Abstract
Efficient phagocytosis of apoptotic cells is important for normal tissue development, homeostasis, and the resolution of inflammation. Although many receptors have been implicated in the clearance of apoptotic cells, the roles of these receptors in the engulfment process have not been well defined. We developed a novel system to distinguish between receptors involved in tethering of apoptotic cells versus those inducing their uptake. Our results suggest that regardless of the receptors engaged on the phagocyte, ingestion does not occur in the absence of phosphatidylserine (PS). Further, recognition of PS was found to be dependent on the presence of the PS receptor (PSR). Both PS and anti-PSR antibodies stimulated membrane ruffling, vesicle formation, and “bystander” uptake of cells bound to the surface of the phagocyte. We propose that the phagocytosis of apoptotic cells requires two events: tethering followed by PS-stimulated, PSR-mediated macropinocytosis.
Keywords: macropinocytosis; phagocytosis; apoptotic cells; phosphatidylserine; phosphatidylserine receptor
Introduction
As cells undergo apoptosis, they are rapidly phagocytosed as intact bodies by cells in the surrounding tissue or by professional phagocytes such as macrophages and immature dendritic cells. This noninflammatory removal process occurs throughout the lifespan of an organism and is the fate of most dying cells. Clearance of apoptotic cells prevents the release of intracellular debris, reducing the likelihood of tissue damage resulting from inflammation or inappropriate autoimmune responses (Wyllie et al., 1980; Savill et al., 1992; Ren and Savill, 1998).
The exposure of phosphatidylserine (PS)* in the outer leaflet of the plasma membrane is one of the most striking changes on the surface of apoptotic cells (Fadok et al., 1992b; Schlegel et al., 1993; Koopman et al., 1994; Homburg et al., 1995; Martin et al., 1995; Vermes et al., 1995; van den Eijnde, 1998; Verhoven et al., 1999). Recent evidence has led to the hypothesis that PS exposure is crucial for the uptake of unopsonized, apoptotic cells (Fadok et al., 2001). Stereo-specific PS recognition has long been included in the list of apoptotic cell removal mechanisms (Fadok et al., 1992a), and we have recently identified and cloned a candidate PS receptor (PSR) that appears to participate in uptake of apoptotic cells and is found on the surface of many cell types (Fadok et al., 2000).
Many receptors have been proposed to mediate the phagocytosis of apoptotic cells (for reviews see Duvall et al., 1985; Savill, 1998; Savill and Fadok, 2000; Savill et al., 1993). These receptors include scavenger receptors (Pearson, 1996; Platt et al., 1996; Shiratsuchi et al., 1999; Zhou et al., 2001), CD14 (Flora and Gregory, 1994; Devitt et al., 1998), CD68 (Ramprasad et al., 1995; Sambrano and Steinberg, 1995; Sambrano et al., 1997), αvβ5 (Albert et al., 1998, 2000), and a receptor system involving CD36, a class B scavenger receptor, in conjunction with thrombospondin and an integrin, such as αvβ3 (vitronectin receptor [VnR]; Savill et al., 1992). Additionally, members of the collectin family, such as mannose binding lectin and C1q, are thought to mediate apoptotic cell recognition through opsonization (Balasubramanian et al., 1997; Balasubramanian and Schroit, 1998; Ishimoto et al., 2000; Taylor et al., 2000; Schagat et al., 2001).
On the basis of inhibitor or antibody-blocking studies, phagocytic cells show significant redundancy in apoptotic cell recognition and are apparently able to use many receptors at the same time. Expression of individual receptors in a variety of cell lines has usually led to increased binding of apoptotic cells with minimal effects on uptake (Navazo et al., 1996; Devitt et al., 1998; Hamon et al., 2000). Even genetic deletion of scavenger receptor A (SRA) or C1q has generated only a partial reduction in clearance (Platt et al., 1996; Taylor et al., 2000). These results suggest that some receptors may act as a complex (Pradhan et al., 1997), a concept further supported by the apparent requirement for CD36 in the uptake seen with either the VnR–thrombospondin system (Savill et al., 1992) or that involving the PSR (Fadok et al., 2000).
The individual roles of these receptors in binding, phagocytosis, or transduction of antiinflammatory signals is currently unclear. To address these questions, we have developed a novel system to target individual receptors using erythrocytes conjugated to specific protein ligands or antibodies. Here we demonstrate that ligation of many receptors, including CD14, CD68, CD36, and αvβ3 integrin, resulted in particle binding. However, bound particles were not ingested unless PS was present. The PS-dependent uptake of target particles was stereo specific and inhibited by anti-PSR antibodies. Further, we found that PSR stimulation resulted in membrane ruffling and formation of large, fluid-filled vesicles. We propose that engulfment of apoptotic cells is a two-step process involving an initial tethering event followed by PS-stimulated, PSR-mediated uptake by a process akin to macropinocytosis.
Results
Ligation of many putative phagocytic receptors results in particle adhesion, but not ingestion
To determine which phagocytic receptors may act to promote apoptotic cell binding and which induce engulfment, we developed a novel system using defined ligands on the surface of erythrocytes. The surface proteins of erythrocytes (E) were biotinylated (Eb) and coated with avidin (Eba). These erythrocytes were then bound to a biotinylated protein or antibody for the desired receptor (e.g., Ebab–vitronectin or Ebab–anti-CD36). The use of erythrocytes as target particles has several advantages. First, erythrocytes without surface alterations do not bind to phagocytes. Second, hypotonic lysis can be used to distinguish between erythrocytes that are bound to the phagocytes and those that have been ingested (Gigli and Nelson, 1968). Third, PS may be deliberately inserted into the erythrocyte membrane, allowing for recreation of one of the hallmark characteristics of apoptotic cells.
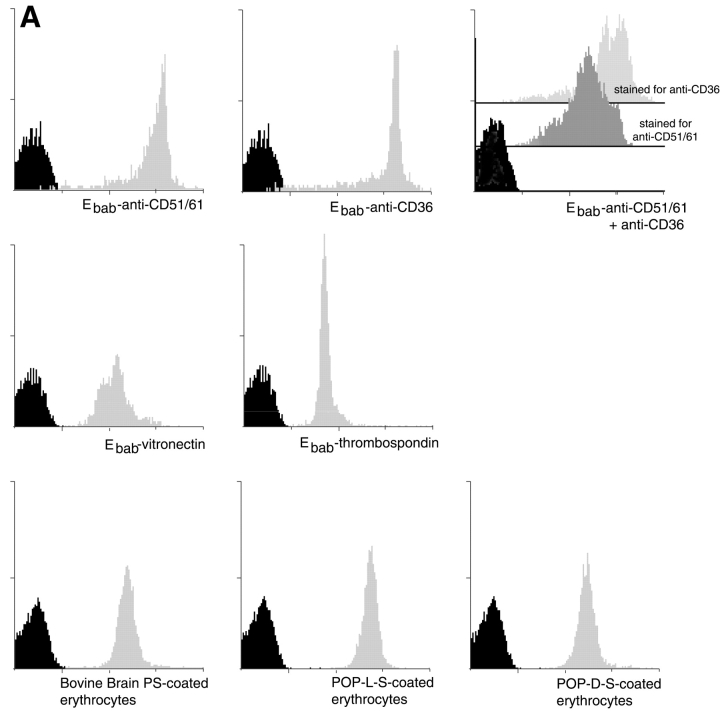
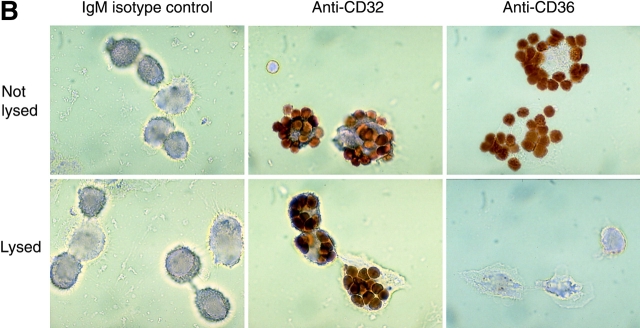
The efficient coating of erythrocytes with antibody, protein ligand, or various forms of PS was verified by flow cytometry (Fig. 1 A). The Ebab were then incubated with human monocyte–derived macrophages (HMDM), murine thioglycolate–elicited peritoneal macrophages (MPM), or other phagocytic cells. The use of FACS®-based uptake assays was explored (Licht et al., 1999; Albert et al., 2000), but was not useful in distinguishing between Ebab that had attached to the outside of the HMDM and those that had been engulfed. Therefore, light microscopy was used to accurately quantitate attachment and engulfment of Ebab (Fig. 1 B). As expected, ligation of the Fcγ receptor IIA (CD32) with Ebab–anti-CD32 induced high levels of both binding and uptake, representing the phagocytic potential of the HMDM under these conditions. The use of antibodies for adequate triggering of uptake was further supported by the observation that Ebab–anti-C3bi receptor (anti-CD18) also demonstrated high levels of binding and uptake (unpublished data). Isotype control IgG or IgM on the erythrocytes induced neither binding nor uptake.
Figure 1.
Erythrocytes can be coated with antibodies, protein ligands, or aminophospholipids targeting specific phagocytic receptors. Binding and uptake of erythrocytes by macrophages can then be assessed quantitatively. (A) Cytofluorographs demonstrate the efficient coating of erythrocytes with antibodies, protein ligands, or different forms of PS. Cy3-conjugated antibodies were used to detect either biotinylated antibodies or biotinylated proteins on the surface of erythrocytes. Phycoerythrin–annexin V was used to detect various forms of PS on the surface of erythrocytes. Histogram in black demonstrates unstained erythrocytes. (B) Light microscopy was used to accurately assess the binding or uptake of Ebab by HMDM. Erythrocytes coated with either an irrelevant IgM isotype control (anti-TNP), anti-CD32, or anti-CD36 were incubated with HMDM for 30 min. After washing away nonadherent Ebab, distilled H2O was used to lyse the Ebab bound but not engulfed by the HMDM. This figure illustrates the high level of both tethering and engulfment of Ebab–anti-CD32 by HMDM, whereas Ebab–anti-CD36 were mostly tethered but not engulfed.
