Abstract
Introduction
Changes in hip structure and geometry during aging contribute to decreased bone strength. Little is known, however, about these characteristics at advanced age, when fragility fractures are common. We examined hip structural geometry in men and women of old (72–84 years) and old-old (85–96 years) age to determine (1) gender differences; 2) whether or not these differences are consistent with the increased occurrence of hip fracture in elderly women, compared to men; and (3) whether or not gender-specific changes are consistent with the increased occurrence of fragility fractures after age 80 in both men and women.
Methods
We used Hip Structure Analysis (HSA) software to analyze bone densitometry scans from 916 community-dwelling men and women aged 72–96 years. We examined gender differences in hip geometry by age group (72–74, 75–79, 80–84, and ≥ 85 years) and between gender-specific age groups using multivariable linear regression.
Results
At the femoral narrow neck, there was no gender difference at age 72–74 in bone mineral density (BMD), cortical thickness (CT), and buckling ratio (BR). In contrast, at age 85 or older women had 13% less BMD and CT than men and 8% higher BR. At the intertrochanteric region, women ≥ 85 years had 25–31% less BMD, cross-sectional bone area (CSA), and CT than men of comparable age, and 38% higher BR. These gender differences were approximately 10–20% greater than those between men and women in their 70s. In gender-specific comparisons, women showed increasing change in structural geometry with increasing age. At both narrow neck and trochanteric regions, women ≥ 85 years had nearly 35% higher BR, 15% less BMD and CT, and 10% less CSA than women aged 72–74 years. At the narrow neck they also had 6% greater outer diameter than the youngest women and 8% lower section modulus (Z), an index of bending strength. In contrast, men showed significant age differences only at the narrow neck region, and only at 85 years or older, including 22% higher BR, 10% less BMD and CT, and 5% greater outer diameter, compared to men in their early 70s. Unlike women, men showed no age-associated decline in section modulus.
Conclusions
Gender differences in hip geometry consistent with increased fragility and fracture risk in elderly women, compared to men, continue into old-old age. Both men and women 85 or older show the most unfavorable features, suggesting a structural basis for the increased occurrence of hip fracture in both sexes at advanced age.
Keywords: Hip structure analysis, bone geometry, aging, gender differences, osteoporosis
Introduction
The greater prevalence of bone fragility [1,2] and increased rates of fragility fractures [3] with advancing age in women, compared to men, are explained in part by the larger skeletal size and bone mass of men, even after adjustment for body size [4,5]. In addition, although women and men both lose bone mineral during aging because of endocrine [6–8], paracrine [9,10], and cellular factors [11,12], the effect is more profound in women, beginning even before menopause [13,14], but notably accelerating afterward with rapid decline in estrogen [15,16]. Bone strength, however, is determined not only by the amount of bone mineral, but also by its spatial distribution with respect to the loading forces that may be encountered [17]. Studies using principles from engineering analysis and biomechanics to explore skeletal structural parameters of bone strength [18–20] have been extended by recent investigations [21–29], and a more complete understanding of factors that may contribute to gender differences in bone fragility and fracture risk in old age is emerging.
In tubular bones such as the femur, sex differences in size and strength result from greater periosteal apposition in males and greater endocortical apposition in females in the peripubertal period [30]. Although this leads to a wider periosteal diameter in males, cortical thickness and volumetric density are no different by sex. Nevertheless, cortical bone mass is greater in males, and placement of the cortex further from the neutral axis of the bone confers greater biomechanical advantage and strength to withstand bending [30]. During aging, periosteal bone formation is slowed, but the process continues in both sexes, together with endocortical resorption, resulting in widening of both periosteal and endocortical diameters. Although cross-sectional studies of aging individuals suggest that periosteal apposition is greater in men than in women [23,27], data from one longitudinal study suggest that the rate may be faster in women [31]. In both sexes, however, periosteal widening occurs more slowly than endocortical widening, leading to increased cortical thinning and susceptibility to local buckling [23]. This structural instability may occur at an earlier point in the life of a woman than of a man because of the sexual dimorphism in bone geometry [23,32].
In spite of increased understanding of structural characteristics and changes that can confer bone fragility during aging, there is a lack of information about these characteristics and gender differences at very advanced ages, when the effects of progressive alterations might be expected to put men and women at greatest fracture risk. Few investigations have included participants older than age 80, and individuals older than 85 years are especially underrepresented, even though they constitute the most rapidly growing population group [33] and are 10–15 times more likely to experience fragility fractures than persons aged 60–65 [3,34]. Furthermore, men 80 years or older are even less well-studied than women of similar age, in spite of recent attention to male osteoporosis [35–37].
Therefore, the purpose of this study was to describe characteristics of hip structural geometry in community-dwelling men and women of old age (72–84 years) and old-old age (85–96 years) to address two incompletely understood questions relative to increased bone fragility in older individuals. First, what are the gender differences in hip structural geometry at advanced ages, and are these differences consistent with the greater rates of hip fracture in elderly women compared to elderly men? Second, do characteristics of hip structural geometry in old-old age differ from those at younger old age, and, if so, are the differences consistent with the accelerated rate of fragility hip fractures that occur after age 80 in both men and women?
Materials and methods
Study population
These analyses used data from participants in the Framingham Study, a population-based longitudinal study of risk factors for heart disease. An original cohort of 2875 Caucasian women and 2334 Caucasian men, aged 28–62 years, was assembled in 1948 from a two-thirds sampling of households in Framingham, MA [38]. Surviving participants have provided extensive health-related information by questionnaire and have undergone physical examination and other testing biennially since baseline. Cohort members who had bone densitometry assessment at examination 22 (1992–1993) in the ancillary Framingham Osteoporosis Study [39] are included in the present analyses. Valid bone mineral density measurements of the hip were obtained in 916 participants (343 men, 573 women), representing 67% of the surviving cohort. The study was approved by the appropriate institutional review boards and written informed consent was obtained from all subjects.
Bone densitometry
Proximal femur (neck and trochanter) bone mineral density (BMD) was measured (g/cm²) using dual x-ray absorptiometry (Lunar DPX-L; Lunar Corporation, Madison, WI). Region area also was measured (cm²) and bone mineral content (BMC; g) derived in conventional manner using the Lunar software. The right hip was scanned (n = 883) unless there was history of fracture or hip joint replacement, in which case the left hip was scanned (n = 33). Standard positioning and quality control procedures were used as previously described [40].
Hip structural analysis
The proximal femur densitometry scans were analyzed for geometric properties of bone structure using the Hip Structure Analysis (HSA) software program developed by Beck and colleagues [18]. The HSA technique extends principles first described by Martin and Burr [41] and calculates dimensions of bone cross-sections at specific locations across the proximal femur using bone mass images generated by absorptiometry scanners. The analysis has been described in detail and illustrated in previous publications [21,23,42]. In brief, the HSA program measures bone mineral density and geometry of cross-sections using distributions of mineral mass traversing the bone axis, averaged for precision over five parallel lines (~ 5 mm) across the bone axis. Two femur regions were analyzed and used in this study: 1) the femoral neck at its narrowest point (“narrow neck”; NN); and 2) the intertrochanteric (IT) region, along the bisector of the shaft and femoral neck axes. Areal BMD (g/cm²), bone cross-sectional area (CSA; cm²), subperiosteal outer diameter (cm), and section modulus (Z; cm³) were determined directly from the bone profile at each region, using algorithms described previously [21]. CSA is equivalent to the amount of bone surface area in the cross-section after excluding soft tissue space and is proportional to conventional bone mineral content in the corresponding cross-section. In mechanical terms CSA is an indicator of resistance to loads directed along the bone axis. Section modulus (Z) is an indicator of strength of the bone to resist bending and torsion. Femoral neck length (cm) also was measured [42].
