 B).
Figure 5.
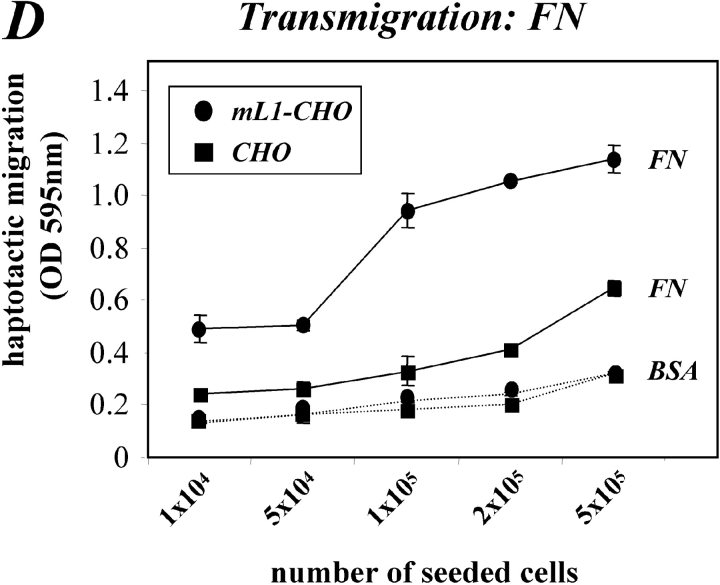
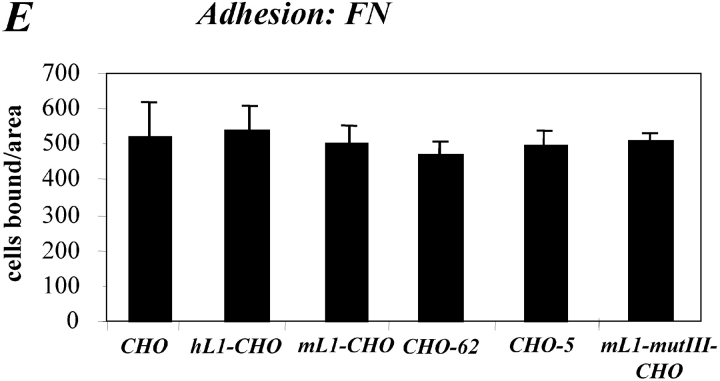
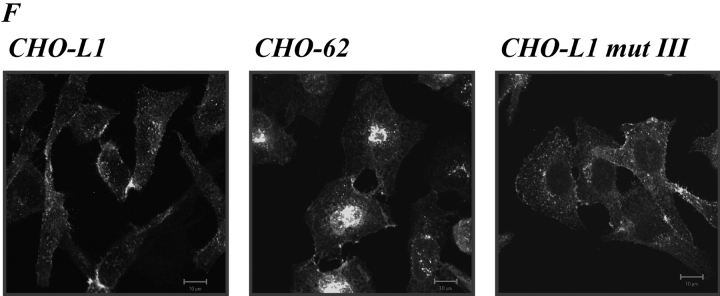
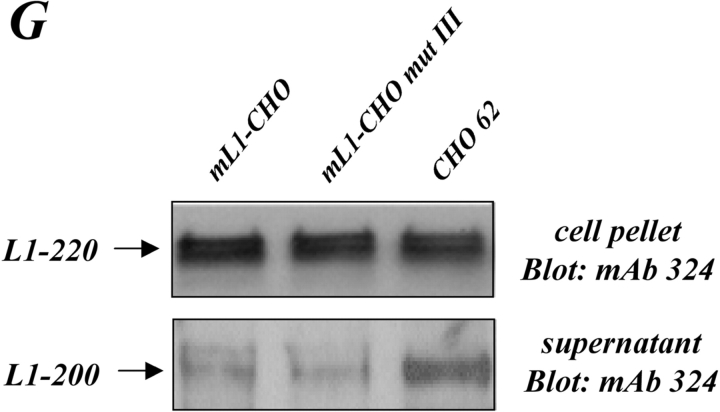
Expression of L1 enhances haptotactic migration of CHO cells. (A) FN at 10 mg/ml or BSA for control were coated on the backside of Transwell chambers. CHO, h/mL1-CHO or mL1-mutIII-CHO cells stably expressing L1 devoid of RGDs were seeded into the top chamber and allowed to transmigrate for 16 h at 37°C. Each determination was done in quadruplicate and transmigrated cells were stained from the back of the filter. The dye was eluted from the filter and measured at 595 nm. The amount of dye is proportional to the number of transmigrated cells. Values for the migration on BSA were below 0.25 OD units for each cell type. (B) Staining of CHO-transfected cells used in (A) with mAbs to human or mouse L1, respectively. (C) Analysis of CHO and mL1-CHO cell migration on vitronectin or laminin (each coated at 10 mg/ml). Analysis of migrated cells was done as described in A. (D) Dose–response curve for the migration of CHO and mL1-CHO cells. The assay was done as described in A. (E) FN or BSA for control were coated on LABTEK chamber slides, and the adhesion of CHO cells and transfectants was determined in the presence of 2 mM Ca 21 and 2 mM Mg21. Binding to BSA was below 50 cells/area. (F) Cells were grown on coverslips, stained with mAb 324 to mouse L1, and analyzed by fluorescence microscopy. (G) Cells were cultivated for 24 h and TCA-precipitated supernatants and cell lysates were analyzed. After SDS-PAGE, L1 was detected by Western blotting and ECL detection using anti L1 antibodies.
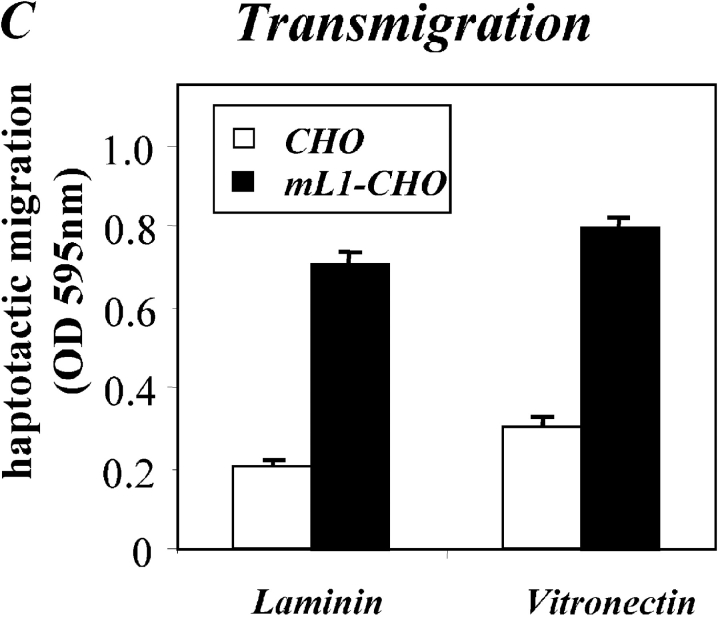
A similar difference in cell migration between mL1-CHO or CHO cells was also observed on vitronectin or laminin as the migration substrate (Fig. 5 C and see below). As shown in the dose–response curve in Fig. 5 D, the difference in migration between CHO and mL1-CHO cells was detectable over a wide range of input cell numbers (between 104 and 5 × 105). To exclude the possibility that the transfection had altered the integrin profiles, the static adhesion to fibronectin was examined. As shown in Fig. 5 E, there was no difference in the adhesion to fibronectin between CHO cells and transfected sublines. Similar observations were made with vitronectin as substrate (unpublished data). The adhesion of CHO and mL1-CHO cells to fibronectin was blocked in both cases with mAb SAM-1 to the α5 integrin that is highly expressed by CHO cells (unpublished data).
To analyze whether the structural changes introduced into L1 had affected the membrane localization, cells were also analyzed by fluorescent microscopy (Fig. 5 F). The membrane localization of mL1-mutIII was not different from wild-type L1. In contrast, CHO-62 cells carrying the cytoplasmic tail of L-selectin showed a different L1 distribution. In addition to surface staining (Fig. 5 B), L1 was localized in a region close to the nucleus. The cells appeared also larger in morphology with a more rounded-up appearance. Despite their inability to migrate, CHO-62 cells released significantly more L1 into the supernatant than wild-type CHO-L1 cells (Fig. 5 G).
The αvβ5 integrin is involved in enhanced cell migration
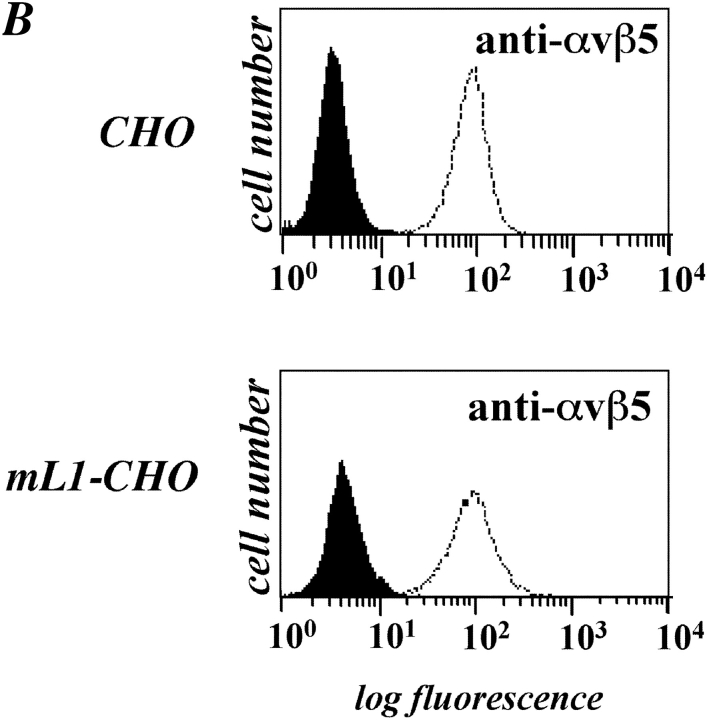
We investigated the difference in migration between mL1-CHO cells and CHO cells in more detail using blocking mAbs. As shown in Fig. 6 A, mAb P1F6 to human αvβ5 crossreacted with hamster integrins and could inhibit the migration of mL1-CHO cells to the level of CHO cells. Importantly, there was no effect of the αvβ5-specific mAb on the migration of CHO cells. Additional FACS analysis with mAb P1F6 showed no difference in the expression level of the αvβ5 integrin (Fig. 6 B). The migration of mL1-CHO was also efficiently blocked in the presence of mAb 324 against mouse L1, but not by the isotype-matched control mAb 79 (Fig. 6 A).