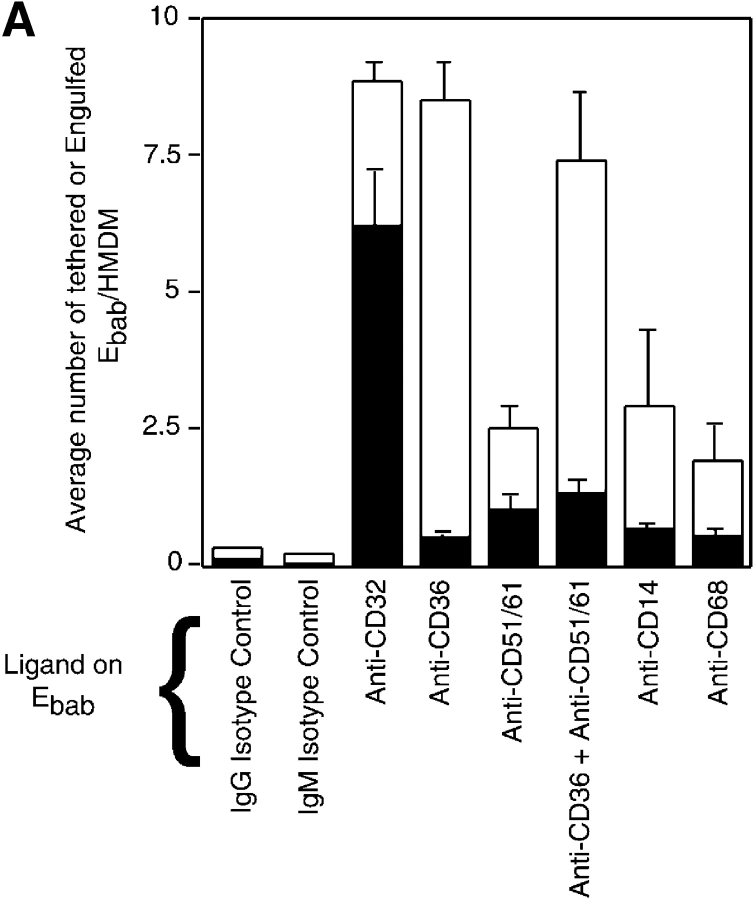
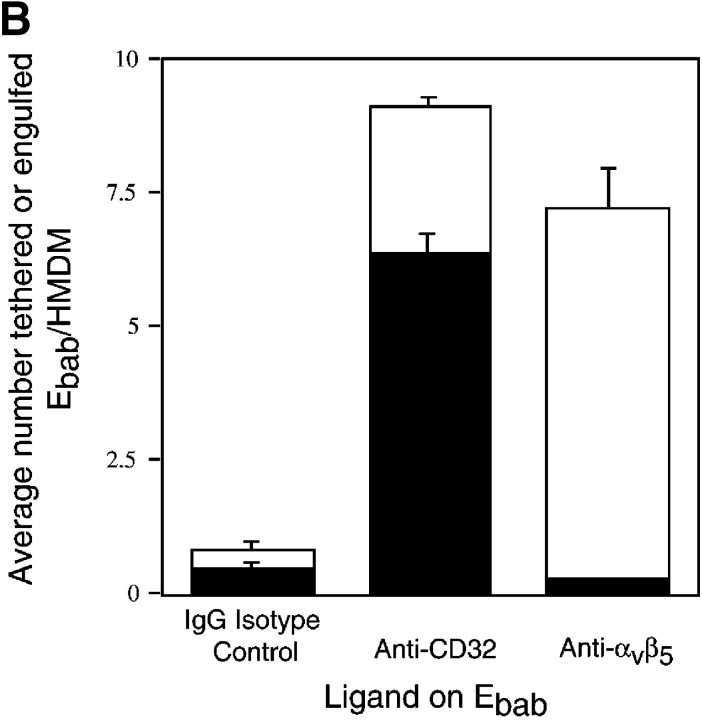
Using this system, it was determined that engagement of putative apoptotic cell receptors including anti-CD36, anti-αvβ3 integrin (CD51/61), anti-CD14, and anti-CD68 resulted in binding of the erythrocytes to the macrophages without substantial uptake. Combining antibodies against CD36 with those against the VnR (CD51/61) did not significantly increase uptake (Fig. 2 A). Recently the αvβ5 integrin has been described as mediating uptake of apoptotic cells by dendritic cells (Albert et al., 1998, 2000). However, engagement of this receptor using our system also promoted tethering with no uptake by HMDM (Fig. 2 B).
Figure 2.
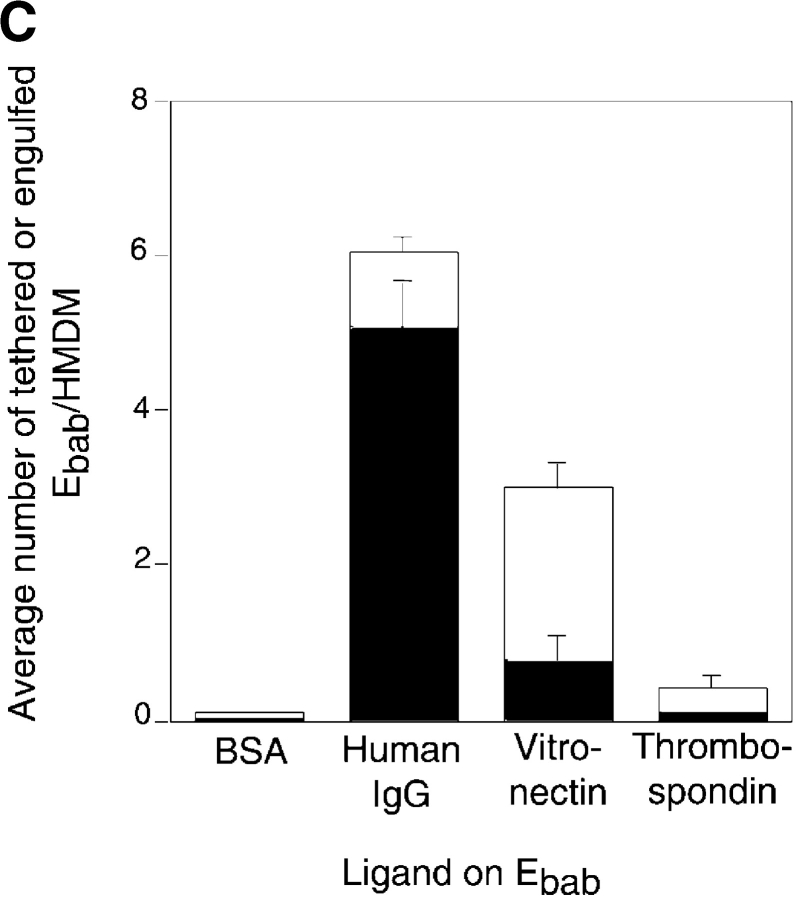
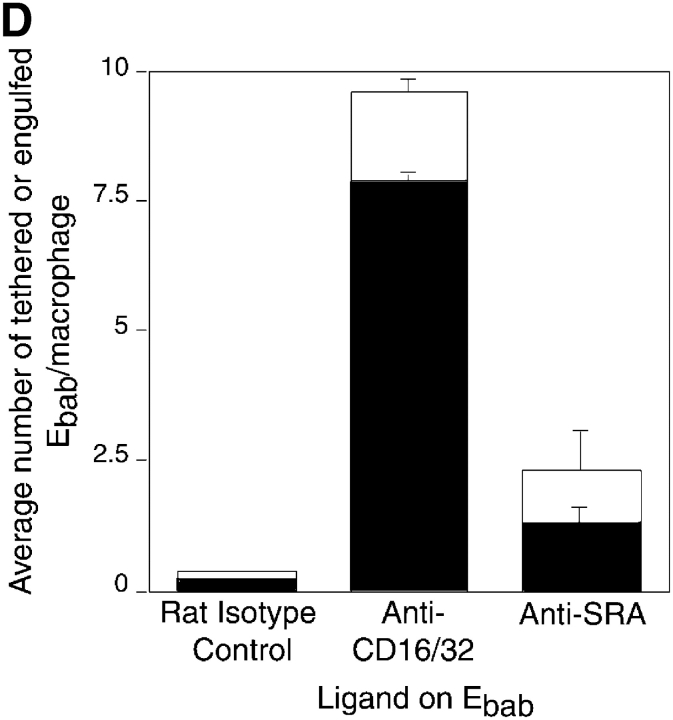
Many proposed phagocytic receptors promote tethering, but not engulfment. Ebab targeting various receptors on the surface of HMDM were used to investigate the role of the receptors in binding versus uptake. All results represent mean ± SEM from three or more experiments. (A) Ebab coated with antibodies against several phagocytic receptors, either individually or in combination, tethered to HMDM, but were not efficiently engulfed. (B) Ebab coated with antibodies against the αvβ5 integrin also demonstrated tethering to HMDM with little uptake. (C) Ebab coated with vitronectin or thrombospondin, natural ligands for αvβ3 integrin or αvβ3/CD36, respectively, were not efficiently engulfed by HMDM. (D) Ebab coated with anti-SRA antibodies, tethered to murine macrophages, but were not efficiently engulfed compared with positive controls. ▪, Engulfed; □, tethered.
Protein ligands were also used to target specific receptors. Ebab–vitronectin and Ebab–thrombospondin, the natural ligands for αvβ3 integrin, and the proposed thrombospondin receptor system, respectively (Pytela et al., 1985), were bound by the phagocytes, but were not substantially ingested (Fig. 2 C). As mentioned above, another candidate receptor for uptake is SRA (Platt et al., 1996). Because antibodies against the human version of this receptor are unavailable, the role of SRA was examined using MPM. Anti-SRA antibodies promoted some uptake of bound Ebab, but were far less efficient than the positive controls (Fig. 2 D).
