Abstract
Hyperosmolarity has been recognized to be a pro-inflammatory stress to the corneal epithelium. The cell signalling pathways linking hyperosmolar stress and inflammation have not been well elucidated. This study investigated whether exposure of human limbal epithelial cells to hyperosmotic stress activates the mitogen-activated protein kinase (MAPK) pathways and induces production of pro-inflammatory cytokines, interleukin (IL) -1β, tumor necrosis factor (TNF) α, and the C-X-C chemokine IL-8. Primary human limbal epithelial cultures in normal osmolar media (312 mOsM) were exposed to media with higher osmolarity (400–500 mOsM) by adding 50–90 mM NaCl, with or without SB202190, an inhibitor of c-Jun N-terminal kinases (JNK) pathway, PD 98059, an inhibitor of extracellular-regulated kinase (ERK) pathway, dexamethasone or doxycycline for different lengths of time. The conditioned media were collected after 24 hr of treatment for ELISA. Total RNA was extracted from cultures treated for 6 hr for semi-quantitative RT-PCR. Cells treated for 15–60 min were lysed in RIPA buffer and subjected to Western blot with phospho (p)-specific antibodies against p-JNK and p-ERK. The concentrations of IL-1β, TNF-α and IL-8 proteins in 24 hr conditioned media of limbal epithelial cells progressively increased as the media osmolarity increased from 312 to 500 mOsM. Active p-JNK-1/p-JNK-2 and p-ERK-1/p-ERK-2 were detected by Western blot and peaked at 60 min in cells exposed to hyperosmolar media. The levels of p-JNK-1/p-JNK-2 and p-ERK1/p-ERK2 were positively correlated with the medium osmolarity. SB202190, PD98059 and doxycycline markedly suppressed the levels of p-JNK-1/p-JNK-2 and/or p-ERK1/p-ERK2, as well as IL-1β, TNF-α and IL-8 mRNAs and proteins stimulated by hyperosmolar media. These findings provide direct evidence that hyperosmolarity induces inflammation in human limbal epithelial cells by increasing expression and production of pro-inflammatory cytokines and chemokines, a process that appears to be mediated through activation of the JNK and ERK MAPK signalling pathways. The efficacy of doxycycline in treating ocular surface diseases may be due to its ability to suppress JNK and ERK signalling activation and inflammatory mediator production in the limbal epithelium.
Keywords: cornea, epithelium, hyperosmolarity, inflammatory cytokine, chemokine, JNK, ERK, MAPK
1. Introduction
Inflammation is a key component in the pathophysiology of many diseases, including corneal and ocular surface diseases. A variety of inflammatory stimuli such as wound healing, ultraviolet light and growth factor stimulation can induce and promote production of inflammatory cytokines, chemokines and matrix metalloproteinases (MMP). Hyperosmolarity has been recognized to be a potent pro-inflammatory stress. There is increasing evidence that osmotic stress, caused by increased extracellular osmolarity, is a highly relevant challenge to normal cell function in a variety of tissues, including human bronchial epithelial cells (Hashimoto et al., 1999; Loitsch et al., 2000), peripheral blood mononuclear cells (Shapiro and Dinarello, 1997) and the corneal epithelium (Katsuyama and Arakawa, 2003). Tear film hyperosmolarity may cause pathological changes in the corneal epithelium, such as increased desquamation, decreased intercellular connections, blunting and loss of microplicae, cell membrane disruptions and cellular swelling with decreased cytoplasmic density (Gilbard et al., 1984). A hyperosmotic tear film has been observed in eyes with keratoconjunctivitis sicca (KCS), the ocular surface epithelial disease of dry eye, and tear film hyperosmolarity has been proposed as one of the key pathogenic factors (Farris, 1994b). In a mouse model, treatment of the ocular surface with hyperosmolar saline (500 mOsM) was found to stimulate expression and production of interleukin (IL) -1β, tumor necrosis factor α (TNF-α) and MMP-9 by the corneal and conjunctival epithelia, when compared with age matched controls and mice treated with normo-osmolar (305 mOsM) balanced salt solution with (Luo et al., in press). In human corneal epithelial cultures, hyperosmolar media induced expression and production of a number of MMPs, including gelatinase MMP-9, collagenases MMP-1 and MMP-13, and stromelysin MMP-3 (Li et al., 2004). The cell signalling pathways linking hyperosmolar stress and inflammation have not been well elucidated.
The mitogen-activated protein kinases (MAPKs) are well conserved signalling pathways that include extracellular signal regulated kinases (ERK), c-Jun N-terminal kinases (JNK) and p38 MAPK. JNK is also known as stress-activated protein kinases (SAPK) for its response to a variety of stressors (Galcheva-Gargova et al., 1994; Kyriakis et al., 1994; Rosette and Karin, 1996). The activated kinases initiate a cascade of protein phosphorylation involving multiple other kinases and activate nuclear transcription factors such as NF-kappa B, AP-1 and ATF (Gupta et al., 1996; Barchowsky et al., 2000), which promote the expression of inflammatory cytokines, chemokines and MMPs.
We hypothesize that hyperosmotic stress may cause inflammation by activating MAPK signalling pathways in human corneal epithelium. The present study investigated the effects of hyperosmotic stress on activation of MAPK signalling pathways and production of pro-inflammatory cytokines and chemokines by primary cultured human limbal epithelial cells.
2. Material and methods
2.1. Materials and reagents
Cell culture dishes, plates, centrifuge tubes and other plastic ware were purchased from Becton Dickinson (Lincoln Park, NJ), polyvinylidine difluoride (PVDF) membranes were from Millipore (Bedford, MA), polyacrylamide gels (PAGE) and sodium dodecyl sulfate (SDS) were from Bio-Rad (Hercules, CA). Dulbecco modified Eagle medium (DMEM), Ham F-12, amphotericin B, and gentamicin were from Invitrogen-GIBCO BRL (Grand Island, NY). Fetal bovine serum (FBS) was from Hyclone (Logan, UT). SB202190 and PD98059 were from CALBIOCHEM, EMD Biosciences, Inc. (San Diego, CA). Protease inhibitor cocktail tablets were from Roche Applied Science (Indianapolis, IN). Micro BCA protein assay kit was from Pierce Chemical (Rockford, IL). ECL advance chemiluminescence reagents were from Amersham Biosciences (Piscataway, NJ). Rabbit polyclonal antibody against ERK and horseradish peroxidase-conjugated goat anti-rabbit IgG were from cell signalling (Beverly, MA). Rabbit antibody against JNK and monoclonal antibodies (mAb) against phosphorylated JNK (p-JNK) and p-ERK were from Santa Cruz Biotechnology (Santa Cruz, CA). Enzyme-linked immunosorbent assay (ELISA) kits for human IL-1β, TNF-α, and IL-8 were from R&D systems (Minneapolis, MN). GeneAmp RNA-PCR kit was from Applied Biosystems (Foster City, CA). Doxycycline, dexamethasone, 100 bp DNA ladders and all other chemicals and reagents were from Sigma (St Louis, MO).
