Abstract
A prospective and controlled study of training after surgery for lumbar disc herniation (LDH). The objective was to determine the effect of early neuromuscular customized training after LDH surgery. No consensus exists on the type and timing of physical rehabilitation after LDH surgery. Patients aged 15–50 years, disc prolapse at L4–L5 or L5–S1. Before surgery, at 6 weeks, 4, and 12 months postoperatively, the following evaluations were performed: low back pain and leg pain estimated on a visual analog scale, disability according to the Roland–Morris questionnaire (RMQ) and disability rating index (DRI). Clinical examination, including the SLR test, was performed using a single blind method. Consumption of analgesics was registered. Twenty-five patients started neuromuscular customized training 2 weeks after surgery (early training group=ETG). Thirty-one patients formed a control group (CG) and started traditional training after 6 weeks. There was no significant difference in pain and disability between the two training groups before surgery. Median preoperative leg pain was 63 mm in ETG and 70 mm in the CG. Preoperative median disability according to RMQ was 14 in the ETG and 14.5 in the CG. Disability according to DRI (33/56 patients) was 5.3 in the ETG vs. 4.6 in the CG. At 6 weeks, 4 months, and 12 months, pain was significantly reduced in both groups, to the same extent. Disability scores were lower in the ETG at all follow-ups, and after 12 months, the difference was significant (RMQ P=.034, DRI P=.015). The results of the present study show early neuromuscular customized training to have a superior effect on disability, with a significant difference compared to traditional training at a follow-up 12 months after surgery. No adverse effects of the early training were seen. A prospective, randomized study with a larger patient sample is warranted to ultimately demonstrate that early training as described is beneficial for patients undergoing LDH surgery.
Keywords: Lumbar disc herniation, Neuromuscular training, Neutral position, Surgery
Introduction
Among patients scheduled for surgery for lumbar disc herniation (LDH), 50% show reduced muscle strength and endurance corresponding to the afflicted nerve root [15, 18, 36]. This impairment can remain after surgical decompression of the nerve root and reduction of pain [9, 33]. Proximal neuromuscular dysfunction seems to have a prognostic value as regards residual sciatic pain and disability [22]. Clinical segmental instability can occur after first lumbar disc surgery and correlates with unsatisfactory long-term outcome [19].
Previous studies of rehabilitation after a first LDH surgery have demonstrated that early [17] and intense training [2, 4, 6, 21] have a positive effect on pain, disability, and lumbar mobility. No postoperative training program has yet proven superior long-term results [24]. No consensus exists on the type and timing of physical rehabilitation after LDH.
Specific stabilizing exercise programs for patients with first episodes of acute low back pain and chronic low back pain patients with spondylosis or spondylolisthesis have a proven long-term effect on pain and disability [10, 25]. This has been interpreted as a result of change in motor program, an automatic feed-forward recruitment of the deep core muscles [12].
The purpose of this study was to determine the effect of early neuromuscular customized training after lumbar disc surgery.
Materials and methods
The inclusion criteria for this study specified patients aged 15–50 years, scheduled to undergo surgery for a symptomatic, MRI-verified disc prolapse at L4–L5 or L5–S1. Patients with a previous spine operation or any other spinal, rheumatological, or neurological disease or lower extremity dysfunction emanating from musculoskeletal disorder, other than the current LDH, were excluded. All patients received verbal and written information about the study and gave informed consent. The patients were allocated to an early training group (ETG) and a control group (CG), based on geographic habitat (see below).
Altogether, 69 patients with MRI-documented LDH managed surgically by open or microscopic technique during a 3-year period were included in the study. All patients had previously undergone nonoperative therapy without pain-relieving effect. The patients were examined the day before surgery and at out-patient visits to the orthopedic department 6 weeks, 4 months, and 12 months after surgery. Follow-up was conducted according to the Swedish National Register of Lumbar Spine Surgery [35]. The study was approved by the local ethics committee.