Figure 6.
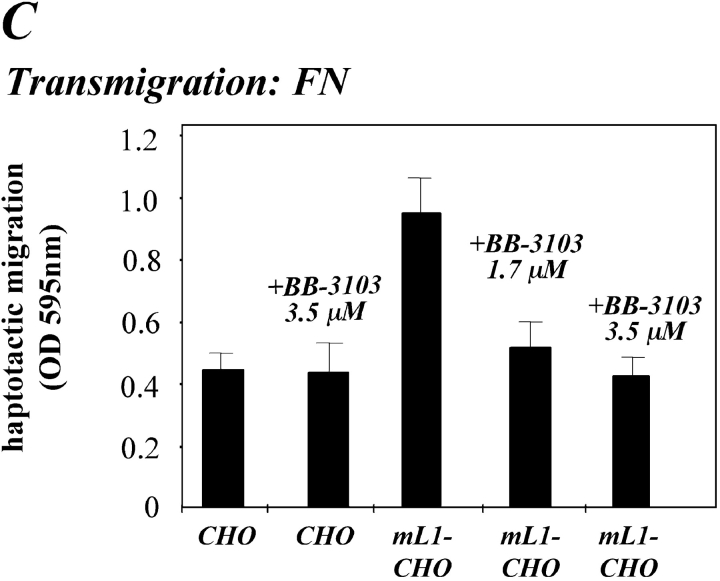
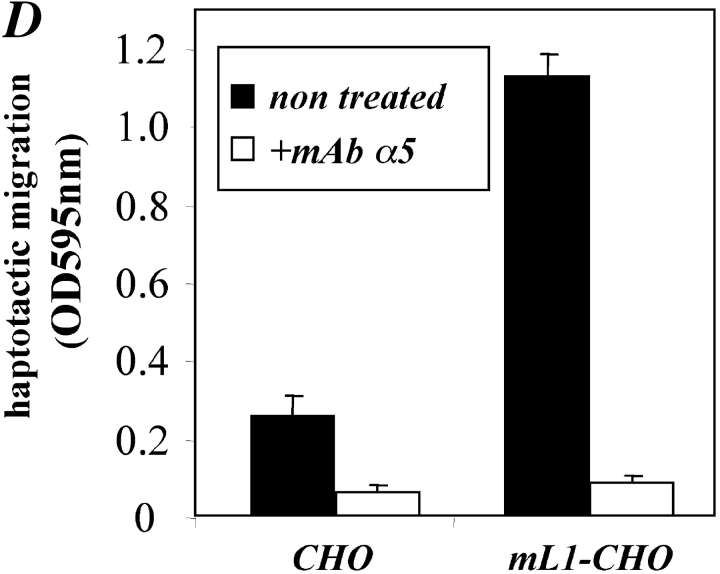
Inhibition of cell migration by antibodies and metalloproteinase inhibitor. (A) ML1-CHO cells or CHO cells were preincubated with the indicated mAbs at 10 mg/ml and added to the upper compartment of the Transwell chamber coated with FN from the backside. Note that antibody was present during the assay time. (B) Staining of CHO and mL1-CHO cells with a mAb to αvβ5 integrin. (C) ML1-CHO cells or CHO cells were preincubated with the indicated amount of BB-3103 inhibitor for 2 h and then transferred to the upper compartment of the Transwell chamber. Each determination was done in quadruplicate and transmigrated cells were stained from the backside of the filter as described in Fig. 5 A. (D) Inhibition of cell migration by a mAb to the α5 integrin. ML1-CHO or CHO cells were preincubated with the mAbs at 10 mg/ml and added to the upper compartment of the Transwell chamber coated with FN.
To rule out the possibility that CHO and mL1-CHO cells used different integrins to migrate on fibronectin, additional blocking experiments were carried out. As shown in Fig. 6 D, mAb SAM-1 to the α5 integrin also could efficiently block the transmigration. Most importantly, both CHO and mL1-CHO cells were equally well blocked by the mAb. These results indicated that altered integrin levels or altered integrin usage were not the reason for the improved cell migration of L1-CHO cells.
We also reinvestigated the migration of CHO and mL1-CHO cells on laminin. The migration of both type of cells was nearly completely blocked by mAb GoH3 against CD49f. Similar to fibronectin, mAb P1F6 to the αvβ5 integrin did not affect the migration of CHO cells, but the enhanced migration of mL1-CHO cells was reduced again to the level of CHO cells (unpublished data). We concluded that in transfected cells the presence of L1 led to an enhanced cell migration on different substrates that specifically involved the αvβ5 integrin, and that the elimination of RGDs in L1 abolished the augmenting effect on cell migration (Figs. 5 A and 6 A).
Inhibition of L1 ectodomain shedding blocks enhanced cell migration
We investigated the possibility that L1 shedding was crucial for the enhanced migration of L1-CHO cells. Cells were pretreated with BB-3103 and the haptotactic migration on FN was analyzed. As shown in Fig. 6 C, the metalloproteinase inhibitor reduced the migration of mL1-CHO cells back to the level of CHO cells in a dose-dependent manner. The inhibitor did not effect the migration of CHO cells, although a slight reduction might have been undetected due to the low level of migration of these cells. Similar results were obtained with the Ro 31-9790 inhibitor (unpublished data). We concluded that for L1-CHO cells, the metalloproteinase inhibitors exerted a similar effect on the haptotactic migration as the mAb to the αvβ5 integrin. Both compounds did not block the migration of the cells completely but rather selectively effected the enhanced cell migration caused by the presence of L1.
Soluble L1 can stimulate migration of CHO cells
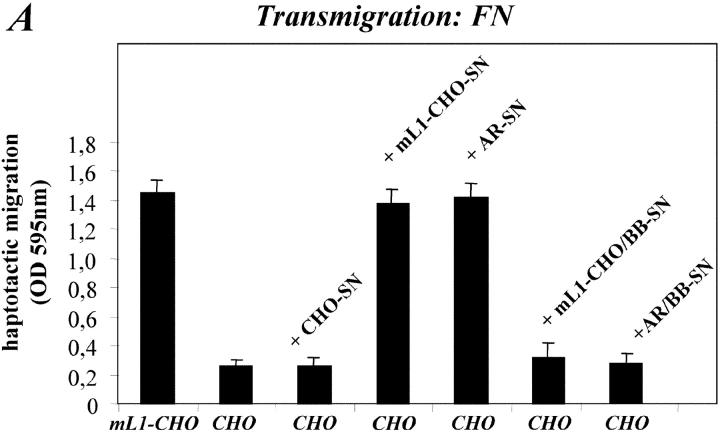
We hypothesized that cleaved L1 might deliver an autocrine signal to the cells and thereby promote enhanced migration. According to this hypothesis, the addition of soluble L1 should enhance the migration of CHO cells. To test this assumption, CHO cells were first exposed to conditioned medium from L1-positive cells, and the effect on the haptotactic migration was analyzed. As shown in Fig. 7 A, the medium from L1-CHO but not CHO cells could stimulate cell migration that was of similar magnitude as L1-CHO cells. The stimulatory activity was also present in the conditioned medium of AR tumor cells but was absent when AR cells or L1-CHO cells were cultivated in the presence of BB-3103 in order to block shedding. To further prove that the activity was mediated by soluble L1, we used a recombinant L1-Fc protein (Fig. 7 B). As shown in Fig. 7 C, the L1-Fc protein but not a control protein (SART-1-Fc) could stimulate CHO migration like conditioned medium containing native L1. We concluded that soluble L1 can stimulate cell migration.
Figure 7.