2.2. Primary cultures of human limbal epithelial cells
Human corneoscleral tissues, which did not meet the criteria for clinical use, from donors aged 21–69 years were obtained from the Lions Eye Bank of Texas (Houston, TX). Corneal epithelial cells were grown from limbal explants using a previously described method (Li et al., 2001; Kim et al., 2004). In brief, after carefully removing the central cornea, excess sclera, iris, corneal endothelium, conjunctiva and Tenon’s capsule, the remaining limbal rim was cut into 12 equal pieces (each about 2×2 mm in size). Two pieces with the epithelial side up were directly placed into each well of 6-well culture plates, and each explant was covered with a drop of FBS overnight. The explants were then cultured in SHEM medium, which was composed of a 1:1 mixture of DMEM and Ham’s F12 medium containing 5 ng mL−1 EGF, 5 μg mL−1 insulin, 5 μg mL−1 transferrin, 5 ng mL−1 sodium selenite, 0.5 μg mL−1 hydrocortisone, 30 ng mL−1 cholera toxin A, 0.5% DMSO, 50 μg mL−1 gentamicin, 1.25 μg mL−1 amphotericin B and 5% FBS, at 37°C under 5% CO2 and 95% humidity. The medium was renewed every 2–3 days. Epithelial phenotype of these cultures was confirmed by characteristic morphology and immuno-fluorescent staining with cytokeratin antibodies (AE-1/AE-3). To avoid possible mixed phenotype of the cultures, each experiment used primary explant cultures established from the same donor limbus. All experiments were performed at least three times using separate sets of cultures that were initiated from different donor limbal tissues.
2.3. Cell treatment
Sub-confluent primary limbal epithelial cultures (grown for 12–14 days, about 4–5×105 cells/well) were washed three times with PBS and switched to a serum-free medium (SHEM media without FBS) for 24 hr before treatment. For cytokine ELISA, the limbal epithelial cells were cultured for an additional 24 hr in an equal volume (1.4 mL/well) of serum-free media with a different osmolarity, ranging from 312–500 mOsM which was achieved by adding 0, 30, 50, 70 or 90 mM sodium chloride (NaCl), with or without SB202190 (10–20 μM, an inhibitor of the JNK pathway), PD98059 (20–40 μM, an inhibitor of the ERK pathway), dexamethasone (1 μM) or doxycycline (10 μg mL−1), which were pre-added 40 min before adding NaCl. The osmolarity of the culture media was measured by a vapour pressure osmometer in the Body Fluid Chemistry Clinical Laboratory of the Methodist Hospital (Houston, TX). The conditioned media were then collected and centrifuged, and the supernatants were stored at −80°C before use. For Western blot, the cultures were treated with the same conditions as above for a shorter time period ranging from 15 to 60 min. The adherent cells were lysed in RIPA buffer containing 50 mM Tris–HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 2 mM sodium fluoride, 2 mM EDTA, 0.1% SDS and protease inhibitor cocktail. The cell extracts were centrifuged at 12 000×g for 15 min at 4°C and the supernatants were store at −80°C before use. The total protein concentrations of the conditioned media and cell extracts were determined by BCA Micro protein assay (Pierce Biotechnology, Rockford, IL). For gene expression, the cultures receiving the same treatments as above for 6 hr were lysed in 4 M guanidium solution and subjected to total RNA extraction. These experiments were performed at least three times on each of three separate sets of cultures that 0were initiated from different donor corneas.
2.4. Enzyme-linked immunosorbent assay (ELISA)
Double-sandwich ELISA for human IL-1β, TNF-α and IL-8 was performed using commercial kits from R&D Systems according to the manufacturer’s protocols. In brief, 200 μL of standard dilutions of recombinant human IL-1β or TNF-α, or culture conditioned media were dispensed into wells of a 96-well plate coated with anti-IL-1β or TNF-α mAb, respectively. After incubation at room temperature (RT) for 2 hr and washing four times, 200 μl of rabbit anti-IL-1β or TNF-α antibodies conjugated with horseradish peroxidase was added into each well and incubated at RT for 1 hr. For IL-8 ELISA, 100 μL of assay buffer, 50 μL of IL-8 standard dilutions or sample media, and 100 μL of IL-8 conjugate were added together into wells of 96-well plate coated with anti-IL-8 mAb and incubated for 2.5 hr at RT. After washing, 200 μL of the substrate reagent 3,3′,5,5′-tetramethylbenzidine (TMB) was applied for 20–30 min to develop a blue colour, and the reaction was stopped by adding 50 μl of 1 M H2SO4. Absorbance was read at 450 nm by a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA) with a reference wavelength of 570 nm.
2.5. Western blot analysis
Western blot was performed using a previously described method (Li et al., 2004; Kim et al., 2005). The cell extract samples (50 μg/lane) were mixed with 6× SDS reducing sample buffer and boiled for 5 min before loading. Proteins were separated by SDS polyacrylamide gel electrophoresis, and transferred electrostatically to PVDF membranes. The membranes were blocked with 5% non-fat milk in TTBS (50 mM Tris, pH 7.5, 0.9% NaCl2 and 0.1% Tween-20) for 1 hr at RT, and then incubated 2 hr at RT with a primary antibody against JNK (1:1000), p-JNK (1:100), ERK (1:1000) or p-ERK (1:1000). The membranes were washed with TTBS and then incubated for 1 hr at RT with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (1:2000 dilution). After washing the membranes for four times, the signals were detected with an ECL advance chemiluminescence reagent using a Kodak image station 2000R (Eastman Kodak, New Haven, CT). The intensity of bands on the images was determined using Kodak 1D v3.6 software. The quantified data were presented as ratios of p-JNK/JNK or ratios of p-ERK/ERK.
2.6. RNA isolation and semi-quantitative RT-PCR
Total RNA was isolated from limbal epithelial cultures with different treatment by acid guanidium thiocyanate–phenol–chloroform extraction using a previously described method (Li and Tseng, 1995). The PCR primers for IL-1β, TNF-α, IL-8 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed from published human gene sequences (Table 1). The semi-quantitative reverse transcription and polymerase chain reaction (RT-PCR) were performed to evaluate the expression of these cytokines by limbal epithelial cells with the house-keeping gene GAPDH as an internal control (Li and Tseng, 1995; Li et al., 2001). In brief, the first-strand cDNAs were synthesized from 1 μg of total RNA at 42°C for 30 min. PCR amplification was performed in a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA) using the following program: first denaturation for 2 min at 95°C followed by 20–40 cycles of denaturation for 1 min at 95°C, annealing for 1 min at 60°C and extension for 1 min at 72°C, and last extension for 7 min at 72°C. Semi-quantitative RT-PCR was established by terminating reactions at intervals of 20, 24, 28, 32, 36 and 40 cycles for each primer pair to ensure that the PCR products formed were within the linear portion of the amplification curve. The fidelity of the RT-PCR product was verified by comparing the size of the amplified products to the expected cDNA bands and by sequencing the PCR products.