Of the 69 patients, 13 did not complete the study. Six of these patients belonged to the training group (two men and four women) and 7 patients to the CG (two men and five women). Two patients in the ETG had repeat surgery, two patients moved to another part of the country after the second control, and two patients refused participation before starting the training. In the CG, one patient had repeat surgery, one patient moved to another part of the country after the second check-up, and five patients could not be motivated for longer-term follow-up evaluation and only attended the first and second check-ups. The demographics and preoperative data of these patients did not differ from the data for those completing the study. The proportion of white- and blue-collar workers was equal.
Thus, 56 patients (20 women and 36 men) participated in the investigation. Their mean age was 38 (range 23–50, median 38 years). Forty patients had a lumbosacral herniation and 16 patients had L4–L5 herniation. Leg pain and low back pain were estimated by the patient on a 100-mm visual analog scale (VAS) [3, 13, 38, 39]. Function in daily activities was measured by the Roland–Morris disability questionnaire [14, 29] and the disability rating index (DRI) [31]. The Roland–Morris questionnaire (RMQ) is a disability measure with a 24-point scale ranging from 0 (the best estimation) to 24 (the worst estimation). DRI is an instrument for assessment of physical function, primarily designed for patients with low back pain. It consists of 12 questions on disability, all graded 0–10 on a VAS, and the mean score is used. Due to a change in routines, preoperative DRI was only given to and completed by the first 33 patients (15/25 in the ETG and 18/31 in the CG) admitted to the study. The VAS, RMQ, and DRI were administered by a secretary. The questionnaires were completed by the patients (after verbal instructions) and returned in sealed envelopes. The envelopes were not opened until after completion of the study. The completeness of data was satisfactory (Table 1).
Table 1.
Preoperative base-line data
| Total (n=56) | ETG (n=25) | CG (n=31) | |
|---|---|---|---|
| Age (mean=median) | 38 | 37 | 39 |
| Sex | |||
| Females | 20 | 6 | 14 |
| Males | 36 | 19 | 17 |
| Occupation | |||
| Sedentary | 23 | 9 | 14 |
| Intermediate | 22 | 10 | 12 |
| Heavy | 11 | 6 | 5 |
| Analgesics | |||
| Regular use | 30 | 14 | 16 |
| Sporadic | 14 | 6 | 8 |
| No | 12 | 5 | 7 |
| VAS leg pain (median) | 70 | 63 | 70 |
| VAS back pain (median) | 30 | 30 | 30 |
| Roland–Morris (median) | 14 | 14 | 14.5 |
| DRI (median) | 4.8 (n=33) | 5.3 (n=15) | 4.6 (n=18) |
No significant difference in pain, disability, consumption of analgesics, age, or type of occupation. The ETG consisted of fewer females than the CG; the number of males was equivalent
Clinical examination of neurological signs, lumbar mobility, and muscle function was performed by a physiotherapist blinded to which group of treatment each patient belonged and all anamnesic and clinical data. The straight leg raising (SLR) test at each investigation was performed with the patient in supine position and no dorsiflexion of the ankle, using an inclinometer for precise data and raising to a maximum of 90°. For a positive SLR test, only pain radiating to the leg, identical to the preoperative leg pain, was accepted. Comparison with SLR test of the contra-lateral leg was performed, and back pain was disregarded. Lumbar flexion was determined by the modified Schober technique.
On discharge from the hospital, all patients were instructed to gradually return to normal life, but to avoid loaded lumbar flexion and prolonged sitting in low chairs during the first 6 weeks. The patients were divided into an ETG and a CG. At our university hospital, patients from a vast area are operated on. As long car rides should be avoided in the first weeks after LDH surgery, randomization was considered risky. Instead, a pragmatic grouping method was used, with the municipality of the patient determining group affiliation. Patients belonging to the local municipality were placed in the ETG, meaning a transportation of maximum 15 km. All patients from other municipalities formed the CG.