Estimates of average cortical thickness (CT; cm) were calculated by modeling cortices of the NN and IT cross-sections as concentric circles and assuming that 60% of the measured mass is cortical and 40% is trabecular in the NN region, and that 70% is cortical in the IT region [21]. Buckling ratio (BR), a mechanical index of wall stability in thin-walled tubes, was calculated as the distance from the center of mass to the medial or lateral cortex (whichever distance was larger) divided by the estimated average cortical thickness. High values of BR can approximate conditions in osteoporotic bone in which the cortex has become structurally unstable.
Repeated bone densitometry scans that could permit an assessment of HSA precision were not collected in this study, but precision error (CV%) for HSA variables at the NN and IT regions by scanner manufacturer and model have been published [42].
Other measurements
Age at time of scan was determined by records of birth date. Height (without shoes) was measured to the nearest one-fourth inch using a stadiometer. Weight was measured using a standardized balance beam scale. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m²).
Statistical analyses
Anthropometric and bone geometric measures were checked for missing data, outliers, and normality. Scans with structural features greater or less than 3 standard deviations (SD) from mean values were examined for sources of error, such as excessive hip anteversion, incomplete (cut-off) image, or scan quality issues. Such scans were reanalyzed by HSA if appropriate, and valid re-analyzed values were used in subsequent statistical analyses. Unadjusted comparisons between genders for age, height, weight, body mass index, and each geometric measure were performed using Student’s t-test. Because bone dimensions and cross-sectional properties are at least partly dependent on body size and are affected by age [5,23,31], differences between genders and among gender-specific age groups then were assessed using general linear models (GLM) adjusting for age, height, and weight. However, because buckling ratio is a ratio of two dimensions, it was not corrected for body size (see discussion). Nevertheless, it is age-dependent, and gender comparisons were therefore age-adjusted. To compare the magnitude of gender differences in geometric parameters, which have different units of measure, normalized values (percent differences) were generated by expressing the gender difference in adjusted least squares (LS) mean value for each parameter as a percentage of the corresponding LS mean value for men (referent). To compare age differences in geometric parameters, we defined four age groups: 72–74, 75–79, 80–84, and ≥ 85 years. For age comparisons according to gender, normalized values (percent differences) were generated using the youngest gender-specific age group (72–74 years) as the referent. Subsequent to analysis of variance, statistical significance of age group comparisons was assessed using Dunnett’s test for multiple comparisons.
Pearson correlation coefficients were calculated to explore relationships between conventional and geometric DXA parameters. We investigated whether changes seen in geometric parameters differed from those apparent in BMD alone by comparing models with and without BMD as a covariate. Model 1 included adjustment for age and body size, as previously described. Model 2 included additional adjustment for conventional femoral neck BMD, measured by the Lunar densitometer. Model 3 included the adjustments of Model 1, as well as adjustment for BMD measured by the HSA software in the region used to derive geometry. Differences between Models 1 and 2 demonstrate the effect of correcting for conventional BMD, although the mineral mass used in estimates of geometric parameters is not the same as that used by the densitometer to estimate conventional BMD. Differences between Models 1 and 3, however, should better explain why bones with equivalent BMD might differ geometrically at the same location.
All analyses were conducted using SAS statistical software (version 9.1; SAS Institute Inc., Cary, NC), and results were considered statistically significant at the 0.05 level.
Results
Bone densitometry scans from 916 men and women were submitted for hip structure analysis. The percent of geometric features that could not be measured was 0.8% in the narrow neck region and 1.1% for most intertrochanteric features, typically because of hip positioning or obscured femoral neck margins. Truncated images of the femur shaft resulted in a failure rate of 9.9% for estimated IT cortical thickness and buckling ratio, parameters which use shaft outer diameter in the estimate. A scan was not excluded entirely from the study for loss of only isolated measurements at one bone region. The total number of scans providing valid structural measurements for analyses was 914.
Correlations between conventional and geometric DXA parameters
Using gender-pooled data, BMD measured by HSA at the narrow neck was highly correlated with femoral neck BMD measured by the Lunar software (r = 0.85, p < 0.0001). Narrow neck CSA was highly correlated with the Lunar-measured bone mineral content (r = 0.89, p < 0.0001), as expected, because the geometric parameter and the conventional parameter both quantify the amount of bone in the femur neck. Conventional femoral neck area is a crude measure of the average outer diameter of the neck because the length of the region along the neck is generally a default value. In our data, correlation of conventional femoral neck area with HSA narrow neck outer diameter was 0.68. Cortical thickness and section modulus of the narrow neck were significantly (p < 0.0001) correlated with conventional neck BMD (CT, r = 0.84; Z, r = 0.72). Buckling ratio has a non-linear and negative relationship with BMD (i.e., varies as 1/BR), and the correlation between BR and conventional BMD was −0.63. In our gender-specific data, the correlations between conventional and geometric DXA parameters were somewhat higher overall in men than in women, likely because the 1.5 cm conventional femoral neck region is more like a narrow cross-section in the larger bones of men, while it encompasses a greater and more dimensionally variable proportion of the whole femoral neck in women.
Gender differences
Characteristics and descriptive statistics of the study population are reported in Table 1. The average age of the sample was 79 years (range 72–96 years), similar for men and women. There were 584 participants in their seventies (233 men, 351 women) and 330 in their eighties or nineties (109 men, 221 women), of whom 109 were age 85 or older (37 men, 72 women). As expected, men on average were taller and heavier than women, but no different from women in body mass index.
Table 1.
Characteristics of participants. Values for age groups are numbers of participants within each group (% of gender-specific population). Values for other characteristics are group mean values (± standard deviation, SD).
| Women | Men | |
|---|---|---|
| (n = 572) | (n = 342) | |
| Age (y) | 78.8 (4.7) | 78.3 (4.4) |
| Age Groups (n, %) | ||
| 72–74 y (175) | 110 (19.2) | 65 (19.0) |
| 75–79 y (409) | 241 (42.1) | 168 (49.1) |
| 80–84 y (221) | 149 (26.1) | 72 (21.1) |
| ≥ 85 y (109) | 72 (12.6) | 37 (10.8) |
| Height (cm) | 155.2 (6.5) | 169.8 (7.0) |
| Weight (kg) | 64.4 (12.7) | 78.2 (13.2) |
| Body Mass Index (kg/m²) | 26.7 (5.0) | 27.0 (4.0) |
Table 2 displays gender differences in geometric measures before and after adjustment for age and body size (height and weight). Buckling ratio was adjusted for age but not body size (see discussion). Group mean values are also given for conventional BMD, BMC, and region area of the femoral neck as measured by the Lunar software. In unadjusted analyses men and women were significantly different in all measures (p < 0.001). Consistent with their larger bones, men had higher values than women except in buckling ratio (BR), for which women had higher values. After adjustment, the magnitude of gender differences was reduced, but remained statistically significant (p < 0.001). The greatest differences between men and women after adjustment were in section moduli (Z), 22% to 27% lower in women (p < 0.0001), cross-sectional areas (CSA), 15–20% less in women (p < 0.0001), and estimated cortical thickness (CT), 7–19% thinner in women (p < 0.001). In contrast, BR was 7% higher in women compared to men at the femoral narrow neck (p < 0.001) and 28% higher at the intertrochanteric region (p < 0.0001).
Table 2.
BMD and hip structural geometry in women and men. Measures are from Hip Structure Analysis (HSA) except for BMD, BMC, and Area at the Lunar femoral neck, which are from the Lunar densitometer. Unadjusted data are mean values (± standard deviation, SD). Adjusted data are group least squares mean values (± standard error of the mean, SEM) after adjustment for each individual’s age, height, and weight. BR, dimensionless, was not adjusted for height and weight.