Table 1.
Human primer sequences used for RT-PCR
| Gene | Accession | Sense primer | Antisense primer | Product |
|---|---|---|---|---|
| IL-1β | M15330 | TGAACTGAAAGCTCTCCACC | CTGATGTACCAGTTGGGGAA | 297 bp |
| TNF-α | M10988 | TCAGCCTCTTCTCCTTCCTG | TGAAGAGGACCTGGGAGTAG | 324 bp |
| IL-8 | XM_031289 | ATGACTTCCAAGCTGGCCGT | TGTGGTCCCTCTCAATCACTC | 179 bp |
| GAPDH | M33197 | GCCAAGGTCATCCATGACAAC | GTCCACCACCCTGTTGCTGTA | 498 bp |
2.7. Statistical analysis
The student t-test was used for statistical comparison of assay results between two groups.
3. Results
3.1. Hyperosmolarity induced production of inflammatory cytokines, IL-1β and TNF-α, and chemokine IL-8 by human limbal epithelial cells
As shown in Fig. 1, the production of inflammatory cytokines, IL-1β and TNF-α, and the C-X-C chemokine IL-8 was induced in a concentration dependent fashion in the conditioned media from human limbal epithelial cells cultured in media of increasing osmolarity (about 400–500 mOsM) by adding 50, 70 or 90 mM NaCl to normal osmolar media (312 mOsM) for 24 hr. Normalized by total cellular protein, the IL-1β concentration in the conditioned media significantly increased from 90.47±24.22 (mean±S.D.) pg mg−1 cellular protein by control cells (312 mOsM) to 145.78±16.70, 203.27±64.14 (both P < 0.05, n = 5) and 260.47±15.19 pg mg−1 (P < 0.01, n = 5) in cells exposed to media with increasing osmolarities of 400, 450 and 500 mOsM, respectively. The TNF-α levels in the media were significantly increased from 22.22±3.74 pg mg−1 in control cells to 52.33±15.16 (P < 0.05, n = 5), 110.03±17.07 and 133.12±28.53 pg mg−1 (both P < 0.01, n = 5) by cells exposed to media with increasing osmolarities of 400, 450 and 500 mOsM, respectively. Production of the chemokine IL-8 was also stimulated from normal levels (1.56±0.33 ng mg−1) to 2.92±0.75, 3.51±1.06 and 3.77±1.13 ng mg−1 (all P < 0.05, n = 4) by cells exposed to media with increasing osmolarities of 400, 450 and 500 mOsM, respectively.
Fig. 1.
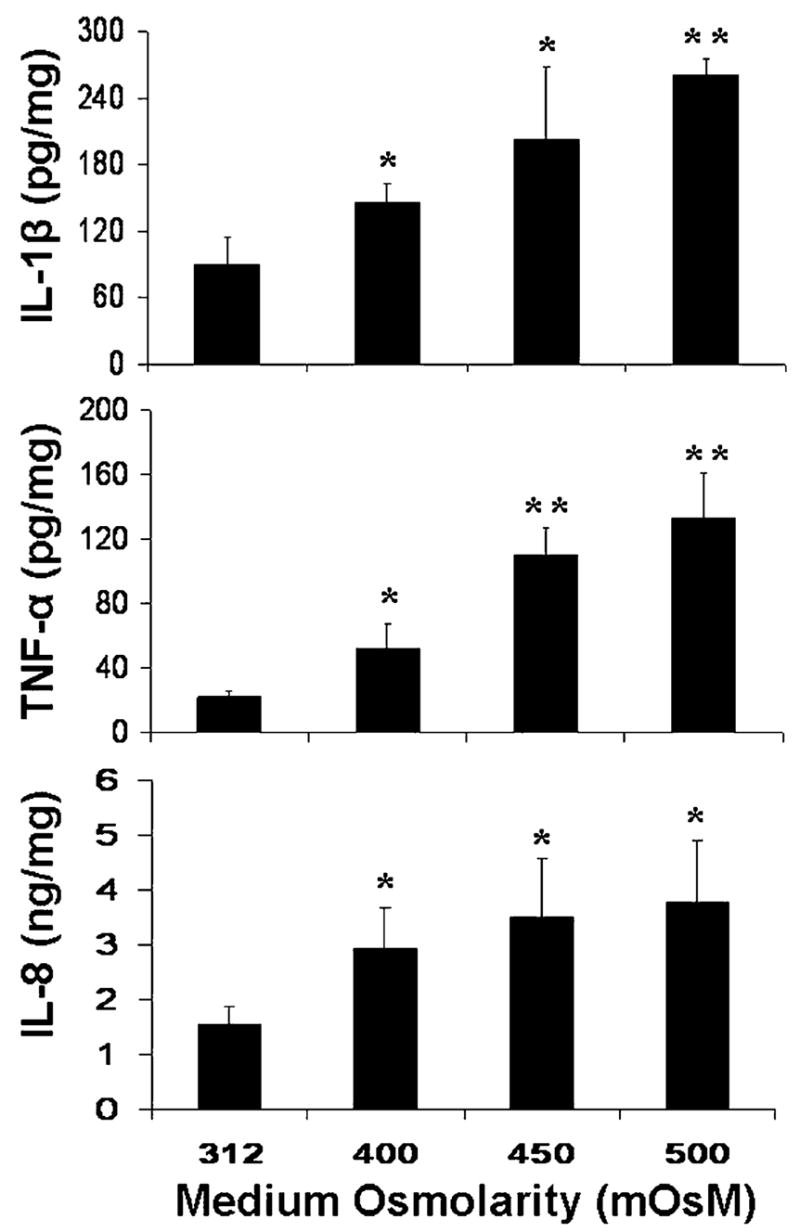
Concentration of IL-1β, TNF-α, and IL-8 proteins in the conditioned media from cells exposed to hyperosmolar media (400–500 mOsM) for 24 hr measured by ELISA and normalized by total cellular protein. Subconfluent primary human limbal epithelial cultures were switched to serum-free media and then treated for 24 hr with normal (312 mOsM) and hyperosmolar (400, 450 and 500 mOsM) media by adding increasing concentrations of NaCl (50, 70 or 90 mM). The supernatants of the conditioned media were collected for ELISAs that were performed according to the manufacturer’s protocols. *P < 0.05, **P < 0.01, n = 5 for IL-1β or TNF-α, and n = 4 for IL-8, compared with controls in normal osmolar media.