Twenty-five patients (6 women and 19 men, mean age 37 and range 23–50 years) started a neuromuscular customized stabilizing exercise program at one clinic 2 weeks after surgery. The early neuromuscular customized training model used in our study is based on data of the biomechanics of the lumbar spine and motor control in healthy subjects and patients with lumbar or extremity joint disorders. The aim was to regain normal neuromuscular control [27] of the active stabilizing system of the spine with the lumbar spine in neutral position [26, 28, 30].
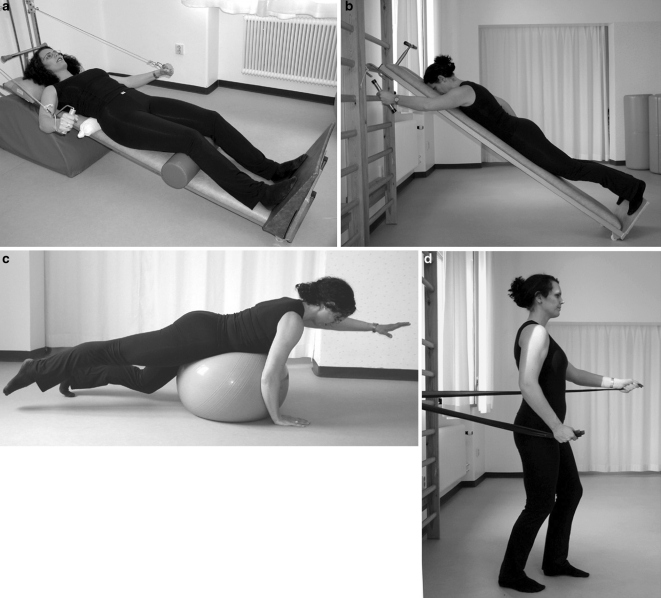
The early training program was supervised by one of two previously instructed physiotherapists and focused on feed-forward co-activation of the deep core muscles. Gravity of body weight and voluntary movements of the extremities, mostly the upper limbs, was used to produce postural reactions [1, 11, 40], without compensatory movements such as altered posture/pelvic position or unwanted extremity movements. Adequate lumbar lordosis and proportion of simultaneous ventral vs. dorsal core muscle support were obtained by positioning the columna and the head and orientation of the extremities. The training was performed with the lower extremities in closed kinetic chain [23, 34], for example, with the patient taking partial body-weight lying on a sloping board with the plantar soles against a support platform for optimal afferent input [5, 40]. Light weights and a high number of repetitions were used to automate feed-forward core muscle activation. Only training equipment with freedom of movement were used, to challenge the center of gravity (Fig. 1a–d).
Fig. 1.
a–d Examples of neuromuscular training with the lumbar spine in neutral position, co-contraction of the deep core muscles and closed kinetic chains
The supervised early training program was performed twice a week for 4 weeks, each training sequence lasting 40–60 min. The patients received information and instruction about individualized home exercises and regime. All 25 patients followed through in this first period. After these 4 weeks, all but two patients in the ETG continued the neuromuscular training supervised by one of three previously informed physiotherapists working at primary health care centers. The total mean number of training sessions in the ETG, starting from 2 weeks, was 22 (5–60, median 21).
Thirty-one patients (14 women and 17 men, mean age 39 and range 26–50 years) formed the CG and started traditional training 6 weeks after surgery at a physiotherapy institute of their own selection, usually a physiotherapist they had been in contact with before surgery. No special advice concerning the training was given, although commencing with trunk stabilizing exercises was recommended. The patients in the CG trained on stabilization exercises mainly using different types of stationary gym equipment and also focused on coordination and mobility. Four patients in the CG did not contact a physiotherapist after surgery. For those who did, the mean number of postoperative training sessions was 26 (1–70, median 17); thus, utilization of physiotherapy resources was similar in the two groups. No group changes occurred.
Statistical analysis
The recorded results of the examinations and coded patient information were registered in the Stat View II program on a Macintosh computer. For the current study, only outcome for pain, disability, consumption of analgesics, lumbar flexion, and SLR test are reported. For calculation of differences between the two groups in leg pain, low back pain, and disability according to the RMQ and the DRI at different times, a nonparametric method, the Mann–Whitney U-test, was used. Fisher’s exact test was used to calculate the difference in level of leg pain and occurrence of positive SLR test 12 months after surgery. A level of P<.05 was chosen for statistical significance.