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| Narrow Neck | ||||
| Neck Length (cm) | 4.58 (0.67 | 5.39 (0.81)**** | 4.68 (0.04) | 5.23 (0.05)**** |
| BMD (g/cm²) | 0.63 (0.14 | 0.75 (0.15)**** | 0.66 (0.01) | 0.71 (0.01)*** |
| Cross Sectional Area (cm²) | 1.76 (0.36) | 2.42 (0.47)**** | 1.88 (0.02) | 2.22 (0.03)**** |
| Outer Diameter (cm) | 2.94 (0.28) | 3.38 (0.27)**** | 3.00 (0.01) | 3.27 (0.02)**** |
| Cortical Thickness (mm) | 1.22 (0.03) | 1.44 (0.03)**** | 1.26 (0.01) | 1.35 (0.02)*** |
| Section Modulus (cm³) | 0.92 (0.24) | 1.43 (0.32)**** | 1.00 (0.01) | 1.29 (0.02)**** |
| Buckling Ratio | 14.40 (5.28) | 13.32 (3.55)*** | 14.35 (0.19) | 13.40 (0.25)*** |
| Lunar Femoral Neck | ||||
| BMD (g/cm²) | 0.70 (0.12) | 0.86 (0.14)*** | 0.73 (0.01) | 0.82 (0.01)**** |
| BMC (g) | 4.79 (0.55) | 5.61 (0.45)**** | 3.61 (0.03) | 4.44 (0.05)**** |
| Area (cm²) | 3.37 (0.72) | 4.84 (0.94)**** | 4.92 (0.02) | 5.39 (0.03)**** |
| Intertrochanteric | ||||
| BMD (g/cm²) | 0.61 (0.15) | 0.81 (0.16)**** | 0.64 (0.01) | 0.76 (0.01)**** |
| Cross Sectional Area (cm²) | 3.04 (0.72) | 4.53 (0.93)**** | 3.28 (0.03) | 4.12 (0.05)**** |
| Outer Diameter (cm) | 5.22 (0.39) | 5.92 (0.46)**** | 5.37 (0.02) | 5.67 (0.03)**** |
| Cortical Thickness (mm) | 2.24 (0.06) | 3.32 (0.07)**** | 2.53 (0.03) | 3.14 (0.06)**** |
| Section Modulus (cm³) | 2.69 (0.78) | 4.68 (1.10)**** | 3.02 (0.04) | 4.13 (0.05)**** |
| Buckling Ratio | 13.49 (5.49) | 10.43 (3.26)**** | 13.46 (0.21) | 10.50 (0.28)**** |
P < 0.001
P < 0.0001
Table 3 illustrates the percent differences between women and men in hip geometry after adjusting for age and body size (Model 1), and after including additional adjustment for conventional femoral neck BMD (Model 2) or BMD of the corresponding region by HSA (Model 3). Adjustment for BMD had little effect on outer diameters (as expected), but interesting effects on other geometric differences. For equivalent BMD, women still demonstrated significantly lower bending resistance (section modulus) at both narrow neck and intertrochanteric regions, although the differences were smaller. Bone cross-sectional area, as an index of resistance to axial loads, also remained smaller in women with BMD equivalent to men. However, the gender difference in buckling ratio reversed after adjustment for BMD, suggesting that men have larger values than women for equivalent levels of BMD (not significant at IT region with Model 2).
Table 3.
Differences (%) between women and men in hip structural geometry, referent to men. Model 1 adjusted for age, height, and weight. Model 2 included adjustments of Model 1, as well as adjustment for femoral neck BMD measured by the Lunar densitometer. Model 3 included adjustments of Model 1, as well as adjustment for BMD measured by HSA at the corresponding region. In all models, the dimensionless Buckling Ratio was not adjusted for height and weight.
| Difference between Women and Men (%) | |||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Narrow Neck | |||
| Cross Sectional Area | −15.2**** | −6.2**** | −10.3**** |
| Outer Diameter | −8.4**** | −9.8**** | −9.6**** |
| Cortical Thickness | −6.7*** | +5.6*** | +0.6**** |
| Section Modulus | −22.2**** | −15.5**** | −17.5**** |
| Buckling Ratio | +7.1*** | −17.6**** | −14.4**** |
| Intertrochanteric | |||
| Cross Sectional Area | −20.5**** | −12.0*** | −7.2**** |
| Outer Diameter | −5.2**** | −6.1**** | −7.2**** |
| Cortical Thickness | −19.3**** | −9.6**** | −3.7**** |
| Section Modulus | −26.82*** | −20.4**** | −15.9**** |
| Buckling Ratio | +28.2**** | −3.00 | −13.4**** |
P < 0.001
P < 0.0001
Gender differences in BMD and hip structural geometry at different ages
Figure 1 illustrates differences in size-adjusted BMD and hip geometry between men and women within the same age group. At the femoral narrow neck, gender differences at age 72–74 years were negligible (< 2%) in BMD, CT, and BR. In contrast, in the oldest age group (≥ 85 y), women had significantly lower BMD and CT than men (−13% for each measure; p ≤ 0.03) and greater BR (8%, but not statistically significant). The oldest group also showed substantial gender difference in narrow neck CSA (21%, p < 0.001), notably greater than the difference in CSA between men and women in the youngest group.
Figure 1.
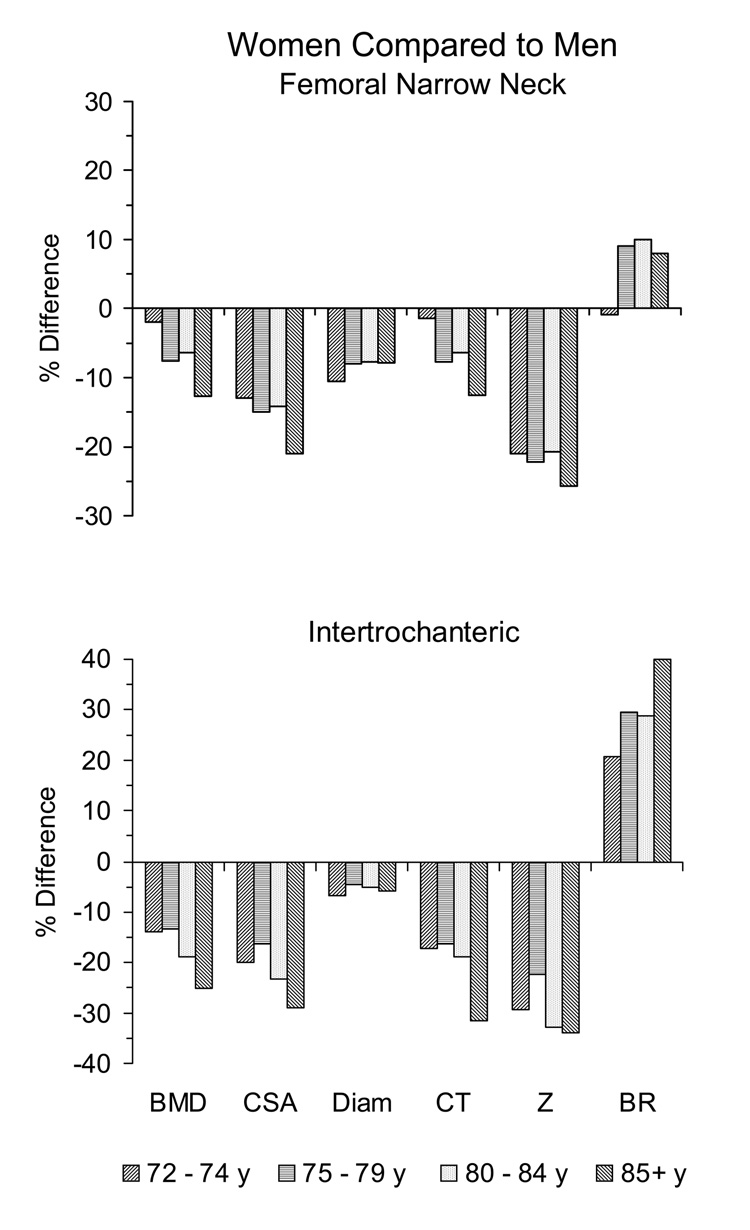
Effects of age on gender differences in hip structural geometry at the femoral narrow neck and intertrochanteric regions. Women are compared to men in four age groups (72–74, 75–79, 80–84, and 85 years or older). For each age group the percent difference between genders, referent to men, is shown for HSA-measured bone mineral density (BMD), cross-sectional area (CSA), outer diameter (Diam), average cortical thickness (CT), and section modulus (Z) after adjustment for age, height, and weight. Buckling ratio (BR) was not adjusted for height and weight. All gender differences were statistically significant (p < 0.05) at the femoral neck except BMD and CT in groups 72–74 and 80–84 years and BR in all groups except 75–79 years. At the IT region all gender differences were statistically significant.