3.2. Hyperosmolarity activated JNK and ERK MAPK signalling pathways in human limbal epithelial cells
Using phospho-specific antibodies, Western blot analysis revealed that the JNK-1 (p-JNK-1) and JNK-2 (p-JNK-2) were activated (phosphorylated) in limbal epithelial cells within 15–60 min of exposure to media of approximately 500 mOsM and peak levels were observed at 60 min (Fig. 2A). Levels of p–JNK-2 were stimulated in a concentration-dependent fashion when the cells were exposed to media of increasing osmolarity (400–500 mOsM) for 60 min (Fig. 2B). Treatment with a JNK pathway inhibitor, SB202190 (10–20 μM), almost abolished the hyperosmolarity induced activation of JNK-1 and JNK-2. There was no change in the intensity of the total JNK1/2 bands in these samples when an antibody that detects total JNK (including both unphosphorylated and phosphorylated forms) was used (Fig. 2). Similarly, ERK-1 (p-ERK-1) and ERK-2 (p-ERK-2) were activated in limbal epithelial cells within 15 min, with the peak levels observed at 60 min after exposure to hyperosmolar media (500 mOsM) (Fig. 3A). Levels of p-ERK-1 and p-ERK-2 were stimulated in a concentration-dependent fashion by increasing osmolarity (400–500 mOsM) for 60 min (Fig. 3B). The ERK pathway inhibitor, PD98059 (20–40 μM), abolished this hyperosmolarity induced phosphorylation of ERK-1 and ERK-2. There was no change in the intensity of the total ERK1/2 bands in these samples (Fig. 3).
Fig. 2.
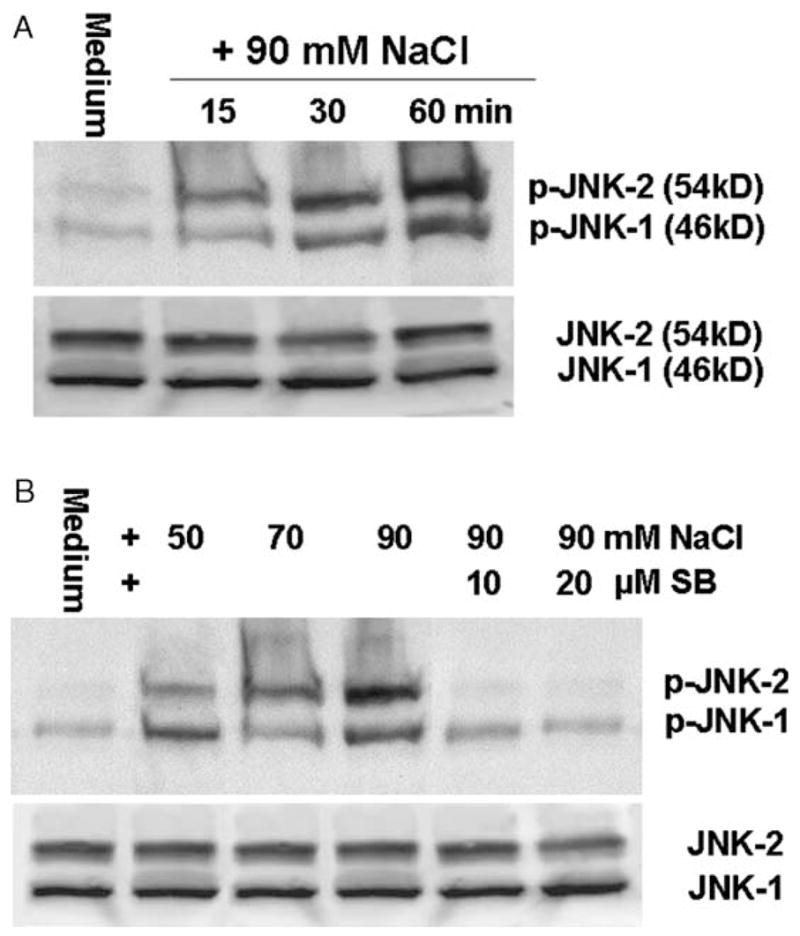
Representative Western blots from three experiments showing the time course (A) and dose-response (B) of increasing medium osmolarity on levels of activated p-JNK1/2 with total JNK1/2 as controls in limbal epithelial cells. Subconfluent primary human limbal epithelial cultures were switched to serum-free media and then treated for 15–60 min with 500 mOsM media for the time course, or treated for 60 min in media with osmolarities of 400, 450 or 500 mOsM that were created by adding increasing concentrations of NaCl (50, 70 or 90 mM) with or without SB202190 (SB, 10–20 μM). The cells were then lysed in RIPA buffer, and the supernatants (50 μg total protein per lane for each sample) of cellular extracts were used for immunoblotting with specific antibodies against p-JNK or total JNK.
Fig. 3.
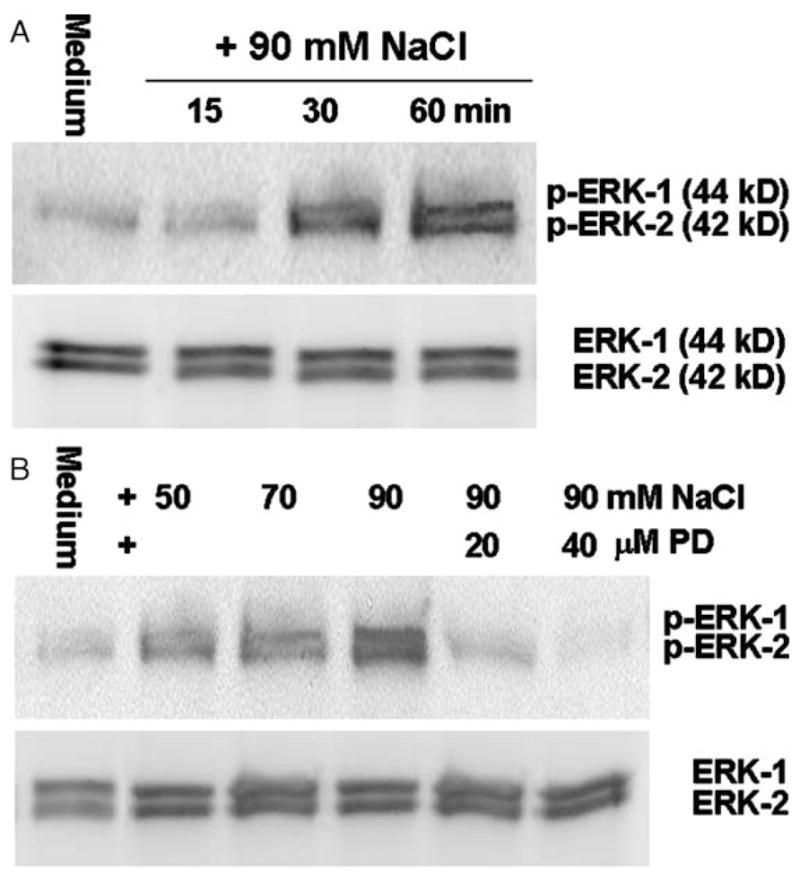
Representative Western blots from three experiments showing the time course (A) and dose-response (B) of increasing medium osmolarity on levels of activated p-ERK1/2 with total ERK1/2 as controls in limbal epithelial cells. Subconfluent primary human limbal epithelial cultures were switched to serum-free media and then treated for 15–60 min with 500 mOsM media for the time course, or treated for 60 min in media with osmolarities of 400, 450 or 500 mOsM that were created by adding increasing concentrations of NaCl (50, 70 or 90 mM) with or without PD98059 (PD, 20–40 μM). The cells were then lysed in RIPA buffer, and the supernatants (50 μg total protein per lane for each sample) of cellular extracts were used for immunoblotting with specific antibodies against p-ERK or total ERK.
