Abstract
The potential benefit of ovarian hormone replacement therapy in cerebrovascular disease is well supported by experimental observations but not by recent large, randomized clinical trials. This discrepancy points out the need for better understanding of the vascular actions of ovarian hormones as well as medroxyprogesterone acetate (MPA), a synthetic analog of progesterone (P) widely prescribed in combination with estrogens. Therefore, we investigated whether in vivo exposure to 17β-estradiol (E) and/or P or MPA modifies inflammation in the cerebral vasculature, a key process in the evolution of ischemic brain injury. Female rats were injected (ip) with LPS to induce inflammation, and 6 h later brains were taken for blood vessel isolation and Western blot analysis of the inflammatory enzymes inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2). In ovariectomized (O) females, LPS induced cerebrovascular iNOS and COX-2; however, this effect was significantly decreased when O animals were treated for 3 wk with E. In contrast, treatment of O females with either MPA or P exacerbated the cerebrovascular inflammatory response to LPS. In intact females, LPS induction of iNOS and COX-2 in cerebral vessels was found to vary with the stage of the estrous cycle: LPS had the greatest effect during estrus, when circulating estrogen is low and progesterone is high. Thus exposure to endogenous or exogenous ovarian hormones appears to modulate cerebrovascular inflammation. Anti-inflammatory effects of estrogen would attenuate ischemic brain injury; however, this vasoprotective benefit may be diminished in the presence of progestagens.
Keywords: Progesterone, medroxyprogesterone, cerebrovasculature, lipopolysaccharide, cyclooxygenase-2, inducible nitric oxide synthase, estrous cycle
Vascular diseases are an enormous health care burden in Western societies, and the incidence increases substantially with aging (31). The relative risk in women also increases with age, diminishing the well-known sex difference in cardiovascular disease (18, 39). One contributing risk factor may be the postmenopausal decrease in ovarian hormones, which impacts many tissues, including the vasculature (15, 31). Observational studies and experimental animal research have consistently shown beneficial effects of estrogen on cerebrovascular function (15). However, recent large, randomized clinical trials raise questions about the benefit of hormone replacement therapy (HRT) and, in fact, found an increase in stroke (2, 14, 20).
These disparate findings point out the need for better understanding of the vascular actions of ovarian hormones as well as medroxyprogesterone acetate (MPA), a synthetic analog of progesterone (P) widely prescribed in combination with estrogen for treatment of perimenopausal symptoms. Not much is known about vascular effects of MPA, but several studies have suggested that MPA may oppose beneficial effects of estrogen on cardiovascular function (4, 34, 35). For instance, MPA negates the beneficial effects of estrogen on lipid metabolism, vascular reactivity, and atherosclerotic progression (4). A better understanding of effects of P and MPA may elucidate ways to mitigate adverse effects of HRT and selectively enhance beneficial effects on the cardiovascular system.
Cerebrovascular inflammation plays a central role in the pathogenesis of cerebral ischemia and secondary effects contributing to blood-brain barrier damage (8), cerebral edema, increased intracranial pressure, and possible brain herniation (7). A key process in cerebrovascular inflammation is the induction of proinflammatory mediators, including inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) (21, 28). During cerebral ischemia, COX-2 is upregulated, resulting in the production of prostanoids, such as PGE2, that are thought to be detrimental to stroke outcome (16). Expression of iNOS is also elevated, and this enzyme has been shown to peak 24–48 h after cerebral ischemic injury (17). NO production is thought to have both beneficial and deleterious effects following ischemia, depending on the cellular compartment, quantities produced and the stage of evolution of cerebral injury (5, 17).
Experimental animal studies have consistently shown that estrogen has beneficial effects in models of cerebrovascular injury (15). We (29) previously found that in vivo estrogen treatment suppresses inflammation induced by interleukin-1β in rat cerebral blood vessels. However, possible effects of progestagens on cerebrovascular inflammation are unknown. To investigate this question, we used rat models of in vivo progestagen treatment, which mirror known clinical blood levels (23). Because of the unexpected adverse effects of combined HRT on stroke (1), we hypothesized that progestagens, P or MPA, would negate the effects of estrogen on inflammation. We also hypothesized that normal changes in endogenous estrogen and P throughout the estrous cycle would cause variation in cerebrovascular inflammation. Specifically, we measured lipopolysaccharide (LPS) induction of both iNOS and COX-2 in cerebral blood vessels isolated from female rats that were either intact, ovariectomized, or treated with 17β-estradiol (E), P, and/or MPA.
MATERIALS AND METHODS
In vivo hormone and LPS treatments
The present study was conducted in accordance with National Institutes of Health guidelines for the care and use of animals in research and under protocols approved by the Institutional Animal Care and Use Committee at the University of California, Irvine. Seven groups of rats were used: intact females, ovariectomized females (O), O treated with E (OE), O treated with P (OP), O treated with both E and P (OE-P), O treated with MPA (O-MPA), and O treated with both E and MPA (OE-MPA). Three-month-old female Fischer 344 rats (Charles River-SASCO Laboratories) were ovariectomized while under anesthesia (46 mg/kg ketamine and 4.6 mg/kg xylazine ip). During ovariectomy, some rats were subcutaneously implanted with Silastic capsules (1.57 mm ID × 3.18 mm OD) containing either E (5 mm length), P (90 mm length), or MPA (3 mm length). Animals were allowed to recover from surgery and then were returned to a vivarium, where they were housed in individual cages with free access to chow and water in a temperature-controlled room (22°C) on a 12:12-h light-dark cycle.
One month after surgery and hormone capsule implantation, animals were injected intraperitoneally with LPS (1 mg/kg) or an equivalent volume of vehicle (0.9% saline). At various time points (1–24 h) after LPS or saline injection, animals were anesthetized with CO2, and blood samples were obtained by cardiac puncture for measurement of serum E and P by radioimmunoassay (Diagnostic Products, CA and MP Biomedicals, respectively). Rats then were killed by decapitation, and their brains were immediately removed, frozen in dry ice, and kept at −80°C until analysis.