Results
Preoperative data
There was no significant difference in pain and disability between the two training groups before surgery (Table 1). The incidence of former problems with low back pain was equivalent in the two groups, as was the incidence of preoperatively positive SLR test and active lumbar flexion (Schober) in standing position. Preoperative consumption of analgesics was similar in both groups, with 30 of 56 patients using analgesics regularly. Five patients in the ETG and seven patients in the CG used no analgesics before surgery (Table 1). Female subjects had more preoperative leg pain (P=.036) and higher disability according to the RMQ (P=.024) than male subjects. There were no gender differences for low back pain or disability according to the DRI.
Postoperative data
Pain
Leg pain and low back pain decreased rapidly after surgery and this improvement persisted over time but with a wide range of VAS scores. At the 6-week and 4-month assessments, leg pain and low back pain were significantly reduced in both groups to the same extent (Table 2).
Table 2.
Median, interquartile range, and mean values for leg pain, low back pain, RMQ, and DRI
| Preoperative | 6 Weeks | 4 Months | 12 Months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Med | Iqr | Mean | Med | Iqr | Mean | Med | Iqr | Mean | Med | Iqr | Mean | ||
| Leg pain | ETG | 63 | 50 | 54 | 5 | 11 | 10 | 3 | 15 | 13 | 1* | 9 | 5 |
| CG | 70 | 34 | 64 | 8 | 32 | 19 | 2 | 18 | 13 | 9 | 24 | 16 | |
| LBP | ETG | 30 | 62 | 34 | 7 | 12 | 10 | 2 | 11 | 10 | 1 | 10 | 10 |
| CG | 30 | 48 | 37 | 7 | 17 | 12 | 3 | 26 | 15 | 2 | 22 | 14 | |
| RMQ | ETG | 14 | 6 | 14 | 8 | 9 | 7.8 | 1 | 8.5 | 4.2 | 1* | 2.5 | 2.5 |
| CG | 14.5 | 9 | 14.6 | 8 | 9 | 9.1 | 4.5 | 8.5 | 5.1 | 4 | 8 | 5.3 | |
| DRI | ETG | 5.3 | 3.1 | 5.3 n=15 | 5.1 | 2.2 | 4.7 | 2.3 | 3.3 | 2.8 | 0.6* | 2.5 | 1.5 |
| CG | 4.6 | 4.3 | 5.5 n=18 | 5.3 | 2.8 | 5.1 | 3.2 | 3.6 | 3.0 | 2.1 | 3.1 | 2.5 | |
Preoperative DRI n=33/56 patients (ETG 15/25 and CG 18/31). Significant differences between the two groups occurred only at 12 months and have been marked with “*”. Leg pain was reduced in both groups, to the same extent
A majority of the patients had satisfactory results from the disc surgery. After 12 months, only 1/25 patients in the ETG estimated leg pain exceeding 30 mm on the VAS scale, compared with 7/31 patients in the CG (P=.063). The female patients, having more leg pain before surgery than the males, reported a postoperative level of leg pain equal to that of the male patients.
As regards low back pain, no difference in mean value between the two training groups was seen at any time during follow-up (Table 2).
Disability
The RMQ and the DRI did not decrease as rapidly after surgery as the VAS leg pain and VAS low back pain but continued to decrease during the whole follow-up period. Disability according to the RMQ reduced more in the ETG than in the CG; the absolute change in median value from before surgery was 13 in the ETG compared to 10.5 in the CG, and showed a significant difference of P=.034 between the groups at 12 months (Table 2).