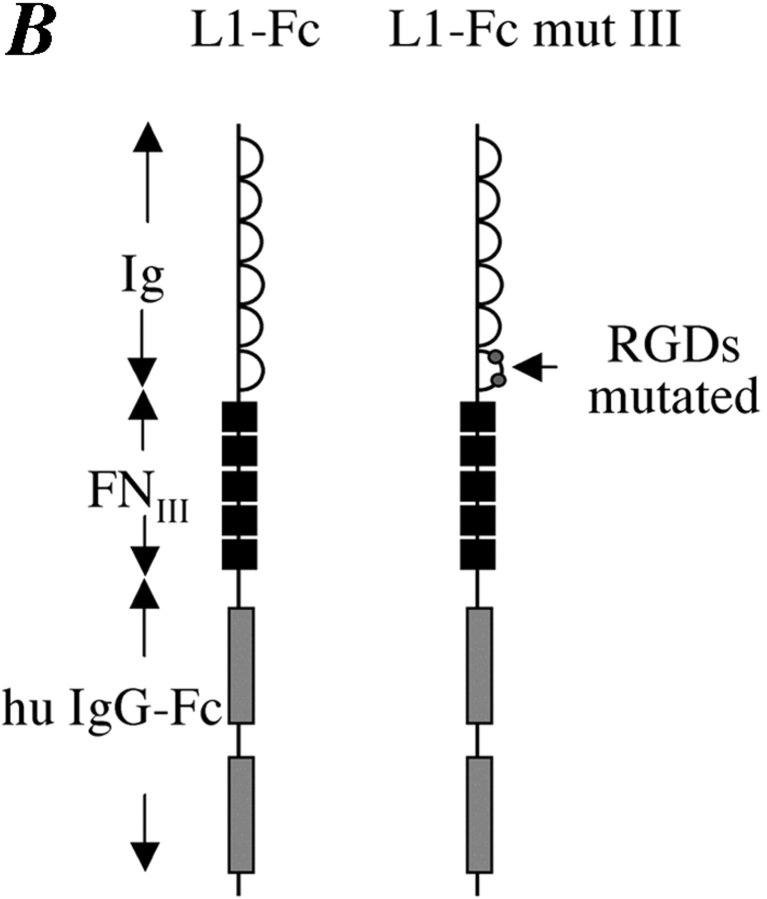
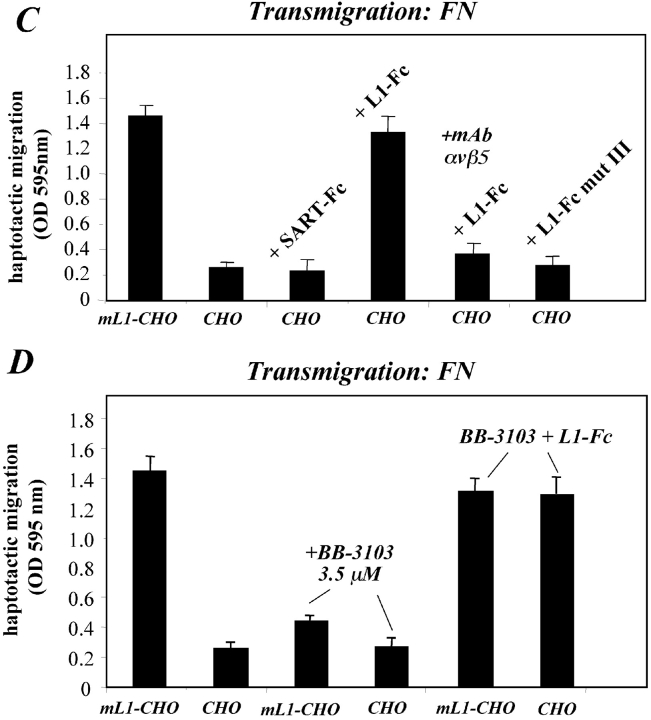
Exogenous addition of soluble L1 enhances migration of CHO cells. (A) CHO cells in the presence of the indicated conditioned media (1:10 final dilution) were seeded into the upper compartment of the Transwell chamber coated with FN from the backside and allowed to transmigrate for 16 h at 37°C. (B) Schematic representation of the L1-Fc and L1-Fc mut III fusion proteins. (C) CHO cells in the presence of the indicated Fc fusion protein (final concentration 0.67 mg/ml) were seeded into the upper compartment of the Transwell chamber. (D) ML1-CHO cells were pretreated with BB-3103 to prevent L1 shedding and haptotactic migration. Cells were then seeded together with the indicated Fc fusion proteins into the upper compartment of the Transwell chamber. Each determination was done in quadruplicates and transmigrated cells were stained from the backside of the filter as described in Fig. 5 A.
The effect of soluble L1 is blocked by a mAb to αvβ5 and requires RGDs in L1
To determine whether the migration promoting effect of soluble L1 required a direct binding to αvβ5 integrin, antibody blocking experiments were performed. Indeed, as shown in Fig. 7 C, the migration-promoting effect of soluble L1 was efficiently blocked in the presence of the antibody to αvβ5.
Previously, it has been shown that conserved RGD sites in Ig domain 6 of L1 can support binding to integrins (Ruppert et al., 1995; Ebeling et al., 1996; Montgomery et al., 1996; Felding-Habermann et al., 1997; Oleszewski et al., 1999). To analyze whether the RGD sites were necessary for the migration-promoting effect of soluble L1, we used an L1-Fc protein in which the RGDs were mutated to RGE (L1-Fc mut III). We have reported before that L1-Fc mut III does not support integrin-mediated cell binding of mouse B16F10 or IH3 cells (Oleszewski et al., 1999). As shown in Fig. 7 C, the L1-Fc mut III proteins were unable to stimulate CHO migration. Additional controls revealed that similar amounts of L1-Fc and L1-Fc mut III fusion proteins were present in the assay and that both Fc proteins could efficiently bind to immobilized neurocan (unpublished data). Like CHO cells, ML1–mutIII CHO cells were able to migrate in response to soluble L1-Fc (unpublished data).
We next investigated whether the metalloproteinase-inhibiting effect on L1-CHO migration could be overcome by the addition of exogenous L1. Indeed, as shown in Fig. 7 D, BB-3103 pretreated L1-CHO cells migrated well on fibronectin in response to L1-Fc protein. Collectively, these results suggested that one effect of the metalloproteinase inhibitor was to prevent the shedding of L1 and the subsequent autocrine/paracrine signaling via αvβ5 integrins. This inhibition was overcome by exogenous addition of soluble L1.
L1 cell surface expression levels affect cell migration
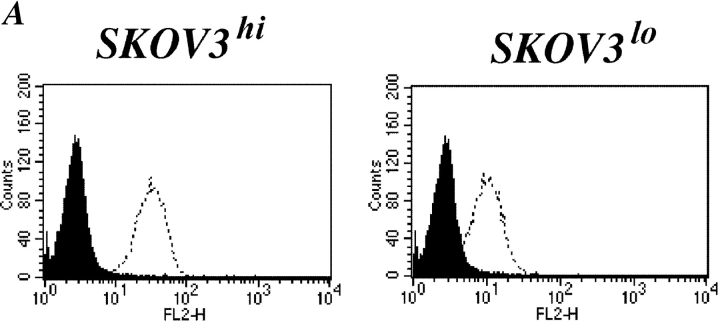
We examined whether the expression level of L1 could influence the rate of cell migration. SKOV3 cells were subjected to several rounds of cell sorting and stable cell populations with elevated or reduced expression of L1 were established. As shown in Fig. 8 A, SKOV3hi expressed approximately threefold higher levels of L1 as compared with SKOV3lo cells (mean fluorescence 24–58). As established by ELISA, SKOV3hi, and SKOV3lo cells also released different amounts of soluble L1 into the medium (unpublished data).
Figure 8.
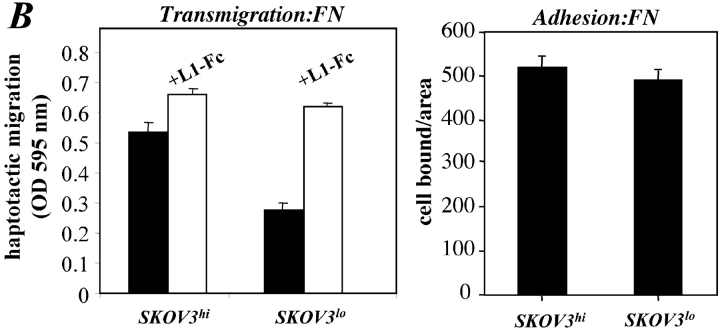
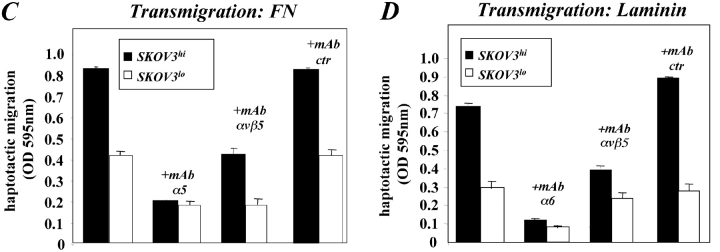
The level of L1 expression affects cell migration. SKOV3 cell variants differing in the expression levels of L1 (SKOV3hi or SKOV3lo) were established by repeated FACS sorting. (A) Cytofluorographic staining of SKOV3hi or SKOV3lo cells with mAb UJ 127.11. (B) Analysis of cell adhesion and haptotactic cell migration of SKOV3hi or SKOV3lo cells. The assays were done as described in Figs. 5 E and 7 C, respectively. (C) Cells were preincubated with the indicated mAbs at 10 mg/ml and transmigration on FN was tested as described in Fig. 6 A. (D) Cells were preincubated with the indicated mAbs at 10 mg/ml and transmigration on laminin was tested as described in Fig. 6 A.