LPS treatment of intact cycling females
The estrous cycle of intact female rats was monitored by daily collection and microscopic inspection of vaginal smears. The types of cells present were used to determine the stage of the estrous cycle (9). Only female rats showing at least two consecutive 4- or 5-day estrous cycles were used. On the morning of diestrus, proestrus, or estrus, animals were injected with LPS (1 mg/kg ip) or an equivalent volume of vehicle (0.9% saline) and euthanized 6 h later for collection of brain and blood samples.
Cerebral blood vessel isolation
Cerebral blood vessels were isolated using procedures previously described (22, 30). Whole brains were gently Dounce homogenized in ice-cold 0.01 mol/l phosphate-buffered saline (PBS, pH 7.4) and centrifuged at 4,500 g for 5 min at 4°C. The pellet was washed several times by resuspension in PBS followed by centrifugation at 4,500 g for 5 min. The pellet was then resuspended in PBS, layered over 15% dextran (MW = 35,000–40,000 Da; Sigma, St. Louis, MO) and centrifuged in a swinging bucket rotor at 4,500 g for 45 min at 4°C. The top layer of the gradient was discarded and the pellet containing blood vessels collected over a 50-μm nylon mesh and washed for several minutes with cold PBS. The vessels isolated by this procedure contain a mixture of arteries, arterioles, veins, venules, and capillaries.
Western blot analysis
Levels of iNOS and COX-2 proteins were measured by Western blot analysis of cerebral vessel lysates. Vessels were glass homogenized in lysis buffer (50 mmol/l β-glycerophosphate, 100 mmol/l NaVO3, 2 mmol/l MgCl2, 1 mmol/l EGTA, 0.5% Triton X-100, 1 mmol/l DL-dithiothreitol, 20 μmol/l pepstatin, 20 μmol/l leupeptin, 0.1 U/ml aprotinin, and 1 mmol/l phenylmethylsulfonyl fluoride) and incubated on ice for 25 min. Samples then were centrifuged at 4,500 g for 10 min at 4°C. Supernatants were collected, and protein content was determined by bicinchoninic acid assay (Pierce).
For every experiment, vessel samples from each of the different animal groups being compared were run together on the same Western blot. Equal amounts of vessel protein (20 μg/lane) were loaded in each lane of 8% Tris-glycine gels, and proteins were separated by SDS-PAGE. Additionally, a positive control, PMA/LPS-induced RAW 264.7 whole cell lysate (Santa Cruz Biotechnology, Santa Cruz, CA), and broad-range biotinylated molecular weight markers (Bio-Rad) were also loaded for identification of protein bands. After electrophoretic separation, proteins were transferred to nitrocellulose membranes (Amersham, Piscataway, NJ) in blocking buffer containing 0.01 mol/l PBS + 0.1% Tween-20 (PBST) and 6.5% nonfat dry milk. Membranes were then incubated with the primary antibody of interest: rabbit polyclonal anti-COX-2 (1:1,000; Cayman Chemical, Ann Arbor, MI), rabbit polyclonal anti-iNOS (1:400; Santa Cruz Biotechnology) or mouse monoclonal anti-α-actin (1:15,000; Sigma) followed by the appropriate secondary antibody: anti-rabbit IgG-horseradish peroxidase (1:5,000) or anti-mouse IgG-horseradish peroxidase (1:5,000; Transduction Laboratories). α-Actin was used to verify equal protein loading of all lanes of the gel. Immunoreactive bands were detected using electrochemiluminescence reagent (Amersham) and Hyperfilm (Amersham), and band density was quantitated with the computer-based electrophoresis analysis program UNSCAN-IT (Silk Scientific, Orem, UT).
Statistical analysis
Data are expressed as means ± SE. Statistical analysis was performed with GraphPad Prism 4.0a software. Differences between groups were determined by one-way ANOVA. Optical density measurements were analyzed by ANOVA with repeated measures. Pairwise comparisons were made using Newman-Keuls post hoc analysis. In all cases, statistical significance was set at P ≤ 0.05.
RESULTS
In vivo models of hormone treatment
Chronic hormone treatment of ovariectomized female rats was used to test the effects of E, MPA, and P on in vivo induction of cerebrovascular iNOS and COX-2 by LPS. To verify our hormone models, serum levels of E and P, whole body weights, and dry uterine weights were measured 1 mo after surgical and hormone treatments (Table 1). Serum levels of E in OE, OE-P, and OE-MPA animals approximate those found in normally cycling female rats (22); these values were significantly greater than those found in O, OP, or OMPA animals. Serum levels of P from OP and OE-P groups were similar to those detected in normally cycling rats (3) and were significantly greater than all other animal groups studied. MPA levels were not measured; however, we have previously demonstrated (23) that implantation of MPA capsules 3 mm in length in ovariectomized female rats results in serum levels that are similar to those observed clinically in humans.
Table 1.
Effect of chronic in vivo steroid treatment on serum levels of 17β-estradiol and progesterone and body and uterine weights
| Rat Group | 17β-Estradiol, pg/ml | Progesterone, ng/ml | Body Weight, g | Uterine Weight, mg |
|---|---|---|---|---|
| O | 9±1* | 12±2* | 203±3* | 10±2* |
| OE | 51±2† | 17±2* | 170±2† | 182±3† |
| OP | 5±3* | 37±2† | 185±1‡ | 52±7‡ |
| OMPA | 7±2* | 9±2* | 183±2‡ | 46±3‡ |
| OE-P | 41±3† | 39±1† | 176±2† | 93±8§ |
| OE-MPA | 47±2† | 6±1* | 177±2† | 90±7§ |
Values represent means ± SE. O, ovariectomized; E, 17β-estradiol; P, progesterone; MPA, medroxyprogesterone acetate. Statistical differences were determined by one-way ANOVA followed by Newman-Keuls post hoc tests. Within each column, groups designated by different symbols are significantly different (P ≤ 0.05), n = 15–30.