The same pattern was seen concerning DRI but with a time delay. The DRI for the whole study group showed no difference between the preoperative (n=33/56) and the first postoperative assessment. No difference was seen between the two groups before surgery (n=33/56) (P=.827) or at the two first postoperative controls. At the 4-month control, the physical function had improved in both groups compared to preoperative conditions. Further improvement was seen at the 12-month control, mainly in the ETG, with an absolute median change from before surgery of 4.7 compared with 2.5 in the CG, thus yielding a significant difference between the two groups (P=.015) (Table 2). There were no significant gender differences in disability after surgery.
Analgesics
Consumption of analgesics was reduced after surgery. Four months after surgery, only four of the 56 patients, two in each group, used analgesics regularly. After 12 months, no patient in the ETG but four patients in the CG reported regular consumption (nonsignificant difference). The sporadic consumption was equal.
Clinical signs
The SLR test was positive among most patients before surgery (ETG 23/25, CG 28/30) but rapidly disappeared later. At the 12-month control, no patient in the ETG but four patients in the CG had a positive SLR test (nonsignificant difference). Lumbar flexion measured with the modified Schober technique increased gradually during the postoperative period. No difference in mobility between the two groups was noted.
There was no significant difference between the groups in the proportion of patients who were fully employed 1 year after surgery, 21/24 (84%) in the training group and 25/31 (80%) in the CG. Four patients in the ETG and six patients in the CG were on partial or full-time sick-leave. No adverse effects of the early training were seen.
Discussion
Several studies on training models after disc surgery have been presented over the years. The content and results have varied. Many have demonstrated good short-term effect [8, 37] but few have proven long-lasting results as in the present study. Current data about the biomechanics of the lumbar spine support postoperative muscle rehabilitation after LDH surgery [10, 12, 20]. No study with a pronounced focus on early neuromuscular customized training for stabilization in neutral position and closed kinetic chain exists to our knowledge.
Study design
The early training started 2 weeks after surgery. This point of time was chosen in order not to interfere with monitoring for postoperative complications. The early training at our institution lasted only 4 weeks; a prolonged training period might have improved the results. A third group of patients, with no training at all, would have provided more knowledge on the subject. This model was difficult to include as postoperative training after disc surgery has been a standard procedure at most institutions, including our hospital, for many years.
Selection of patients
Ideally, this study would be randomized but for logistic reasons this was not feasible. Long-distance transport of recently operated patients would be unsuitable for medical and financial reasons, and might elicit pain. The early onset was considered to be a possible risk and the training method was comparatively new. We wanted all patients in the ETG to be handled by one of two physiotherapists at the same institution, in order to secure equivalent instructions and training within the group.
Pain and disability
Leg pain is the main indication for LDH surgery. In this study, the long-term outcome for the majority of the patients was very good, with the exception of three outliers. There were no specific preoperative features for these patients compared to the remaining study group.
The CG had proportionately more females than the ETG, which might explain the nonsignificant difference in preoperative leg pain between the two training groups. The female subjects had more preoperative leg pain than the males, but after surgery, the difference was no longer present.
A contributory cause of the lower level of preoperative leg pain in the ETG was one patient who was operated on mainly because of disability due to major distal motor loss but had insignificant pain. In a future study, a lowest rate of pain might be stipulated.
One year after surgery, only one patient in the ETG had leg pain exceeding 30 mm on the VAS. At this time, no patient in the ETG showed a positive SLR test or reported regular use of analgesics. With a larger patient sample, the differences between the two groups might have been significant. Postoperative SLR test correlates strongly with clinical outcome, including consumption of analgesics [16].
In the present study, the differences in disability, leg pain at rest, radiating pain at SLR test, and consumption of analgesics arise between 4 and 12 months. It is noteworthy that the reduction of disability exceeds that of pain when the two groups are compared. Whether the beneficial outcome at 12 months is an effect of the early training, or the program as such, cannot be deduced.
Disability outcome scores
The mean age for LDH is around 40 years [32, 35] and most patients with LDH are physically active, at work or in their leisure time. The RMQ is well suited for basic daily activities but might be insufficient when rating higher physical demands. At the first assessment after surgery, it was not only expected but desirable to have 2–3 points on the RMQ, as the patient was still prescribed to adjust his/her life to the recent surgery. A difference in disability score between the two groups at the 6-week assessment would be improbable.
