Abstract
Tissues from males can be regulated by a balance of androgenic and estrogenic effects because of local metabolism of testosterone and expression of relevant steroid hormone receptors. As a critical first step to understanding sex hormone influences in the cerebral circulation of males, we investigated the presence of enzymes that metabolize testosterone to active products and their respective receptors. We found that cerebral blood vessels from male rats express 5α-reductase type 2 and aromatase, enzymes responsible for conversion of testosterone into dihydrotestosterone (DHT) and 17β-estradiol, respectively. Protein levels of these enzymes, however, were not modulated by long-term in vivo hormone treatment. We also showed the presence of receptors for both androgens (AR) and estrogens (ER) from male cerebral vessels. Western blot analysis showed bands corresponding to the full-length AR (110 kDa) and ERα (66 kDa). Long-term in vivo treatment of orchiectomized rats with testosterone or DHT, but not estrogen, increased AR levels in cerebral vessels. In contrast, ERα protein levels were increased after in vivo treatment with estrogen but not testosterone. Fluorescent immunostaining revealed ERα, AR, and 5α-reductase type 2 in both the endothelial and smooth muscle layers of cerebral arteries, whereas aromatase staining was solely localized to the endothelium. Thus, cerebral vessels from males are target tissues for both androgens and estrogen. Furthermore, local metabolism of testosterone might balance opposing androgenic and estrogenic influences on cerebrovascular as well as brain function in males.
Keywords: androgen receptor, cerebral circulation, dihydrotestosterone, testosterone
Introduction
Estrogen and androgens, administered in vivo, have opposing actions on function of cerebral blood vessels (Krause et al, 2006). For example, exogenous estrogen treatment decreases both vascular tone and inflammation, whereas androgens increase these processes. To assess the impact of endogenous sex steroid hormones, the effects of gonadectomy, with and without subsequent hormone replacement, have been studied. In ovariectomized female animals, estrogen replacement generally restores cerebrovascular function to that found in cerebral vessels from intact, untreated females (Geary et al, 1998, 2000; McNeill et al, 1999). However, in males, the situation is more complex. For endothelial-dependent responses as well as modulation of inflammatory responses, testosterone replacement in castrated male rats does not mimic responses found in cerebral vessels of intact, untreated males (Geary et al, 2000; Razmara et al, 2005). Moreover, certain responses in arteries in males, such as nitric oxide-dependent dilation, are reproduced by estrogen treatment of castrated males (Geary et al, 2000). These findings suggest that active hormone metabolites and/or local testosterone metabolism, which may differ depending on the source of testosterone, contribute to the overall influence of gonadal hormones in the cerebrovasculature in males.
According to popular belief, testosterone is considered to be the ‘male’ hormone, whereas estrogen is associated with females. However, this is well known to be an oversimplification, as both estrogen and testosterone have important roles to play in individuals of either sex (Simpson 2003). Testosterone acts directly on the androgen receptor (AR) but is also metabolized by 5α-reductase to the more potent AR ligand, 5α-dihydrotestosterone (DHT) (Fujimoto et al, 1994). Alternatively, testosterone is metabolized by aromatase (CYP 19) to the primary form of estrogen, 17β-estradiol (Bulun et al, 2003). Thus, in both males and females, the balance between estrogen and testosterone production through various life stages influences the function of both reproductive and nonreproductive organs.
Investigation of the localization in various tissues of the testosterone-metabolizing enzymes, 5α-reductase and aromatase, is critical to understand the balance of androgenic and estrogenic effects in specific tissues. For example, 5α-reductase in the prostate converts testosterone into DHT, a critical step for effective promotion of prostate growth and function (Steimer 2003). In contrast, in bone the conversion of testosterone into estrogen by aromatase promotes mineralization and prevents osteoporosis; men with mutations of genes encoding either estrogen receptor alpha (ERα) or aromatase have a markedly altered bone phenotype (Smith et al, 1994; Carani et al, 1997). In fact, local estrogen production in bone cells is thought to be critical to the maintenance of bone mineralization in both men and women (Simpson et al, 2000). Estrogen has also been shown to have a physiological role in male reproductive organs, regulating the re-absorption of luminal fluid in the head of the epididymis. Disruption of this function results in infertility (Hess et al, 1997).
The ability of estrogen to enhance endothelial-dependent vasodilator function has been well described in females (Geary et al, 1998; Gislard et al, 1988; Gonzales et al, 2001). However, endogenous estrogen also influences vascular function in males. For example, a young man with an ERα mutation showed both coronary artery disease and endothelial dysfunction (Sudhir et al, 1997a, b). In healthy young men, inhibition of aromatase by administration of anastrozole impaired flow-mediated dilation, suggesting that endogenous estrogen production directly affects endothelial function in men (Lew et al, 2003).
Endogenous production of estrogen has also been shown to be important in brain function in males. At a critical point in development, sexual dimorphism of the brain of the male rat fetus is thought to be dependent on estrogen generated from testosterone by aromatase (Reisert and Pilgrim, 1991). Estrogen also appears to modulate synaptic plasticity (Von Schassen et al, 2006; Mukai et al, 2006), and the presence of neuronal aromatase suggests local testosterone metabolism to estrogen (Von Schassen et al, 2006). In fact, synthesis of estrogen has been demonstrated in adult male rat hippocampus, and interestingly, local concentrations of estrogen were higher than those measured in the circulation (Mukai et al, 2006). The well-described neuroprotective effects of estrogen have been shown to occur in brains of both males and females (McCullough and Hurn, 2003; Suzuki et al, 2006), and there is evidence that aromatase and extragonadal estrogen play a role in ischemic neuroprotection in females (McCullough et al, 2003). However, the tissues involved in extragonadal steroid metabolism that is critical for neuroprotective effects of estrogen have not been determined. Recently, men with the CC phenotype for estrogen receptor ERα were found to have a substantial increase in risk of stroke, independent of established risk factors (Shearman et al, 2005). This suggests an important influence of endogenous estrogen on cerebrovascular pathophysiology and ischemic brain disease in males and underscores the importance of a better understanding of the role of sex steroids in cerebrovascular physiology in males.
We previously showed that estrogen and testosterone alter function of cerebral blood vessels in both males and females by a variety of mechanisms dependent on alterations in endothelial function (Geary et al, 1998, Gonzales et al, 2004, 2005). However, as mentioned above, testosterone administration to castrated males generally does not reproduce the intact state. Therefore, to comprehend the actions of gonadal steroids on cerebrovascular function in males, it is critical to determine whether testosterone can be locally converted into estrogen and/or DHT, and whether the relevant receptors are also present. Besides the importance of this knowledge for understanding cerebrovascular function, such an investigation is also critical for understanding the actions of gonadal hormones on the brain. Although these hormones can be locally produced in some brain regions, circulating hormones may also have access to the brain, and the balance between estrogenic and androgenic effects could be altered by the presence of aromatase and/or 5α-reductase in the cerebral vasculature.