At the intertrochanteric region, older age also was associated with greater gender differences in BMD and hip geometry measures. This was particularly evident for men and women 85 years or older in BMD (25% less in women; p < 0.0001), CSA (29% less in women; p < 0.0001), and CT (31% less in women; p < 0.0001). Most dramatically, women 85 or older had IT buckling ratios 38% higher than comparably aged men (p = 0.001), a difference almost 20% greater than gender differences in BR for men and women in their early 70s.
Although women in all age groups differed significantly (< 0.0001) from men in outer diameter and section modulus at both narrow neck and IT regions, the differences were approximately the same across age groups (7–11% smaller diameter and 21–26% lower Z at the neck, compared to men in the same age group; 4–7% smaller diameter and 22–34% lower Z at the IT region).
The effects of BMD on gender differences in hip geometry are shown in Table 4. Outer diameters were 8–11% smaller in women across age groups whether or not they were corrected for BMD (Model 1 compared to Models 2 and 3). Cross-sectional area and section modulus also remained significantly smaller in women across age groups even after adjustment for BMD (Models 2 and 3), with only two exceptions. Without BMD adjustment (Model 1), femoral narrow neck buckling ratios differed significantly only in the 75–79 y age group, although gender differences in BR in all age groups were highly significant at the IT region. Adjustment for conventional femoral neck BMD (Model 2) eliminated the gender difference in BR at the IT region but not at the femoral neck. Adjustment for BMD measured by HSA (Model 3) led to significant gender differences in buckling ratios in all age groups (lower values in women) at both narrow neck and IT regions.
Table 4.
Gender differences in hip structural geometry at older ages. Differences (%) in each age group were calculated referent to the men. In Model 1 data were adjusted for age, height, and weight. Model 2 included adjustments of Model 1, as well as adjustment for femoral neck BMD measured by the Lunar densitometer. Model 3 included adjustments of Model 1, as well as adjustment for BMD measured by HSA at the corresponding region. In all models, dimensionless Buckling Ratio was not adjusted for height and weight.
| Difference (%) between Women and Men | ||||
|---|---|---|---|---|
| 72–74 y | 75–79 y | 80–84 y | ≥ 85 y | |
| Narrow Neck | ||||
| Cross Sectional Area | ||||
| Model 1 | −13.0*** | −15.0**** | −14.1**** | −21.0**** |
| Model 2 | −7.4** | −7.0**** | −2.3 | −8.6** |
| Model 3 | −11.6**** | −9.2**** | −9.6**** | −13.0**** |
| Outer Diameter | ||||
| Model 1 | −10.6**** | −8.0**** | −7.7**** | −7.9*** |
| Model 2 | −11.2**** | −9.2**** | −9.5**** | −12.4**** |
| Model 3 | −10.9**** | −9.2**** | −8.9**** | −10.4**** |
| Cortical Thickness | ||||
| Model 1 | −1.4 | −7.7* | −6.3 | −12.5* |
| Model 2 | +6.1* | +2.6 | +9.1** | +9.9* |
| Model 2 | +0.8*** | +0.4*** | +0.5** | +1.1**** |
| Section Modulus | ||||
| Model 1 | −21.1**** | −22.2**** | −20.7**** | −25.7**** |
| Model 2 | −16.7**** | −16.2**** | −11.6** | −18.6*** |
| Model 3 | −19.7**** | −16.7**** | −16.3**** | −19.5**** |
| Buckling Ratio | ||||
| Model 1 | −0.9 | +9.1* | +10.0 | +8.0 |
| Model 2 | −18.5**** | −17.9**** | −16.7*** | −19.7**** |
| Model 3 | −14.7**** | −16.1**** | −12.8**** | −13.0**** |
| Intertrochanteric | ||||
| Cross Sectional Area | ||||
| Model 1 | −20.1**** | −16.4**** | −23.4**** | −29.1**** |
| Model 2 | −15.2**** | −8.7**** | −13.6**** | −16.3*** |
| Model 3 | −9.0**** | −5.2**** | −8.3**** | −11.7*** |
| Outer Diameter | ||||
| Model 1 | −7.0**** | −4.5**** | −5.1*** | −6.0** |
| Model 2 | −7.1**** | −5.3**** | −6.3**** | −8.8*** |
| Model 3 | −8.3**** | −6.1**** | −7.4**** | −10.2**** |
| Cortical Thickness | ||||
| Model 1 | −17.3**** | −16.3**** | −18.7**** | −31.4**** |
| Model 2 | −11.0** | −7.0** | −8.6* | −17.7** |
| Model 2 | −3.4 | −3.1* | −2.2 | −9.3** |
| Section Modulus | ||||
| Model 1 | −29.2**** | −22.4**** | −28.3**** | −33.9**** |
| Model 2 | −25.4**** | −16.6**** | −21.0**** | −23.6**** |
| Model 3 | −20.4 | −13.4**** | −15.1**** | −18.6**** |
| Buckling Ratio | ||||
| Model 1 | +20.6**** | +29.6**** | +28.8**** | +38.2**** |
| Model 2 | −1.7 | −5.4 | −0.8 | −1.6 |
| Model 3 | −11.6**** | −15.5**** | −11.5**** | −10.6* |
P < 0.05
P < 0.01
P < 0.001
P < 0.0001
Gender-specific differences in BMD and hip structural geometry with increasing age
Figure 2 illustrates age trends in size-adjusted BMD and hip geometry assessed by HSA at the narrow neck and IT regions in women and men separately. The three older age groups are compared (percent difference) to the 72–74 y group. Table 5 summarizes the differences between the oldest (≥ 85 y) and youngest groups and shows the effects of adjustment for BMD within each gender.
Figure 2.