It can also be discussed whether DRI is suitable at the 6-week assessment, as some of the questions concern activities not recommended at that time point, such as prolonged sitting, heavy lifting, and running. The fact that the two disability scores show similar results but with a time delay for the DRI indicates the benefit of recording both scores.
Due to a change in preoperative routines, unfortunately only the first 33 of the 56 patients filled in the DRI before surgery. The data for these patients concerning preoperative leg pain, low back pain, and disability according to the RMQ, however, are equivalent to those who missed filling the form. As the Roland–Morris score and the DRI show similar development through the follow-up period, we may anticipate the preoperative DRI results to correlate to the preoperative Roland–Morris scores, and the justification for including the DRI score, as mentioned above, is its reflection of higher physical strain.
Training model
Training in neutral position is thought to minimize the risk of posterior disc protrusion or relapse as lumbar flexion provokes posterior migration of the nucleus [7]. Active muscular stabilization in dynamic situations is an essential part of our training model as the lumbar spine has the least passive stiffness in neutral position [26, 27], and postoperative training should prepare the patient for return to active life with varying physical demands. The low levels on the postoperative disability scores might be a result of our training model.
No adverse effect of the early training was seen in this study. Judging by the mean number of treatment sessions, there was no increased economic burden induced by the early training. Utilization of physiotherapy resources varied more in the CG, which probably reflects the lack of homogeneity in postoperative training after surgery for LDH in our country. We thus suggest early start of stabilization training after lumbar disc surgery, to be performed with the lumbar spine in neutral position and in closed kinetic chain, aiming to regain and automatize the feed-forward recruitment of deep core muscles.
Conclusion
The results of the present study show early neuromuscular customized training to have a superior effect on disability, with a significant difference compared to traditional training at follow-up 12 months after surgery. No adverse effects of the early training were seen. A prospective, randomized study with a larger patient sample is warranted to ultimately demonstrate that early training as described is beneficial for patients undergoing LDH surgery.
Acknowledgements
The study was supported by grants from Vårdrådet, Lund University Hospital and the Anne-Marie and Ragnar Hemborg Minnesfond, Lund, Sweden. This study has been performed within the current laws of our country and was approved by the ethics committee of the Lund University Hospital, Sweden.
References
- 1.Bouisset S, Zattara M. Biomechanical study of the programming of anticipatory postural adjustments associated with voluntary movement. J Biomech. 1987;20(8):735–742. doi: 10.1016/0021-9290(87)90052-2. [DOI] [PubMed] [Google Scholar]
- 2.Brennan G, Schultz B, Hood R, et al. The effects of aerobic exercise after lumbar microdiscectomy. Spine. 1994;19:735–739. doi: 10.1097/00007632-199404000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson AM. Assessment of chronic pain: aspects of the reliability and validity of the visual analog scale. Pain. 1983;16:87–101. doi: 10.1016/0304-3959(83)90088-X. [DOI] [PubMed] [Google Scholar]
- 4.Danielsen J, Johnsen R, Kibsgaard S. Early, aggressive exercise for postoperative rehabilitation after discectomy. Spine. 2000;25(8):1015–1020. doi: 10.1097/00007632-200004150-00017. [DOI] [PubMed] [Google Scholar]