Therefore, we investigated whether aromatase and 5α-reductase type 2, both testosterone-metabolizing enzymes, are present in cerebral blood vessels in males, and whether receptors for androgens and estrogen are also present. In particular, we measured AR and ERα. The latter was chosen based on our previous work in females showing the importance of this ER subtype in cerebral artery function (Geary et al, 2001; Stirone et al, 2003). In addition, we have previously found that treatment with estrogen upregulates the level of ERα in female rats (Stirone et al, 2003). Therefore, we also investigated whether long-term estrogenic or androgenic treatment would affect levels of sex steroid enzymes and receptors in male rats, as an indication of the potential physiological function of these receptors.
Materials and methods
Animal Hormone Treatment
Experimental animal protocols were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine. Male Fischer-344 rats (3-month old; Harlan, Indianapolis, IN, USA) were anesthetized with ketamine (91 mg/kg; intramuscularly) and xylazine (9 mg/kg; intramuscularly). Animals were either left intact or orchiectomized (ORX) under aseptic surgical conditions. At the time of testes removal, rats were divided into four groups and chronically implanted with pellets containing testosterone propionate (testosterone; Silastic tubing 1.57 × 15 mm, Geary et al, 2000; Gonzales et al, 2004), 5α-androstan-17β-ol-3-one (DHT; 25 mg/pellet/21 days; Innovative Research of America, Sarasota, FL, USA), 17β-estradiol (Silastic tubing 1.57 × 5 mm; Geary et al, 1998, 2000) or blank pellets (ORX; empty 5 or 15 mm Silastic tubing or placebo pellets purchased from Innovative Research). Preliminary experiments indicated that the type of blank pellet used for the sham implant in the ORX animals did not affect the data given in Table 1, and therefore all ORX animals were combined. All pellets were implanted subcutaneously at the base of the neck, and post-surgery animals were treated with a single injection (intramuscularly) of penicillin (penicillin G benzathine/penicillin G procaine, 30,000 U). After recovery from anesthesia, rats were returned to vivarium housing (1200:1200 light/dark cycle) with fresh water, food, and bedding.
Table 1.
Serum hormone levels in intact, orchiectomized (ORX), and ORX rats chronically treated with testosterone (+T), dihydrotestosterone (+DHT), or 17β-estradiol (+E)
|
Animal treatment groups |
|||||
|---|---|---|---|---|---|
| Serum levels | Intact (n = 6) | ORX (n = 40) | +T (n = 31) | +DHT (n = 20) | +E (n = 20) |
| T (ng/mL) | 4.1 ± 1.0 | ND | 5.9 ± 0.6 | 0.4 ± 0.01* | ND |
| DHT (pg/mL) | 812 ± 53 | 74 ± 5* | 692 ± 137 | 417 ± 32* | ND |
| E (pg/mL) | ND | ND | ND | ND | 43 ± 4 |
Values represent means ± s.e.
P ≤ 0.05 versus intact.
ND, below the level of detection (T, 0.03 ng/mL; DHT, 6 pg/mL; and E, 10 pg/mL).
Three weeks after surgery/implant, animals were deeply anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally), and the thoracic cavity exposed. A direct cardiac puncture was used to collect blood samples for serum hormone measurements using either an enzyme-linked immunosorbent assay (ALPCO Diagnostics kits, Salem, NH, USA, for DHT or total testosterone; levels of detection, DHT 6 pg/mL and testosterone 0.03 ng/mL) or radioimmunoassay (Alpha Diagnostics kit, Owings Mills, MD, USA, for 17β-estradiol, level of detection, 10 pg/mL). After blood samples were collected, animals were heparinized and exsanguinated. Next, brains were removed and placed in a Sylgard-coated dissection dish containing ice-cold phosphate-buffered saline (PBS) or rapidly frozen and stored at −80°C for future processing.
Confocal Laser Scanning Microscopy
Confocal imaging was used to determine the localization of AR, ERα, aromatase, and 5α-reductase type 2 in middle cerebral arteries (MCAs) segments. Using a microscope, MCAs were freshly dissected and collected from brains of the following groups: intact males, castrated males (ORX), and castrated males treated with testosterone or DHT. Arteries were cut into small segments (2 mm in length), fixed in 3% formaldehyde (30 mins), and permeabilized with Triton X-100 (0.1%) for 5 mins. After addition of 10% horse serum and/or 1% bovine serum albumin, arterial segments were coincubated with primary antibodies specific for the N20 region of AR (AR N20) (1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), the N-terminal region of ERα (H-184) (1:100; Santa Cruz Biotechnology), aromatase (1:100; Acris, Herford, Germany), or 5α-reductase type 2 (1:100; Santa Cruz Biotechnology) for 3 h at room temperature. After incubation, vessels were washed (30 mins, buffer change every 5 mins) and incubated with a fluorescent secondary antibody at 10 μg/mL (Oregon Green, Alexa Fluor Green, or Texas Red; Molecular Probes, Eugene, OR, USA) overnight at 4°C followed by a final wash before mounting. Using forceps, small MCA segments were arranged on microscope slides that were coverslipped with Vecta-Shield mounting medium containing 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Vector Laboratories, Burlingame, CA, USA) to counterstain cellular nuclei, then sealed with clear fingernail polish. Images were obtained using a Carl Zeiss Meta Laser Scanning Systems LSM 510 microscope equipped with standard and ultra-violent lasers. We conducted immunohistochemical experiments on at least three tissues taken from the different groups of male rats (ORX, testosterone, DHT, and intact) and we observed that, for each protein of interest, there were no apparent differences in the localization among the treatment groups. Representative arteries in Figures 1, 3, and 5 were from testosterone-treated castrated males. Representative arteries illustrated for ERα in Figure 7 are from intact males. In control staining conditions, vessels were incubated with the secondary antibody alone, but showed no fluorescence.
Figure 1.
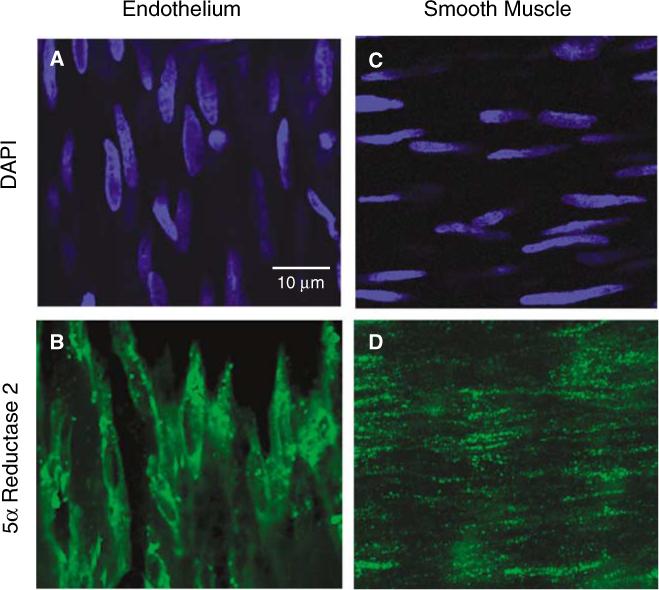
The presence of 5α-reductase type 2 in middle cerebral artery (MCA) was determined using confocal immunofluorescence (40×). A representative MCA isolated from an ORX male rat treated with testosterone (T) is shown. Arterial segments were incubated with anti-5α-reductase type 2 antibody (green) and cellular orientation of the endothelial (A) and smooth muscle (C) layers within the MCA segments was determined with the nuclear stain, 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; blue). 5α-reductase type 2 (green) was found in both the intimal (B) and medial (D) layers.