Whole body weights of O rats were significantly higher compared with any of the five hormone treatment groups. In addition, the body weights of both OP and OMPA groups were higher than those of OE rats. As expected, E increased uterine weight, but this effect was attenuated in the presence of P or MPA in combination with E. Together, the results in Table 1 indicate that implanted pellets achieved physiological levels of E and P for the 1-mo treatment period and that the E, P, and MPA treatments were biologically active.
LPS induction of cerebrovascular iNOS and COX-2
We initially examined the time course of COX-2 and iNOS protein expression in cerebral vessels at different time points, from 1 to 24 h, following administration of LPS or vehicle. Western blots using antibodies to iNOS and COX-2 showed, respectively, a single band migrating at 130 kDa (Fig. 1) and a characteristic doublet at ~72 kDa (Fig. 2). These bands corresponded to the expected molecular masses of these proteins and comigrated with the appropriate positive controls. In vessels from vehicle-injected animals, COX-2 protein was detected at low levels, whereas iNOS protein was undetectable. In contrast, LPS significantly increased the levels of both COX-2 and iNOS proteins in cerebral vessels. Maximal induction of both enzymes was observed at 6 h after LPS injection, and this time point was used in all subsequent studies.
Fig. 1.
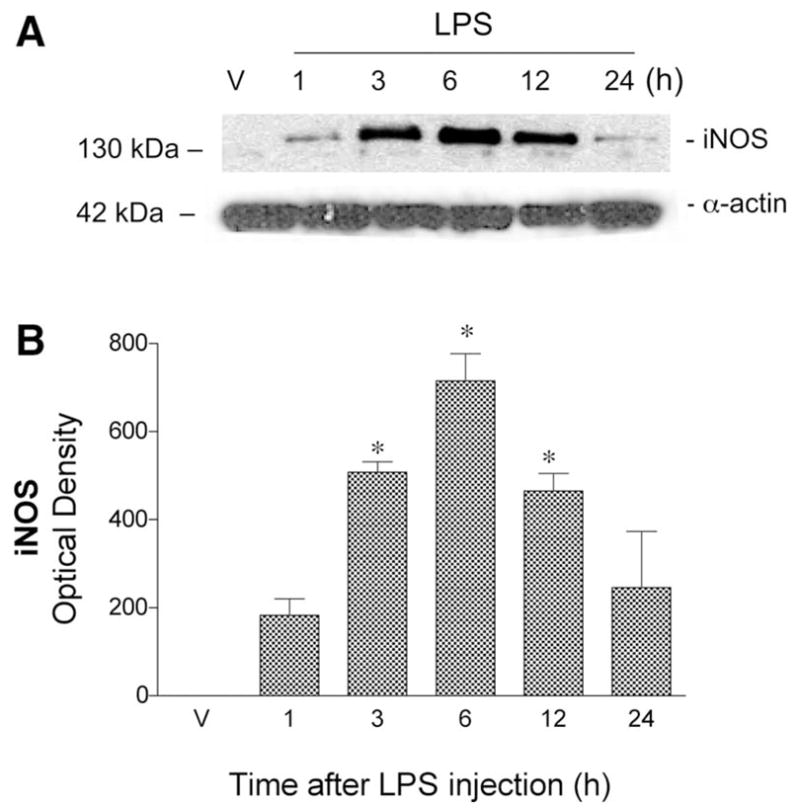
Twenty-four-hour time course of cerebrovascular inducible NO synthase (iNOS) protein expression after LPS or saline (vehicle, V) administration (ip) to ovariectomized (O) rats. A: representative Western blot illustrates iNOS immunoreactivity in cerebral vessel lysates at different time points. Bands migrating at 130 and 42 kDa were detected using antibodies directed against iNOS and α-actin, respectively. B: iNOS optical density (OD) values were quantitated from Western blots. Values represent means ± SE; n = 3 rats. *Significantly different from 1-h time point, P ≤ 0.05.
Fig. 2.
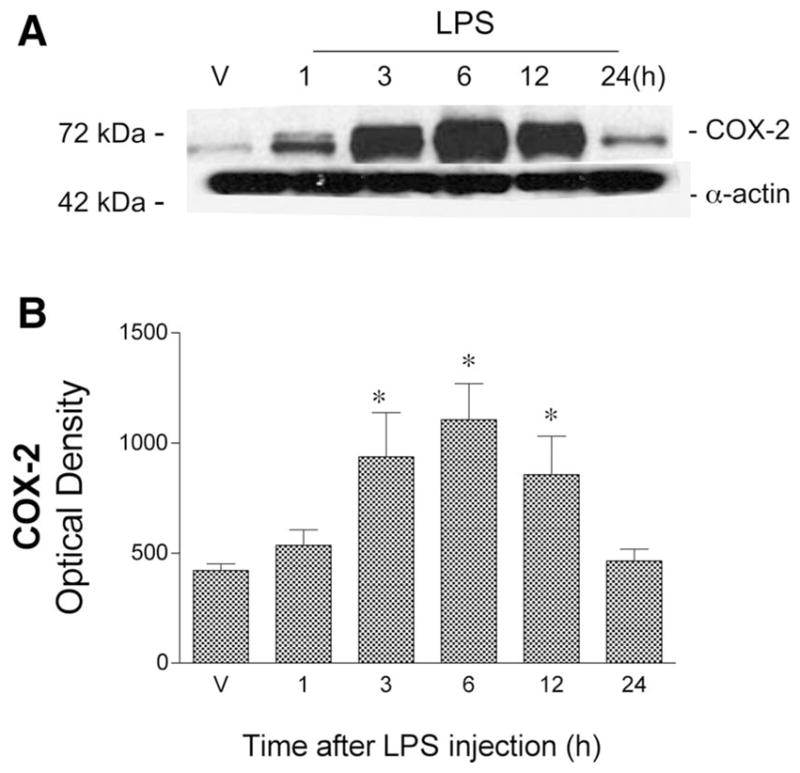
Twenty-four-hour time course of cerebrovascular cyclooxygenase-2 (COX-2) protein expression after ip injection of LPS or saline (V) in O rats. A: representative Western blot illustrates COX-2 immunoreactivity in cerebral vessel lysates at different time points. Bands migrating at 72 and 42 kDa were detected using antibodies directed against COX-2 and α-actin, respectively. B: COX-2 ODs were quantitated from Western blots. Values represent means ± SE; n = 3 rats. *Significantly different from V values, P ≤ 0.05.