PS induces uptake of tethered particles in a stereo-specific manner and is mediated by the PSR
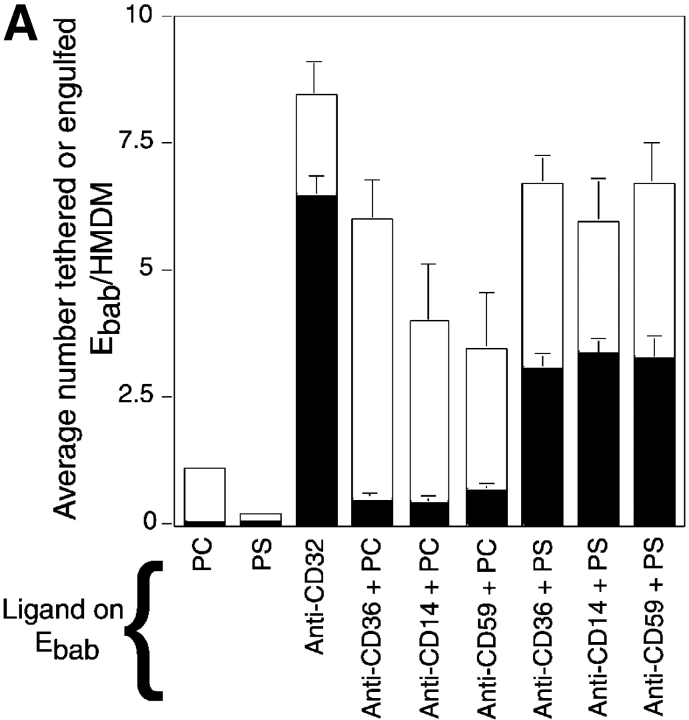
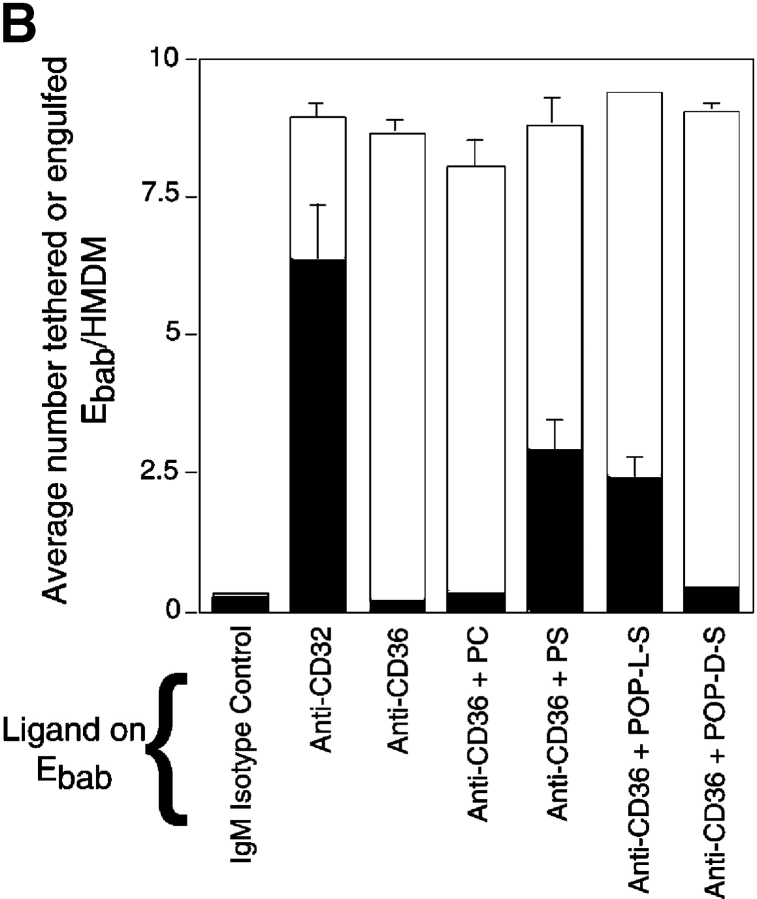
Construction of Ebab–anti-PSR was not possible because the biotinylation of our monoclonal antibody (mAb 217) destroyed its ability to bind the receptor (unpublished data). Therefore, we used erythrocytes coated with PS to investigate the role of the PSR in binding versus uptake of particles. PS alone was not sufficient to mediate adhesion or engulfment of the erythrocytes. However, when membrane PS was combined with any of the ligands capable of tethering the Ebab to macrophages, adhesion was converted into engulfment (Fig. 3 A). PS on the surface of Ebab was sufficient to drive uptake even when Ebab were tethered to CD59, a surface molecule not thought to be involved in phagocytosis. This effect was also seen when PS-coated Ebab were tethered to NIH 3T3 fibroblasts (Ebab–anti-major histocompatibility complex [MHC]-I) or MPM (or Ebab–anti-F4/80-antigen; unpublished data). These results suggest that even though PS on the surface of a particle alone is not sufficient to confer particle binding, its presence is sufficient to trigger the engulfment of a tethered particle regardless of the initial adhesive ligand.
Figure 3.
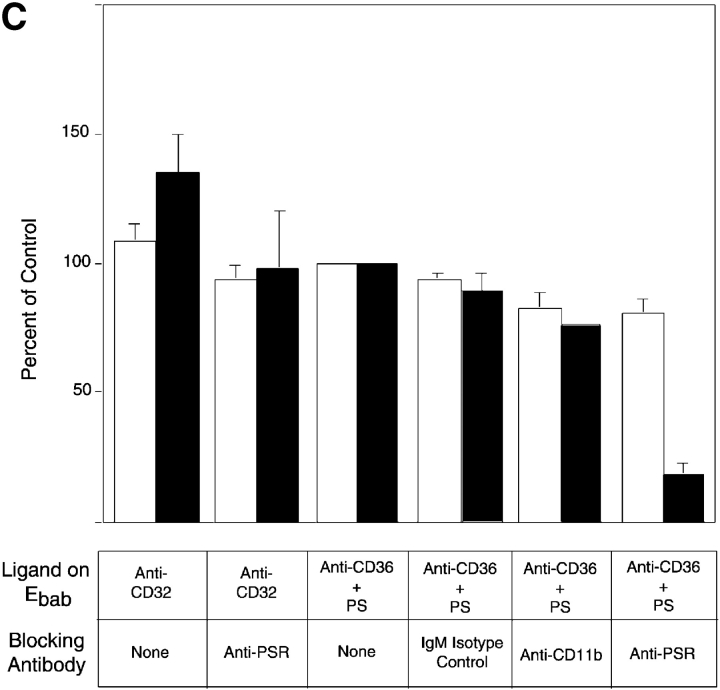
Presence of PS on the surface of tethered Ebab results in their uptake in a stereo-specific manner. Erythrocytes coated with PS (or PC, as a control) were incubated with HMDM. All results represent mean ± SEM. from three or more experiments. (A) Addition of PS, but not PC, to erythrocytes resulted in little tethering or uptake by HMDM. Addition of PS to various Ebab induced their engulfment by HMDM, whereas PC had no effect. (B) To address the stereo specificity of PS-mediated Ebab engulfment, POP-L-S or POP-D-S was added to the erythrocyte membrane. Addition of POP-L-S induced uptake of tethered Ebab by HMDM to similar levels observed with bovine brain PS, whereas POP-D-S did not. (C) Preincubation of HMDM with anti-PSR antibodies blocked the engulfment of PS-coated Ebab, but minimally affected engulfment of Ebab via FcγR. Preincubation of HMDM with other IgM antibodies did not block engulfment of PS-coated Ebab. ▪, Engulfed; □, tethered.
To examine whether the presence of PS on the membrane of Ebab promoted uptake in a specific manner, we used synthetic, stereo-isomeric PS in combination with tethering antibodies. The ability of PS to induce uptake of particles was found to be stereo specific in that the L isoform of PS, 1-palmitoyl-2-oleoyl-sn-3-glycerphospho-l-serine (POP-L-S; see Materials and methods), promoted uptake similar to the natural form (PS isolated from bovine brain), whereas the D isoform, 1-palmitoyl-2-oleoyl-sn-3-glycerphospho-d-serine (POP-D-S), had no effect on ingestion of antibody-tethered erythrocytes (Fig. 3 B).
Essential role for the PSR in PS-stimulated engulfment
The stereo-specific, PS-induced engulfment of various tethered Ebab suggested the involvement of a PS-specific receptor. In addition to the recently identified PSR (Fadok et al., 2000), other receptors for apoptotic cell engulfment have been reported to recognize PS (Rigotti et al., 1995; Sambrano and Steinberg, 1995; Devitt et al., 1998; Oka et al., 1998). To determine whether the PSR was involved in recognition of PS on erythrocytes, anti-PSR antibody blocking studies were performed. Preincubation of HMDM with anti-PSR antibodies minimally affected uptake through the Fcγ receptor, indicating that the antibody did not inhibit phagocytosis in general. However, preincubation of HMDM with anti-PSR antibodies before the addition of PS-coated Ebab–anti-CD36 inhibited the uptake of those particles (Fig. 3 C). This effect was not seen when HMDM were preincubated with an irrelevant isotype control antibody or an IgM that binds to CD11b.
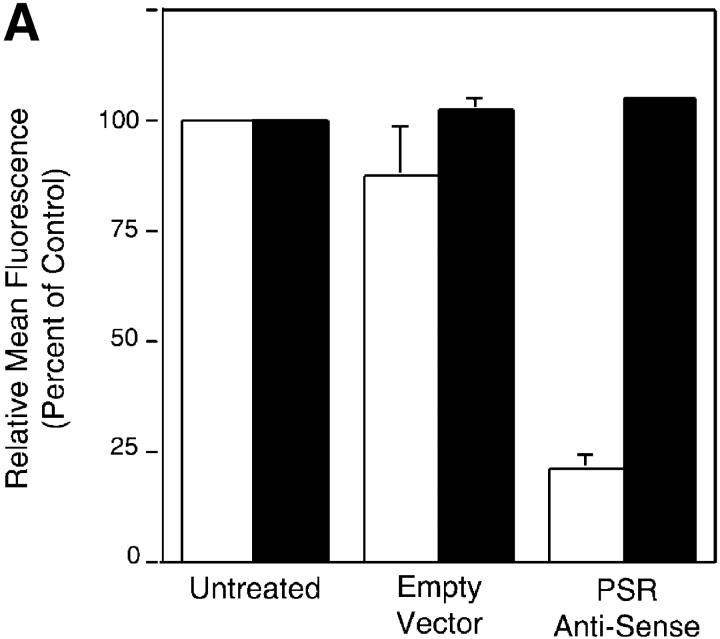
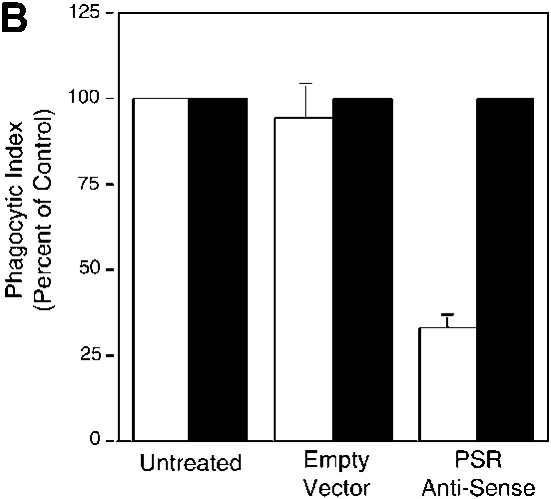
To directly address the requirement for the PSR in the uptake of apoptotic cells, NIH 3T3 fibroblasts, which express the PSR, were transfected with a PSR antisense DNA construct, a sense construct, or an empty vector control. Apoptotic Jurkat cells were fed to transfected fibroblasts and their uptake was determined by light microscopy (see Materials and methods for induction and characterization of Jurkat apoptosis). When expression of the PSR was reduced with antisense transfection to below 25% of control levels (Fig. 4 A), the uptake of apoptotic cells was diminished by a nearly equivalent amount (Fig. 4 B). Transfection did not affect the phagocyte membrane or phagocytosis in a general manner, as both expression of the α chain of the VnR and uptake of latex beads were unaffected.
Figure 4.