Figure 3.
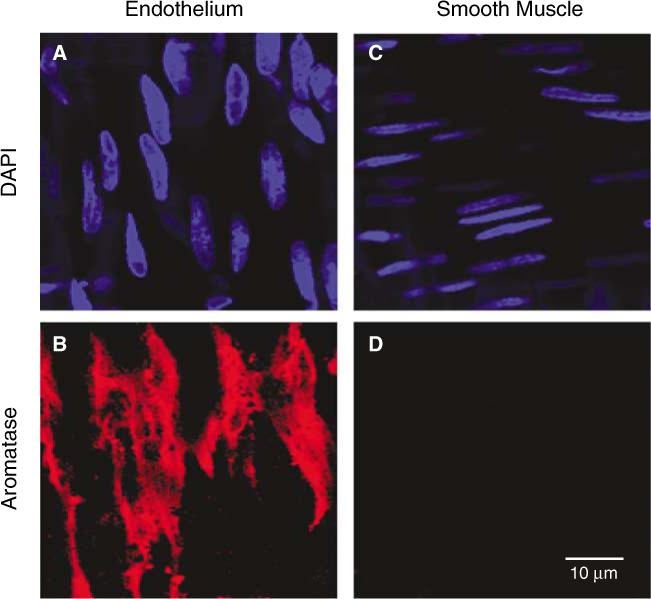
Confocal immunofluorescence (40×) revealed the presence and localization of aromatase in the MCA of males. Representative MCA isolated from an ORX male treated with testosterone (T) is shown. Nuclear staining with DAPI was used to identify the focal plane of the endothelial (A) and smooth muscle (C) layers within MCA segments. Fluorescent labeling with an anti-aromatase antibody (red) is found in the endothelial layer (B); however, no staining for aromatase was observed in the smooth muscle layer (D).
Figure 5.
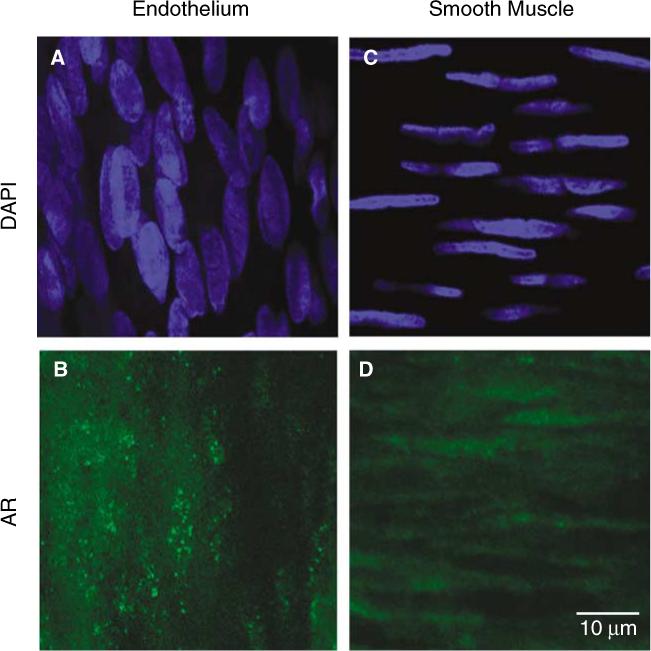
Laser scanning confocal microscopic imaging (40×) of androgen receptor (AR) immunofluoresence in middle cerebral artery (MCA; representative artery dissected from an ORX rat treated with T is shown) in males. Arterial segments were incubated with anti-AR antibody N20 (green) and mounted in medium containing the nuclear stain, 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; blue). The orientation of the nuclei was used to confirm whether the confocal plane was in the endothelial (A) or smooth muscle (C) layer. Selective AR staining (green) was found in both the endothelial (B) and smooth muscle (D) layers.
Figure 7.
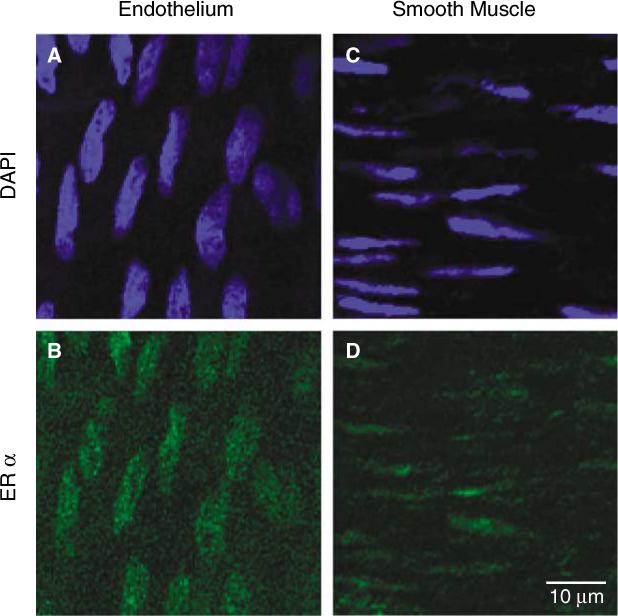
The presence of ERα immunofluorescence in the MCA of males. Representative artery from an intact male rat is shown using laser scanning confocal microscopy (40×). Nuclear staining with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; blue) was used to identify endothelial (A) and smooth muscle (C) layers. ERα staining with Oregon green was observed in both the endothelial (B) and smooth muscle (D) layers.
Western Analysis
Before Western blot, brains from all groups of rats were thawed on ice, and cerebral vessels were isolated using a procedure published previously (McNeill et al, 1999). In brief, whole brain was gently homogenized (glass Dounce) in cold PBS (pH 7.4) and centrifuged (720 g; 10 mins at 4°C). The pellets were resuspended in cold PBS and layered over a 15% dextran solution (35 to 45 kDa). After centrifugation (1300 g; 30 to 40 mins at 4°C), the pellet containing blood vessels was poured over a nylon mesh (50 μm) and the vessels washed with ice-cold PBS and collected. Light microscopy verified that the vessel fraction consisted of a mixture of arteries, arterioles, venules, veins, and capillaries.
Vessels were homogenized in lysis buffer (20 mins), and the lysate clarified by low-speed centrifugation (4500 g for 10 mins at 4°C). An aliquot of the supernatant was analyzed for protein concentration using a bicinchoninic acid protein assay (Pierce, Rockford, IL, USA). Lysates were then added to Tris-glycine sodium dodecyl sulfate sample buffer and boiled for 6 mins. Equal amounts of protein (20 to 40 μg/lane) from each animal group were then loaded and separated in 8% (AR, ERα, and aromatase) or 16% (5α-reductase type 2) polyacrylamide gels using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. In addition to tissue samples, gels were loaded with a standard molecular weight marker (BioRad, Hercules, CA, USA). Separated proteins were transferred to polyvinylidene difluoride membranes and blocked overnight at 4°C in PBS (0.1% Tween and 6.5% nonfat dry milk). Membranes were probed with antibodies specific for AR N20 (1:250 dilution; Santa Cruz Biotechnology), aromatase (1:500; Acris), ERα (1:250; H-184, Santa Cruz Biotechnology), or 5α-reductase type 2 (1:250; Santa Cruz Biotechnology). α-Actin antibody (1:20,000) was used to verify loading. Enhanced chemiluminescence development in conjunction with a horseradish peroxidase-labeled secondary antibody (1:10,000) was used to visualize the proteins of interest. Densitometry of bands was analyzed with UN-SCAN-it software (Silk Scientific; Orem, UT, USA).