- 5.Dietz V, Duysens J. Significance of load receptor input during locomotion: a review. Gait Posture. 2000;11(2):102–110. doi: 10.1016/S0966-6362(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 6.Dolan P, Greenfield K, Nelson R. Can exercise therapy improve the outcome of microdiscectomy? Spine. 2000;25(12):1523–1532. doi: 10.1097/00007632-200006150-00011. [DOI] [PubMed] [Google Scholar]
- 7.Fennel A, Jones A, Hukins D. Migration of the nucleus pulposus within the intervertebral disc during flexion and extension of the spine. Spine. 1996;21:2753–2757. doi: 10.1097/00007632-199612010-00009. [DOI] [PubMed] [Google Scholar]
- 8.Filiz M, Cakmak A, Ozcan E. The effectiveness of exercise programmes after lumbar disc surgery: a randomized controlled study. Clin Rehabil. 2005;19(1):4–11. doi: 10.1191/0269215505cr836oa. [DOI] [PubMed] [Google Scholar]
- 9.Frymoyer JW, Hanley E, Howe, et al. Disc excision and spine fusion in the management of lumbar disc disease. Spine. 1978;3(1):1–6. doi: 10.1097/00007632-197803000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Hides J, Jull G, Richardson C. Long-term effects of specific stabilizing exercises for first-episode low back pain. Spine. 2001;26(11):E243–E248. doi: 10.1097/00007632-200106010-00004. [DOI] [PubMed] [Google Scholar]
- 11.Hodges PW, Richardson CA. Feedforward contraction of transversus abdominis is not influenced by the direction of arm movement. Exp Brain Res. 1997;114(2):362–370. doi: 10.1007/PL00005644. [DOI] [PubMed] [Google Scholar]
- 12.Hodges PW, Moseley GL. Pain and motor control of the lumbopelvic region: the effect and possible mechanisms. J Electromyogr Kinesiol. 2003;13(4):361–370. doi: 10.1016/S1050-6411(03)00042-7. [DOI] [PubMed] [Google Scholar]
- 13.Huskisson EC. Measurement of pain. Lancet. 1974;2(7889):1127–1131. doi: 10.1016/S0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 14.Johansson E, Lindberg P. Subacute and chronic low back pain: reliability and validity of a Swedish version of the Roland and Morris disability questionnaire. Scand J Rehabil Med. 1998;30:139–143. doi: 10.1080/003655098444066. [DOI] [PubMed] [Google Scholar]
- 15.Junge A, Dvorak J, Ahrens St. Predictors of bad and good outcomes of lumbar disc surgery: a prospective clinical study with recommendations for screening to avoid bad outcomes. Spine. 1995;20:460–468. doi: 10.1097/00007632-199502001-00009. [DOI] [PubMed] [Google Scholar]
- 16.Jönsson B, Strömqvist B. The straight leg raising test and the severity of symptoms in lumbar disc herniation. Spine. 1995;20(1):27–30. doi: 10.1097/00007632-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Kjellby-Wendt G, Styf J. Early active training after lumbar discectomy; a prospective, randomized and controlled study. Spine. 1998;23:2345–2351. doi: 10.1097/00007632-199811010-00019. [DOI] [PubMed] [Google Scholar]
- 18.Kortelainen P, Puranen J, Koivisto, et al. Symptoms and signs of sciatica and their relation to the location of the lumbar disc herniation. Spine. 1985;10:88–92. doi: 10.1097/00007632-198501000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Kotilainen E, Valtonen S. Clinical instability of the lumbar spine after microdiscectomy. Acta Neurochir (Wien) 1993;125(1–4):120–126. doi: 10.1007/BF01401838. [DOI] [PubMed] [Google Scholar]
- 20.Leinonen V, Kankaanpaa M, Luukkonen M, et al. Lumbar paraspinal muscle function, perception of lumbar position, and postural control in disc herniation-related back pain. Spine. 2003;28(8):842–848. doi: 10.1097/00007632-200304150-00019. [DOI] [PubMed] [Google Scholar]
- 21.Manniche C, Skall HF, Braendholt L, et al. Clinical trial of postoperative dynamic back exercises after first lumbar discectomy. Spine. 1993;18:92–97. doi: 10.1097/00007632-199301000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Millisdotter M, Strömqvist B, Jönsson B. Proximal neuromuscular impairment in lumbar disc herniation. Spine. 2003;28:1281–1289. doi: 10.1097/00007632-200306150-00013. [DOI] [PubMed] [Google Scholar]