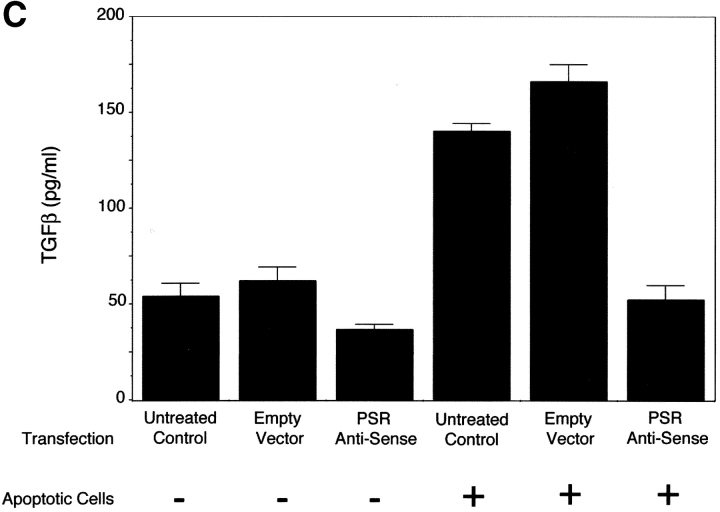
TGF-β secretion and PS-dependent engulfment of targeted erythrocytes and apoptotic cells is mediated by PSR. (A) Transfection of NIH 3T3 fibroblasts with vectors containing PSR antisense DNA resulted in a decrease of PSR expression to 25% of control levels, as measured by flow cytometry. Antisense transfection did not alter the levels of other phagocytic receptors, shown here by flow cytometric analysis of CD51 (αv integrin) expression. Transfection with sense PSR (unpublished data) or empty vector controls did not alter levels of PSR expression. □, PSR expression; ▪, αv expression. (B) The decreased expression of PSR induced by antisense transfection (Fig. 3 A) resulted in a decrease of apoptotic cell engulfment compared with control levels. However, antisense PSR DNA transfection did not alter the ability of these cells to engulf latex beads. Data were normalized to control (unmanipulated) cells. All results represent mean ± SEM from at least three experiments. □, Apoptotic cell uptake; ▪, latex bead uptake. (C) PSR is essential for TGF-β release by phagocytes during uptake of apoptotic cells. NIH 3T3 fibroblasts showed decreased production of TGF-β after an overnight feeding of apoptotic cells, as compared with unmanipulated fibroblasts and fibroblasts transfected with empty vector controls. All results represent mean ± SEM from at least two experiments.
Finally, an important issue regarding the uptake of apoptotic cells versus other opsonized or nonself particles is the consequential release of antiinflammatory or inflammatory mediators, respectively. TGF-β is released from phagocytes after the uptake of apoptotic cells (Fadok et al., 1998) and is thought to play a key role in downregulating inflammatory responses (Shull et al., 1992; Kulkarni et al., 1993). Transfection of PSR antisense DNA inhibited the TGF-β response in 3T3 cells, after the uptake of apoptotic Jurkat cells (Fig. 4 C).
Stimulation of phagocytes with PS or anti-PSR antibody triggers “bystander uptake” of adherent particles
The above data suggests that the phagocytosis of apoptotic cells involves independent and separable events: particle tethering and engulfment. To investigate this further, we attempted to induce phagocytosis of adherent erythrocytes by secondary stimulation of the PSR. Erythrocytes were created that bound to the surface of NIH 3T3 fibroblasts by coating Eba with biotinylated anti-Dq/Lq MHC class I (Ebab–anti-MHC-I). These Ebab were not internalized in the absence of additional stimulation. Many growth factors, including EGF, PDGF, and macrophage colony–stimulating factor (M-CSF), stimulate the process of macropinocytosis (Brunk et al., 1976; Racoosin and Swanson, 1989; Hewlett et al., 1994), resulting in the ingestion of fluid, solutes, and macromolecules, as well as particles adhered to the cell surface (Galan et al., 1992). We observed that treatment of NIH 3T3 fibroblasts with EGF (or PDGF; unpublished data) stimulated the uptake of adherent Ebab–anti-MHC-I (Fig. 5 A). Similarly, treatment of HMDM with M-CSF triggered the uptake of Ebab–anti-CD36 (unpublished data).
Figure 5.
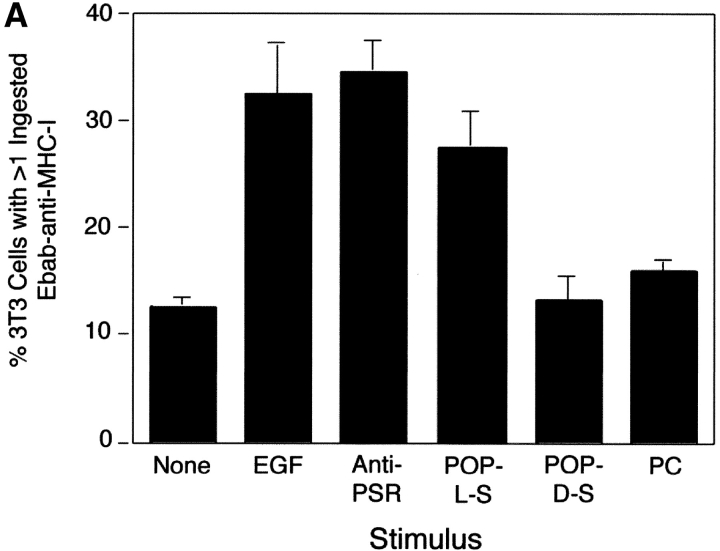
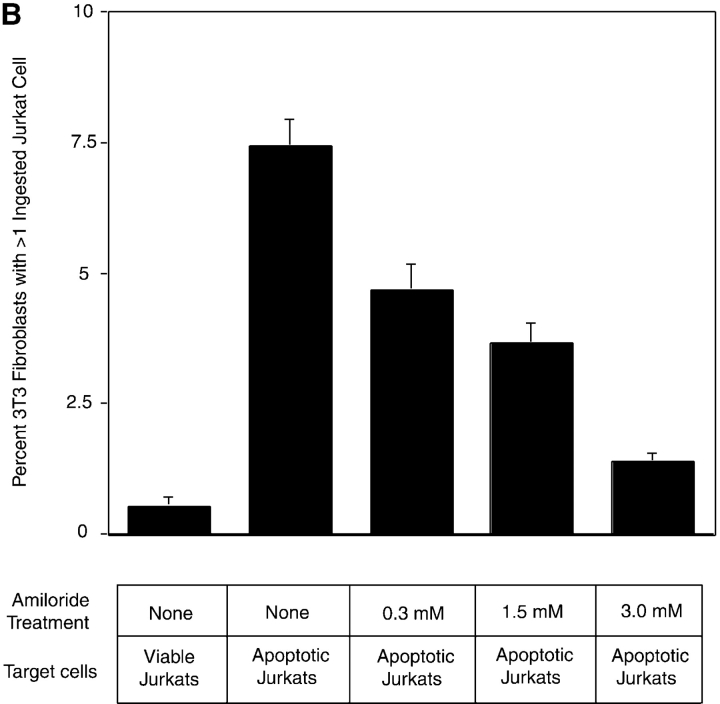
PS- and PSR-mediated uptake of apoptotic cells occurs by a process akin to macropinocytosis. (A) To separately examine the tethering and engulfment events, Ebab were first allowed to tether to MHC-I on the phagocyte surface for 15 min, followed by stimulation with various forms of PS, anti-PSR antibody, or EGF (positive control) for 45 min. Ebab–anti-MHC-I were engulfed by fibroblasts when stimulated with EGF, anti-PSR, or POP-L-S, but not when stimulated with POP-D-S stereoisomer or PC. Results represent mean ± SEM from three experiments. (B) Amiloride, a specific inhibitor of macropinocytosis, inhibited the engulfment of apoptotic Jurkat T cells by NIH 3T3 fibroblasts at concentrations as low as 0.3 mM. Results represent mean ± SEM from three experiments.
To stimulate the PSR, liposomes containing PS or a control phospholipid, phosphatidylcholine (PC), were added to the culture media after Ebab–anti-MHC-I were allowed to adhere to the phagocytes. The addition of POP-L-S liposomes, but not liposomes containing POP-D-S or PC, resulted in internalization of the bound erythrocytes. The same result was obtained when HMDM were stimulated with PS after binding of Ebab–anti-CD36; addition of antibodies to the Fcγ receptor on these macrophages did not stimulate the ingestion of tethered erythrocytes (unpublished data). We hypothesized that the anti-PSR antibody, an IgM, may aggregate the PSR and result in its activation. In support of this hypothesis, we found that stimulation of the phagocytes with anti-PSR antibodies, but not an IgM isotype control (unpublished data), also triggered the uptake of tethered erythrocytes (Fig. 5 A). Importantly, the stimulatory effects of the anti-PSR antibody cease after 30 min, allowing for its dual use as a blocking antibody. These results suggest that ligation of the PSR with exogenous PS or anti-PSR antibody is capable of triggering the engulfment of any particle tethered to the surface of the phagocyte.
The stimulated ingestion of tethered particles is characteristic of a macropinocytosis-mediated engulfment mechanism. Amiloride, an inhibitor of the Na+/H+ antiporter, is known to selectively inhibit the process of macropinocytosis (Swanson, 1989; West et al., 1989; Dowrick et al., 1993). To assess whether the engulfment of apoptotic cells was related to the process of macropinocytosis, we attempted to inhibit the uptake of apoptotic cells with amiloride. When NIH 3T3 fibroblasts were incubated in amiloride for 15 min before the addition of apoptotic Jurkat cells, uptake was inhibited at concentrations as low as 0.3 mM (Fig. 5 B). This result suggests that the phagocytosis of apoptotic cells, regardless of the receptors involved, is an amiloride-sensitive endocytic process.
Apoptotic cells, PS, and anti-PSR antibody stimulate membrane ruffling
The process of macropinocytosis is subsequent to the induction of membrane ruffling (Francis et al., 1993). To further examine the ability of PS and the PSR to stimulate the uptake of apoptotic cells by the process of macropinocytosis, we tested their ability to induce membrane ruffling in Swiss 3T3 fibroblasts. Cultured fibroblasts were briefly serum starved, and then cultured with viable or apoptotic Jurkat cells or PDGF as a positive control. When apoptotic cells were incubated with Swiss 3T3 fibroblasts, membrane ruffling was induced within 15 min, even when apoptotic cells were not obviously in contact with the phagocytes. Consistent with this observation, we also found that liposomes containing PS, but not PC, induced membrane ruffling (unpublished data). Membrane ruffling was rarely observed in unstimulated cultures or when viable Jurkat cells were added to fibroblast cultures (unpublished data). Anti-PSR antibody, but not an IgM isotype control antibody, induced ruffles in the plasma membrane of quiescent Swiss 3T3 fibroblasts at time points as short as 2 min, with increasing formation of stress fibers over a 20-min period (Fig. 6 A).