Solutions and Chemicals
Phosphate-buffered saline was prepared from pre-made packets. Lysis buffer for Western blots, containing β-glycerophosphate (50 mmol/L), sodium orthovanadate (100 μmol/L), magnesium chloride (2 mmol/L), ethylene glycol-bis (b-aminoethyl ether) (1 mmol/L), and Triton X-100 (0.5%), was prepared fresh, and then dl-dithiothreitol (1 mmol/L), phenylmethylsulfonyl fluoride (1 mmol/L), pepstatin (20 μmol/L), leupeptin (20 μmol/L), and aprotinin (0.1 U/mL) were added from stock solutions (dissolved in solvent, double-distilled water or dimethylsulfoxide, and stored at −20°C). Unless noted otherwise, all drugs and chemicals were purchased from Sigma Chemical (St Louis, MO, USA).
Data Analysis
Data obtained by Western blot included group comparisons (e.g., ORX versus DHT, testosterone, or estrogen) on the same blot. Band densities were compared using analysis of variance with repeated measures, and the Student-Newman-Keuls test was used post hoc when analysis of variance indicated statistical differences. For presentation in figures, density values from each Western blot were expressed as an optical density ratio relative to the ORX control, and then the means±s.e.m. from all blots determined. Change in body weight was calculated as [(final weight 3 weeks post-implant–initial weight pre-implant)/final weight 3 weeks post-implant] × 100. Changes in body weight were compared using analysis of variance and a Student's-Newman-Keuls post hoc test. Data presented in results section are expressed as mean±s.e.m. P≤0.05 was considered statistically significant for analysis.
Results
Serum Hormone Levels and Body Weights
In ORX rats, serum hormone levels of testosterone and estrogen were below the radioimmunoassay level of detection. However, after 3 weeks of in vivo hormone treatment (Table 1), serum hormone levels were similar to those of intact males and within the physiological range previously reported for intact male and female rats (Geary et al, 1998, 2000; Gonzales et al, 2004). Interestingly, DHT was also detected in the ORX group receiving testosterone, suggesting that the exogenous source of testosterone administered was being biologically metabolized in the animal. Over the 3-week treatment period, ORX animals showed a 3±1% increase in body weight, whereas animals treated with androgen had significantly greater weight gains (T = 8±2% increase; DHT = 7±1% increase; P≤0.05 versus ORX). In contrast, estrogen treatment prevented weight gain in ORX animals (E = −1±2% change in weight; P≤0.05 versus ORX). Changes in body weight were not determined for intact males used in this study.
5α-Reductase Type 2
Testosterone is converted into DHT by two isoforms of 5α-reductase, type 1 and 2, which are differentially expressed in a variety of tissues (Normington and Russell, 1992). In this study, we focused on type 2, as it has been found in vascular tissues (Eicheler et al, 1994) and is thought to be more involved in production of active metabolites of testosterone (Normington and Russell, 1992). To investigate whether cerebral blood vessels have the capacity to metabolize testosterone to DHT locally, the presence of 5α-reductase type 2 was first tested using confocal immunofluorescence. Costaining with the nuclear specific stain, DAPI (blue), was used to identify the focal plane of the endothelial (Figure 1A) and smooth muscle (Figure 1C) layers within MCA segments. Distinction between the vascular cell layers was observed by noting that the endothelial cell nuclei were oriented in the direction of blood flow, whereas smooth muscle nuclei are arranged perpendicular to the direction of flow. As shown in a MCA isolated from an ORX rat treated with testosterone, selective staining for 5α-reductase type 2 (green) is detected in both the endothelial (Figure 1B) and smooth muscle layers (Figure 1D). Similar staining for 5α-reductase type 2 was observed in MCA isolated from ORX animals (data not shown).
We also used Western blot analysis to verify the presence of 5α-reductase type 2 protein in cerebral blood vessels isolated from whole brain (Figure 2A), and then used this method to measure the influence of long-term in vivo treatment with testosterone, DHT, or estrogen on levels of this enzyme. Figure 2A is a representative Western blot illustrating 5α-reductase type 2 as a single band at the expected molecular weight of 27 kDa. No other bands were visualized under the conditions used for the Western blot. Densitometric analysis revealed that protein levels of 5α-reductase type 2 were not significantly altered by long-term in vivo androgen or estrogen treatment (Figure 2B).
Figure 2.
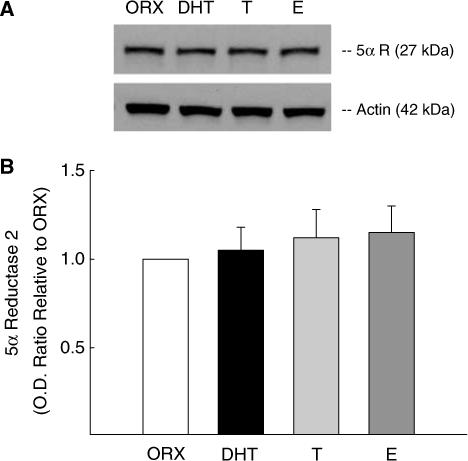
Effect of chronic androgen and estrogen treatment on 5α-reductase type 2 protein levels in cerebral vessels in males. (A) represents Western blots for 5α-reductase type 2 protein in blood vessels isolated from the brains of ORX rats, or ORX treated chronically with either dihydrotestosterone (DHT), testosterone (T), or 17β-estradiol (E). Anti-5α-reductase type 2 antibody confirmed the presence of a band at 27 kDa in the cerebral vessel lysates. Protein levels of α-actin (42 kDa) were used to determine protein loading in each lane. (B) Densitometric values for enzyme protein levels were calculated and presented as optical density ratio (OD) relative to ORX. Data are expressed as means±s.e., and there was no statistically significant differences among the animal groups. n = 8 for ORX; n = 5 for T and DHT-treated groups; and n = 3 for E-treated group.
Aromatase
The presence of aromatase in MCA was investigated using confocal imaging. Costaining with DAPI once again was used to identify the focal plane of the endothelial and smooth muscle layers (Figures 3A and 3C). Figure 3 shows a representative segment of a MCA from a testosterone-treated ORX animal. Interestingly, anti-aromatase fluorescent staining (red) was only found in the endothelial layer (Figure 3B) with no staining observed in the focal plane corresponding to the smooth muscle layer (Figure 3D). A similar endothelial localization of aromatase was found in MCA isolated from ORX and testosterone-treated ORX males. These data indicate that, unlike 5α-reductase, the presence of aromatase is localized to intimal layers in MCA in males.