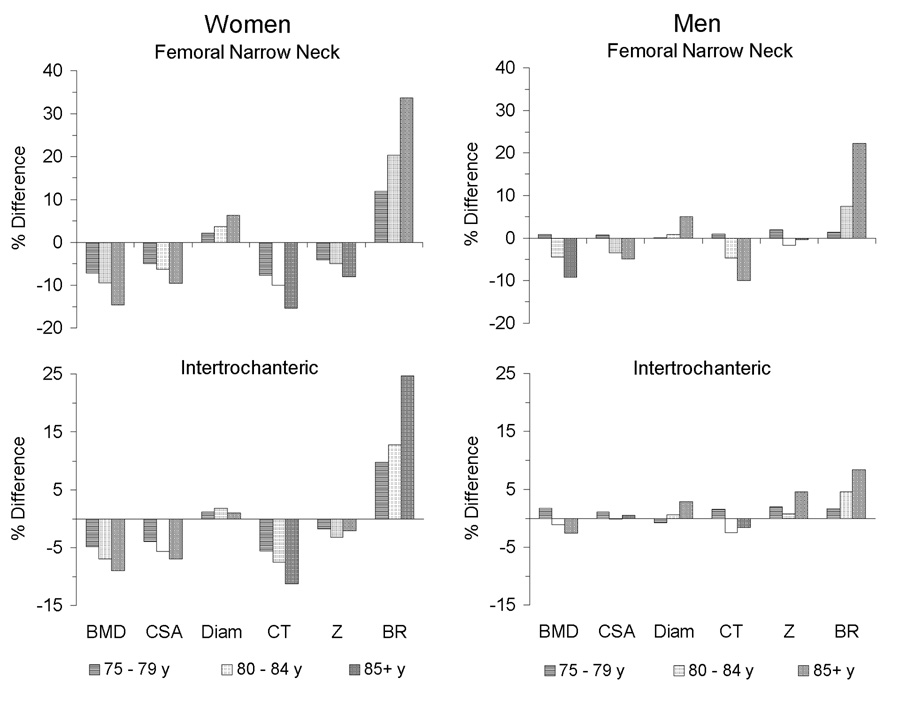
Gender-specific effects of age on hip structural geometry at the femoral narrow neck and intertrochanteric regions. The three older groups (75–79, 80–84, and 85 years or older) are compared to the youngest (72–74 y) for women (left panels) and men (right panels) in HSA-measured bone mineral density (BMD), cross-sectional area (CSA), outer diameter (Diam), average cortical thickness (CT), and section modulus (Z) after adjustment for age, height, and weight. Buckling ratio (BR) was not adjusted for height and weight. Percent differences in measures within each gender-specific age group are referent to the youngest group (72–74 y). Among women, all measures showed statistically significant age-associated differences except intertrochanteric outer diameter and Z. Among men, significant age differences were seen only for men 85 or older, and only at the femoral neck, in BMD, outer diameter, CT, and BR.
Table 5.
Gender-specific differences in hip structural geometry with age. For simplicity, data are shown only for differences (%) between the oldest (≥ 85 y) and youngest (72–74 y) age groups, referent to the youngest. In Model 1 data were adjusted for age, height, and weight. Model 2 included adjustments of Model 1, as well as adjustment for femoral neck BMD measured by the Lunar densitometer. Model 3 included adjustments of Model 1, as well as adjustment for BMD measured by HSA at the corresponding region. In all models, dimensionless Buckling Ratio was not adjusted for height and weight.
| Women | Men | |||||
|---|---|---|---|---|---|---|
| ≥ 85 y vs. 72–74 y (% difference) | ≥ 85 y vs. 72–74 y (% difference) | |||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Narrow Neck | ||||||
| Cross Sectional Area | −9.6*** | −1.7 | +2.5 | −4.9 | +0.4 | +3.2* |
| Outer Diameter | +6.3**** | +4.6*** | +3.1* | +5.00** | +4.4** | +3.7** |
| Cortical Thickness | −15.4**** | −6.5** | +0.0 | −9.9* | −4.0 | −0.1 |
| Section Modulus | −8.0* | −1.8 | +5.9* | −0.4 | +4.3 | +7.5** |
| Buckling Ratio | +33.7**** | +9.9* | +0.8 | +22.3**** | +8.0* | +2.6 |
| Intertrochanteric | ||||||
| Cross Sectional Area | −7.0* | +1.4 | +0.6 | +0.5 | +5.0* | +2.8 |
| Outer Diameter | +1.1 | +0.9 | +0.9 | +2.9 | +2.6 | +2.7 |
| Cortical Thickness | −11.2* | −2.4 | −2.8* | −1.6 | +4.7 | +1.4 |
| Section Modulus | −2.1 | +5.1 | +5.0* | +4.6 | +8.3* | +6.6* |
| Buckling Ratio | +24.8**** | +3.3 | +3.5 | +8.3 | −6.8 | −6.1 |
P < 0.05
P < 0.01
P < 0.001
P < 0.0001
Among women, consistent trends in geometric parameters occurred with increasing age (ptrend 0.03 for NN section modulus, ptrend ≤ 0.006 all other measures except IT outer diameter and section modulus [no significant trend]). Women 85 or older exhibited the greatest differences, with BR 25% (IT region) to 34% (neck region) higher than values for women aged 72–74 years. Compared to the youngest women, the oldest also had approximately 10–15% less BMD and CT, 7–10% less CSA, and 6% greater neck outer diameter. Furthermore, compared even to women in their younger 80s, women 85 or older showed significant (p < 0.05) structural differences at the femoral neck, including 6% less BMD, 3% increased outer diameter, and 10% higher BR. Thus the oldest women had wider femurs (n.s. at IT) containing less bone tissue (CSA), thinner cortices, less bending resistance (n.s. at IT), and significantly greater buckling ratios. After adjustment for BMD (Table 5), CSA and BR differences became non-significant, although section moduli were significantly higher in the oldest group at both regions for equivalent BMD measured by HSA (Model 3).
Among men (Figure 2 and Table 5), significant age differences in structural parameters were apparent only at the femoral narrow neck, and only in men 85 or older. Compared to men in their early 70s, the oldest men demonstrated patterns and magnitudes of differences in hip structural geometry that were similar to differences seen in comparisons of women 85 or older to younger women, including lower BMD (10%; p = 0.04), smaller CSA (5%, but not statistically significant), increased outer diameter (5%; p = 0.005), thinner cortex (10%; p = 0.04), and higher BR (22%; p < 0.0001). Men age 85 or older also showed structural differences at the femoral neck compared even to men in their younger 80s, including higher BR (14%, p = 0.008) and increased outer diameter (4%, p = 0.007). Men in their early 80s exhibited patterns of structural change at the femoral neck similar to the oldest men, but they were not statistically different from men in their early 70s. Men showed no age-associated difference in femoral neck section modulus, unlike women.
At the intertrochanteric region, men displayed a trend (p = 0.03) toward increased bone outer diameter with increasing age, but no other significant age-related differences in hip geometry. Similar to women, section modulus was significantly higher in the oldest group of men compared to the youngest after adjustment for BMD estimated by HSA.
As one would expect in adult bones, femoral neck length showed no age-associated change in either women or men (data not shown).
Discussion
The community-dwelling elderly men and women in this study exhibited significant gender differences in hip structural geometry. Furthermore, the differences increased with age. These results support the hypothesis that elderly women, compared to men of similar age, have increased hip fracture risk because of greater cortical instability (thinner cortex and higher buckling ratio) and reduced structural capacity to resist compressive or bending forces (smaller bone cross-sectional area and diminished section modulus). The gender differences in features of structural fragility were greatest between men and women in the oldest-old age group (85–96 years). This finding supports the observation that elderly women have greater hip fracture risk than elderly men, but it also suggests that the gender difference in fracture risk increases even more at advanced age. In this study women had diminished bone mineral density compared to men, but the difference generally was less than that for geometric measures of bone structure, even after adjustment for body size, especially in women in their seventies.
The gender differences in BMD and inferences that might be drawn are worthy of specific comment. In the femoral neck region these differences were influenced by the opposing effects of the amount of bone in the region (CSA and BMC, determined by HSA and conventional methods, respectively) and the external dimensions of the bone (as apparent in outer diameter and region area, respectively). For example, compared to the women, the femoral necks of the men contained 15% more bone surface (CSA). However, because their femoral necks were 8% wider in diameter, the adjusted BMD of the men was only 7% greater than that of the women. This means that in absolute terms the strength difference between genders is underestimated by the BMD difference. The size-adjusted BMD data would suggest that the femoral necks of the women were 7% (measured at the HSA site) and 11% (measured at the conventional site) weaker than those of the men. However, the resistance to axial loads (CSA) was 15% lower in the women compared to the men. Furthermore, because of the non-linear effect of outer diameter, the gender difference in bending resistance (section modulus) was even greater, with the women having 22% lower value in Z than the men. The gender difference in load resistance was even larger at the intertrochanteric region. When we examined gender differences in geometry adjusted for BMD, we found that women with the same BMD as men, independent of body size, had 7–10% lower resistance to axial loads and 16–18% lower bending resistance. While women had higher buckling ratios in absolute terms, for an equivalent BMD their BR were actually lower than the BR in men. This would suggest that compared to the femurs of women, the femurs of men would have a greater susceptibility to buckling at a similar BMD level.