3.3. SB202190, PD98059 and doxycycline inhibited activation of JNK and ERK pathways and expression and production of IL-1β, TNF-α and IL-8 in human limbal epithelial cells exposed to hyperosmolar media
To evaluate whether hyperosmotic stress induces production of pro-inflammatory mediators via JNK and/or ERK MAPK pathways, a JNK pathway inhibitor, SB202190, an ERK pathway inhibitor, PD98059, and two clinical anti-inflammatory compounds, doxycycline and dexamethasone, were added individually to the culture media 40 min before adding NaCl to elevate media osmolarity. As shown in Fig. 4A, the Western blot clearly demonstrates that the addition of 20 μM of SB202190 almost abolished the activation of JNK-1 and JNK-2 by hyperosmolar media (500 mOsM). Doxycycline at 10 μg mL−1 showed an inhibitory effect comparatively close to that of 20 μM of SB202190, while 1 μM of dexamethasone showed much less inhibition of JNK activation in osmotically challenged limbal epithelial cells. In contrast, similar intensities of total JNK-1 and JNK-2 were detected in these samples by Western blot using a JNK antibody that detects both unphosphorylated and phosphorylated forms of JNK (Fig. 4A). When normalized by total JNK, the quantified ratio of p-JNK2/JNK2 indicates that the hyperosmolar media (500 mOsM) increased p-JNK2 to 3.4-fold. SB202190 or doxycycline almost abolished this activation (P < 0.05, n = 3) and dexamethasone treated cells showed only 22.2% inhibition (P > 0.05, n = 3). The ratio of p-JNK1/JNK1 was increased 2.1 fold by hyperosmolar media. SB202190 abolished this activation of JNK1 (P < 0.05, n = 3) while doxycycline and dexamethasone showed little inhibitory effects (P > 0.05, n = 3, Fig. 4A).
Fig. 4.
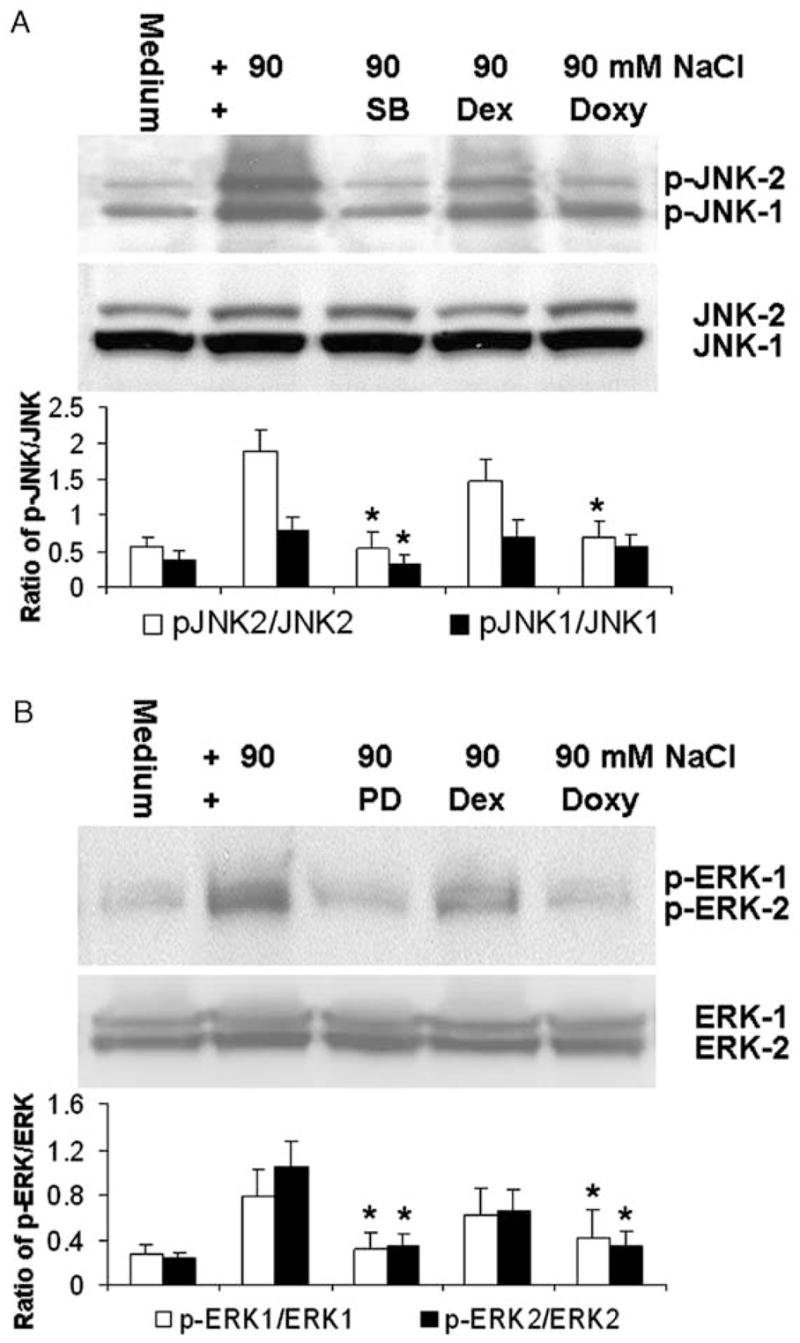
Representative Western blots from three experiments showing (A) the bands of p-JNK-1/p-JNK-2, total JNK-1/JNK-2, and quantified ratios (mean±S.D.) of the band densities of p-JNK1/JNK1 and p-JNK2/JNK2; and (B) the bands of p-ERK-1/p-ERK-2, total ERK-1/ERK-2, and quantified ratios (mean±S.D.) of p-ERK1/ERK1 and p-ERK2/ERK2 in primary human limbal epithelial cultures exposed to normal (312 mOsM) and hyperosmolar media (500 mOsM) created by adding 90 mM NaCl with or without 20 μM SB202190 (SB), 40 μM PD98059 (PD), 1 μM dexamethasone (Dex) or 10 μg mL−1 doxycycline (Doxy). *P < 0.05, **P < 0.01, n = 3, compared with the levels stimulated by the hyperosmolar media.