Neither type of cell differed in their adhesion to fibronectin (Fig. 8 B). However, the haptotaptic migration on fibronectin was different. Fig. 8 B shows that SKOV3hi cells migrated much better compared with SKOV3lo cells, but this difference was no longer detectable when L1–Fc fusion protein was added to the cells before the assay. The migration was blocked in the presence of metalloproteinase inhibitors (unpublished data). Additional mAb inhibition experiments revealed that on fibronectin the migration of SKOV3hi and SKOV3lo cells was inhibited mAb SAM-1 to the α5 integrin and on laminin by mAb GoH3 to the α6 integrin, respectively (Fig. 8, C and D). Most importantly, and in agreement with the results obtained with CHO cells, a partial inhibition by mAb P1F6 to the αvβ5 integrin was seen on both substrates (Fig.8, C and D). Results similar to those presented here for SKOV3 cells were obtained with high and low L1-expressing variants of the ovarian tumor line OAW 42 (unpublished data). Thus, the level of cell surface expression of L1 and hence the amount of L1 released by the cells affected the haptotactic migration on two different substrates.
L1 shedding in AR cells involves ADAM10
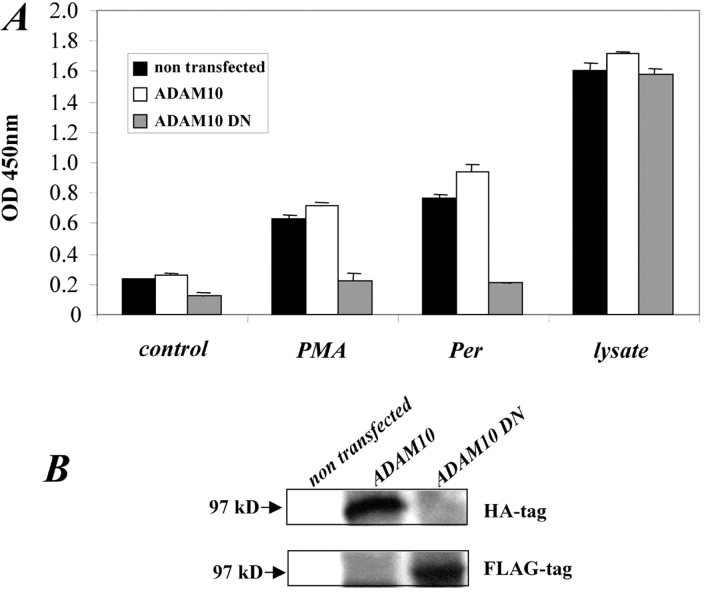
ADAM10 has been identified as the principal α-secretase for APP, and ADAM10 activity can be upregulated by treatment of cells with MCD or the hydroxymethyl glutaryl-CoA reductase inhibitor lovastatin (Lammich et al., 1999; Kojiro et al., 2001). Given the observation that MCD treatment of AR cells also drastically enhanced L1 shedding, we investigated whether ADAM10 was a candidate sheddase for L1 release. Fig. 9 A shows that transient expression of a dominant-negative form of ADAM10 (ADAM10 DN) could nearly completely abolish the pervanadate or PMA-induced release of L1 from AR cells. Overexpression of ADAM10 only marginally enhanced L1 release, suggesting that the shedding of L1 was controlled by additional factors beyond the expression level of shedding enzyme. Additional experiments using antibodies to hemagglutinin and Flag epitopes confirmed that ADAM10 and ADAM10 DN were expressed by the transfected cells (Fig. 9 B). Further studies using mL1-CHO cells showed a similar downregulation of L1 release by the ADAM10 DN construct (unpublished data). Collectively, these results strongly imply that in both AR and CHO cells the L1 release involved ADAM10.
Figure 9.
ADAM10 is involved in L1 shedding. (A) AR cells were transiently transfected with 10 mg plasmid DNA encoding HA-tagged ADAM10 or Flag-tagged dominant-negative ADAM10 (ADAM10 DN). 48 h after transfection, cells were stimulated with PMA or pervanadate to induce shedding. After 2 h, the supernatants were harvested and the cells lysed in lysis buffer. Cell lysates and conditioned media were analysed by ELISA for soluble L1. (B) Detection of HA-tagged ADAM10 or FLAG-tagged ADAM10 DN in the lysate of transfected cells.
The L1–150 plasmin fragment augments migration of CHO cells
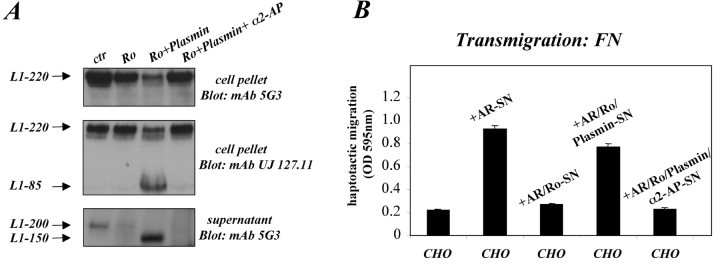
It has been shown recently that the addition of plasmin to L1-positive cells results in a dose-dependent loss of surface L1 expression with the concomitant appearance of a 140–150-kD soluble L1 species (L1–150) into the supernatant (Nayeem et al., 1999). Cleavage occurs at a dibasic site in the middle of the third FNIII repeat, thus, the released fragment contains the RGD site(s) in the 6.Ig-domain. We asked whether the plasmin fragment could substitute for the metalloproteinase-released ectodomain of L1. AR cells in serum-free medium were treated with a predetermined concentration of plasmin or plasmin plus the specific inhibitor α2-antiplasmin, and supernatants and cell pellets were taken for biochemical and functional analysis. To suppress the metalloproteinase-mediated L1 release, all supernatants were generated in the presence of Ro 31-9790 inhibitor. Fig. 10 A shows that in the presence of Ro1plasmin, a substantial loss of L1 from the cell pellet was observed which could be detected with both L1 mAbs 5G3 and UJ 127.11. The latter mAb also detected the L1-85 fragment remaining associated with the cell pellet after plasmin cleavage. This is in agreement with the notion that mAb UJ 127.11 recognizes an epitope between the dibasic cleavage site and the plasma membrane (Fig. 1 B), whereas mAb 5G3 reportedly binds to the NH2 terminus of L1 (Silletti et al., 2000). Analysis of the supernatant from treated cultures indicated that in the presence of the metalloproteinase inhibitor, the formation of soluble L1-200 was drastically reduced, and that plasmin addition resulted in the appearance of an expected 150-kD L1 fragment (Fig. 10 A). The formation of the 150-kD plasmin fragment was blocked in the presence of α2-antiplasmin.
Figure 10.
The L1–150-kD plasmin fragment can also stimulate cell migration. AR cell were treated with Ro 31-9790 inhibitor (Ro) in combination with plasmin and α2-antiplasmin for 6 h at 37°C. (A) Cells and supernatants were collected and analyzed by Western blotting using the indicated antibodies. (B) Supernatants of treated cultures were investigated for the ability to induce haptotactic migration of CHO cells as described in Fig. 7 A.
To analyze whether the plasmin fragment also could trigger cell migration, the supernatants of treated cultures were tested for the ability to induce haptotactic migration of CHO cells. As shown in Fig. 10 B, supernatants containing L1-200 or the plasmin fragment L1-150 could indeed induce migration whereas control supernatants containing no cleaved L1 could not. It should be noted that the L1-150 fragment was derived directly from the cell surface, and that there was apparently more L1 protein present than in the L1-200–containing supernatant. Therefore, it is possible that on a molar basis, the L1-200 fragment is more potent in the induction of migration than the L1-150 fragment.
Discussion
This report presents the first evidence that ectodomain cleavage of L1 has a biological role by providing soluble L1, which in turn can trigger cell migration. We find that L1-32, a fragment comprising the transmembrane and cytoplasmic region of L1, remains associated with the membrane. Not only is the fragment detected in varying amounts in human L1-positive carcinoma cell lines, but it is also in mouse brain of stage P1. The presence of L1-32 declines with advancing age of the mice. Therefore, ectodomain cleavage of L1 is not only a property of tumor cells, but it occurs in vivo during brain development. In cultured cells, the cleavage could be augmented by treatment with PMA and pervanadate and was blocked by hydroxamate based metalloproteinase inhibitors, suggesting that a metalloproteinase was involved. Transfection studies indicated that in established cell lines the shedding of L1 required ADAM10.
The starting point of our study was the observation that L1-transfected CHO cells showed a three- to fivefold better migration on fibronectin than CHO cells, and that this enhanced migration was blocked by the BB-3103 metalloproteinase inhibitor, suggesting a role of L1 shedding in the migration process. It was possible that metalloproteinase inhibitors of the hydroxamate family might cause effects beyond the inhibition of ectodomain shedding. A recent study has shown that batimastat (BB-94) can alter gene expression levels in vivo (Krüger et al., 2001). Additional data revealed that mAbs to αvβ5 and L1 also inhibited migration of L1-CHO cells to control levels. CHO cells are low in αvβ5, but express high levels of α5β1 which are used for migration on fibronectin. Indeed, the migration and adhesion of both types of cells on fibronectin were efficiently blocked by a mAb to the α5 integrin. Thus, membrane-released L1 exerted an enhancing effect on the basal migration of CHO cells which involved the αvβ5 integrin. We reasoned that αvβ5 could become directly engaged in migration or indirectly by transmitting signals which accelerated the existing migration machinery. The first option appeared less likely, as several studies have shown that αvβ5 only participates in migration when cells receive additional signals like PMA or growth factors (Klemke et al., 1994; Filardo et al., 1996). A direct role of αvβ5 in migration was also not consistent with the complete blocking of the α5-specific mAb. In addition, the mAb to αvβ5 blocked partially the α6 integrin–mediated migration on laminin, which is RGD independent. We observed that soluble L1, either in a native form or as an ectodomain Fc protein, could stimulate migration of CHO cells, and that this could also be blocked by the mAb to αvβ5. The stimulation of migration required the presence of RGDs in L1, consistent with the involvement of an integrin in the signaling pathway. Moreover, the inhibition of CHO-L1 migration by BB-3103 was fully restored by the addition of soluble L1. Collectively, these data supported the notion that soluble L1 was driving cell migration by binding to αvβ5, and that inhibition of L1 cleavage interrupted this autocrine/paracrine loop. The results in the CHO system were further corroborated by the analysis of SKOV3 cells differing in expression levels of L1. Cell variants with a higher level of L1 migrated better than cells with lower level on both fibronectin and laminin as substrate. As shown for fibronectin, the addition of soluble L1 could restore the defect of SKOV3lo cells. Thus, the amounts of L1 at the cell surface and released by the cells appeared to be an important factor in determining the rate of cell migration.