Among women, comparisons across age groups revealed a consistent and progressively worsening pattern of hip structural geometry with increasing age. Women age 85 years or older had the most unfavorable features, but the changes that were obvious at advanced age also were apparent at age 75. Among men, however, age group differences in structural features were significant only at the femoral neck and only among men age 85 or older, although men in their earlier 80s showed similar patterns. Except for section modulus, the features of increased fragility at the femoral neck seen in the oldest men were similar to those seen in the oldest women. Our finding that these men did not have decreased section modulus, in contrast to the decrease seen at the femoral neck with increasing age in the women, is particularly noteworthy, because it may explain the higher resilience of the male femur to bending forces generated by falls. When geometric features were adjusted for BMD assessed by HSA, we found that in both men and women the bending resistance was actually larger in the oldest group for an equivalent BMD. This is consistent with the observation that expansion of outer diameter can preserve this parameter with a smaller amount of bone tissue. Age effects on buckling ratio became non-significant in both men and women after adjusting for BMD. This suggests that buckling ratio and BMD capture similar geometric information.
Our data confirm findings reported by other population-based cohorts of community dwelling, predominantly white elders with regard to gender differences in hip structural characteristics and trends with aging toward increased fragility [5,21,28,31,43], while substantially extending the age range of these types of data to 96 years. The gender differences we found are similar to those reported by Kaptoge et al. [31] and are consistent with NHANES III data on analyses at the femoral neck [5]. However, the magnitudes of differences between men and women in our study differ modestly from these previous studies, most probably because their populations were younger (59–69 years [5] and 67–79 years [31]) and because one study [31] adjusted for body size using average, mixed gender population data rather than individual data. Nevertheless, both of these other studies as well as ours found bone cross-sectional area and section modulus to be the greatest discriminators between genders in hip structural characteristics. In our study intertrochanteric buckling ratio and cortical thickness also differed significantly between men and women, which may partially explain the pronounced gender difference in hip fracture incidence at older age. Buckling ratio estimates have been noted to be significantly higher in hip fracture cases compared to sex- and age-matched controls [23,24,44]. Similarly, cortical thinning has been observed in femoral fracture cases, including disproportional thinning in cortical regions likely to be loaded maximally on impact from a fall [45–47].
In comparing buckling ratio between genders, we adjusted for age but not body size. In precise terms, buckling ratio is not a strength measurement, but rather a dimensionless geometric configuration that describes threshold behavior beyond which strength may be compromised under compressive loads because of local buckling of thin regions. Unlike section modulus or CSA, one would not adjust buckling ratio for body size to correct for the fact that larger bones must be stronger in bigger subjects.
In both men and women, there were no strong age trends in section moduli at either the femoral neck or the intertrochanteric region, consistent with the observation that expansion of bone outer diameter tends to preserve resistance to pure bending [47,48]. However, expansion of outer diameter combined with cortical thinning means that the compensatory changes are accomplished with progressively less bone, i.e., a wider bone requires less bone tissue for the same section modulus. The potential compromise from this effect is an increase in susceptibility to local cortical buckling. The significant upward trends in buckling ratio with increasing age (higher BR values increase susceptibility) suggest a structural basis for the gender difference in hip fracture type in old age. Among women, femoral neck fractures from low trauma occur more frequently than trochanteric fractures before age 60, but increasing numbers of trochanteric fractures occur in older age [49]. The elderly women in our study showed significant age-associated increases in buckling ratio at both the femoral neck and the intertrochanteric region, suggesting that strength is compromised at both sites. The elderly men, however, showed a significant age-associated increase in buckling ratio only at the femoral neck. This suggests that with aging, the femoral neck in men is less able to withstand bending forces without buckling and thus is less resistant to fracture. Indeed, among men, cervical fractures occur much more frequently than trochanteric fractures in older age [49].
With regard to hip structure in aging, our data extend previous studies by demonstrating that changes in geometric features consistent with increased fragility and fracture risk continue into old-old age, beyond 85 years, an age range that has been excluded from or minimally represented in other investigations. Furthermore, these changes occur in very elderly men as well as in women. One of the most significant findings that emerged by including older participants was the observation that men and women age 85 or older showed the most unfavorable hip geometry. In many measures these differences were significantly greater than among men and women even in their early 80s, and dramatically worse than among those in their 70s. These differences parallel the markedly increased hip fracture rates among both men and women age 80 or older, compared to persons in their seventies, both in the United States and many other countries [3,50–52].
A major strength of this study is the use of a large, population-based cohort of older men and women, including substantial numbers of both men and women over age 80, with many older than 85 years. The participants were generally healthy, ambulatory, community-dwelling elders carrying out routine levels of activities.
Several potential limitations must be acknowledged. Although our data are population-based, they are drawn from a mostly non-Hispanic white cohort and may not be generalizable to other ethnic populations. In addition, the bone densitometry scans used in the hip structure analyses were obtained in 1992–1993, and secular trends as well as period-cohort effects may limit extrapolating our findings to different generations. However, if such a trend did exist in our study sample, it should have been demonstrated by a longer and wider femoral neck in the younger subjects (age in lower 70s), compared to the older (age in higher 80s). As discussed and shown in Table 5, this was not the case.
This was a cross-sectional study and we can only infer that geometric features seen among groups of individuals of different ages reflect the natural history of aging bone. Nevertheless, the pattern of our results is in agreement with the few studies [22,31,53] that have obtained longitudinal data to describe the evolution of hip structural parameters with age, although those data are available only over relatively short times (mean 2.7–3.5 years).
We did not have measures of skeletal muscle or lean body mass to directly assess the contribution of skeletal loading, which affects mechanical properties of bones and perhaps accounts for decline in strength of aged bones [54]. As other studies have done [5,28,31,53], we adjusted for body mass and size using weight and standing height, although variations in height because of vertebral collapse in the very old can be confounding. A related issue is that CSA and section modulus tend to scale better with lean body mass [22,55] than with total weight, and men are proportionately leaner than women [56]. This may mean that some of the residual gender differences in geometry after correction for height and weight may be due in part to the smaller lean mass fraction in women. On the other hand, the forces generated in falls are a function of total body mass, not lean mass, inferring that relative strength to resist trauma still would be lower in women than in men.
Our study of bone geometric features is restricted by the inherent limitations of DXA technology. Current scanners cannot reliably measure small changes in dimensions in an individual that are structurally important. Thus large populations, such as included in this study, are required to provide statistical power in analyses of structure. Furthermore, subperiosteal bone margins may not be detected accurately in osteoporotic bone, thereby underestimating bone outer diameter, especially in old persons with very low bone mineral density and cortical porosity. Finally, estimates of average cortical thickness and buckling ratio in the HSA method include several assumptions about shape of tubular bones and the assigned fractions of cortical and trabecular bone. Nevertheless, until new methods of quantifying bone structural morphology are available and practical for application in population-based studies, the analysis of hip structural geometry with bone absorptiometry provides at least an insight into geometric changes that may have clinical importance.