Effect of E and P treatments
We next investigated the effects of chronic E and/or P treatment on levels of cerebrovascular iNOS and COX-2 following LPS or vehicle administration. As shown in Figs. 3 and 4, chronic E treatment significantly attenuated induction of both proinflammatory enzymes compared with levels in O animals. In contrast, P alone significantly exacerbated the cerebrovascular inflammatory response to LPS compared with O. When given in combination, E significantly reversed the effect observed with P alone.
Fig. 3.
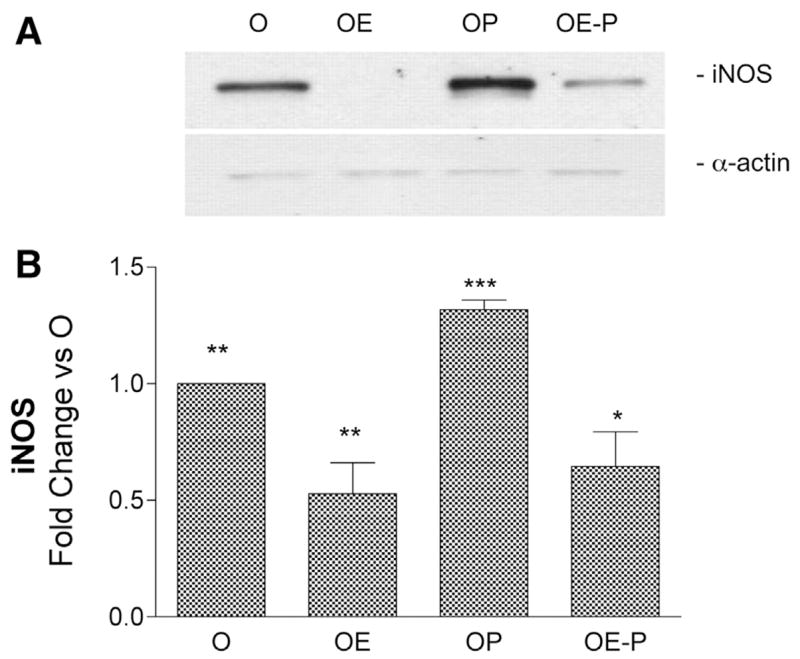
Effects of in vivo 17β-estradiol (E) and progesterone (P) treatments on levels of cerebrovascular iNOS protein after LPS injection. A: representative Western blot illustrates differences in iNOS immunoreactivity in cerebral vessel lysates from O, OE, OP, and OE-P groups injected with LPS (6 h). Bands migrating at 130 and 42 kDa were detected with antibodies directed against iNOS and α-actin, respectively. B: ODs of iNOS bands were quantitated and expressed as fold change vs. O values. Means ± SE are plotted; n = 4 rats. Significance was determined using OD values by ANOVA with repeated measures. *Significantly different from 1 other group, **2 other groups, ***3 other groups, P ≤ 0.05.
Fig. 4.
Effects of in vivo E and P treatments on levels of cerebrovascular COX-2 protein after LPS injection. A: representative Western blot illustrates differences in COX-2 immunoreactivity in cerebral vessel lysates from O, OE, OP, and OE-P groups injected with LPS (6 h). Bands migrating at 72 and 42 kDa were detected using antibodies directed against COX-2 and α-actin, respectively. B: ODs of COX-2 bands were quantitated and expressed as fold change vs. O values. Means ± SE are plotted; n = 4 rats. Significance was determined using OD values by ANOVA with repeated measures. *Significantly different from 1 other group, **2 other groups, ***3 other groups, P ≤ 0.05.
Because of its clinical use in humans, we determined whether a synthetic progestagen, MPA, would have effects similar to those produced by P. As shown in Figs. 5 and 6, MPA treatment alone also significantly increased the levels of iNOS and COX-2 after LPS administration. When E was given in combination with MPA, it significantly lowered the levels of iNOS and COX-2 from those observed with MPA alone. In all groups studied, there were no significant differences in the levels of inflammatory factors iNOS and COX-2 among groups treated with saline control.
Fig. 5.

Effects of in vivo E and medroxyprogesterone acetate (MPA) treatments on levels of cerebrovascular iNOS protein after LPS injection. A: representative Western blot illustrates differences in iNOS immunoreactivity in cerebral vessel lysates from O, OE, O-MPA, and OE-MPA groups injected with LPS (6 h). B: ODs of iNOS bands were quantitated and expressed as fold change vs. O values. Means ± SE are plotted; n = 4 rats. Significance was determined using OD values by ANOVA with repeated measures. **Significantly different from 2 other groups, ***3 other groups, P ≤ 0.05.
Fig. 6.