We verified the presence of aromatase protein in cerebral blood vessels isolated from whole brain using Western blot analysis and also determined if long-term treatment with testosterone, DHT, or estrogen modulates levels of cerebrovascular aromatase. Anti-aromatase antibody detected a single band on the blot at the expected molecular weight of 66 kDa; however, the levels of aromatase protein were not altered by long-term androgen or estrogen treatment of ORX males (Figure 4).
Figure 4.
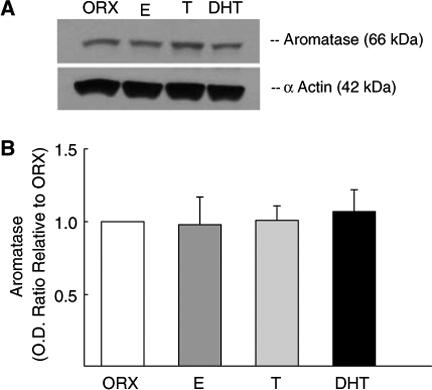
The effect of long-term sex steroid hormone treatment on levels of aromatase in cerebral blood vessels in males. Vessel fractions were isolated from the brains of orchiectomized male rats (ORX) and ORX treated with either dihydrotestosterone (DHT), testosterone (T), or 17β-estradiol (E). α-Actin (42 kDa) was used to verify equal protein loading of samples (A). Densitometry data for aromatase protein levels are illustrated as a fold increase compared with the ORX sample run on the same blot (B). Values are means±s.e. (n = 5), and there was no significant difference among the animal treatment groups.
Androgen Receptor
We investigated whether AR was present in endothelial and/or smooth muscle cell layers in MCA in males. Figure 5 shows a representative arterial segment isolated from an ORX rat chronically treated with testosterone. Staining for AR was also performed in MCA isolated from ORX and DHT-treated ORX animals, and the results were similar to testosterone-treated ORX (data not shown). Costaining with DAPI (blue) was used to distinguish between the endothelial and smooth muscle layers based on nuclear orientation (Figures 5 A and 5C). Colabeling with an antibody selective for the N20 region of AR (green) resulted in fluorescent staining in both the endothelial (Figure 5B) and smooth muscle layers (Figure 5D).
Western blots were used to verify the presence of AR protein in blood vessels isolated from whole brain, and the effect of long-term treatment with testosterone, DHT, or estrogen on cerebrovascular AR levels was also assessed. In each Western blot experiment, α-actin was visualized to verify equal protein loading of the samples, and testes or ovary lysate was used to verify AR band migration at 110 kDa. Figure 6A illustrates a representative Western blot of vessel homogenates isolated from ORX males and ORX males treated with either DHT, testosterone, or estrogen. Densitometric analysis demonstrated that AR protein levels were significantly increased in the cerebral vasculature after long-term in vivo testosterone or DHT treatment (Figure 6B). In contrast, long-term treatment with estrogen did not alter AR protein levels in ORX male rats.
Figure 6.
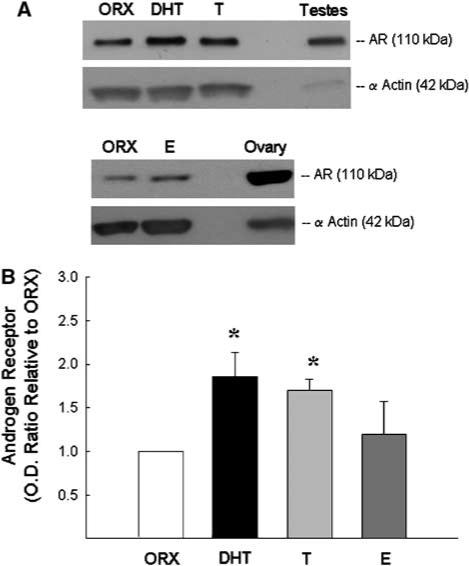
Effect of long-term sex steroid hormone treatment on androgen receptor (AR) protein levels in cerebral vessels. (A) Representative Western blots for AR protein in blood vessels isolated from ORX male rats and ORX treated with either dihydrotestosterone (DHT), testosterone (T), or 17β-estradiol (E). Rat testes or ovary lysate (5 μg/lane) was used as positive control to confirm the specific band for AR migrating at 110 kDa. Protein levels of α-actin (42 kDa) were used to determine protein loading. Calculated densitometric values for AR protein levels are shown in (B). Data are presented as optical density ratio (OD) relative to ORX and expressed as means±s.e. *P≤0.05 versus ORX; n = 7 rats.
Estrogen Receptor-α
We have previously shown in female rats that ERα is present in both the endothelial and smooth muscle layers of cerebral arteries, and the level of cerebrovascular receptor protein is increased in female animals exposed to estrogen (Stirone et al, 2003). The finding of aromatase in the MCA of males suggests a role for estrogen produced by local metabolism of circulating testosterone. Thus, we determined if cerebrovascular tissue in males is receptive to estrogen by assessing the presence of ERα in cerebral blood vessels in males, and, if so, whether receptor levels are affected by long-term androgen or estrogen treatment. Similar to our observations in females (Stirone et al, 2003), ERα immunofluorescence is found in both the smooth muscle and endothelium of MCAs in males using an antibody against the N-terminal of ERα (H-184) (Figure 7). Western blots confirm the presence of ERα protein in blood vessels isolated from the brains of male rats (Figure 8A). We have previously reported that multiple forms of ERα protein are found in female rat cerebral vessels (Stirone et al, 2003). Although similar bands could be observed in vessel lysates from male rats (data not shown), we chose to analyze the band migrating at 66 kDa, which corresponds to the putative unmodified, full-length form of ERα. Figure 8B shows that levels of ERα (66 kDa) were significantly greater in vessel homogenate from ORX male rats treated with long-term estrogen compared with vessels of males receiving either testosterone or no hormone (ORX).
Figure 8.
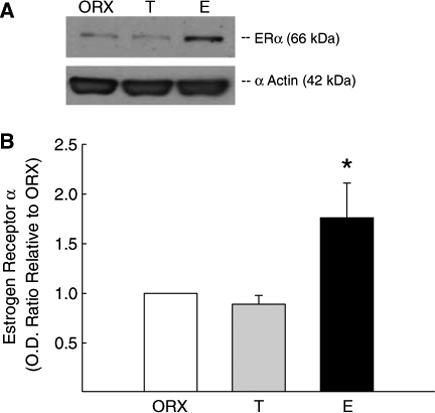
The effect of long-term T and E treatment on ERα levels in cerebral blood vessels in males. (A) Representative Western blot of ERα protein in blood vessels isolated from the brains of ORX male rats and ORX rats treated with either T or E. Results for ERα protein levels are expressed as densitometric values calculated as optical density ratio (OD) relative to ORX. (B) A band for ERα was detected at 66 kDa using an N-terminal antibody (H-184). Data are expressed as means±s.e. *Significantly different from ORX (P≤0.05); n = 5 rats.