Figure 6.
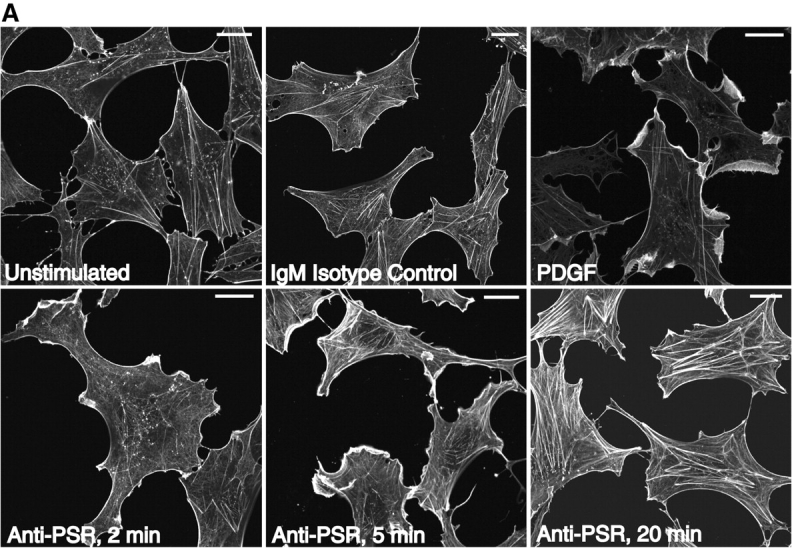
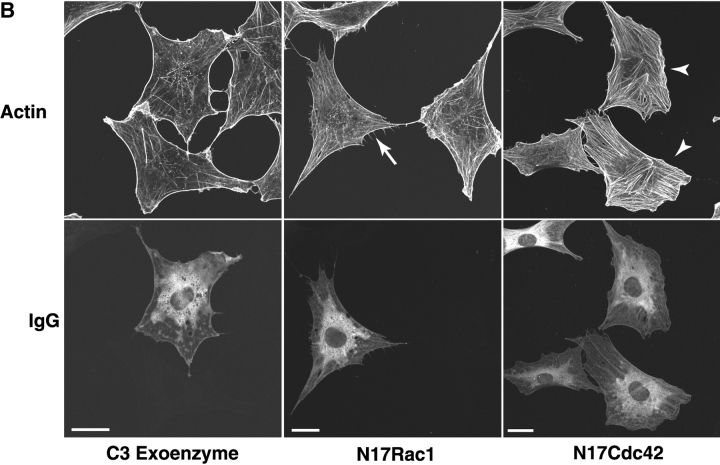
Anti-PSR antibody stimulates membrane ruffling in Swiss 3T3 fibroblasts. (A) Anti-PSR antibodies induce membrane ruffling in Swiss 3T3 fibroblasts within 2 min of antibody stimulation. Membrane ruffling is optimally induced by 5 min, and stress fiber formation is observed by 20 min (bottom). These results are not seen with an IgM isotype control antibody (top, center) or in unstimulated cells (top, left). PDGF-induced membrane ruffling at 10 min is shown as a positive control (top, right). (B) Quiescent Swiss 3T3 fibroblasts were co-microinjected with rat IgG and C3 exoenzyme (a specific inhibitor of RhoA), N17Cdc42, or N17Rac, and stimulated with IgM anti-PSR for 10 min. Cells were fixed and stained for injected antibody (bottom) and actin (top). Representative injected cells for each experiment are shown. Microinjection of C3 exoenzyme did not inhibit anti-PSR–induced membrane ruffling (left), although it inhibited FCS-induced stress fiber formation (not shown). (Center) N17Rac1 inhibited anti-PSR–induced membrane ruffling and resulted in formation of filopodia (arrow). (Right) Cells injected with N17Cdc42 also did not ruffle in response to anti-PSR stimulation, and had enhanced stress fiber formation (arrowheads). Bars: (A) 2 μm; (B) 20 μm.
PSR-mediated membrane ruffling requires Cdc42 and Rac1, but not RhoA activity
Microinjection techniques were used to investigate the role of the low molecular weight GTPases Cdc42, Rac1, and RhoA in PSR-mediated membrane ruffling. Although inhibiting RhoA with the microinjection of C3 transferase did not affect anti-PSR antibody-stimulated membrane ruffling, the microinjection of dominant negative recombinant protein for Cdc42 and Rac1 (N17Cdc42 and N17Rac1) resulted in the inhibition of membrane ruffling. Representative pictures of injected cells are shown, with neighboring uninjected cells shown for comparison (Fig. 6 B). Injected cells were visually inspected for the presence or absence of membrane ruffles. Inhibition of membrane ruffling by microinjection of N17Cdc42 or N17Rac1 was estimated at 20–40% inhibition and 40–90% inhibition, respectively, in four separate experiments. These results indicate that PS and PSR are capable of signaling changes in the actin cytoskeleton and these changes require both Cdc42 and Rac1. These findings are consistent with the requirement for Cdc42 and Rac1 in the uptake of apoptotic cells (Leverrier and Ridley, 2001) and macropinocytosis (Ridley et al., 1992; Swanson and Watts, 1995; Chen et al., 1996; Garrett et al., 2000).
PS and anti-PSR antibodies stimulate macropinocytosis
Even though membrane ruffling is closely associated with the process of macropinocytosis, stimulation of membrane ruffling does not always result in vesicle formation (Li et al., 1997; Bretscher and Aguado-Velasco, 1998). HMDM were stimulated with either the anti-PSR antibody or PS-containing liposomes and examined for uptake of a water-soluble, membrane-impermeable fluorescent dye, Lucifer yellow (LY). Whereas untreated macrophages showed little or no uptake of LY, treatment of HMDM with M-CSF, which has been shown to induce macropinocytosis in macrophages (Racoosin and Swanson, 1989, 1992), resulted in uptake of LY into large macropinocytotic vesicles (Fig. 7 A). Large vesicles containing the dye were also detectable in PS liposome–treated cells. However, little uptake of dye was seen when cells were stimulated with liposomes containing only PC. We also attempted to induce macropinocytosis with an anti-CD36 IgM antibody and saw little or no LY uptake. As observed for M-CSF and PS, anti-PSR antibody also stimulated the uptake of LY into large vesicles.
Figure 7.
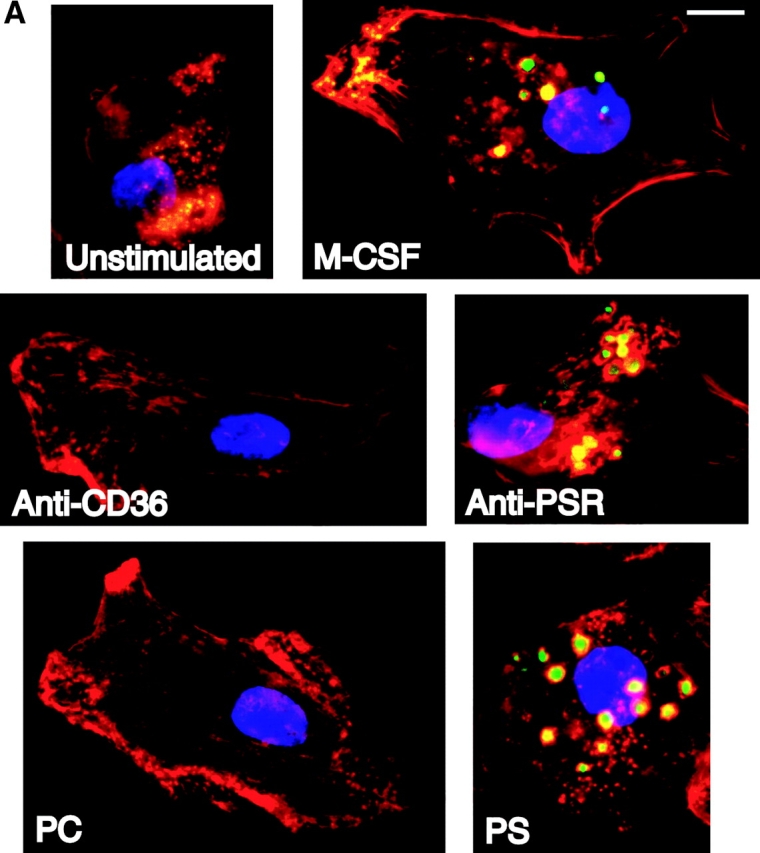
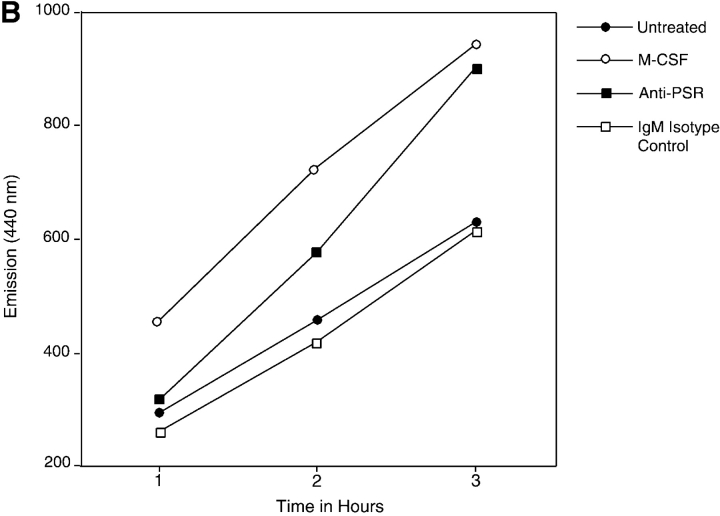
Anti-PSR antibodies and PS stimulate macropinocytosis. (A) HMDM were stimulated with M-CSF (as a positive control), antibodies to the PSR or CD36, PS, or PC. Vesicle formation was visualized using LY (green). Nuclei were stained with DAPI (blue), and cells were stained for actin with rhomadine–phalloidin (red). Macrophages stimulated with either anti-PSR antibodies or PS formed vesicles similar to those formed after stimulation with M-CSF, a known inducer of macropinocytosis. Representative images are shown. (B) Accumulation of Cascade blue was quantified by fluorimetry. MEM were untreated or stimulated with M-CSF, anti-PSR antibody, or an IgM isotype control antibody, in the presence of dye. At the designated time point, cells were washed, lysed, and fluorescence of the lysate was measured. Similar results were obtained on four separate occasions. Bar, 5 μm.
Macropinocytosis was assessed quantitatively using MPM due to donor-to-donor variability inherent with the use of HMDM. Cells were serum starved for 2 h and stimulated in the presence of dye. Cells were then washed free of extracellular dye, lysed, and dye content was measured by fluorimetry (Racoosin and Swanson, 1989, 1992; Swanson, 1989). Anti-PSR antibodies stimulated dye uptake to levels similar to M-CSF, whereas IgM isotype control antibodies had no effect on dye uptake (Fig. 7 B), similar to the results shown above.