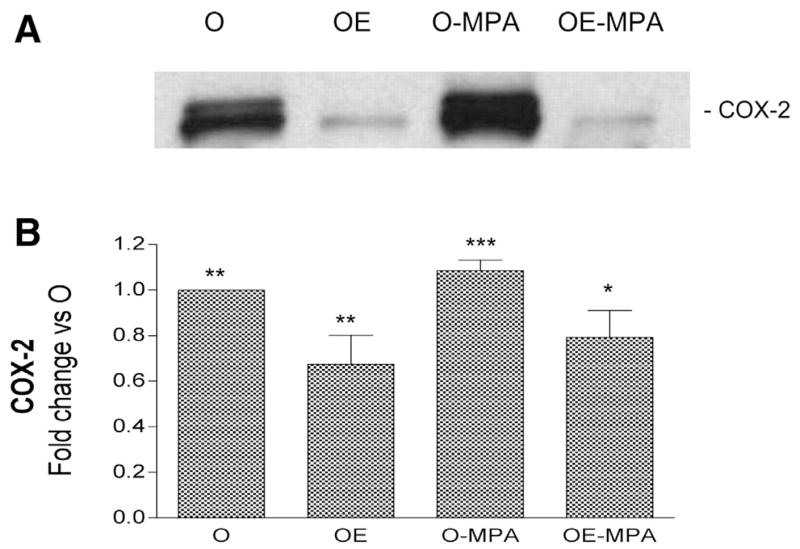
Effects of in vivo E and MPA treatments on levels of cerebrovascular COX-2 protein after LPS injection. A: representative Western blot illustrates differences in COX-2 immunoreactivity in cerebral vessel lysates from O, OE, O-MPA, and OE-MPA groups injected with LPS (6 h). B: ODs of COX-2 bands were quantitated and expressed as fold change vs. O values. Means ± SE are plotted; n = 4 rats. Significance was determined using OD values by ANOVA with repeated measures. *Significantly different from 1 other group, **2 other groups, ***3 other groups, P ≤ 0.05.
Effect of the estrous cycle on cerebrovascular inflammation
We verified the stage of the estrous cycle for intact females by examining the cytology of vaginal smears and measuring serum levels of E and P at the time of euthanasia (Table 2). As expected, E was highest during the proestrus stage, whereas P was highest during estrus (3).
Table 2.
Serum levels of 17β-estradiol and progesterone at different stages of the estrous cycle in female rats
| Rat Group | n | 17β-Estradiol, pg/ml | Progesterone, ng/ml |
|---|---|---|---|
| Diestrus | 12 | 31±1* | 22±2† |
| Proestrus | 16 | 82±10† | 5±2† |
| Estrus | 16 | 24±6* | 44±4† |
Values represent means ± SE. Statistical differences were determined by one-way ANOVA followed by Newman-Keuls post hoc tests.
Significantly different from 1 group;
significantly different from 2 groups within the same column, P < 0.05.
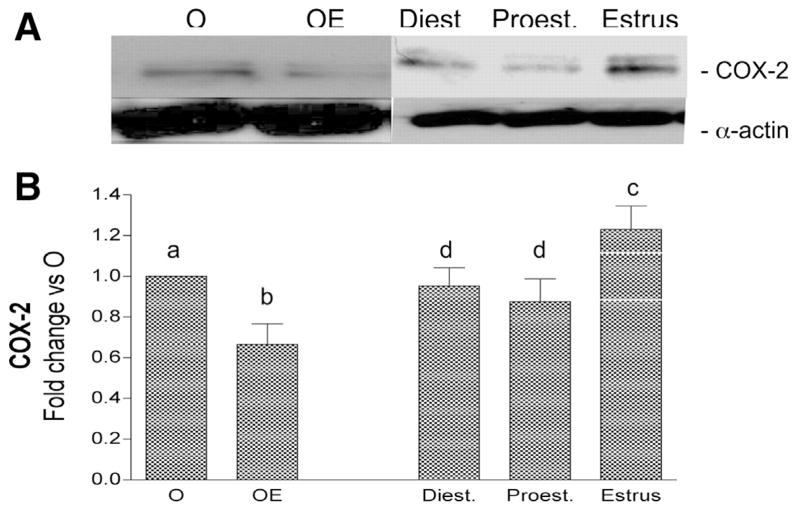
Cerebrovascular levels of iNOS and COX-2 were determined after LPS administration at each stage of the cycle. In all cases there was an increase in cerebral vessel iNOS and COX-2 at 6 h after LPS injection. However, the level of induction of these inflammatory markers varied significantly depending on the stage of the estrous cycle (Figs. 7 and 8). Protein levels of iNOS and COX-2 were greatest in cerebral vessels from animals injected with LPS during estrus. In contrast, LPS injection on the previous day of the cycle, proestrus, resulted in significantly lower levels of iNOS and COX-2, similar to levels found in OE animals. LPS injection on the day of diestrus also resulted in lower levels of iNOS and COX-2 compared with estrus.
Fig. 7.
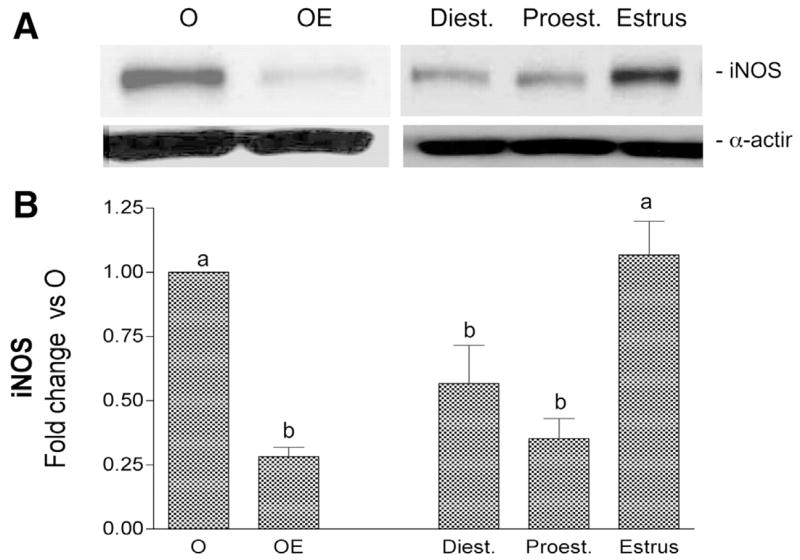
Effect of estrous cycle on LPS induction of iNOS protein in cerebral blood vessels. A: representative Western blot illustrates differences in cerebrovascular iNOS immunoreactivity when LPS (6 h) is injected at different stages of the rat estrous cycle. LPS-injected O and OE groups were also run for comparison. B: ODs of iNOS bands were quantitated and expressed as fold change vs. O values. Means ± SE are shown; n = 16 rats. Significance was determined using OD values by ANOVA with repeated measures; groups designated by different letters are significantly different, P ≤ 0.05.