The results obtained with L1/L-selectin mutants deserve a further notion. Whereas it is conceivable that CHO-5 (L-selectin/L1) cells did not migrate because the L-selectin ectodomain could not stimulate, the situation with CHO-62 cells (L1/L-selectin) is less clear. We observed that these cells released significantly more L1 into the medium than CHO-L1 cells, yet did not show haptotactic migration. Additional experiments showed that the conditioned medium of CHO-62 cells was able to stimulate vigorous migration of CHO cells, suggesting that the ectodomain was functionally active (unpublished data). However, in CHO-62, the L1 distribution was different compared with CHO-L1 cells. In addition to surface expression, L1 was localized in a region close to the nucleus. Moreover, the cells were altered in morphology. It is quite possible that secondary effects like changes in cytoskeletal organization are responsible for the reduced haptotactic migration of CHO-62 cells compared with L1-CHO wildtype cells. Another possibility could be that the L-selectin cyttail actively inhibited cell migration. Further analysis is needed to solve this issue.
Several previous studies have shown that soluble L1 can influence axon growth and cell migration. It is well established that immobilized, purified L1 or L1 presented on a cell monolayer can serve as substrate for neurite outgrowth. Several studies have provided evidence that soluble L1 can stimulate neurite outgrowth of cerebellar neurons (Doherty et al., 1995) or explants from embryonic chick brain (Sugawa et al., 1997). Indeed, soluble L1 has been suggested as a potential pharmaceutical for the promotion of nerve regeneration (Sugawa et al., 1997). In most cases, the growth- and migration-promoting effects of L1 have been attributed to L1 homophilic binding. However, Yip et al. (1998) have demonstrated that the RGD motif in L1 also contributes to neurite outgrowth by interacting with the αvβ3 integrin (Yip et al., 1998). Interestingly, in this study both homophilic and integrin binding promoted outgrowth of dorsal root ganglion cells, whereas neural retinal cells responded only via homophilic binding (Yip et al., 1998). Soluble L1 is also involved in the invasion and migration of malignant gliomas (Izumoto et al., 1996). It was shown that C6 glioma cells are stimulated to migrate by soluble L1 or L1 released from transfected fibroblasts (Izumoto et al., 1996). Another study from this group indicated that soluble L1, but not fibronectin or tenascin, stimulated the invasion and migration of C6 cells into cultured brain slices (Ohnishi et al., 1998). Antibody inhibition experiments suggested that homophilic binding of L1 was involved in stimulating glioma migration, but a contribution of RGD motifs and integrin binding was not tested (Izumoto et al., 1996). In our experiments, the enhanced migration of L1-CHO was blocked by αvβ5-specific mAbs and by mAb 324 to mouse L1. The latter antibody was previously found to block α5β1-mediated binding of murine cells suggesting that the epitope recognized by this mAb partially overlaps with the Ig domain 6 (Ruppert et al., 1995). Also, the migration of CHO cell stimulated by soluble L1 was inhibited by the αvβ5-specific mAbs, and was not observed with soluble L1 devoid of RGDs. This suggested that the triggering signal involved binding to integrins. We concluded that soluble L1 can stimulate cellular responses like cell migration by binding and signaling through integrins like αvβ5.
Recent work has provided evidence that protein ectodomain cleavage regulates the function of membrane-anchored growth factors and cytokines and is involved in signal transduction processes (Blobel, 2000). The EGF receptor–mediated cell proliferation and migration of human mammary epithelial cells can be blocked with metalloproteinase inhibitors, demonstrating that relevant EGF receptor ligands need to be cleaved from the membrane to become activated (Dong et al., 1999). Also, the EGF receptor transactivation caused by G protein–coupled receptors requires metalloproteinase cleavage of the EGF-ligand heparin-binding EGF-like growth factor (Prenzel et al., 1999). Earlier work has implicated ectodomain shedding as an important element for EGF receptor signaling in development (Peschon et al., 1998). Mice lacking TACE/ADAM17 have phenotypes that resemble those seen in TGF-α and EGF receptor deficient animals (Peschon et al., 1998). Thus, the membrane-anchored form of TGF-α has to be released by proteolysis before it can fulfill its function as EGF receptor ligand. ADAM mediated cleavage is also involved in Notch signaling, which is important in regulating cell fate during development (Artavanis-Tsakonas et al., 1999). The extracellular domain of Notch can be cleaved by ADAM10 or ADAM17 (Brou et al., 2000; Mumm et al., 2000). The cytoplasmic domain then enters the nucleus and, in cooperation with a transcription factor, activates the Notch-dependent genetic program (Artavanis-Tsakonas et al., 1999). These examples support the notion that regulated proteolysis is an important element in cell signaling and in the cross-communication between different signaling systems (Prenzel et al., 1999; Blobel, 2000). Our results show that cleavage of a membrane-bound adhesion molecule can initiate integrin signaling via its RGD-containing ectodomain. Many of the intracellular signaling cascades influenced by soluble growth factors and their receptors are also regulated by integrins (Giancotti and Ruoslahti, 1999). In fact, growth factors and integrin-mediated signals often act synergistically on cell functions (Sieg et al., 2000).
Ectodomain cleavage of membrane molecules is accelerated under pathological conditions such as inflammation and apoptosis and by diverse stimuli (Hooper et al., 1997; Subramanian et al., 1997). In addition to PMA and pervanadate, other agents that also induce shedding include calcium ionophores, chemotactic peptides, cytokines, and growth factors. Recently, it was shown that in A549 cells integrin ligation could augment the shedding of the hepatocyte growth factor receptor Met (Nath et al., 2001). Cells grown on collagen released significantly more Met than when cultivated on uncoated plastic and this induction involved activation of the erk signaling pathway (Nath et al., 2001). Although we have not yet tested any influence of integrin engagement in our system, it seems reasonable to assume that shedding of L1 has to be maintained during cell migration. Extracellular matrix components serving as substrate for cell migration might induce signals that lead to sustained cleavage. If indeed this is the case, it could explain our observation that the enhanced migration of L1-CHO cells was observed on diverse substrates like fibronectin, laminin, and vitronectin. Indeed, in previous work we have observed a link between the rate of L1 shedding and cytoskeletal changes (Gutwein et al., 2000). The cytoplasmic domain of L1 can associate with several kinases as well as with the membrane skeletal protein ankyrin (Kamiguchi and Lemmon, 1997). It is possible that for the regulated cleavage these interactions are indispensable.
How then can the cleavage of L1 be enhanced in MCD treated cells? A recent study has shown that the cleavage of APP by the α-secretase ADAM10 was augmented in MCD treated cells. The increase in enzymatic activity of ADAM10 was correlated with enhanced membrane fluidity (Kojiro et al., 2001). The results presented in this study provide first evidence that at least in cultured cell lines ADAM10 was involved in the cleavage of L1, as a dominant-negative form of ADAM10, that was shown before to suppress cleavage of APP (Lammich et al., 1999), also affected the release of L1. These data confirm and extend previous data showing that ADAM10 activity can be augmented by cholesterol efflux of the membrane. The exact molecular mechanism of this enhanced cleavage remains to be investigated. The similarity with the APP system supports the notion that ADAM10 is involved in L1 cleavage, very likely as the executing enzyme. Direct biochemical evidence for the cleavage of L1 by ADAM10 is presently not available. It is possible that ADAM10 is inhibited by cholesterol itself or by a cholesterol-dependent associated protein not yet identified. Perhaps depletion of cholesterol increases encounter rates between L1 and the metalloproteinase. It remains to be seen whether other ectodomain cleavage processes are also sensitive to MCD treatment, or whether this is a particular feature of ADAM10. A further important question is whether ADAM10 mediates L1 cleavage also during brain development. We observed that cleavage of L1 in mouse brain was highest around birth. Indeed, a coordinated expression of APP and the α-secretase ADAM10 was reported in mouse brain development (Marcinkiewicz and Seidah, 2000).
The study of Nayeem et al. has provided the first evidence that in addition to metalloproteinase(s) the release of a large portion of the L1 ectodomain can be mediated by the plasmin/plasminogen proteolytic system (Nayeem et al., 1999). The activation of plasminogen to plasmin involves a cascade of inhibitors and activators and requires binding to the cell surface (Plow et al., 1995). In the brain, plasminogen and its proteolytic fragment are abundant in the hippocampus (Chen and Strickland, 1997). It has been known for a long time that growth cones and migrating neurons release plasmin-like proteases (Krystosek and Seeds, 1981). Proteolysis is required during neurite outgrowth and neuronal migration is retarded in mice lacking the tissue-type plasminogen activator (tPA) gene (Seeds et al., 1999). The proteolytic activation of plasminogen by the urokinase-type plasminogen activator, which is synthesized and secreted by many tumor cells, plays an important role in tumor cell migration and invasion into the surrounding tissue (Reuning et al., 1998). We demonstrate here that the 150-kD fragment of L1 generated and released following plasmin cleavage can also promote cell migration as it contains the RGDs in the 6.Ig domain. This raises the possibility that L1-150 can substitute for the complete ectodomain of L1. The generation of L1-150 is evidently dependent on the availability of plasminogen and a cellular source of plasminogen activators and may therefore be restricted. In contrast, as shown in this study the release of L1 via metalloproteinase-mediated cleavage appears to be more abundant, at least in cultured human tumor cells.