- 23.Nyland J, Brosky T, Currier D, et al. Review of the afferent neural system of the knee and its contribution to motor learning. J Orthop Sports Phys Ther. 1994;19(1):2–11. doi: 10.2519/jospt.1994.19.1.2. [DOI] [PubMed] [Google Scholar]
- 24.Ostelo RW, de Vet HC, Waddell G et al (2002) Rehabilitation after lumbar disc surgery. Cochrane Database Syst Rev (2):CD003007 [DOI] [PubMed]
- 25.O’Sullivan P, Thomey L, Allison G. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylosis or spondylolisthesis. Spine. 1997;22(24):2959–2967. doi: 10.1097/00007632-199712150-00020. [DOI] [PubMed] [Google Scholar]
- 26.Panjabi M. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord. 1992;5:390–396. doi: 10.1097/00002517-199212000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Panjabi M. Clinical spinal instability and low back pain. J Electromyogr Kinesiol. 2003;13(4):371–379. doi: 10.1016/S1050-6411(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 28.Richardson CA, Jull GA, Hodges PW, et al. Therapeutic exercises for spinal segmental stabilization in LBP; scientific evidence and clinical approach. Edinburgh: Churchill Livingstone; 1999. [Google Scholar]
- 29.Roland MA, Morris R (1983) A study of the natural history of back pain. Part 1: Development of a reliable and sensitive measure of disability in low-back pain. Spine pp 8141–8150 [DOI] [PubMed]
- 30.Saal JA, Saal JS. Nonoperative treatment of herniated lumbar intervertebral disc with radiculopathy: an outcome study. Spine. 1989;14:431–437. doi: 10.1097/00007632-198904000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Salén BA, Spangfort EV, Nygren ÅL, et al. The Disability Rating Index: an instrument for the assessment of disability in clinical settings. J Clin Epidemiol. 1994;47(12):1423–1434. doi: 10.1016/0895-4356(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 32.Saruhashi Y, Mori K, Katsuura A, et al. Evaluation of standard nucleotomy for lumbar disc herniation using the Love method: results of follow-up studies after more than 10 years. Eur Spine J. 2004;13:626–630. doi: 10.1007/s00586-004-0690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spangfort E. The lumbar disc herniation. A computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl. 1972;142:1–95. doi: 10.3109/ort.1972.43.suppl-142.01. [DOI] [PubMed] [Google Scholar]
- 34.Steindler A. Kinesiology of the human body: under normal and pathological conditions. 3. Springfield, IL: Charles C Thomas; 1955. [Google Scholar]
- 35.Strömqvist B, Jönsson B, Fritzell P. The Swedish National Register for Lumbar Spine Surgery: Swedish Society for Spinal Surgery. Acta Orthop Scand. 2001;72:99–106. doi: 10.1080/000164701317323327. [DOI] [PubMed] [Google Scholar]
- 36.Woertgen C, Rothoerl RD, Breme K, et al. Variability of outcome after lumbar disc surgery. Spine. 1999;24(8):807–811. doi: 10.1097/00007632-199904150-00013. [DOI] [PubMed] [Google Scholar]
- 37.Yilmaz F, Yilmaz A, Merdol F, Parlar D, Sahin F, Kuran B. Efficacy of dynamic lumbar stabilization exercise in lumbar microdiscectomy. J Rehabil Med. 2003;35(4):163–167. doi: 10.1080/16501970306125. [DOI] [PubMed] [Google Scholar]
- 38.Zanoli G, Strömqvist B, Jönsson B. Visual analog scales for interpretation of back and leg pain intensity in patients operated for degenerative lumbar spine disorders. Spine. 2001;26:2375–2380. doi: 10.1097/00007632-200111010-00015. [DOI] [PubMed] [Google Scholar]
- 39.Zanoli G, Strömqvist B, Jönsson B, et al. Pain in low back pain. Problems in measuring outcomes in musculoskeletal disorders. Acta Orthop Scand Suppl. 2002;73(305):54–57. doi: 10.1080/000164702760379576. [DOI] [PubMed] [Google Scholar]
- 40.Zätterström R (1999) The injured anterior cruciate ligament and neuromuscular training. Thesis, University of Lund