Fig. 8.
Effect of estrous cycle on LPS induction of COX-2 protein in cerebral blood vessels. A: representative Western blot illustrates differences in cerebrovascular COX-2 immunoreactivity when LPS (6 h) is injected at different stages of the rat estrous cycle. LPS-injected O and OE groups were also run for comparison. B: ODs of COX-2 bands are quantitated and expressed as fold change vs. O values. Means ± SE are shown; n = 4 rats. Significance was determined using OD values by ANOVA with repeated measures; groups designated by different letters are significantly different, P ≤ 0.05.
DISCUSSION
The most striking finding of this study is that estrogen and progestagens have significant, but opposite, effects on induction of cerebrovascular inflammation. The proinflammatory enzymes iNOS and COX-2 were induced in cerebral blood vessels following in vivo injection of the bacterial endotoxin LPS. Chronic treatment of ovariectomized animals with either P or its synthetic analog MPA exacerbated this inflammatory response, whereas E treatment suppressed it. Endogenous hormones appear to exert similar actions on the cerebral vessels. LPS induction of iNOS and COX-2 proteins varied over the estrous cycle, with the greatest effect observed during estrus, when circulating P is high and E is low. Effects of endogenous hormones on susceptibility to cerebrovascular inflammation could be a factor in conditions such as menstrual migraine (19). The anti-inflammatory effect of estrogen on cerebral blood vessels also may contribute to the ability of estrogen to attenuate ischemic brain injury (20, 40). However, this protective effect of estrogen appears to be opposed by the presence of progestagens. This type of interaction could explain, in part, the recent negative clinical results with combination hormone therapy (1).
The hormone-treated rat models that we used resulted in physiological serum levels of E and P and levels of MPA seen clinically (23). We also verified the physiological actions of these hormones on body and uterine weights (22). Estrogen-treated animals showed a consistent reduction in body weight that may be due to increased resting energy expenditure (6). Recent observations in our laboratory have shown that estrogen increases the rate-limiting steps in energy production (38). Alternatively, estrogen may have hypophagic effects (26). The physiological relevance of the hormone administration regimens that we have used is also supported by trophic effects of E on the uterus, an effect partially blocked by P or MPA (4).
The inflammatory response to LPS is a well-established model for upregulation of iNOS and COX-2 both in vitro and in vivo in brain and peripheral tissues (13). In our study, we administered LPS peripherally and demonstrated upregulation of iNOS and COX-2 in the cerebral vasculature. We (29) have recently demonstrated a similar vascular upregulation of COX-2 after peripheral administration of the cytokine interleukin-1β. The induction of these inflammatory markers has been implicated in the pathogenesis of cerebral ischemic injury in experimental models, and inhibition of these factors protects against cerebral injury (8, 13). Production of inflammatory mediators might be beneficial or deleterious during ischemic stroke depending on the cellular compartment in which they are produced, the quantities produced, and the stage of evolution of cerebral injury (5, 17). Nevertheless, defining the effects of gonadal steroids on this process provides important new information.
As we have previously demonstrated (29), exogenous chronic estrogen treatment suppressed the vascular inflammatory response. In contrast to what we observe in cerebral blood vessels, estrogen has been shown to upregulate basal COX-2-derived prostacyclin production in the mouse aortic smooth muscle cells both in vivo and in vitro (10). Estrogen also upregulated iNOS both in vitro and in vivo in ovine coronary arteries (24). We (23, 30) have previously shown that estrogen upregulates basal levels of the constitutive isoforms endothelial (e)NOS and COX-1; thus it is possible that the effects outlined above represent regulation of basal production of mediators unrelated to inflammatory responses. Indeed, the effects of estrogen to suppress induction of inflammatory mediators appear to represent a general suppression of the inflammatory response rather than actions on specific mediators. Estrogen has been shown to suppress NF-kB activation in cerebral blood vessels (29) as well as in cerebral endothelial cells (11), suggesting that estrogen acts to suppress the inflammatory response as a whole and not the upregulation of specific mediators (29). Interestingly, we have also recently shown (32) that estrogen also suppresses the cerebrovascular inflammatory response to LPS in male rats. Furthermore, testosterone has the opposite effect, exacerbating the induction of proinflammatory factors in cerebral vessels (32).
An important aspect of our study was examination of the effects of progestagens. MPA is a synthetic analog of P, commonly used in combination with estrogen in hormone replacement therapy (34). We found that either MPA or P alone exacerbated the inflammatory response to LPS. In combination with estrogen, P or MPA partially reversed the anti-inflammatory effects of estrogen. Some have speculated that addition of MPA or P may negate the beneficial cardiovascular actions of estrogen, and this might be a confounding factor that counteracts potential beneficial effects of estrogen (4, 37).
Complexities in the biological actions of these compounds have been reported, either showing synergistic actions with estrogen or alternatively negating the effects of estrogen. These discrepancies might be explained by tissue/cell specificity, receptor heterogeneity, or nonspecific interactions with other signaling pathways or androgen receptors (36). In fact, MPA has been shown to negate some effects of estrogen on the cardiovascular system. For instance, MPA negates the beneficial effect of estrogen on lipid metabolism, vascular reactivity, vascular injury response, and atherosclerotic progression (4, 37, 41). In contrast to our study, others have shown that MPA does not block the suppressive actions of estrogen on PGE2 production (29) and does not affect estrogen-induced increases in cerebrovascular eNOS (23) or suppression by estrogen of intercellular adhesion molecule-1 and E-selectin induction in cultured cells (25). There are also conflicting studies concerning the effect of P on cardiovascular and cerebrovascular function (37). Some have shown beneficial effects of P, whereas others show little or no effect (36). For example, P has been shown to have a positive effect on exercise-induced myocardial ischemia and on the outcome of experimental stroke (12, 33). Of course, the cerebral vasculature is only one component involved in stroke; neuroprotective effects of of P and its neurosteroid metabolites must also be considered in the overall effects of P. Clearly, further work must be done to understand the cellular and molecular mechanisms by which gonadal steroids and their analogs modulate inflammatory responses. Emerging concepts of the mechanisms of action of nuclear hormones suggest that the cellular context, including patterns of expression of transcription factors, may modify hormone action, greatly increasing the possibility for hormonal action complexity and specificity (27).