In conclusion, in this study of elderly men and women of old age (72–84 years) and old-old age (85–96 years), significant gender differences in hip structural geometry were clearly apparent. These differences suggest that elderly women may have greater risk of hip fracture than elderly men because of inherent structural features of the proximal femur that lead to increased bone fragility. Furthermore, both men and women of oldest-old age have indices of bone strength that are notably worse than those of men and women in their seventies and early eighties. Thus the accelerated rates of hip fracture after age 80 both in men and in women may be explained at least in part by unfavorable distribution of bone tissue with ensuing increased bone fragility. Because these structural changes are consistent with imbalances in processes of bone modeling and remodeling, including periosteal apposition and endocortical resorption, continued focus is warranted on developing therapeutic agents to regulate cellular aspects of bone formation and destruction and thereby maintain or restore bone strength.
Acknowledgements
We thank Sophia Menn and Dr. Serkalem Demissie for assembling and cleaning data for statistical analysis.
This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195) and by grants R01-AR/AG 41398 and R01-AR050066 from the National Institutes of Health. Dr. Yates received support in the form of a Junior Faculty Development Award from the Hartford Foundation and Harvard Medical School Center of Excellence in Geriatric Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Beck’s institution (The Johns Hopkins University) has licensed the Hip Structure Analysis software to Hologic Inc. The other authors have no conflicts of interest.
References
- 1.Looker AC, Orwoll ES, Johnston CC, Jr, Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12:1761–1768. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 2.Melton LJ, 3rd, Khosla S, Achenbach SJ, O'Connor MK, O'Fallon WM, Riggs BL. Effects of body size and skeletal site on the estimated prevalence of osteoporosis in women and men. Osteoporos Int. 2000;11:977–983. doi: 10.1007/s001980070037. [DOI] [PubMed] [Google Scholar]
- 3.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 4.Nieves JW, Formica C, Ruffing J, Zion M, Garrett P, Lindsay R, Cosman F. Males have larger skeletal size and bone mass than females, despite comparable body size. J Bone Miner Res. 2005;20:529–535. doi: 10.1359/JBMR.041005. [DOI] [PubMed] [Google Scholar]
- 5.Looker AC, Beck TJ, Orwoll ES. Does body size account for gender differences in femur bone density and geometry? J Bone Miner Res. 2001;16:1291–1299. doi: 10.1359/jbmr.2001.16.7.1291. [DOI] [PubMed] [Google Scholar]
- 6.Manolagas SC, Kousteni S, Jilka RL. Sex steroids and bone. Recent Prog Horm Res. 2002;57:385–409. doi: 10.1210/rp.57.1.385. [DOI] [PubMed] [Google Scholar]
- 7.Mellstrom D, Johnell O, Ljunggren O, Eriksson AL, Lorentzon M, Mallmin H, Holmberg A, Redlund-Johnell I, Orwoll E, Ohlsson C. Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res. 2006;21:529–535. doi: 10.1359/jbmr.060110. [DOI] [PubMed] [Google Scholar]
- 8.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 9.Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev. 2005;208:207–227. doi: 10.1111/j.0105-2896.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 10.Barrett-Connor E, Goodman-Gruen D. Gender differences in insulin-like growth factor and bone mineral density association in old age: the Rancho Bernardo Study. J Bone Miner Res. 1998;13:1343–1349. doi: 10.1359/jbmr.1998.13.8.1343. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein RS, Manolagas SC. Apoptosis and osteoporosis. Am J Med. 2000;108:153–164. doi: 10.1016/s0002-9343(99)00420-9. [DOI] [PubMed] [Google Scholar]
- 12.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 13.Riggs BL, Wahner HW, Melton LJ, 3rd, Richelson LS, Judd HL, Offord KP. Rates of bone loss in the appendicular and axial skeletons of women. Evidence of substantial vertebral bone loss before menopause. J Clin Invest. 1986;77:1487–1491. doi: 10.1172/JCI112462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slemenda C, Longcope C, Peacock M, Hui S, Johnston CC. Sex steroids, bone mass, and bone loss. A prospective study of pre-, peri-, and postmenopausal women. J Clin Invest. 1996;97:14–21. doi: 10.1172/JCI118382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahlborg HG, Johnell O, Nilsson BE, Jeppsson S, Rannevik G, Karlsson MK. Bone loss in relation to menopause: a prospective study during 16 years. Bone. 2001;28:327–331. doi: 10.1016/s8756-3282(00)00451-8. [DOI] [PubMed] [Google Scholar]
- 16.Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. N Engl J Med. 2003;349:327–334. doi: 10.1056/NEJMoa022464. [DOI] [PubMed] [Google Scholar]
- 17.Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14 Suppl 3:S13–S18. doi: 10.1007/s00198-002-1345-4. [DOI] [PubMed] [Google Scholar]
- 18.Beck TJ, Ruff CB, Warden KE, Scott WW, Jr., Rao GU. Predicting femoral neck strength from bone mineral data. A structural approach. Invest Radiol. 1990;25:6–18. doi: 10.1097/00004424-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Beck TJ, Ruff CB, Bissessur K. Age-related changes in female femoral neck geometry: implications for bone strength. Calcif Tissue Int. 1993;53 Suppl 1:S41–S46. doi: 10.1007/BF01673401. [DOI] [PubMed] [Google Scholar]
- 20.Beck TJ, Ruff CB, Scott WW, Jr, Plato CC, Tobin JD, Quan CA. Sex differences in geometry of the femoral neck with aging: a structural analysis of bone mineral data. Calcif Tissue Int. 1992;50:24–29. doi: 10.1007/BF00297293. [DOI] [PubMed] [Google Scholar]
- 21.Beck TJ, Looker AC, Ruff CB, Sievanen H, Wahner HW. Structural trends in the aging femoral neck and proximal shaft: analysis of the Third National Health and Nutrition Examination Survey dual-energy X-ray absorptiometry data. J Bone Miner Res. 2000;15:2297–2304. doi: 10.1359/jbmr.2000.15.12.2297. [DOI] [PubMed] [Google Scholar]
- 22.Beck TJ, Oreskovic TL, Stone KL, Ruff CB, Ensrud K, Nevitt MC, Genant HK, Cummings SR. Structural adaptation to changing skeletal load in the progression toward hip fragility: the study of osteoporotic fractures. J Bone Miner Res. 2001;16:1108–1119. doi: 10.1359/jbmr.2001.16.6.1108. [DOI] [PubMed] [Google Scholar]
- 23.Duan Y, Beck TJ, Wang XF, Seeman E. Structural and biomechanical basis of sexual dimorphism in femoral neck fragility has its origins in growth and aging. J Bone Miner Res. 2003;18:1766–1774. doi: 10.1359/jbmr.2003.18.10.1766. [DOI] [PubMed] [Google Scholar]
- 24.Filardi S, Zebaze RM, Duan Y, Edmonds J, Beck T, Seeman E. Femoral neck fragility in women has its structural and biomechanical basis established by periosteal modeling during growth and endocortical remodeling during aging. Osteoporos Int. 2004;15:103–107. doi: 10.1007/s00198-003-1539-4. [DOI] [PubMed] [Google Scholar]
- 25.Kaptoge S, Dalzell N, Jakes RW, Wareham N, Day NE, Khaw KT, Beck TJ, Loveridge N, Reeve J. Hip section modulus, a measure of bending resistance, is more strongly related to reported physical activity than BMD. Osteoporos Int. 2003;14:941–949. doi: 10.1007/s00198-003-1484-2. [DOI] [PubMed] [Google Scholar]