In summary, membrane-released L1 can promote cell migration in two ways. First, soluble L1 can be immobilized into the surrounding matrix by the specific binding to laminin (Montgomery et al., 1996) or the proteoglycan neurocan (Oleszewski et al., 2000). As shown before, immobilized L1 can serve as a potent substrate for cell migration. Second, as presented in this publication, soluble L1 fragments can directly interact with cells and stimulate cell migration. The metalloproteinase-released ectodomain was able to stimulate migration through autocrine/paracrine binding to integrins. This is reminiscent of recent studies on ephrins and deleted in colorectal cancer (DCC) showing that proteolytic cleavage plays a critical role in regulating cell movements. The ability of soluble L1 to stimulate cell migration could be relevant for migratory processes under many physiological and pathophysiological conditions.
Materials and methods
Cells
Human breast tumor cell lines AR, MDA-231, MDA-435, SKBr3, and the ovarian tumor cell lines SKOV3 and OAW42 were cultivated in DME supplemented with 10% FBS at 37°C, 5% CO2, and 100% humidity. All cell lines were obtained from the tumor bank of the German Cancer Research Center (Heidelberg, Germany). CHO cells stably transfected with human (hL1-CHO) or mouse L1 (mL1-CHO) were described before (Gutwein et al., 2000). Ectodomain swapped fusion proteins between human L-selectin (CD69L) and mouse L1 were constructed using PCR. In brief, the L1 ectodomain (amino acids M1 to E1123) were amplified and fused to the transmembrane and cytoplasmic part of CD69L (amino acids P346 to Y385). The ectodomain of CD69L (amino acids M1 to L319) was amplified and fused to the COOH-terminal L1 fragment (amino acids I1090 to E1260). The latter fragment comprises the 34 membrane-proximal amino acids, the transmembrane and the cytoplasmic part of mouse L1. Mouse L1 devoid of RGDs (D555E and D564E) was constructed by site-directed mutagenesis as described previously (Oleszewski et al., 1999). All cDNA constructs were cloned into pCDNA3 and confirmed by DNA sequencing. Plasmid DNAs were transfected into CHO cells. G418 resistant colonies were stained for L1 or CD69L followed by the respective secondary antibodies, and positive cells were selected by several rounds of FACS sorting (Beer et al., 1999). SKOV3 cells with high or low expression of L1 (SKOV3hi or SKOV3lo) were established by three rounds of FACS sorting using an antibody to L1 and regrowth of sorted cells. A similar protocol was applied to OAW42 cells. Cells were kept in DME and cultivated at 37°C, 5% CO2, and at 100% humidity. Plasmids encoding bovine HA-tagged ADAM10 and a dominant-negative form of ADAM10 carrying a E384A substitution (Flag-tagged ADAM10 DN) were described previously (Lammich et al., 1999). Transient expression was achieved by transfection of AR cells with superfect (Stratagene). Analysis of shed L1 in the culture supernatants by ELISA was done 48 h later (see below).
Chemicals and antibodies
mAb 324 against the mouse L1 adhesion molecule, mAb UJ 127.11 against human L1, and mAb 79 against mouse CD24 were described before (Ebeling et al., 1996; Beer et al., 1999).
mAb DREG56 against human CD69L was obtained from E.C. Butcher (Stanford University, Stanford, CA). The mAb to αvβ5 (P1F6) was obtained from Chemicon, the mAb to human CD49e (SAM-1) was from Dianova, the human L1 mAb 5G3 was obtained from Becton Dickinson, and the mAb to CD49f (GoH3) was obtained from Beckman Coulter. Polyclonal antibodies to the human L1 cytoplasmic domain (pcytL1) were produced by immunizing a rabbit with a recombinant L1 cytoplasmic fragment expressed in bacteria. PMA, sodium vanadate, MCD, and laminin from pepsinized human placenta (laminin-10/11) were obtained from Sigma-Aldrich. Vitronectin and bovine serum fibronectin were purchased from Life Technologies, and human plasmin and α2-antiplasmin were obtained from Calbiochem. BB-3103 was a gift of British Biotech, and Ro 31-9790 was obtained from Roche.
Immunfluorescence
The surface staining of cells with saturating amounts of mAbs, either hybridoma supernatants or purified antibodies, and phenylephrine-conjugated goat antibodies to mouse Ig (Dianova) has been described elsewhere (Ebeling et al., 1996). Stained cells were analyzed with a FACScan (Becton Dickinson) or sorted using a FACS Vantage. Where indicated, cells were grown on coverslips and fixed with 2% paraformaldehyde/PBS before fluorescent staining and confocal microscopy analysis as previously described (Krauss and Altevogt, 1999).
Analysis of L1 shedding
These assays were described previously (Gutwein et al., 2000). In brief, 2 × 106 cells were plated in 6-cm tissue culture plates in 5 ml of complete medium. After 24 h at 37°C, the complete medium was replaced by 1.7 ml serum-free culture medium for the indicated length of time. Cells remained firmly attached to the plastic surface. Where indicated, PMA (50 ng/ml) or pervanadate (200 mmolar) were included in the medium. A stock solution of pervanadate was freshly prepared by mixing a solution of H2O2 and sodium vanadate (Gutwein et al., 2000). MCD treatment (10 mM final concentration) for 30 min at 37°C was done as described before (Krauss and Altevogt, 1999). BB-3103 inhibitor was added 30 min before the addition of PMA or pervanadate or before MCD treatment. Aliquots of the medium were TCA precipitated or immunoprecipitated with mAb to L1 and analyzed for the presence of L1 by Western blotting. Alternatively, cells were removed from the tissue culture plastic surface by PBS/5 mM EDTA treatment for 5 min. Shedding was analyzed by resuspending the cells in serum-free medium in 1.5 ml microfuge tubes at 37°C for 1 h in the presence or absence of inducers and inhibitors. BB-3103 was used at 7 mM final concentration. In some experiments Ro 31-9790 at a final concentration of 30 mM was used with essentially similar results. Cell pellets and culture medium were separated by 10 min centrifugation in an Eppendorf centrifuge at 13,000 × g. Culture supernatants from analyzed cells were TCA precipitated and redissolved in twofold-concentrated, nonreducing SDS-sample buffer. Cell pellets were lysed in lysis buffer (20 mM Tris/HCl, pH 8.0, containing 1% Triton X-100, 150 mM NaCl, 1 mM PMSF), cleared by centrifugation, and mixed with twofold-concentrated reducing SDS sample buffer. Mouse brains of different developmental stages were homogenized in 1% Triton X-100/TBS/1mM orthovanadate, 1mM NaF/protease inhibitors (leupeptin, pepstatin, aprotinin 10 mg/ml each, 10 mM EDTA, 2 mM 1,10 phenanthroline) at 4°C. The protein content of cell lysates was determined using the Biorad protein assay kit, and 20 mg protein was analyzed by SDS-PAGE and blotting.
Plasmin treatment of cell cultures
2 × 106 adherent AR cells in serum-free medium were treated with plasmin (0.6 U/ml) or plasmin plus α2-antiplasmin (1.2 U/ml) to neutralize plasmin activity. Where indicated, Ro 31-9790 was included. Cells were incubated for 6 h and supernatants were collected and protease inhibitors (Roche completeZ mini) were added to prevent further cleavage. Aliquots of each supernatant were TCA precipitated and analyzed for the presence of L1 by Western blotting. For mAb 5G3 blots, samples were prepared by heating at 80°C in nonreducing sample buffer as described (Nayeem et al., 1999). Supernatants were also tested for the stimulation of CHO haptotactic migration (see below).
Recombinant proteins
Fusion proteins containing the Fc portion of human IgG1 and the ectodomain of mouse L1 or human L1 have been described in previous publications (Oleszewski et al., 1999). The mouse L1–Fc mut III protein in which both RGDs in the 6.Ig-like domain are mutated to RGE has also been described (Oleszewski et al., 1999). The production and characterization of SART-1-Fc will be reported in a separate publication (unpublished data). Plasmid-DNA was transfected into COS-7 cells and supernatants containing the Fc fusion protein were collected. Fc-fusion proteins were purified by protein A Sepharose chromatography and analyzed and quantified using a human Fc-specific capture ELISA with purified human IgG as standard (Oleszewski et al., 1999).
ELISA for the detection of soluble human L1
For the detection of soluble L1, 96-well flexible microtiter plates (Falcon 3912; Becton Dickinson) were coated with 1 mg/ml of capturing antibody (anti-L1 mAb 5G3) overnight. Wells were blocked with 3% BSA/PBS for 45 min and then incubated for 1 h with tissue culture supernatants containing soluble L1. After five washes with BSA/PBS, the wells were incubated for 1 h with biotin-conjugated mAb UJ127.11 followed by streptavidin-conjugated peroxidase (Dianova) using BMP as substrate. The color reaction was stopped by the addition of 10 mM H2SO4 and analyzed at 450 nm using an ELISA reader. Human L1–Fc protein served as an internal standard for the assay.