Discussion
An important first step to determining whether the cerebral circulation in males is subject to a balance of androgenic and estrogenic effects is to assess the presence of testosterone-metabolizing enzymes and sex steroid receptors. In this study, we demonstrated clearly that 5α-reductase and aromatase, enzymes that metabolize testosterone to active products, are both localized to cerebral blood vessels in males. This finding emphasizes the potential for local transformation of circulating testosterone to the potent androgen, DHT, and to the estrogenic compound, 17β-estradiol. We also show that receptors for both steroids, AR and ERα, are present in cerebral blood vessels in males and that protein levels of each receptor are increased by administration of androgens or estrogen, respectively. These findings clearly suggest a potential physiological function for each receptor type in the cerebral circulation in males.
Indeed, we have showed previously that long-term in vivo administration of sex steroids affects cerebral vascular function. Long-term androgen treatment in castrated male rats increases the tone of pressurized cerebral arteries (Geary et al, 1998; Gonzales et al, 2004, 2005). Mechanisms involved in testosterone-enhanced tone include modulation of endothelial-derived vascular metabolites, such as thromboxane and endothelial hyperpolarizing factor (EDHF), that affect smooth muscle function (Gonzales et al, 2004, 2005). Long-term androgen treatment also enhances the inflammatory response of cerebral blood vessels (Razmara et al, 2005). In contrast, long-term estrogen treatment has opposite effects, decreasing cerebral arterial tone and vascular inflammation in both males and females (Krause et al, 2006; Geary et al, 1998, 2000; Ospina et al, 2004; Razmara et al, 2005). Estrogen influences cerebral endothelium to increase production of endothelial-derived vasodilators such as nitric oxide (Geary et al, 2000; Stirone et al, 2003) and prostacyclin (Ospina et al, 2002). These functional studies of vessels from male and female rodents treated with hormones in vivo indicate that cerebral vascular function is regulated by sex steroids.
In addition to showing that the cerebral vasculature is a target tissue for sex steroids, the findings of this study suggest that males may indeed be subject to estrogenic as well as androgenic effects. Indeed, assessment of nitric oxide dilation in cerebral arteries showed that responses in vessels from intact males were affected by castration and that the effect of gonad removal could be reversed by administration of estrogen, but not testosterone (Geary et al, 2000). Similarly in brain, we recently found that estrogen treatment decreased brain mitochondrial oxidative stress, whereas DHT had no effect in ORX male rats (Razmara et al, 2007). Of relevance to the current study, intact male rats also showed a decrease in brain mitochondrial oxidative stress compared with ORX, suggesting that endogenous estrogen plays a role in the male brain. Taken together, these data suggest that testosterone may indeed undergo local metabolic transformation to estrogen as well as DHT.
5α-Reductase has been found in the prostate gland, skin, and liver (Andersson and Russell, 1990; Eicheler et al, 1995) and in several vascular tissues (Eicheler et al, 1994; Fujimoto et al, 1994; Milewich et al, 1987; Martel et al, 1994). Inhibition of 5α-reductase has been shown to attenuate the effect of testosterone to increase thromboxane receptor density in isolated rat aortic smooth muscle cells and decrease thromboxane receptor density in the aorta (Higashiura et al, 1996). Although there is a wide distribution in the body of both isoforms of 5α reductase, types 1 and 2, in this study we focused on type 2. Type 2, with a lower Km, is thought to be more important for the conversion of low concentrations of testosterone into the active metabolite DHT in mediating anabolic effects. In contrast, type 1, with a higher Km, is believed to mainly be critical for catabolism of higher levels of testosterone to breakdown products (Normington and Russell, 1992). Thus, the current finding that 5α-reductase type 2 is localized to both endothelial and smooth muscle cells of cerebral arteries supports the concept that local metabolism of testosterone to DHT could promote androgenic effects of testosterone on cerebrovascular function or influence the hormonal milieu of the brain.
In addition to 5α-reductase, we also investigated the presence of aromatase in the cerebral blood vessel preparations. Aromatase P450 is the key enzyme for estrogen biosynthesis, and, owing to alternative use of multiple promoters, the gene for aromatase (CYP19) shows tissue-specific expression (Bulun et al, 2003). One of these promoters is vascular endothelial cell specific, the endothelial-type promoter I.7. It has been hypothesized that activity of this promoter may be important for paracrine effects of estrogen on blood vessel physiology (Bulun et al, 2003). In our studies, we found aromatase staining exclusively in the endothelium and not in the smooth muscle layer of cerebral blood vessels from males. Although immunohistochemical methods cannot absolutely rule out the presence of aromatase in smooth muscle cells, our observations certainly suggest that aromatase plays a prominent role in endothelial cells of cerebral blood vessels compared with smooth muscle cells. In contrast, Harada et al (1999) showed localized staining of aromatase in both the intimal and medial layers of aortic preparations as well as in cultured endothelial and smooth muscle cells. Differences in staining locale may be specific for various vascular beds.
The presence of aromatase suggests that aromatase conversion of testosterone into estrogen may influence production of estrogen-regulated endothelial vasoactive factors such as prostacyclin and nitric oxide, thus indirectly modulating the underlying smooth muscle. The importance of the local conversion of testosterone into estrogen in males has been shown in several studies (Mendelsohn and Rosano, 2003). For example, after administration of an aromatase inhibitor to healthy young men, endothelium-dependent, flow-mediated dilation of the brachial artery significantly decreased (Lew et al, 2003). This finding suggests that suppression of the production of endogenous estrogen resulted in a decrease in flow-induced vasodilation, most likely by modulating endothelial nitric oxide synthase levels and/or activity. Studies of aromatase knockout mice also show the importance of estrogen production in male mice. Vasodilator responses to the endothelium-dependent vasodilator, acetylcholine, were significantly blunted in isolated aorta from male aromatase knockout mice compared with wild type (Kimura et al, 2003). All of these studies support the concept that endogenous estrogen contributes to the normal vascular function of males.
Gender differences in physiological and pathological responses might be due in part to the level of sex steroid receptors in various cellular populations (McCrohon et al, 2000). Receptor binding assays along with immunohistochemical studies have shown that AR is ubiquitously expressed in a variety of tissues throughout the body including the wall of blood vessels (Chang et al, 1989; Kimura et al, 1993; Fujimoto et al, 1994; McCrohon et al, 2000; Hanke et al, 2001). In agreement with these earlier studies, we show that AR is present in both endothelial and smooth muscle layers of cerebral blood vessels from male rats. Furthermore, we show that ERα is also present, opening up the possibility of a mix of androgenic and estrogenic effects in the cerebral circulation of males.
An important question is whether gender differences in gonadal steroid hormone expression may be due to genetic causes or current hormonal regulation. In this regard, there is evidence that levels of sex steroid receptors themselves can be modulated by hormone treatment. Stirone et al (2003) showed that long-term exposure to estrogen in gonadectomized female rats increased ERα protein levels in cerebral blood vessels. Additionally, levels of AR in human skeletal muscle were increased after androgen treatment suggesting that these differences are hormonally, rather than genetically, controlled (Sinha-Hikim et al, 2004). Such studies suggest that gonadal hormones, by binding to their respective receptor, modulate levels of that receptor. In this study in cerebral blood vessels from males, we clearly show that AR protein levels were significantly increased by androgenic stimulation, because treatment with either testosterone or DHT had the same effect. In contrast, long-term treatment with estrogen increased levels of ERα. However, there were no effects of long-term androgen treatment on levels of ER, nor did long-term estrogen treatment affect levels of AR. Thus, our findings support the concept that AR and ER can be activated by binding of endogenous androgens or estrogens, respectively, suggesting the possibility of significant physiological effects, both estrogenic and androgenic, in cerebral blood vessels in males.