Discussion
Apoptosis occurs throughout the lifetime of an organism, from embryogenesis to death. Recent evidence lends further support to the concept that efficient removal of apoptotic cells and cell bodies prevents exposure of surrounding tissue to potentially cytotoxic, immunogenic, or inflammatory cellular contents (Albert et al., 1998; Sauter et al., 2000). The removal process is completed by a wide variety of cell types and appears to involve multiple receptors and apoptotic cell ligands, yet the phagocytic mechanism has not been clearly defined. The data presented here suggest that uptake of apoptotic cells involves two separate steps: an initial tethering event followed by PS-stimulated macropinocytosis.
Many putative phagocytic receptors appear to mediate tethering of the apoptotic cell to the phagocyte. In contrast, PS by itself appears to promote poor binding of particles to phagocytes, but plays a critical role in stimulating the actual uptake process through the PSR. The ability of PS to drive internalization of any tethered particle may, in part, explain the recent reports that apoptotic and necrotic cells appear to be recognized and engulfed in a similar manner (Chung et al., 2000; Cocco and Ucker, 2001). In this proposed scenario, binding and ingestion are separable events. However, the requirement for both a tethering receptor and an engulfment receptor is indispensable. It is plausible that the ability of macrophages, or professional phagocytes, to rapidly and efficiently clear apoptotic cells compared with their nonprofessional counterparts may be due to the wide repertoire of adhesion receptors, not to differences in engulfment mechanisms (Parnaik et al., 2000). In addition, we have recently suggested that the collectin family of innate immune system molecules can mediate uptake of apoptotic cells (Ogden et al., 2001), perhaps because they can enhance adhesion to phagocyte surfaces. However, surface calreticulin, a receptor for the collagenous domains common to the collectin family, also appears to be capable of stimulating macropinocytosis, perhaps through CD91 (α2 macroglobulin receptor/LRP), and may provide another example of apoptotic cell removal by this mechanism (Ogden et al., 2001).
The requirement for Rac1 during anti-PSR antibody-mediated membrane ruffling suggests that the PSR utilizes signaling pathways for apoptotic cell engulfment similar to those defined in the Caenorhabditis elegans system (Gumienny et al., 1999; Liu and Hengartner, 1999; Chung et al., 2000; Zhou et al., 2001). In addition, the PSR appears to require the activity of Cdc42 for the induction of membrane ruffling. How the PSR transmits signals to the low molecular weight GTPases is currently unclear. Although our results suggest that receptors such as SRA, αv integrins, and CD36 do not transmit engulfment signals, these studies do not rule out the possible formation of a complex, where tethering receptors and the PSR act in concert to transmit engulfment signals. The integrin, αvβ5, has been shown to recruit Rac1 and Dock180 via the adaptor protein CrkII in HEK293 cells in the presence of apoptotic lymphocytes (Albert et al., 2000). It is interesting to speculate that the integrins implicated in apoptotic cell engulfment may be activated by the binding of target ligands on the apoptotic cell, resulting in the recruitment of Rac1 to the engulfment site and the induction or amplification of actin cytoskeleton rearrangement.
Surprisingly, PS alone on the surface of erythrocytes resulted in limited adhesion to the phagocyte surface. The lack of particle binding by PS–PSR interactions could reasonably be explained by either low surface expression of the PSR on the phagocytes, i.e., low avidity, or by low affinity between PS and the PSR. HMDM from many donors have been shown to express the PSR at low levels unless stimulated with TGF-β and β-glucan (Fadok et al., 2000). However, preliminary studies using HMDM, stimulated to upregulate PSR expression, have not resulted in increased erythrocyte binding, suggesting that low avidity is not responsible for the lack of PS-coated erythrocyte binding. Affinity studies are currently being conducted to determine the strength and specificity of the PS–PSR interactions. It is imagined that relatively low affinity PSR interactions with PS are markedly enhanced by localized expression of PS on the apoptotic cell and, in particular, by the binding of the apoptotic cell by recognition receptors.
As macropinocytosis represents a nearly universal mechanism for ingestion by eukaryotic cells, it follows that PS may be capable of initiating this mechanism in a wide variety of cell types. It is tempting to speculate that the presence of PS is a powerful and ubiquitous engulfment signal, capable of stimulating the ingestion of apoptotic cells by many different types of phagocytes, regardless of the initial tethering ligand. These data suggest that PS stimulation of macropinocytosis and apoptotic cell engulfment is mediated in majority by the PSR. The PSR appears to be expressed by nearly all tissue types, and PSR homologues have been identified not only in man and mouse but also in Drosophila melanogaster and C. elegans (Fadok et al., 2000). Although playing a minor role in the binding of apoptotic cells, the PSR is critical for their ingestion, as indicated by antisense transfection data. In addition to conducting a signal that contributes to apoptotic cell uptake, the PSR appears to be responsible for inducing the release of a key antiinflammatory, antiimmunogenic molecule, TGF-β. The structure, localization, and characterization of domains on the PSR that mediate signaling for phagocytosis and cytokine production are currently under investigation.
Materials and methods
Cells and cell lines
Human monocytes were obtained from normal donors and cultured in X-Vivo 10 (BioWhittaker) containing 10% human serum. The differentiated macrophages (HMDM) were used on days 6 and 7 of culture. MPM were elicited with thioglycolate, harvested, and plated as previously described (Fadok et al., 1992a). NIH 3T3 fibroblasts were obtained from the American Type Culture Collection and cultured in DME (Cellgro®; Mediatech, Inc.), containing 10% fetal calf serum, sodium pyruvate, glutamine, penicillin, and streptomycin. Swiss 3T3 fibroblasts were obtained and cultured as previously described (Ridley and Hall, 1992; Ridley et al., 1992).
The Jurkat human leukemia T cell line was obtained from the American Type Culture Collection and cultured in RPMI 1640 (Cellgro®), containing 20% fetal calf serum (Gemini Bioproducts), glutamine, penicillin, and streptomycin. Apoptosis was induced by exposure to UV irradiation at 254 nm for 10 min, followed by 3 h of culture as previously described (Fadok et al., 2001). Loss of phospholipid asymmetry and exposure of PS were evaluated by analysis of annexin V–FITC binding. Propidium iodide was used as a control to determine the level of secondary necrosis, according to the manufacturer's instructions for using the annexin kit (R&D Systems). Under these conditions, cells are <5% propidium iodide positive and 50–80% annexin V positive. Apoptosis was also quantified by evaluation of nuclear morphology at the light microscopic level. By this method, these cells are typically 70–90% apoptotic.
Antibodies and protein reagents
Antibodies against human macrophage receptors included anti-CD32 (FL18.26; PharMingen), anti-CD36 (CB38; PharMingen), anti-CD51/61 (23C6; PharMingen), anti-CD68 (KP1; Dako), anti-CD59 (p282; PharMingen), anti-αvβ5 (P1F6; Chemicon), and anti-CD14 (MEM-18; Monosan®). Anti–human PSR antibody (mAb 217; Fadok et al., 2000) was used for all cell types in this study. Antibodies against MPM receptors included anti-CD16/32 (2.4G2; PharMingen) and anti-SRA (2F8; Serotec). Isotype control antibodies used included murine IgG (28–14–8, anti-H-2Db; provided by T. Potter, National Jewish Medical and Research Center, Denver, CO) or IgM (G155–228, anti-TNP; PharMingen [or mouse myeloma; CalBiochem]) for HMDM experiments and rat IgG (A95–1, anti-TNP; PharMingen) for murine macrophage experiments. Anti–H-2Dq/H-2Lq (anti-MHCI, KH117; PharMingen) was used in experiments with NIH 3T3 cells. Antibodies used to block Fcγ receptors in phagocytosis assays included anti–human CD16, anti–human CD32, anti–human CD64, and anti–murine CD16/32 (3G8, FL18.26, 10.1, and 2.4G2, respectively; PharMingen). Proteins used as ligands for phagocytic receptors included human vitronectin (Life Technologies) and thrombospondin (Calbiochem). All antibodies and ligands were biotinylated (Boehringer) and resuspended in PBS before coating of erythrocytes.
Construction of Ebab
Human erythrocytes were obtained from normal donors and washed in PBS (pH 8.0). 3 × 107 erythrocytes were resuspended in 800 μl PBS and incubated with EZ-Link-Sulfo-NHS-LC-Biotin (Pierce Chemical Co.) according to the manufacturer's instructions. The biotinylated erythrocytes (Eb) were washed with PBS, resuspended in PBS, pH 8.0, and incubated with 120 μg streptavidin (Sigma-Aldrich) at room temperature for 30 min. Avidin-coated, biotinylated erythrocytes (Eba) were washed, resuspended in PBS, pH 7.4, and incubated with 5–10 μg of biotinylated antibody or protein. Ebab were then washed and resuspended in DME before addition to phagocytes. Efficient coating of Ebab was confirmed by flow cytometry using the appropriate fluorochrome-conjugated secondary antibody (Jackson ImmunoResearch Laboratories).
Coating of erythrocytes with phospholipids
Bovine brain PS, bovine liver PC, 1-palmitoyl-2-oleoyl-sn-3-glycerphosphorylcholine (POPC), and POP-L-S were obtained from Avanti Polar Lipids Inc. POP-D-S was synthesized from POPC by phospholipase D–catalyzed headgroup exchange in the presence of D-serine (Comfurius and Zwaal, 1977; Juneja et al., 1989). Unilamellar liposomes containing brain PS, liver PC, POPC, POP-L-S, and POP-D-S were prepared as previously described (Fadok et al., 2001). Association of liposomes containing the various forms of PS with erythrocytes was confirmed by flow cytometry after staining with phycoerythrin-annexin V (PharMingen).
Phagocytosis assays
For phagocytosis assays involving Ebab, macrophages were plated in 24-well plates at 5 × 105 cells/well. Culture media was washed from macrophages and replaced with serum-free DME. Fcγ receptors on macrophages were blocked by preincubating the cells with a cocktail of monoclonal antibodies directed against Fcγ receptors for 10 min at room temperature. In some experiments, monoclonal antibodies against HMDM surface molecules were added at 100 μg/ml and incubated at 4°C for 30 min before addition of target Ebab. Target Ebab were added to macrophages at a ratio of 15:1 and allowed to incubate at 37°C for 30 min. Unbound Ebab were washed away with HBSS. Lysis of uningested Ebab was performed by adding deionized H2O for 10 s, followed by immediate replacement with DME. Cells were fixed with 0.75% gluteraldehyde, stained with dianisidine and H2O2 (Sigma-Aldrich), and counterstained with eosin. Binding and engulfment of Ebab were quantified by light microscopy before and after hypotonic lysis, respectively. 200 cells were scored per well and experiments were repeated at least three times. Assays using apoptotic Jurkat cells as targets and NIH 3T3 fibroblasts as phagocytes were performed as previously described (Fadok et al., 2001). For some experiments, amiloride (Sigma-Aldrich) was added at varying concentrations in serum-free DME for 15 min before the addition of apoptotic target cells.