The hyperosmolarity induced activation of ERK-1 and ERK-2 was markedly inhibited by 40 μM of PD98059 and doxycycline 10 μg mL−1. Less inhibition of ERK activation was observed with 1 μM of dexamethasone (Fig. 4B). No change in the density of total ERK-1 and ERK-2 bands was observed in these samples when using an antibody that recognized both unphosphorylated and phosphorylated ERKs (Fig. 4B). The quantified ratio of p-ERK2/ERK2 indicates that the hyperosmolar media increased p-ERK2 to 4.3-fold. PD98059 or doxycycline significantly inhibited this activation (P < 0.05, n = 3), while dexamethasone produced only 37% inhibition (P > 0.05, n = 3, Fig. 4B). The ratio of p-ERK1/ERK1 was increased 2.8 fold by hyperosmolar media. PD98059 and doxycycline inhibited this activation of ERK1 (P < 0.05, n = 3) while dexamethasone produced only 20% inhibition (P > 0.05, n = 3, Fig. 4B).
Evaluated by ELISA and normalized by total cellular protein (Fig. 5), the concentration of IL-1β in 24 hr conditioned media markedly increased from 71.68±19.80 pg mg−1 cellular protein in normal osmolar media (312 mOsM) to 220.09±59.99 pg mg−1 (P < 0.01, n = 5) in hyperosmolar media (500 mOsM). These stimulated IL-1β levels were significantly decreased to 86.99±28.19 (P < 0.01, n = 5), 109.27±23.54 (P < 0.05, n = 5) and 125.43±17.87 pg mg−1 (P < 0.05, n = 5) by 20 μM SB202190, 40 μM PD98059 and 10 μg mL−1 doxycycline, respectively, while 1 μM dexamethasone did not produce an inhibitory effect (170.54±72.23 pg mg−1, P > 0.05, n = 5). The concentrations of TNF-α were dramatically stimulated 5.4-fold, from 20.52±2.64 pg mg−1 in control media to 109.89±39.20 pg mg−1 (P < 0.01, n = 5) in hyperosmolar media. A significant decrease to 33.82±10.53 (P < 0.01, n = 5), 46.29±7.48 (P < 0.05, n = 5), 58.58±20.63 (P < 0.05, n = 5) and 49.87±25.38 pg mg−1 (P < 0.05, n = 5) was observed in cultures treated with SB202190, PD98059, dexamethasone and doxycycline, respectively. The concentrations of the chemokine IL-8 (Fig. 5) also markedly increased from 1.66±0.39 ng mg−1 in normal media to 3.99±0.74 ng mg−1 (P < 0.01, n = 5) in hyperosmolar media. Significant inhibition to 1.94±0.51, 1.89±0.30 (both P < 0.01, n = 5), 2.58±0.54 and 2.18±0.38 ng mg−1 (both P < 0.05, n = 5) was observed with SB202190, PD98059, dexamethasone and doxycycline treatment, respectively.
Fig. 5.
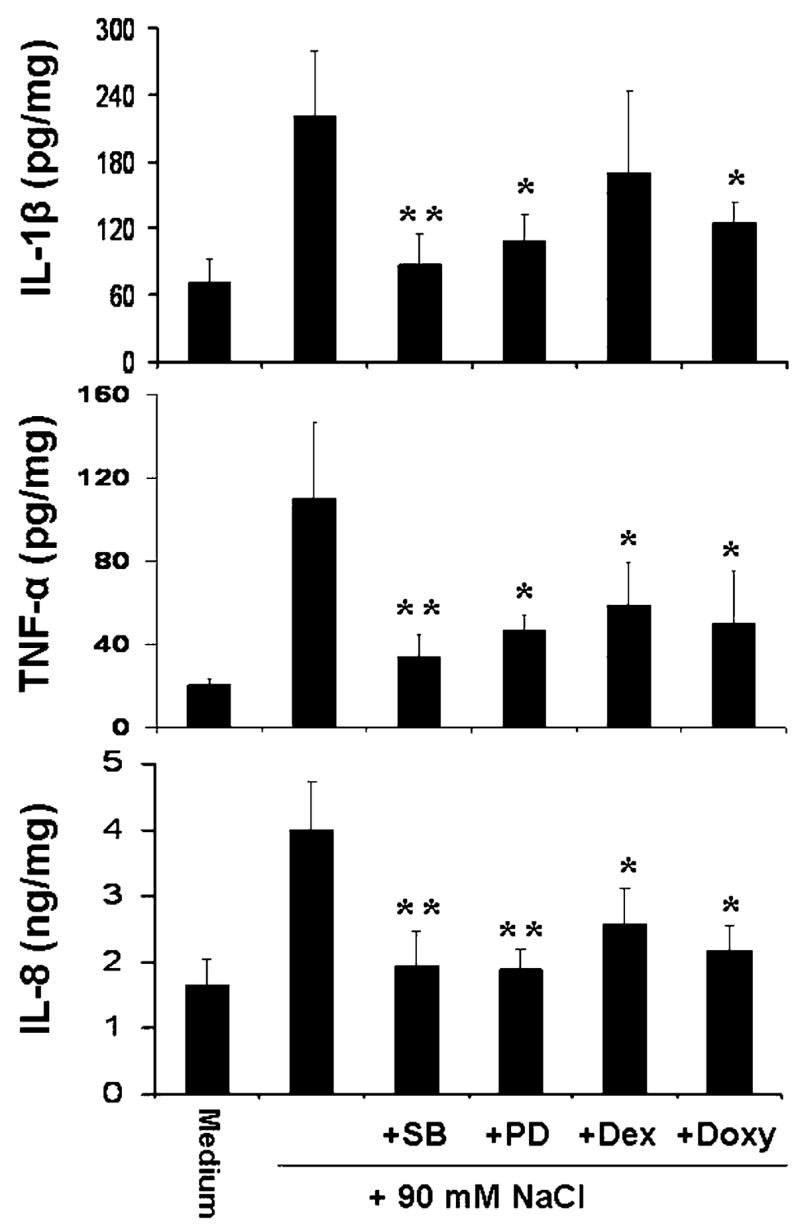
Results of ELISAs form five experiments showing the production of IL-1β, TNF-α, and IL-8 proteins in the 24 hr conditioned media of primary human limbal epithelial cultures exposed to normal (312 mOsM) and hyperosmolar media (500 mOsM) created by adding 90 mM NaCl with or without 20 μM SB202190 (SB), 40 μM PD98059 (PD), 1 μM dexamethasone (Dex) or 10 μg mL−1 doxycycline (Doxy). *P < 0.05, **P < 0.01, n = 5, compared with the levels stimulated by the hyperosmolar media.
Semi-quantitative RT-PCR (Fig. 6) showed that the expression of IL-1β, TNF-α, and IL-8 mRNA was stimulated in limbal epithelial cells exposed to hyperosmolar media with 90 mM NaCl added, and these stimulations were inhibited by SB202190, PD98059 and doxycycline. Dexamethasone produces less inhibitory effect on the levels of these mRNAs.
Fig. 6.