Biochemical analysis
Samples were separated by SDS-PAGE under nonreducing conditions and transferred to an Immobilon membrane using semi-dry blotting. After blocking with 5% skim milk in TBS, the blots were developed with the respective primary antibody (mAbs UJ127.11, 5G3 or pcytL1) followed by peroxidase conjugated secondary antibody and ECL detection.
Transmigration assays
For the haptotactic cell migration assay, fibronectin, vitronectin, laminin, or BSA (each at 10 mg/ml) were coated for 90 min on the backside of Transwell chambers (Costar, 6.5 mm diam, 5-mm pore size). Cells (105 or as otherwise indicated) in RPMI 1640 medium containing 0.5% BSA were seeded into the upper chamber and allowed to transmigrate to the lower compartment for 16 h at 37°C. For mAb blocking the cells were preincubated at 10 mg/ml with the respective mAb for 30 min at 37°C before onset of the assay. To quantify transmigrated cells, the inner chamber was removed and cleaned carefully with a cotton swab to remove the non-migrated cells. Migrated cells adherent to the bottom of the membrane were stained with crystal violet solution. The membranes were extensively washed in water and the remaining stain was eluted with 10% acidic acid. The eluted dye was measured at 595 nm in an ELISA reader. Each determination was done in quadruplicate and the data are given as mean values ± SEM.
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Christine Schlösser and Daniel Straub and Klaus Hexel for help with FACS sorting.
This work was supported by a grant from the German-Israeli Cooperation in Cancer Research (P. Gutwein and P. Altevogt), a grant from MINERVA (N. Agmon-Levin), and by a guest scientist stipend from DKFZ (M. Fogel). V. Lemmon was supported by a grant from the National Eye Institute (EY05285).
S. Mechtersheimer and P. Gutwein contributed equally to this work.
Footnotes
Abbreviations used in this paper: ADAM, a disintegrin and metalloproteinase; APP, amyloid precursor protein; AR, androgen receptor; FN, fibronectin; MCD, methyl-β-cyclodextrin; RGD, Arg-Gly-Asp.
References
- Artavanis-Tsakonas, S., M.D. Rand, and R.J. Lake. 1999. Notch signaling: cell fate control and signal integration in development. Science. 284:770–776. [DOI] [PubMed] [Google Scholar]
- Beer, S., M. Oleszewski, P. Gutwein, C. Geiger, and P. Altevogt. 1999. Metalloproteinase-mediated release of the ectodomain of L1 adhesion molecule. J. Cell Sci. 112:2667–2675. [DOI] [PubMed] [Google Scholar]
- Black, R.A., and J.M. White. 1998. ADAMs: focus on the protease domain. Curr. Opin. Cell Biol. 10:654–659. [DOI] [PubMed] [Google Scholar]
- Blobel, C.P. 2000. Remarkable roles of proteolysis on and beyond the cell surface. Curr. Opin. Cell Biol. 12:606–612. [DOI] [PubMed] [Google Scholar]
- Brou, C., F. Logeat, N. Gupta, C. Bessia, O. LeBail, J.R. Doedens, A. Cumano, P. Roux, R.A. Black, and A. Israel. 2000. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol. Cell. 5:207–216. [DOI] [PubMed] [Google Scholar]
- Brümmendorf, T., S. Kenwrick, and F.G. Rathjen. 1998. Neural cell recognition molecule L1: from cell biology to human hereditary brain malformations. Curr. Opin. Neurobiol. 8:87–97. [DOI] [PubMed] [Google Scholar]
- Chen, Z.L., and S. Strickland. 1997. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 91:917–925. [DOI] [PubMed] [Google Scholar]
- De Angelis, E., J. MacFarlane, J.S. Du, G. Yeo, R. Hicks, F.G. Rathjen, S. Kenwrick, and T. Brummendorf. 1999. Pathological missense mutations of neural cell adhesion molecule L1 affect homophilic and heterophilic binding activities. EMBO J. 18:4744–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec, H., E.I. Christensen, and P.M. Ronco. 1998. The cell adhesion molecule L1 is developmentally regulated in the renal epithelium and is involved in kidney branching morphogenesis. J. Cell Biol. 143:2067–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty, P., E. Williams, and F.S. Walsh. 1995. A soluble chimeric form of the L1 glycoprotein stimulates neurite outgrowth. Neuron. 14:57–66. [DOI] [PubMed] [Google Scholar]
- Doherty, P., G. Williams, and E.J. Williams. 2000. CAMs and axonal growth: a critical evaluation of the role of calcium and the MAPK cascade. Mol. Cell. Neurosci. 16:283–295. [DOI] [PubMed] [Google Scholar]
- Dong, J., L.K. Opresko, P.J. Dempsey, D.A. Lauffenburger, R.J. Coffey, and H.S. Wiley. 1999. Metalloprotease-mediated ligand release regulates autocrine signaling through the epidermal growth factor receptor. Proc. Natl. Acad. Sci. USA. 96:6235–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling, O., A. Duczmal, S. Aigner, C. Geiger, S. Schöllhammer, J.T. Kemshead, P. Möller, A.R. Schwartz, and P. Altevogt. 1996. L1 adhesion molecule on human lymphocytes and monocytes: expression and involvement in binding to alpha v beta 3 integrin. Eur. J. Immunol. 26:2508–2516. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann, B., S. Silletti, F. Mei, C.H. Siu, P.M. Yip, P.C. Brooks, D.A. Cheresh, T.E. O'Toole, M.H. Ginsberg, and A.M. Montgomery. 1997. A single immunoglobulin-like domain of the human neural cell adhesion molecule L1 supports adhesion by multiple vascular and platelet integrins. J. Cell Biol. 139:1567–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler, I.J. 1990. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 50:6130–6138. [PubMed] [Google Scholar]
- Filardo, E.J., S.L. Deming, and D.A. Cheresh. 1996. Regulation of cell migration by the integrin beta subunit ectodomain. J. Cell Sci. 109:1615–1622. [DOI] [PubMed] [Google Scholar]
- Galko, M.J., and M. Tessier-Lavigne. 2000. Function of an axonal chemoattractant modulated by metalloprotease activity. Science. 289:1365–1367. [DOI] [PubMed] [Google Scholar]
- Gechtman, Z., J.L. Alonso, G. Raab, D.E. Ingber, and M. Klagsbrun. 1999. The shedding of membrane-anchored heparin-binding epidermal-like growth factor is regulated by the Raf/mitogen-activated protein kinase cascade and by cell adhesion and spreading. J. Biol. Chem. 274:28828–28835. [DOI] [PubMed] [Google Scholar]
- Giancotti, F.G., and E. Ruoslahti. 1999. Integrin signaling. Science. 285:1028–1032. [DOI] [PubMed] [Google Scholar]
- Gutwein, P., M. Oleszewski, S. Mechtersheimer, N. Agmon-Levin, K. Krauss, and P. Altevogt. 2000. Role of Src kinases in the ADAM-mediated release of L1 adhesion molecule from human tumor cells. J. Biol. Chem. 275:15490–15497. [DOI] [PubMed] [Google Scholar]
- Hattori, M., M. Osterfield, and J.G. Flanagan. 2000. Regulated cleavage of a contact-mediated axon repellent. Science. 289:1360–1365. [DOI] [PubMed] [Google Scholar]
- Hortsch, M. 1996. The L1 family of neural cell adhesion molecules: old proteins performing new tricks. Neuron. 17:587–593. [DOI] [PubMed] [Google Scholar]
- Hooper, N.M., E.H. Karran, and A.J. Turner. 1997. Membrane protein secretases. Biochem. J. 321:265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes, R.O. 1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 69:11–25. [DOI] [PubMed] [Google Scholar]
- Izumi, Y., M. Hirata, H. Hasuwa, R. Iwamoto, T. Umata, K. Miyado, Y. Tamai, T. Kurisaki, F.A. Sehara, S. Ohno, and E. Mekada. 1998. A metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKCdelta are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO J. 17:7260–7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumoto, S., T. Ohnishi, N. Arita, S. Hiraga, T. Taki, and T. Hayakawa. 1996. Gene expression of neural cell adhesion molecule L1 in malignant gliomas and biological significance of L1 in glioma invasion. Cancer Res. 56:1440–1444. [PubMed] [Google Scholar]
- Kamiguchi, H., and V. Lemmon. 1997. Neural cell adhesion molecule L1: signaling pathways and growth cone motility. J. Neurosci. Res. 49:1–8. [DOI] [PubMed] [Google Scholar]
- Katayama, M., A. Iwamatsu, H. Masutani, K. Furuke, K. Takeda, H. Wada, T. Masuda, and K. Ishii. 1997. Expression of neural cell adhesion molecule L1 in human lung cancer cell lines. Cell. Struct. Funct. 22:511–516. [DOI] [PubMed] [Google Scholar]
- Klemke, R.L., M. Yebra, E.M. Bayna, and D.A. Cheresh. 1994. Receptor tyrosine kinase signaling required for integrin alpha v beta 5-directed cell motility but not adhesion on vitronectin. J. Cell Biol. 127:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowitz, A., G. Kadmon, M. Eckert, V. Schirrmacher, M. Schachner, and P. Altevogt. 1992. Expression and function of the neural cell adhesion molecule L1 in mouse leukocytes. Eur. J. Immunol. 22:1199–1205. [DOI] [PubMed] [Google Scholar]