- 26.Wang XF, Duan Y, Beck TJ, Seeman E. Varying contributions of growth and ageing to racial and sex differences in femoral neck structure and strength in old age. Bone. 2005;36:978–986. doi: 10.1016/j.bone.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Russo CR, Lauretani F, Bandinelli S, Bartali B, Di Iorio A, Volpato S, Guralnik JM, Harris T, Ferrucci L. Aging bone in men and women: beyond changes in bone mineral density. Osteoporos Int. 2003;14:531–538. doi: 10.1007/s00198-002-1322-y. [DOI] [PubMed] [Google Scholar]
- 28.Crabtree N, Lunt M, Holt G, Kroger H, Burger H, Grazio S, Khaw KT, Lorenc RS, Nijs J, Stepan J, Falch JA, Miazgowski T, Raptou P, Pols HA, Dequeker J, Havelka S, Hoszowski K, Jajic I, Czekalski S, Lyritis G, Silman AJ, Reeve J. Hip geometry, bone mineral distribution, and bone strength in European men and women: the EPOS study. Bone. 2000;27:151–159. doi: 10.1016/s8756-3282(00)00300-8. [DOI] [PubMed] [Google Scholar]
- 29.Peacock M, Liu G, Carey M, Ambrosius W, Turner CH, Hui S, Johnston CC., Jr Bone mass and structure at the hip in men and women over the age of 60 years. Osteoporos Int. 1998;8:231–239. doi: 10.1007/s001980050059. [DOI] [PubMed] [Google Scholar]
- 30.Seeman E. Clinical review 137: Sexual dimorphism in skeletal size, density, and strength. J Clin Endocrinol Metab. 2001;86:4576–4584. doi: 10.1210/jcem.86.10.7960. [DOI] [PubMed] [Google Scholar]
- 31.Kaptoge S, Dalzell N, Loveridge N, Beck TJ, Khaw KT, Reeve J. Effects of gender, anthropometric variables, and aging on the evolution of hip strength in men and women aged over 65. Bone. 2003;32:561–570. doi: 10.1016/s8756-3282(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 32.Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359:1841–1850. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- 33.Kinsella K, Velkoff VA. The demographics of aging. Aging Clin Exp Res. 2002;14:159–169. doi: 10.1007/BF03324431. [DOI] [PubMed] [Google Scholar]
- 34.Melton LJ., 3rd Epidemiology of hip fractures: implications of the exponential increase with age. Bone. 1996;18:121S–125S. doi: 10.1016/8756-3282(95)00492-0. [DOI] [PubMed] [Google Scholar]
- 35.Kaufman JM, Johnell O, Abadie E, Adami S, Audran M, Avouac B, Sedrine WB, Calvo G, Devogelaer JP, Fuchs V, Kreutz G, Nilsson P, Pols H, Ringe J, Van Haelst L, Reginster JY. Background for studies on the treatment of male osteoporosis: state of the art. Ann Rheum Dis. 2000;59:765–772. doi: 10.1136/ard.59.10.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Szulc P, Munoz F, Duboeuf F, Marchand F, Delmas PD. Bone mineral density predicts osteoporotic fractures in elderly men: the MINOS study. Osteoporos Int. 2005;16:1184–1192. doi: 10.1007/s00198-005-1970-9. [DOI] [PubMed] [Google Scholar]
- 38.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hannan MT, Felson DT, Anderson JJ. Bone mineral density in elderly men and women: results from the Framingham osteoporosis study. J Bone Miner Res. 1992;7:547–553. doi: 10.1002/jbmr.5650070511. [DOI] [PubMed] [Google Scholar]
- 40.Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PW, Kiel DP. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:710–720. doi: 10.1359/jbmr.2000.15.4.710. [DOI] [PubMed] [Google Scholar]
- 41.Martin RB, Burr DB. Non-invasive measurement of long bone cross-sectional moment of inertia by photon absorptiometry. J Biomech. 1984;17:195–201. doi: 10.1016/0021-9290(84)90010-1. [DOI] [PubMed] [Google Scholar]
- 42.Khoo BC, Beck TJ, Qiao QH, Parakh P, Semanick L, Prince RL, Singer KP, Price RI. In vivo short-term precision of hip structure analysis variables in comparison with bone mineral density using paired dual-energy X-ray absorptiometry scans from multi-center clinical trials. Bone. 2005;37:112–121. doi: 10.1016/j.bone.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Russo CR, Lauretani F, Seeman E, Bartali B, Bandinelli S, Di Iorio A, Guralnik J, Ferrucci L. Structural adaptations to bone loss in aging men and women. Bone. 2006;38:112–118. doi: 10.1016/j.bone.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 44.Szulc P, Duboeuf F, Schott AM, Dargent-Molina P, Meunier PJ, Delmas PD. Structural determinants of hip fracture in elderly women: re-analysis of the data from the EPIDOS study. Osteoporos Int. 2006;17:231–236. doi: 10.1007/s00198-005-1980-7. [DOI] [PubMed] [Google Scholar]
- 45.Bell KL, Loveridge N, Power J, Garrahan N, Stanton M, Lunt M, Meggitt BF, Reeve J. Structure of the femoral neck in hip fracture: cortical bone loss in the inferoanterior to superoposterior axis. J Bone Miner Res. 1999;14:111–119. doi: 10.1359/jbmr.1999.14.1.111. [DOI] [PubMed] [Google Scholar]
- 46.Power J, Loveridge N, Lyon A, Rushton N, Parker M, Reeve J. Bone remodeling at the endocortical surface of the human femoral neck: a mechanism for regional cortical thinning in cases of hip fracture. J Bone Miner Res. 2003;18:1775–1780. doi: 10.1359/jbmr.2003.18.10.1775. [DOI] [PubMed] [Google Scholar]
- 47.Mayhew PM, Thomas CD, Clement JG, Loveridge N, Beck TJ, Bonfield W, Burgoyne CJ, Reeve J. Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet. 2005;366:129–135. doi: 10.1016/S0140-6736(05)66870-5. [DOI] [PubMed] [Google Scholar]
- 48.Ruff CB. Body size, body shape, and long bone strength in modern humans. J Hum Evol. 2000;38:269–290. doi: 10.1006/jhev.1999.0322. [DOI] [PubMed] [Google Scholar]
- 49.Baudoin C, Fardellone P, Sebert JL. Effect of sex and age on the ratio of cervical to trochanteric hip fracture. A meta-analysis of 16 reports on 36,451 cases. Acta Orthop Scand. 1993;64:647–653. doi: 10.3109/17453679308994590. [DOI] [PubMed] [Google Scholar]
- 50.Lonnroos E, Kautiainen H, Karppi P, Huusko T, Hartikainen S, Kiviranta I, Sulkava R. Increased incidence of hip fractures. A population based-study in Finland. Bone. 2006 doi: 10.1016/j.bone.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7:407–413. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 52.Sanders KM, Nicholson GC, Ugoni AM, Pasco JA, Seeman E, Kotowicz MA. Health burden of hip and other fractures in Australia beyond 2000. Projections based on the Geelong Osteoporosis Study. Med J Aust. 1999;170:467–470. doi: 10.5694/j.1326-5377.1999.tb127845.x. [DOI] [PubMed] [Google Scholar]
- 53.Uusi-Rasi K, Sievanen H, Heinonen A, Beck TJ, Vuori I. Determinants of changes in bone mass and femoral neck structure, and physical performance after menopause: a 9-year follow-up of initially peri-menopausal women. Osteoporos Int. 2005;16:616–622. doi: 10.1007/s00198-004-1724-0. [DOI] [PubMed] [Google Scholar]
- 54.Szulc P, Beck TJ, Marchand F, Delmas PD. Low skeletal muscle mass is associated with poor structural parameters of bone and impaired balance in elderly men--the MINOS study. J Bone Miner Res. 2005;20:721–729. doi: 10.1359/JBMR.041230. [DOI] [PubMed] [Google Scholar]
- 55.Petit MA, Beck TJ, Lin HM, Bentley C, Legro RS, Lloyd T. Femoral bone structural geometry adapts to mechanical loading and is influenced by sex steroids: the Penn State Young Women's Health Study. Bone. 2004;35:750–759. doi: 10.1016/j.bone.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 56.Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB, Lukaski HC, Friedl K, Hubbard VS. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26:1596–1609. doi: 10.1038/sj.ijo.0802167. [DOI] [PubMed] [Google Scholar]