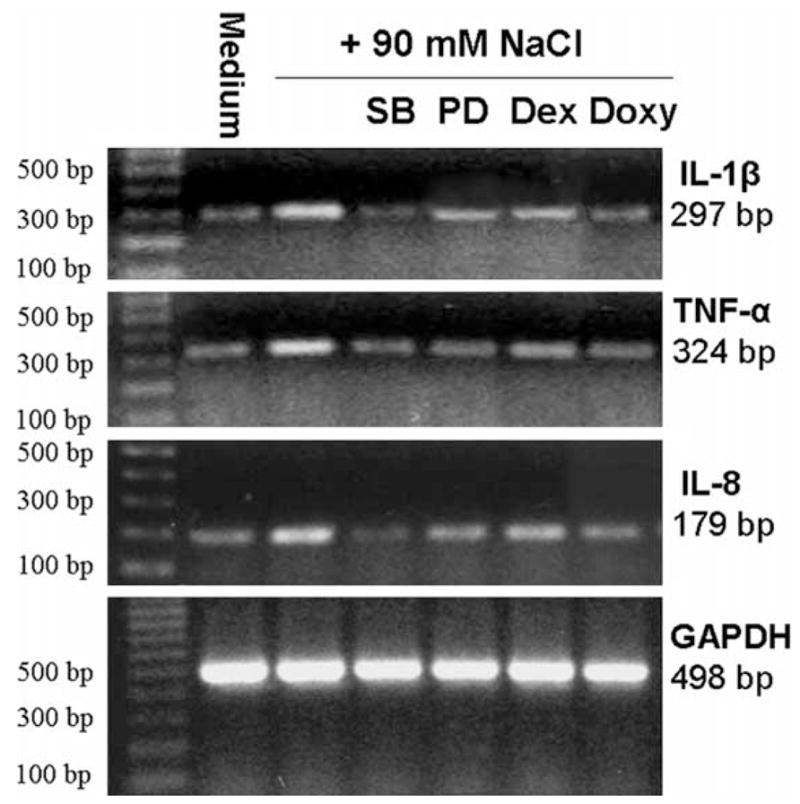
Representative semi-quantitative RT-PCR profiles from three experiments showing the mRNA expressions of IL-1β, TNF-α and IL-8 with GAPDH as an internal control by human limbal epithelial cells treated for 6 hr in normal and hyperosmolar media (500 mOsM) created by adding 90 mM NaCl with or without 20 μM SB202190 (SB), 40 μM PD98059 (PD), 1 μM dexamethasone (Dex) or 10 μg mL−1 doxycycline (Doxy).
4. Discussion
4.1. Hyperosmolarity induces expression and production of IL-1β, TNF-α and IL-8 by human limbal epithelial cells
Elevated tear fluid osmolarity has been recognized as a common feature of dry eye for decades (Farris, 1994a); however, the relationship between hyperosmolar stress and the induction of inflammation on the ocular surface has not been well elucidated. We have recently evaluated the stimulatory effects of hyperosmolarity on MMP production by human corneal epithelial cells (Li et al., 2004) and on IL-1β, TNF-α and MMP-9 in the ocular surface epithelium of mice (Luo et al., 2005). The present study demonstrates for the first time that hyperosmotic stress induces expression and production of the pro-inflammatory cytokines, IL-1β, TNF-α, and the C-X-C chemokine IL-8 by primary cultured human limbal epithelial cells. As demonstrated in Fig. 1, the concentrations of IL-1β, TNF-α and IL-8 increased significantly in a concentration-dependent manner in the conditioned media from limbal epithelial cells exposed to hyperosmolar media compared with control cells in normal osmolar media. Hyperosmolar stimulated transcription of these three cytokines was also observed by semi-quantitative RT-PCR (Fig. 6).
4.2. Hyperosmolarity activates the JNK and ERK signalling pathways in human limbal epithelial cells
Mitogen-activated protein kinases are important cell signalling mediators that play vital roles in the cellular response to stress. The different MAPKs can be activated in response to specific stimuli and they also regulate specific substrates. The JNK and p38 MAPK cascades are strongly activated by cellular stresses, as well as by pro-inflammatory agents such as endotoxin, IL-1 and TNF-α (Raingeaud et al., 1995; Roberts and Cowsert, 1998; Finch et al., 2001). In contrast, ERK MAPK is strongly activated by growth factors such as PDGF, hepatocyte growth factor (HGF), epidermal growth factor (EGF), transforming growth factor (TGF) α and other stimuli that mediate cell proliferation, differentiation, and survival (Zeigler et al., 1999; Cho et al., 2000; Hayashida et al., 2003). The ERK signalling pathway has been also observed to mediate stress induced inflammation (Kyriakis et al., 1995). In experimental cerebral ischemia, ERK1/2 pathway activation was linked to pro-inflammatory cytokine IL-1β signalling, which induced an inflammatory response and exacerbated the severity of the ischemic brain injury (Wang et al., 2004). The mechanism of activation and the functional role of each MAPK cascade are dependent on cell type and the type of the stimulus used (Suzuki et al., 2001). It is well established that phosphorylation of the transcription factor c-Jun by JNK is a key event in the cellular response to stress (Derijard et al., 1994; Kyriakis et al., 1994). The role of the JNK cascade pathway in response to hyperosmolarity is not well understood, although a few studies have reported activation of JNK by hyperosmotic stress in mammalian cells (Galcheva-Gargova et al., 1994). There is no report of ERK signalling activation by hyperosmolarity to date. In our study, Western blot was performed using antibodies specific for the phosphorylated active forms of JNK and ERK. The results showed that JNK1/2 (Fig. 2) and ERK1/2 (Fig. 3) were activated in human limbal epithelial cells exposed to hyperosmolar media (400–500 mOsM). No effect on total levels of the JNKs and ERKs was observed. This phenomenon was confirmed by treatment with SB202190, a JNK pathway inhibitor, which was found to dramatically inhibit the hyperosmolarity stimulated phosphorylation of JNK1/2, and with PD98059, an ERK signalling inhibitor, that blocked the hyperosmolarity induced activation of ERKs.
4.3. Activation of JNK and ERK signalling pathways may mediate the induction of IL-1β, TNF-α and IL-8 by hyperosmolarity in human limbal epithelial cells
MAPK signalling pathways are known to regulate production and activity of inflammatory mediators through activation of transcription factors such as NFkB, AP-1 and ATF in different target cells (Liacini et al., 2002). The JNK and ERK signalling pathways were reported to be activated by inflammatory cytokines, such as IL-1β and TNF-α (Rosette and Karin, 1996; Roberts and Cowsert, 1998; McDermott et al., 2003). The link between the activation of JNK and ERK MAPK and the induction of IL-1β, TNF-α and IL-8 by hyperosmolar stress has not been established, although JNK activation by hyperosmotic stress in mammalian cells was observed ten years ago (Galcheva-Gargova et al., 1994). To establish this connection, the effects of the JNK and ERK pathway inhibitors, SB202190 and PD98059, and the anti-inflammatory agents, doxycycline and dexamethasone, were evaluated on MAPK activation and inflammatory cytokine production in limbal epithelial cells exposed to hyperosmolar media.