- Kojiro, E., G. Gimpl, S. Lammich, W. Marz, and F. Fahrenholz. 2001. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha-secretase ADAM 10. Proc. Natl. Acad. Sci. USA. 98:5815–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss, K., and P. Altevogt. 1999. Integrin leukocyte function-associated antigen-1-mediated cell binding can be activated by clustering of membrane rafts. J. Biol. Chem. 274:36921–36927. [DOI] [PubMed] [Google Scholar]
- Krüger, A., R. Soeltl, I. Sopov, C. Kopitz, M. Arlt, V. Magdolen, N. Harbeck, B. Gansbacher, and M. Schmitt. 2001. Hydroxamate-type matrix metalloproteinase inhibitor batimastat promotes liver metastasis. Cancer Res. 61:1272–1275. [PubMed] [Google Scholar]
- Krystosek, A., and N.W. Seeds. 1981. Plasminogen activator release at the neuronal growth cone. Science. 213:1532–1534. [DOI] [PubMed] [Google Scholar]
- Lammich, S., E. Kojro, R. Postina, S. Gilbert, R. Pfeiffer, M. Jasionowski, C. Haass, and F. Fahrenholz. 1999. Constitutive and regulated alpha-secretase cleavage of Alzheimer's amyloid precursor protein by a disintegrin metalloprotease. Proc. Natl. Acad. Sci. USA. 96:3922–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljelund, P., P. Ghosh, and A.N. van den Pol. 1994. Expression of the neural axon adhesion molecule L1 in the developing and adult rat brain. J. Biol. Chem. 269:32886–32895. [PubMed] [Google Scholar]
- Linnemann, D., A. Raz, and E. Bock. 1989. Differential expression of cell adhesion molecules in variants of K1735 melanoma cells differing in metastatic capacity. Int. J. Cancer. 43:709–712. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz, M., and N.G. Seidah. 2000. Coordinated expression of beta-amyloid precursor protein and the putative beta-secretase BACE and alpha-secretase ADAM10 in mouse and human brain. J. Neurochem. 75:2133–2143. [DOI] [PubMed] [Google Scholar]
- Montgomery, A.M., J.C. Becker, C.H. Siu, V.P. Lemmon, D.A. Cheresh, J.D. Pancook, X. Zhao, and R.A. Reisfeld. 1996. Human neural cell adhesion molecule L1 and rat homologue NILE are ligands for integrin αvβ3. J. Cell Biol. 132:475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm, J.S., E.H. Schroeter, M.T. Saxena, A. Griesemer, X. Tian, D.J. Pan, W.J. Ray, and R. Kopan. 2000. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol. Cell. 5:197–206. [DOI] [PubMed] [Google Scholar]
- Nath, D., N.J. Williamson, R. Jarvis, and G. Murphy. 2001. Shedding of c-Met is regulated by crosstalk between a G-protein coupled receptor and the EGF receptor and is mediated by a TIMP-3 sensitive metalloproteinase. J. Cell Sci. 114:1213–1220. [DOI] [PubMed] [Google Scholar]
- Nayeem, N., S. Silletti, X. Yang, V.P. Lemmon, R.A. Reisfeld, W.B. Stallcup, and A.M. Montgomery. 1999. A potential role for the plasmin(ogen) system in the posttranslational cleavage of the neural cell adhesion molecule L1. J. Cell Sci. 112:4739–4749. [DOI] [PubMed] [Google Scholar]
- Ohnishi, T., H. Matsumura, S. Izumoto, S. Hiraga, and T. Hayakawa. 1998. A novel model of glioma cell invasion using organotypic brain slice culture. Cancer Res. 58:2935–2940. [PubMed] [Google Scholar]
- Oleszewski, M., S. Beer, S. Katich, C. Geiger, Y. Zeller, U. Rauch, and P. Altevogt. 1999. Integrin and neurocan binding to L1 involves distinct Ig domains. J. Biol. Chem. 274:24602–24610. [DOI] [PubMed] [Google Scholar]
- Oleszewski, M., P. Gutwein, W. von der Lieth, U. Rauch, and P. Altevogt. 2000. Characterization of the L1-neurocan-binding site. Implications for L1-L1 homophilic binding. J. Biol Chem. 275:34478–34485. [DOI] [PubMed] [Google Scholar]
- Pancook, J.D., R.A. Reisfeld, N. Varki, A. Vitiello, R.I. Fox, and A.M. Montgomery. 1997. Expression and regulation of the neural cell adhesion molecule L1 on human cells of myelomonocytic and lymphoid origin. J. Immunol. 158:4413–4421. [PubMed] [Google Scholar]
- Patel, K., F. Kiely, E. Phimister, G. Melino, F. Rathjen, and J.T. Kemshead. 1991. The 200/220 kD antigen recognized by monoclonal antibody (MAb) UJ127.11 on neural tissues and tumors is the human L1 adhesion molecule. Hybridoma. 10:481–491. [DOI] [PubMed] [Google Scholar]
- Peschon, J.J., J.L. Slack, P. Reddy, K.L. Stocking, S.W. Sunnarborg, D.C. Lee, W.E. Russell, B.J. Castner, R.S. Johnson, J.N. Fitzner, R.W. Boyce, and N. Nelson, et al.1998. An essential role for ectodomain shedding in mammalian development. Science. 282:1281–1284. [DOI] [PubMed] [Google Scholar]
- Plow, E.F., T. Herren, A. Redlitz, L.A. Miles, and P.J. Hoover. 1995. The cell biology of the plasminogen system. Faseb J. 9:939–945. [DOI] [PubMed] [Google Scholar]
- Prenzel, N., E. Zwick, H. Daub, M. Leserer, R. Abraham, C. Wallasch, and A. Ullrich. 1999. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 402:884–888. [DOI] [PubMed] [Google Scholar]
- Reid, R.A., and J.J. Hemperly. 1992. Variants of human L1 cell adhesion molecule arise through alternate splicing of RNA. J. Mol. Neurosci. 3:127–135. [DOI] [PubMed] [Google Scholar]
- Reuning, U., V. Magdolen, O. Wilhelm, K. Fischer, V. Lutz, H. Graeff, and M. Schmitt. 1998. Multifunctional potential of the plasminogen activation system in tumor invasion and metastasis. Int. J. Oncol. 13:893–906. [DOI] [PubMed] [Google Scholar]
- Ruppert, M., S. Aigner, M. Hubbe, H. Yagita, and P. Altevogt. 1995. The L1 adhesion molecule is a cellular ligand for VLA-5. J. Cell Biol. 131:1881–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoul, K., R. Sadoul, A. Faissner, and M. Schachner. 1988. Biochemical characterization of different molecular forms of the neural cell adhesion molecule L1. J. Neurochem. 50:510–521. [DOI] [PubMed] [Google Scholar]
- Schachner, M. 1997. Neural recognition molecules and synaptic plasticity. Curr. Opin. Cell Biol. 9:627–634. [DOI] [PubMed] [Google Scholar]
- Schlöndorff, J., and C.P. Blobel. 1999. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J. Cell Sci. 112:3603–3617. [DOI] [PubMed] [Google Scholar]
- Schwartz, M.A., and S.J. Shattil. 2000. Signaling networks linking integrins and rho family GTPases. Trends Biochem. Sci. 25:388–391. [DOI] [PubMed] [Google Scholar]
- Seeds, N.W., M.E. Basham, and S.P. Haffke. 1999. Neuronal migration is retarded in mice lacking the tissue plasminogen activator gene. Proc. Natl. Acad. Sci. USA. 96:14118–14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg, D.J., C.R. Hauck, D. Ilic, C.K. Klingbeil, E. Schaefer, C.H. Damsky, and D.D. Schlaepfer. 2000. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2:249–256. [DOI] [PubMed] [Google Scholar]
- Silletti, S., F. Mei, D. Sheppard, and A.M. Montgomery. 2000. Plasmin-sensitive dibasic sequences in the third fibronectin-like domain of L1-cell adhesion molecule (CAM) facilitate homomultimerization and concomitant integrin recruitment. J. Cell Biol. 149:1485–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian, S.V., M.L. Fitzgerald, and M. Bernfield. 1997. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J. Biol. Chem. 272:14713–14720. [DOI] [PubMed] [Google Scholar]
- Sugawa, M., K. Ono, Y. Yasui, T. Kishi, and T. Tsumori. 1997. Enhancement of neurite outgrowth by the soluble form of human L1 (neural cell adhesion molecule). Neuroreport. 8:3157–3162. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne, M., and C.S. Goodman. 1996. The molecular biology of axon guidance. Science. 274:1123–1133. [DOI] [PubMed] [Google Scholar]
- Tokumaru, S., S. Higashiyama, T. Endo, T. Nakagawa, J.I. Miyagawa, K. Yamamori, Y. Hanakawa, H. Ohmoto, K. Yoshino, Y. Shirakata, Y. Matsuzawa, K. Hashimoto, and N. Taniguchi. 2000. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J. Cell Biol. 151:209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, A.J., and N.M. Hooper. 1999. Role for ADAM-family proteinases as membrane protein secretases. Biochem. Soc. Trans. 27:255–259. [DOI] [PubMed] [Google Scholar]
- Yip, P.M., X. Zhao, A.M. Montgomery, and C.H. Siu. 1998. The Arg-Gly-Asp motif in the cell adhesion molecule L1 promotes neurite outgrowth via interaction with the alphavbeta3 integrin. Mol. Biol. Cell. 9:277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]