Besides investigating the actions of exogenous hormone, we also determined the impact of endogenous gonadal steroids on the vascular inflammatory response to LPS. To do so, we followed the response to LPS throughout the estrous cycle. LPS induction of these inflammatory mediators in the cerebral vasculature varied markedly with estrous cycle stage, with lowest expression during proestrus and highest expression during estrus. Because proestrus is the stage with the highest endogenous estrogen whereas estrus has the lowest estrogen levels, one interpretation of our findings is that endogenous estrogen in intact female rats suppresses LPS-induced induction of vascular iNOS and COX-2. However, because levels of P rise just before estrus, proinflammatory effects of P might also contribute to the variation in inflammatory response through the estrous cycle. Future experiments would be important in sorting out these two effects. Nevertheless, this finding underscores the variability of the cerebrovascular inflammatory response over the ovarian cycle. These changes may contribute to conditions associated with the menstrual cycle such as menstrual migraine (19).
In conclusion, systemic LPS upregulated iNOS and COX-2 expression in cerebral blood vessels; endogenous estrogen or chronic 17β-estradiol treatment attenuated this effect. In contrast, either MPA or progesterone alone exacerbated the inflammatory response to LPS and partially reversed the anti-inflammatory effect of estrogen. Reaction products of the proinflammatory mediators iNOS and COX-2 have been implicated in the evolution of cerebral ischemic injury (8, 16). The dual roles of these mediators in modulating cerebral resistance or sensitivity to ischemic injury makes them interesting and challenging pharmacological targets to study, and the cerebral vasculature is an important site for initiation and evolution of responses to injury and other inflammatory stimuli. Understanding possible effects of ovarian steroid hormones on cerebrovascular inflammatory pathways may clarify reasons for the well-known sex difference in stroke outcome and incidence when premenopausal women are compared with age-matched males, as well as the increase in cardiovascular diseases associated with decline in ovarian hormones in post menopausal women (18).
Acknowledgments
We thank Jonnie Stevens for performing surgeries and radioimmunoassays.
GRANTS
This project was supported by National Heart, Lung, and Blood Institute Grant R01 HL-50775.
References
- 1.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 2.Brass LM. Hormone replacement therapy and stroke: clinical trials review. Stroke. 2004;35:2644–2647. doi: 10.1161/01.STR.0000143218.20061.ac. [DOI] [PubMed] [Google Scholar]
- 3.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 4.Clarkson TB. Progestogens and cardiovascular disease. A critical review. J Reprod Med. 1999;44:180–184. [PubMed] [Google Scholar]
- 5.Dawson VL, Dawson TM. Nitric oxide in neurodegeneration. Prog Brain Res. 1998;118:215–229. doi: 10.1016/s0079-6123(08)63210-0. [DOI] [PubMed] [Google Scholar]
- 6.Day DS, Gozansky WS, Van Pelt RE, Schwartz RS, Kohrt WM. Sex hormone suppression reduces resting energy expenditure and (beta)-adrenergic support of resting energy expenditure. J Clin Endocrinol Metab. 2005;90:3312–3317. doi: 10.1210/jc.2004-1344. [DOI] [PubMed] [Google Scholar]
- 7.de Vries HE, Kuiper J, de Boer AG, Van Berkel TJ, Breimer DD. The blood-brain barrier in neuroinflammatory diseases. Pharmacol Rev. 1997;49:143–155. [PubMed] [Google Scholar]
- 8.del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doolen S, Krause DN, Duckles SP. Estradiol modulates vascular response to melatonin in rat caudal artery. Am J Physiol Heart Circ Physiol. 1999;276:H1281–H1288. doi: 10.1152/ajpheart.1999.276.4.h1281. [DOI] [PubMed] [Google Scholar]
- 10.Egan KM, Lawson JA, Fries S, Koller B, Rader DJ, Smyth EM, Fitzgerald GA. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004;306:1954–1957. doi: 10.1126/science.1103333. [DOI] [PubMed] [Google Scholar]
- 11.Galea E, Santizo R, Feinstein DL, Adamsom P, Greenwood J, Koenig HM, Pelligrino DA. Estrogen inhibits NF kappa B-dependent inflammation in brain endothelium without interfering with I kappa B degradation. Neuroreport. 2002;13:1469–1472. doi: 10.1097/00001756-200208070-00024. [DOI] [PubMed] [Google Scholar]
- 12.Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab. 2004;24:805–813. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- 13.Han HS, Qiao Y, Karabiyikoglu M, Giffard RG, Yenari MA. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. J Neurosci. 2002;22:3921–3928. doi: 10.1523/JNEUROSCI.22-10-03921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 15.Hurn PD, Brass LM. Estrogen and stroke: a balanced analysis. Stroke. 2003;34:338–341. doi: 10.1161/01.str.0000054051.88378.25. [DOI] [PubMed] [Google Scholar]
- 16.Iadecola C, Forster C, Nogawa S, Clark HB, Ross ME. Cyclooxygenase-2 immunoreactivity in the human brain following cerebral ischemia. Acta Neuropathol (Berl) 1999;98:9–14. doi: 10.1007/s004010051045. [DOI] [PubMed] [Google Scholar]