For stimulated phagocytosis assays, NIH 3T3 fibroblasts were plated in 24-well plates at 104 cells/well. Ebab were allowed to adhere to fibroblasts for 15 min before stimulation with either 10 ng/ml EGF, 100 μM liposomes containing 100% PC, a 50:50 molar ratio of PC/PS, a 50:50 molar ratio PC/POP-D-PS, a 50:50 molar ratio PC/POP-L-PS, 100 μg/ml anti-PSR, or IgM isotype control for 20 min at 37°C in serum-free DME. Phagocytes were washed with PBS and uningested erythrocytes were lysed as described above. Phagocytes were fixed with methanol and stained with a modified Wright-Giemsa stain. Phagocytosis was determined as described above.
Construction of antisense plasmids and transfections
The entire open reading frame of the human PSR (Fadok et al., 2000) was excised from pcDNA 3.1 (+) using HindIII and NotI. The fragment was ligated in the reverse orientation into pcDNA 3.1 (−) (Invitrogen). NIH 3T3 fibroblasts were transfected with sense and antisense constructs, as well as empty vector using Lipofectamine Plus (GIBCO BRL), and the cells were assessed for receptor expression, uptake of apoptotic cells (as described above), and release of TGF-β (as previously described; Fadok et al., 1998) in response to apoptotic cells 12–15 h later.
Microinjection
Swiss 3T3 fibroblasts were plated on coverslips in complete DME supplemented with sodium pyruvate for 30 h and serum starved (18 h) before microinjection. Cells were coinjected with rabbit IgG (Sigma-Aldrich) and recombinant proteins C3 transferase (0.5 mg/ml), N17Rac (3.2 mg/ml), and N17cdc42 (1.5 mg/ml). Approximately 100 cells per coverslip were injected within 15 min using an Eppendorf Transjector 5246 and a Zeiss Axiovert 100 microscope fitted with a 37°C heated chamber. The cells were incubated for an additional 20 min after microinjection and were then stimulated with either 100 μg/ml anti-PSR, 5 ng/ml PDGF (Amersham Pharmacia Biotech), or 20% FCS for the appropriate time period. Stimulated cells were fixed in 2% paraformaldehyde, permeabilized with 0.5% Triton X-100, and stained for IgG with a FITC-conjugated secondary antibody (goat anti–rabbit IgG; Sigma-Aldrich) and for actin with TRITC-phalloidin (Molecular Probes). A minimum of four separate experiments were completed for each injected protein.
Stimulation of fluid uptake
Serum starved (2 h) HMDM were stimulated with either anti-PSR, anti-CD36 IgM (PharMingen), 100 μM liposomes containing PC or a 50:50 molar ratio of PS/PC (prepared as described above), or 3,000 U/ml M-CSF (Upstate Biotechnology) in serum-free culture media for 20 min. Ingested fluid was labeled with 1.5 mg/ml LY (Molecular Probes), which was added to the culture media for the last 2 min of the incubation. Cells were washed in PBS and fixed in PBS containing 4% paraformaldehyde. Nuclei were stained with DAPI (CalBiochem), and permeabilized cells were stained for actin with TRITC-phalloidin.
Quantitative macropinocytosis
Pinocytosis of the fluorescent dye, Cascade blue (Molecular Probes), was conducted with slight modifications of reported procedures (Racoosin and Swanson, 1989, 1992; Swanson, 1989). In brief, MPM were plated in 24-well plates, and serum starved (2 h) in DME, 0.2% FCS. Before the experiments were initiated, equivalent cell numbers between individual wells were confirmed by the addition 5% WST-1 reagent (Roche Molecular Biochemicals) in macrophage culture media for 1 h during serum starvation. Supernatants were read at 450 nm in an ELISA plate reader. Using this method, relative cell number between untreated and stimulated cells was found to be consistent throughout the 3-h time course. Cells were washed in PBS and stimulated with M-CSF (3,000 U/ml), mAb 217 (100 μg/ml), or IgM isotype control (10 μg/ml; PharMingen) in PBS containing 0.2% FCS in the presence of 0.5 mg/ml Cascade blue. To terminate dye uptake, the media was aspirated and plates were washed by immersion in 1.0 liter ice cold PBS (three times, 5 min per wash). Cells were subsequently lysed with 300 μl of 0.1% Triton X-100 (Bio-Rad Laboratories) in distilled H2O. 100 μl of lysate was diluted into 2.9 ml ultrapure distilled H2O and read in a SLM 8000 fluorimeter (Spectronic Analytical Instruments) at 400 nm excitation, 440 nm emission.
Acknowledgments
We thank T. Potter for providing mouse mAb 28-14-8, F.W. Hoffmann (National Jewish Medical Center) for providing assistance with tissue culture issues, and Jay Weskott for assistance with the TGF-β ELISAs.
This work was funded by grants from the National Institutes of Health (GM61031 and GM60449).
Submitted: 16 August 2001 Revised: 27 September 2001 Accepted: 27 September 2001
P.R. Hoffmann and A.M. deCathelineau contributed equally to this work.
Footnotes
Abbreviations used in this paper: HMDM, human monocyte–derived macrophage; LY, Lucifer yellow; M-CSF, macrophage colony–stimulating factor; MHC, major histocompatibility complex; MPM, murine thioglycolate–elicited peritoneal macrophages; PC, phosphatidylcholine; POPC, 1-palmitoyl-2-oleoyl-sn-3-glycerphosphorylcholine; POP-D-S, 1-palmitoyl-2-oleoyl-sn-3-glycerphospho-d-serine; POP-L-S, 1-palmitoyl-2-oleoyl-sn-3-glycerphospho-l-serine; PS, phosphatidylserine; PSR, phosphatidylserine receptor; SRA, scavenger receptor A; VnR, vitronectin receptor (αvβ3).
References
- Albert, M.L., S.F. Pearce, L.M. Francisco, B. Sauter, P. Roy, R.L. Silverstein, and N. Bhardwaj. 1998. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188:1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert, M.L., J.I. Kim, and R.B. Birge. 2000. alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat. Cell Biol. 2:899–905. [DOI] [PubMed] [Google Scholar]
- Balasubramanian, K., and A.J. Schroit. 1998. Characterization of phosphatidylserine-dependent beta2-glycoprotein I macrophage interactions. Implications for apoptotic cell clearance by phagocytes. J. Biol. Chem. 273:29272–29277. [DOI] [PubMed] [Google Scholar]
- Balasubramanian, K., J. Chandra, and A.J. Schroit. 1997. Immune clearance of phosphatidylserine-expressing cells by phagocytes. The role of beta2-glycoprotein I in macrophage recognition. J. Biol. Chem. 272:31113–31117. [DOI] [PubMed] [Google Scholar]
- Bretscher, M.S., and C. Aguado-Velasco. 1998. Membrane traffic during cell locomotion. Curr. Opin. Cell Biol. 10:537–541. [DOI] [PubMed] [Google Scholar]
- Brunk, U., J. Schellens, and B. Westermark. 1976. Influence of epidermal growth factor (EGF) on ruffling activity, pinocytosis and proliferation of cultivated human glia cells. Exp. Cell Res. 103:295–302. [DOI] [PubMed] [Google Scholar]
- Chen, L.M., S. Hobbie, and J.E. Galan. 1996. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science. 274:2115–2118. [DOI] [PubMed] [Google Scholar]
- Cocco, R.E., and D.S. Ucker. 2001. Distinct modes of macrophage recognition for apoptotic and neurotic cells are not specified exclusively by phosphatidylserine exposure. Mol. Biol. Cell. 12:919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, S., T.L. Gumienny, M.O. Hengartner, and M. Driscoll. 2000. A common set of engulfment genes mediates removal of both apoptotic and necrotic cell corpses in C. elegans. Nat. Cell Biol. 2:931–937. [DOI] [PubMed] [Google Scholar]
- Comfurius, P., and R.F. Zwaal. 1977. The enzymatic synthesis of phosphatidylserine and purification by CM-cellulose column chromatography. Biochim. Biophys. Acta. 467:146–164. [DOI] [PubMed] [Google Scholar]
- Devitt, A., O.D. Moffatt, C. Raykundalia, J.D. Capra, D.L. Simmons, and C.D. Gregory. 1998. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 392:505–509. [DOI] [PubMed] [Google Scholar]
- Dowrick, P., P. Kenworthy, B. McCann, and R. Warn. 1993. Circular ruffle formation and closure lead to macropinocytosis in hepatocyte growth factor/scatter factor-treated cells. Eur. J. Cell Biol. 61:44–53. [PubMed] [Google Scholar]
- Duvall, E., A.H. Wyllie, and R.G. Morris. 1985. Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunology. 56:351–358. [PMC free article] [PubMed] [Google Scholar]
- Fadok, V.A., J.S. Savill, C. Haslett, D.L. Bratton, D.E. Doherty, P.A. Campbell, and P.M. Henson. 1992. a. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J. Immunol. 149:4029–4035. [PubMed] [Google Scholar]
- Fadok, V.A., D.R. Voelker, P.A. Campbell, J.J. Cohen, D.L. Bratton, and P.M. Henson. 1992. b. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148:2207–2216. [PubMed] [Google Scholar]
- Fadok, V.A., D.L. Bratton, A. Konowal, P.W. Freed, J.Y. Westcott, and P.M. Henson. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 101:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok, V.A., D.L. Bratton, D.M. Rose, A. Pearson, R.A. Ezekewitz, and P.M. Henson. 2000. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 405:85–90. [DOI] [PubMed] [Google Scholar]
- Fadok, V.A., A. de Cathelineau, D.L. Daleke, P.M. Henson, and D.L. Bratton. 2001. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 276:1071–1077. [DOI] [PubMed] [Google Scholar]
- Flora, P.K., and C.D. Gregory. 1994. Recognition of apoptotic cells by human macrophages: inhibition by a monocyte/macrophage-specific monoclonal antibody. Eur. J. Immunol. 24:2625–2632. [DOI] [PubMed] [Google Scholar]
- Francis, C.L., T.A. Ryan, B.D. Jones, S.J. Smith, and S. Falkow. 1993. Ruffles induced by Salmonella and other stimuli direct macrophinocytosis of bacteria. Nature. 364:639–642. [DOI] [PubMed] [Google Scholar]
- Galan, J.E., J. Pace, and M.J. Hayman. 1992. Involvement of the epidermal growth factor receptor in the invasion of cultured mammalian cells by Salmonella typhimurium. Nature. 357:588–589. [DOI] [PubMed] [Google Scholar]
- Garrett, W.S., L.M. Chen, R. Kroschewski, M. Ebersold, S. Turley, S. Trombetta, J.E. Galan, and I. Mellman. 2000. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 102:325–334. [DOI] [PubMed] [Google Scholar]