As mentioned previously we have shown that estrogen decreases cerebral vascular tone and suppresses vascular inflammation whereas androgens increase cerebrovascular tone and augment vascular inflammation (Gonzales et al, 2004, 2005; Ospina et al, 2004; Razmara et al, 2005). The presence in cerebral blood vessels of both aromatase and 5α reductase as well as AR and ER suggests that testosterone can be metabolized to either estrogen or the more potent androgen, DHT, in the cerebrovascular wall. These findings suggest the intriguing possibility that the local balance of androgenic and estrogenic effects can be controlled in the vicinity of the cerebral blood vessel itself. They also imply that cerebrovascular concentrations of sex steroids may be different from circulating levels, thus confounding interpretations based on plasma measurements. Interestingly, sex steroid enzymes and receptors are targets for a number of inhibitors that are used clinically (e.g., finrozole, finasteride, fulvestrant, and flutamide); however, little is known about how these treatments may affect the cerebral circulation or possibly brain function.
In summary, we found that 5α-reducatase and aromatase were present in cerebral blood vessels; however, protein levels of either enzyme were unaffected by long term androgen or estrogen treatment. In addition, we have shown that AR is present in cerebral blood vessels and is localized to both smooth muscle and endothelium. We also determined that androgen treatment increased AR protein, which may promote the production of vasoconstrictors to enhance vascular tone. Because of the presence of steroid receptors and metabolic enzymes in cerebral blood vessels, additional studies regarding the regulation and activity of AR and ER and testosterone-metabolizing enzymes are necessary to understand better the role of androgens in modulating cerebrovascular function during both physiological and pathophysiological conditions.
Acknowledgements
The authors thank Nick Barker, Bebe Ehsan, and Kevin Marx for technical help with the Western blots and blood vessel isolation.
This work was supported by the National Heart, Lung and Blood Institute grant HL50775 and by a postdoctoral fellowship from the American Heart Association, Western States Affiliate (RJG).
References
- Andersson S, Russell DW. Structural and biochemical properties of cloned and expressed human and rat steroid 5 alpha-reductases. Proc Natl Acad Sci USA. 1990;87:3640–4. doi: 10.1073/pnas.87.10.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Sebastian S, Takayama K, Suzuki T, Sasano H, Shozu M. The human CYP19 (aromatase P450) gene: update on physiological roles and genomic organization of promoters. J Steroid Biochem Mol Biol. 2003;86:219–24. doi: 10.1016/s0960-0760(03)00359-5. [DOI] [PubMed] [Google Scholar]
- Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER. Effect of testosterone and estradiol in a man with aromatase deficiency. New Eng J Med. 1997;337(2):91–5. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- Chang C, Chodak G, Sarac E, Takeda H, Liao S. Prostate androgen receptor: immunohistochemical localization and mRNA characterization. J Steroid Biochem. 1989;34:311–3. doi: 10.1016/0022-4731(89)90099-x. [DOI] [PubMed] [Google Scholar]
- Eicheler W, Dreher M, Hoffmann R, Happle R, Aumuller G. Immunohistochemical evidence for differential distribution of 5α-reductase isoenzymes in human skin. Br J Dermatol. 1995;133:371–6. doi: 10.1111/j.1365-2133.1995.tb02663.x. [DOI] [PubMed] [Google Scholar]
- Eicheler W, Tuohimaa P, Vilja P, Adermann K, Forssmann WG, Aumuller G. Immunocytochemical localization of human 5α-reductase 2 with polyclonal antibodies in androgen target and non-target human tissues. J Histochem Cytochem. 1994;42:667–75. doi: 10.1177/42.5.8157936. [DOI] [PubMed] [Google Scholar]
- Fujimoto R, Morimoto I, Morita E, Sugimioto H, Ito Y, Eto S. Androgen receptors, 5α-reductase activity and androgen-dependent proliferation of vascular smooth muscle cells. J Steroid Biochem Mol Biol. 1994;50:169–74. doi: 10.1016/0960-0760(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Geary GG, Krause DN, Duckles SP. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am J Physiol. 1998;275:H292–300. doi: 10.1152/ajpheart.1998.275.1.H292. [DOI] [PubMed] [Google Scholar]
- Geary GG, Krause DN, Duckles SP. Gonadal hormones affect diameter of male rat cerebral arteries through endothelial-dependent mechanisms. Am J Physiol. 2000;279:H610–8. doi: 10.1152/ajpheart.2000.279.2.H610. [DOI] [PubMed] [Google Scholar]
- Geary GG, McNeill AM, Ospina JA, Krause DN, Korach KS, Duckles SP. Selected contribution: cerebrovascular NOS and cyclooxygenase are unaffected by estrogen in mice lacking estrogen receptor α. J Appl Physiol. 2001;91:2391–9. doi: 10.1152/jappl.2001.91.5.2391. [DOI] [PubMed] [Google Scholar]
- Gislard V, Miller VM, Vanhoutte PM. Effect of 17β-estradiol on endothelium-dependent responses in the rabbit. J Pharmacol Exp Ther. 1988;244:19–22. [PubMed] [Google Scholar]
- Gonzales RJ, Ghaffari AA, Duckles SP, Krause DN. Testosterone treatment increases thromboxane function in rat cerebral arteries. Am J Physiol Heart Circ Physiol. 2005;289:H578–85. doi: 10.1152/ajpheart.00958.2004. [DOI] [PubMed] [Google Scholar]
- Gonzales RJ, Krause DN, Duckles SP. Testosterone suppresses endothelial-dependent dilation of rat middle cerebral arteries. Am J Physiol. 2004;286:552–60. doi: 10.1152/ajpheart.00663.2003. [DOI] [PubMed] [Google Scholar]
- Gonzales RJ, Walker BR, Kanagy NL. 17β-Estradiol increases nitric oxide-dependent dilation in rat pulmonary arteries and thoracic aorta. Am J Physiol Lung Cell Mol Physiol. 2001;280:L555–64. doi: 10.1152/ajplung.2001.280.3.L555. [DOI] [PubMed] [Google Scholar]
- Hanke H, Lenz C, Hess B, Spindler KD, Weidemann W. Effect of testosterone on plaque development and androgen receptor expression in the arterial vessel wall. Circulation. 2001;103:1382–5. doi: 10.1161/01.cir.103.10.1382. [DOI] [PubMed] [Google Scholar]
- Harada N, Sasano H, Murakami H, Ohkuma T, Nagura H, Takagi Y. Localized expression of aromatase in human vascular tissues. Circ Res. 1999;84:1285–91. doi: 10.1161/01.res.84.11.1285. [DOI] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB. A role for oestrogens in the male reproductive system. Nature. 1997;390:509–12. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiura K, Blaney B, Morgan E, Mathur RS, Halushka PV. Inhibition of testosterone 5α-reductase: evidence for tissue-specific regulation of thromboxane A2 receptors. J Pharmacol Exp Ther. 1996;279:1386–91. [PubMed] [Google Scholar]