- 17.Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997;17:9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isles CG, Hole DJ, Hawthorne VM, Lever AF. Relation between coronary risk and coronary mortality in women of the Renfrew and Paisley survey: comparison with men. Lancet. 1992;339:702–706. doi: 10.1016/0140-6736(92)90599-x. [DOI] [PubMed] [Google Scholar]
- 19.Marcus DA. Sex hormones and chronic headache in women. Expert Opin Pharmacother. 2001;2:1839–1848. doi: 10.1517/14656566.2.11.1839. [DOI] [PubMed] [Google Scholar]
- 20.McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14:228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 21.McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 22.McNeill AM, Kim N, Duckles SP, Krause DN, Kontos HA. Chronic estrogen treatment increases levels of endothelial nitric oxide synthase protein in rat cerebral microvessels. Stroke. 1999;30:2186–2190. doi: 10.1161/01.str.30.10.2186. [DOI] [PubMed] [Google Scholar]
- 23.McNeill AM, Zhang C, Stanczyk FZ, Duckles SP, Krause DN. Estrogen increases endothelial nitric oxide synthase via estrogen receptors in rat cerebral blood vessels: effect preserved after concurrent treatment with medroxyprogesterone acetate or progesterone. Stroke. 2002;33:1685–1691. doi: 10.1161/01.str.0000016325.54374.93. [DOI] [PubMed] [Google Scholar]
- 24.Mershon JL, Baker RS, Clark KE. Estrogen increases iNOS expression in the ovine coronary artery. Am J Physiol Heart Circ Physiol. 2002;283:H1169–H1180. doi: 10.1152/ajpheart.00397.2000. [DOI] [PubMed] [Google Scholar]
- 25.Mueck AO, Seeger H, Wallwiener D. Medroxyprogesterone acetate versus norethisterone: effect on estradiol-induced changes of markers for endothelial function and atherosclerotic plaque characteristics in human female coronary endothelial cell cultures. Menopause. 2002;9:273–281. doi: 10.1097/00042192-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Mystkowski P, Schwartz MW. Gonadal steroids and energy homeostasis in the leptin era. Nutrition. 2000;16:937–946. doi: 10.1016/s0899-9007(00)00458-5. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson S, Gustafsson JA. Estrogen receptor transcription and trans-activation: basic aspects of estrogen action. Breast Cancer Res. 2000;2:360–366. doi: 10.1186/bcr81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nogawa S, Forster C, Zhang F, Nagayama M, Ross ME, Iadecola C. Interaction between inducible nitric oxide synthase and cyclooxygenase-2 after cerebral ischemia. Proc Natl Acad Sci USA. 1998;95:10966–10971. doi: 10.1073/pnas.95.18.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ospina JA, Brevig HN, Krause DN, Duckles SP. Estrogen suppresses IL-1β-mediated induction of COX-2 pathway in rat cerebral blood vessels. Am J Physiol Heart Circ Physiol. 2004;286:H2010–H2019. doi: 10.1152/ajpheart.00481.2003. [DOI] [PubMed] [Google Scholar]
- 30.Ospina JA, Krause DN, Duckles SP. 17Beta-estradiol increases rat cerebrovascular prostacyclin synthesis by elevating cyclooxygenase-1 and prostacyclin synthase. Stroke. 2002;33:600–605. doi: 10.1161/hs0202.102732. [DOI] [PubMed] [Google Scholar]
- 31.Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 32.Razmara A, Krause DN, Duckles SP. Testosterone augments endotoxin-mediated cerebrovascular inflammation in male rats. Am J Physiol Heart Circ Physiol. 2005;289:H1843–H1850. doi: 10.1152/ajpheart.00465.2005. [DOI] [PubMed] [Google Scholar]
- 33.Rosano GM, Webb CM, Chierchia S, Morgani GL, Gabraele M, Sarrel PM, de Ziegler D, Collins P. Natural progesterone, but not medroxyprogesterone acetate, enhances the beneficial effect of estrogen on exercise-induced myocardial ischemia in postmenopausal women. J Am Coll Cardiol. 2000;36:2154–2159. doi: 10.1016/s0735-1097(00)01007-x. [DOI] [PubMed] [Google Scholar]
- 34.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 35.Simon JA, Hsia J, Cauley JA, Richards C, Harris F, Fong J, Barrett-Connor E, Hulley SB. Postmenopausal hormone therapy and risk of stroke: the Heart and Estrogen-Progestin Replacement Study (HERS) Circulation. 2001;103:638–642. doi: 10.1161/01.cir.103.5.638. [DOI] [PubMed] [Google Scholar]
- 36.Simoncini T, Mannella P, Fornari L, Caruso A, Varone G, Genazzani AR. In vitro effects of progesterone and progestins on vascular cells. Steroids. 2003;68:831–836. doi: 10.1016/j.steroids.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Simoncini T, Mannella P, Fornari L, Caruso A, Willis MY, Garibaldi S, Baldacci C, Genazzani AR. Differential signal transduction of progesterone and medroxyprogesterone acetate in human endothelial cells. Endocrinology. 2004;145:5745–5756. doi: 10.1210/en.2004-0510. [DOI] [PubMed] [Google Scholar]
- 38.Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol. 2005;68:959–965. doi: 10.1124/mol.105.014662. [DOI] [PubMed] [Google Scholar]
- 39.Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. doi: 10.1161/01.cir.90.1.583. [DOI] [PubMed] [Google Scholar]
- 40.Wise PM. Estrogens and neuroprotection. Trends Endocrinol Metab. 2002;13:229–230. doi: 10.1016/s1043-2760(02)00611-2. [DOI] [PubMed] [Google Scholar]
- 41.Xing D, Miller A, Novak L, Rocha R, Chen YF, Oparil S. Estradiol and progestins differentially modulate leukocyte infiltration after vascular injury. Circulation. 2004;109:234–241. doi: 10.1161/01.CIR.0000105700.95607.49. [DOI] [PubMed] [Google Scholar]