- Gigli, I., and R.A. Nelson, Jr. 1968. Complement dependent immune phagocytosis. I. Requirements for C'1, C'4, C'2, C'3. Exp. Cell Res. 51:45–67. [DOI] [PubMed] [Google Scholar]
- Gumienny, T.L., E. Lambie, E. Hartwieg, H.R. Horvitz, and M.O. Hengartner. 1999. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development. 126:1011–1022. [DOI] [PubMed] [Google Scholar]
- Hamon, Y., C. Broccardo, O. Chambenoit, M.F. Luciani, F. Toti, S. Chaslin, J.M. Freyssinet, P.F. Devaux, J. McNeish, D. Marguet, and G. Chimini. 2000. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat. Cell Biol. 2:399–406. [DOI] [PubMed] [Google Scholar]
- Hewlett, L.J., A.R. Prescott, and C. Watts. 1994. The coated pit and macropinocytic pathways serve distinct endosome populations. J. Cell Biol. 124:689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homburg, C.H., M. de Haas, A.E. von dem Borne, A.J. Verhoeven, C.P. Reutelingsperger, and D. Roos. 1995. Human neutrophils lose their surface Fc gamma RIII and acquire Annexin V binding sites during apoptosis in vitro. Blood. 85:532–540. [PubMed] [Google Scholar]
- Ishimoto, Y., K. Ohashi, K. Mizuno, and T. Nakano. 2000. Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J. Biochem. 127:411–417. [DOI] [PubMed] [Google Scholar]
- Juneja, L.R., T. Kazuoka, N. Goto, T. Yamane, and S. Shimizu. 1989. Conversion of phosphatidylcholine to phosphatidylserine by various phospholipases D in the presense of L- or D-serine. Biochim. Biophys. Acta. 1003:227–283. [Google Scholar]
- Koopman, G., C.P. Reutelingsperger, G.A. Kuijten, R.M. Keehnen, S.T. Pals, and M.H. van Oers. 1994. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 84:1415–1420. [PubMed] [Google Scholar]
- Kulkarni, A.B., C.G. Huh, D. Becker, A. Geiser, M. Lyght, K.C. Flanders, A.B. Roberts, M.B. Sporn, J.M. Ward, and S. Karlsson. 1993. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA. 90:770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverrier, Y., and A.J. Ridley. 2001. Requirement for Rho GTPases and PI 3-kinases during apoptotic cell phagocytosis by macrophages. Curr. Biol. 11:195–199. [DOI] [PubMed] [Google Scholar]
- Li, G., C. D'Souza-Schorey, M.A. Barbieri, J.A. Cooper, and P.D. Stahl. 1997. Uncoupling of membrane ruffling and pinocytosis during Ras signal transduction. J. Biol. Chem. 272:10337–10340. [PubMed] [Google Scholar]
- Licht, R., C.W. Jacobs, W.J. Tax, and J.H. Berden. 1999. An assay for the quantitative measurement of in vitro phagocytosis of early apoptotic thymocytes by murine resident peritoneal macrophages. J. Immunol. Methods. 223:237–248. [DOI] [PubMed] [Google Scholar]
- Liu, Q.A., and M.O. Hengartner. 1999. The molecular mechanism of programmed cell death in C. elegans. Ann. NY Acad. Sci. 887:92–104. [DOI] [PubMed] [Google Scholar]
- Martin, S.J., C.P. Reutelingsperger, A.J. McGahon, J.A. Rader, R.C. van Schie, D.M. LaFace, and D.R. Green. 1995. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 182:1545–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazo, M.D., L. Daviet, J. Savill, Y. Ren, L.L. Leung, and J.L. McGregor. 1996. Identification of a domain (155-183) on CD36 implicated in the phagocytosis of apoptotic neutrophils. J. Biol. Chem. 271:15381–15385. [DOI] [PubMed] [Google Scholar]
- Ogden, C.A., A. deCathelineau, P.R. Hoffmann, D. Bratton, B. Ghebrehiwet, V.A. Fadok, and P.M. Henson. 2001. C1q and mannose binding lectin (MBL) engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 194:781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka, K., T. Sawamura, K. Kikuta, S. Itokawa, N. Kume, T. Kita, and T. Masaki. 1998. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc. Natl. Acad. Sci. USA. 95:9535–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnaik, R., M.C. Raff, and J. Scholes. 2000. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr. Biol. 10:857–860. [DOI] [PubMed] [Google Scholar]
- Pearson, A.M. 1996. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 8:20–28. [DOI] [PubMed] [Google Scholar]
- Platt, N., H. Suzuki, Y. Kurihara, T. Kodama, and S. Gordon. 1996. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc. Natl. Acad. Sci. USA. 93:12456–12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan, D., S. Krahling, P. Williamson, and R.A. Schlegel. 1997. Multiple systems for recognition of apoptotic lymphocytes by macrophages. Mol. Biol. Cell. 8:767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela, R., M.D. Pierschbacher, and E. Ruoslahti. 1985. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc. Natl. Acad. Sci. USA. 82:5766–5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racoosin, E.L., and J.A. Swanson. 1989. Macrophage colony-stimulating factor (rM-CSF) stimulates pinocytosis in bone marrow-derived macrophages. J. Exp. Med. 170:1635–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racoosin, E.L., and J.A. Swanson. 1992. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J. Cell Sci. 102:867–880. [DOI] [PubMed] [Google Scholar]
- Ramprasad, M.P., W. Fischer, J.L. Witztum, G.R. Sambrano, O. Quehenberger, and D. Steinberg. 1995. The 94- to 97-kDa mouse macrophage membrane protein that recognizes oxidized low density lipoprotein and phosphatidylserine-rich liposomes is identical to macrosialin, the mouse homologue of human CD68. Proc. Natl. Acad. Sci. USA. 92:9580–9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, Y., and J. Savill. 1998. Apoptosis: the importance of being eaten. Cell Death Differ. 5:563–568. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., and A. Hall. 1992. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 70:389–399. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., H.F. Paterson, C.L. Johnston, D. Diekmann, and A. Hall. 1992. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 70:401–410. [DOI] [PubMed] [Google Scholar]
- Rigotti, A., S.L. Acton, and M. Krieger. 1995. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J. Biol. Chem. 270:16221–16224. [DOI] [PubMed] [Google Scholar]
- Sambrano, G.R., and D. Steinberg. 1995. Recognition of oxidatively damaged and apoptotic cells by an oxidized low density lipoprotein receptor on mouse peritoneal macrophages: role of membrane phosphatidylserine. Proc. Natl. Acad. Sci. USA. 92:1396–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrano, G.R., V. Terpstra, and D. Steinberg. 1997. Independent mechanisms for macrophage binding and macrophage phagocytosis of damaged erythrocytes. Evidence of receptor cooperativity. Arterioscler. Thromb. Vasc. Biol. 17:3442–3448. [DOI] [PubMed] [Google Scholar]
- Sauter, B., M.L. Albert, L. Francisco, M. Larsson, S. Somersan, and N. Bhardwaj. 2000. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191:423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill, J. 1998. Apoptosis. Phagocytic docking without shocking. Nature. 392:442–443. [DOI] [PubMed] [Google Scholar]
- Savill, J., and V. Fadok. 2000. Corpse clearance defines the meaning of cell death. Nature. 407:784–788. [DOI] [PubMed] [Google Scholar]
- Savill, J., N. Hogg, Y. Ren, and C. Haslett. 1992. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Invest. 90:1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill, J., V. Fadok, P. Henson, and C. Haslett. 1993. Phagocyte recognition of cells undergoing apoptosis. Immunol. Today. 14:131–136. [DOI] [PubMed] [Google Scholar]
- Schagat, T.L., J.A. Wofford, and J.R. Wright. 2001. Surfactant protein A enhances alveolar macrophage phagocytosis of apoptotic neutrophils. J. Immunol. 166:2727–2733. [DOI] [PubMed] [Google Scholar]
- Schlegel, R.A., M. Stevens, K. Lumley-Sapanski, and P. Williamson. 1993. Altered lipid packing identifies apoptotic thymocytes. Immunol. Lett. 36:283–288. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi, A., Y. Kawasaki, M. Ikemoto, H. Arai, and Y. Nakanishi. 1999. Role of class B scavenger receptor type I in phagocytosis of apoptotic rat spermatogenic cells by Sertoli cells. J. Biol. Chem. 274:5901–5908. [DOI] [PubMed] [Google Scholar]
- Shull, M.M., I. Ormsby, A.B. Kier, S. Pawlowski, R.J. Diebold, M. Yin, R. Allen, C. Sidman, G. Proetzel, D. Calvin, et al. 1992. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 359:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, J.A. 1989. Phorbol esters stimulate macropinocytosis and solute flow through macrophages. J. Cell Sci. 94:135–142. [DOI] [PubMed] [Google Scholar]
- Swanson, S.J., and C. Watts. 1995. Macropinocytosis. Trends Cell Biol. 5:424–428. [DOI] [PubMed] [Google Scholar]
- Taylor, P.R., A. Carugati, V.A. Fadok, H.T. Cook, M. Andrews, M.C. Carroll, J.S. Savill, P.M. Henson, M. Botto, and M.J. Walport. 2000. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J. Exp. Med. 192:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Eijnde, S. 1998. Phosphatidylserine exposure by apoptoic cells is phylogenetically conserved. Apoptosis. 3:9–16. [DOI] [PubMed] [Google Scholar]
- Verhoven, B., S. Krahling, R.A. Schlegel, and P. Williamson. 1999. Regulation of phosphatidylserine exposure and phagocytosis of apoptotic T lymphocytes. Cell Death Differ. 6:262–270. [DOI] [PubMed] [Google Scholar]
- Vermes, I., C. Haanen, H. Steffens-Nakken, and C. Reutelingsperger. 1995. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods. 184:39–51. [DOI] [PubMed] [Google Scholar]
- West, M.A., M.S. Bretscher, and C. Watts. 1989. Distinct endocytotic pathways in epidermal growth factor–stimulated human carcinoma A431 cells. J. Cell Biol. 109:2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie, A.H., J.F. Kerr, and A.R. Currie. 1980. Cell death: the significance of apoptosis. Int. Rev. Cytol. 68:251–306. [DOI] [PubMed] [Google Scholar]
- Zhou, Z., E. Hartwieg, and H.R. Horvitz. 2001. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell. 104:43–56. [DOI] [PubMed] [Google Scholar]