- Kimura M, Sudhir K, Jones M, Simpson E, Jefferis A-M, Chin-Dusting JPF. Impaired acetylcholine-induced release of nitric oxide in the aorta of male aromatase-knockout mice. Circ Res. 2003;93:1267–71. doi: 10.1161/01.RES.0000103172.98986.25. [DOI] [PubMed] [Google Scholar]
- Kimura N, Mizokami A, Oonuma T, Sasano H, Nagura H. Immunocytochemical localization of androgen receptor with polyclonal antibody in paraffin-embedded human tissue. J Histochem Cytochem. 1993;41:671–8. doi: 10.1177/41.5.8468448. [DOI] [PubMed] [Google Scholar]
- Krause DN, Duckles SP, Pelligrino DA. The influence of sex steroid hormones on cerebrovascular function. Invited review. J Appl Physiol. 2006;101:1252–61. doi: 10.1152/japplphysiol.01095.2005. [DOI] [PubMed] [Google Scholar]
- Lew R, Komesaroff P, Williams M, Dawood T, Sudhir K. Endogenous estrogens influence endothelial function in young men. Circ Res. 2003;93:1127–33. doi: 10.1161/01.RES.0000103633.57225.BC. [DOI] [PubMed] [Google Scholar]
- Martel C, Melner MH, Gagne D, Simard J, Labrie F. Wide-spread tissue distribution of steroid sulfatase, 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD), 17β-HSD 5α-reductase and aromatase activities in the rhesus monkey. Mol Cell Endocrinol. 1994;104:103–11. doi: 10.1016/0303-7207(94)90056-6. [DOI] [PubMed] [Google Scholar]
- McCrohon JA, Death AK, Nakhla S, Jessup W, Handelsman DJ, Stanley KK, Celermajer DS. Androgen receptor expression is greater in macrophages from male than from female donors. A sex difference with implications for atherogenesis. Circulation. 2000;101:224–6. doi: 10.1161/01.cir.101.3.224. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003;23:8701–5. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14:228–35. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- McNeill AM, Kim N, Duckles SP, Krause DN. Chronic estrogen treatment increases levels of endothelial nitric oxide synthase protein in rat cerebral microvessels. Stroke. 1999;30:2186–90. doi: 10.1161/01.str.30.10.2186. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Rosano GMC. Hormonal regulation of normal vascular tone in males. Circ Res. 2003;93:1142–5. doi: 10.1161/01.RES.0000108694.68635.1C. [DOI] [PubMed] [Google Scholar]
- Milewich L, Kaimal V, Johnson AR. Steroid 5α-reductase activity in endothelial cells from human umbilical cord vessels. J Steroid Biochem. 1987;26:561–7. doi: 10.1016/0022-4731(87)90008-2. [DOI] [PubMed] [Google Scholar]
- Mukai H, Takata N, Ishii H-t, Tanabe N, Hojo Y, Furukawa A, Kimoto T, Kawato S. Hippocampal synthesis of estrogens and androgens which are paracrine modulators of synaptic plasticity: synaptocrinology. Neuroscience. 2006;138:757–64. doi: 10.1016/j.neuroscience.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Normington K, Russell DW. Tissue distribution and kinetic characterization of rat steroid 5α reductase isozymes. J Biol Chem. 1992;267:19548–54. [PubMed] [Google Scholar]
- Ospina JA, Brevig HN, Krause DN, Duckles SP. Estrogen suppresses IL-1beta-mediated induction of COX-2 pathway in rat cerebral blood vessels. Am J Physiol Heart Circ Physiol. 2004;286:H2010–9. doi: 10.1152/ajpheart.00481.2003. [DOI] [PubMed] [Google Scholar]
- Ospina JA, Krause DN, Duckles SP. 17beta-Estradiol increases rat cerebrovascular prostacyclin synthesis by elevating cyclooxygenase-1 and prostacyclin synthase. Stroke. 2002;33:600–5. doi: 10.1161/hs0202.102732. [DOI] [PubMed] [Google Scholar]
- Razmara A, Krause DN, Duckles SP. Testosterone augments endotoxin-mediated cerebrovascular inflammation in male rats. Am J Physiol Heart Circ Physiol. 2005;289:H1843–50. doi: 10.1152/ajpheart.00465.2005. [DOI] [PubMed] [Google Scholar]
- Razmara A, Procaccio V, Krause DN, Duckles SP. Sex hormone modulation of brain mitochondrial ROS in male and female rats: a possible mechanism for neuroprotection. Stroke (Abstract accepted for presentation at the International Stroke Conference) 2007;38:452. [Google Scholar]
- Reisert I, Pilgrim C. Sexual differentiation of monoaminergic neurons—genetic or epigenetic? TINS. 1991;14:468–73. doi: 10.1016/0166-2236(91)90047-x. [DOI] [PubMed] [Google Scholar]
- Shearman AM, Cooper JA, Kotwinski PJ, Humphries SE, Mendelsohn ME, Housman DE, Miller GJ. Estrogen receptor alpha gene variation and the risk of stroke. Stroke. 2005;36:2281–2. doi: 10.1161/01.STR.0000181088.76518.ec. [DOI] [PubMed] [Google Scholar]
- Simpson E, Rubin G, Colin C, Robertson K, O'Donnell L, Jones M, Davis S. The role of local estrogen biosynthesis in males and females. TEM. 2000;11:184–8. doi: 10.1016/s1043-2760(00)00254-x. [DOI] [PubMed] [Google Scholar]
- Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225–30. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Taylor WE, Gonzales-Cadavid NF, Zheng W, Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endcrinol Metab. 2004;89:5245–55. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- Smith EP, Boys J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. New Eng J Med. 1994;331:1056–61. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- Steimer T. Geneva Foundation for Medical Education and Research. 2003 http://www.gfmer.ch/Books/Reproductive_health/Steroid_hormone_metabolism.html [Google Scholar]
- Stirone C, Duckles SP, Krause DN. Multiple forms of estrogen receptor-alpha in cerebral blood vessels: regulation by estrogen. Am J Physiol Endocrinol Metab. 2003;284:E184–92. doi: 10.1152/ajpendo.00165.2002. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Chou TM, Chatterjee K, Smith EP, Williams TC, Kane JP, Malloy MJ, Korach KS, Rubanyi GM. Premature coronary artery disease associated with a disruptive mutation in the estrogen receptor gene in a man. Circulation. 1997a;96:3774–7. doi: 10.1161/01.cir.96.10.3774. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Chou TM, Messina LM, Hutchison SJ, Korach KS, Chatterjee K, Rubanyi GM. Endothelial dysfunction in a man with disruptive mutation in oestrogen-receptor gene. Lancet. 1997b;349:1146–7. doi: 10.1016/S0140-6736(05)63022-X. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–215. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- von Schassen C, Fester L, Prange-Kiel J, Lohse C, Huber C, Bottner M, Rune GM. Oestrogen synthesis in the hippocampus: role in axon outgrowth. J Neuroendocrinol. 2006;18:847–56. doi: 10.1111/j.1365-2826.2006.01484.x. [DOI] [PubMed] [Google Scholar]