SB202190, a chemical originally reported as a p38 MAPK pathway inhibitor, has also been recognized to inhibit JNK activation (Chen et al., 1998; Ming et al., 1998; Pages et al., 2000). We observed that phosphorylation of JNK in limbal epithelial cultures exposed to 500 mOsM media was inhibited by SB202190 in a range of 10–20 μM (Fig. 2B). Thus, 20 μM of SB202190 was used for all subsequent inhibition experiments in this study. Interestingly, SB202190 also dramatically suppressed the expression and production of IL-1β, TNF-α and IL-8 by the cells exposed to hyperosmolar media as shown by ELISA (Fig. 5) and semi-quantitative RT-PCR (Fig. 6).
PD98059, an ERK pathway inhibitor, was observed to inhibit the phosphorylation of ERK1/2 in limbal epithelial cultures exposed to 500 mOsM media in a range of 20–40 μM (Fig. 3b). Thus, 40 μM of PD98059 was used for all subsequent inhibition experiments in this study. PD98059 was also found to markedly inhibit the expression and production of IL-1β, TNF-α and IL-8 by limbal epithelial cells exposed to hyperosmolar media as shown by ELISA (Fig. 5) and semi-quantitative RT-PCR (Fig. 6).
Doxycycline is a semi-synthetic tetracycline that has been used to treat ocular surface diseases, including meibomian gland disease, ocular rosacea and recurrent corneal epithelial erosion. It has been reported to inhibit the expression and production of MMP-9, -1, -13, -3 and -10 induced by IL-1β and TNF-α in human corneal epithelial cells (Sobrin et al., 2000; Li et al., 2001; Li et al., 2003). Here, we show that doxycycline exhibits similar inhibitory properties to SB202190 and PD98059 on hyperosmolarity activation of JNK1/2 and ERK1/2, respectively. Doxycycline also significantly reduced the expression and production of IL-1β, TNF-α and IL-8 by osmotically challenged limbal epithelial cells (Figs. 2–6). It suggests that doxycycline may inhibit the production of IL-1β, TNF-α and IL-8 through blocking the activation of the JNK and ERK MAPK signalling pathways. In deed, the minocycline, a member of tetracyclines, has been reported to inhibit hypoxic activation of rat microglia by inhibiting p38 MAPK (Suk, 2004), and we previous reported that doxycycline inhibited hyperosmolarity induced activation of JNK (Li et al., 2004) and TGF-β1 induced activation of JNK, ERK and p38 MAPK (Kim et al., 2005) in human limbal epithelial cells.
The corticosteroid dexamethasone showed less inhibitory effect on activation of JNKs and ERKs (Fig. 4), and stimulation of IL-1β expression and production by hyperosmolarity in these limbal epithelial cells (Figs. 4 and 5). Interestingly, dexamethasone did inhibit the stimulated expression and production of TNF-α and IL-8 by the cells exposed to hyperosmolar media (Figs. 5 and 6). This suggests that dexamethasone may regulate the production of inflammatory mediators through other mechanism, such as NFkB signalling pathways.
In the present study, the experiments were conducted at 400–500 mOsM, because lower osmolar media (340–370 mOsM) did not significantly stimulate production of IL-1β, TNF-α and IL-8 and activation of MAPKs by cultured limbal epithelial cells. This could be due to sensitivity of ELISA and Western blot we used, or due to greater resistance of cultured limbal epithelial cells to hyperosmolar stress than ocular surface epithelial cells in vivo. Using a highly sensitive Luminex immunoassay, we were able to detect activation of JNK1 by media with osmolarity of 370 mOsM in cultured human limbal epithelial cells (Li et al., 2004). We have observed that human limbal epithelial cells grow very well in a wide range of medium osmolarities ranging from 312 mOsM SHEM without HEPES to 340 mOsM SHEM with HEPES in our culture system. Gilbard and colleagues (Gilbard et al., 1978) have reported that normal human subjects have a tear osmolarity of 302±6 (mean±S.D.) mOsM, while dry eye patients have an osmolarity of 343±32 (306–441) mOsM. A hyperosmotic tear film has been observed in KCS with peak osmolarities of approximately 450 mOsm (Gilbard et al., 1978; Mathers et al., 1996). A significant increase in tear osmolarity from normal 285 to 559 mOsm was observed in BALB/c mice with experimental dry eye (Stewart et al., 2005). Our findings in the current study were obtained at osmolarities of at least 400 mOsM, which is at the high end of the range of tear osmolarity found in dry eye patients and far above the average tear osmolarity in KCS. Although the data we have presented shows that hyperosmolarity induces inflammatory cytokine expression and activates MAPK pathways, further studies are necessary to establish a direct linkage between elevated tear osmolarity and ocular surface inflammation in human dry eye patients with KCS.
In conclusion, our findings provide direct evidence that hyperosmolarity is a pro-inflammatory stimulus on the corneal epithelium, increasing expression and production of pro-inflammatory cytokines (IL-1β and TNF-α) and the C-X-C chemokine IL-8, a process that may be mediated through activation of the JNK and ERK MAPK signalling pathways in these cells. The efficacy of doxycycline in treating ocular surface diseases may be due to its ability to suppress MAPK signalling activation in the corneal epithelium.
Acknowledgments
The authors thank the Lions Eye Bank of Texas for their great support in providing human corneoscleral tissues.
This study was supported in part by NIH grants, EY11915 (SCP) and EY014553 (DQL), from National Eye Institute, Bethesda, MD, an unrestricted grant from Research to Prevent Blindness, the Oshman Foundation, and the William Stamps Farish Fund.
Footnotes
Presented in part as an abstract at the annual meeting of the Association for Research in Vision and Ophthalmology, May 5–10, 2002, Fort Lauderdale, Florida.
References
- Barchowsky A, Frleta D, Vincenti MP. Integration of the NF-kappaB and mitogen-activated protein kinase/AP-1 pathways at the collagenase-1 promoter: divergence of IL-1 and TNF-dependent signal transduction in rabbit primary synovial fibroblasts. Cytokine. 2000;12:1469–1479. doi: 10.1006/cyto.2000.0743. [DOI] [PubMed] [Google Scholar]
- Chen CY, Gatto-Konczak F, Wu Z, Karin M. Stabilization of interleukin-2 mRNA by the c-Jun NH2-terminal kinase pathway. Science. 1998;280:1945–1949. doi: 10.1126/science.280.5371.1945. [DOI] [PubMed] [Google Scholar]
- Cho A, Graves J, Reidy MA. Mitogen-activated protein kinases mediate matrix metalloproteinase-9 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:2527–2532. doi: 10.1161/01.atv.20.12.2527. [DOI] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and ha-ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Farris RL. Contact lenses and the dry eye. Int Ophthalmol Clin. 1994a;34:129–136. doi: 10.1097/00004397-199403410-00012. [DOI] [PubMed] [Google Scholar]
- Farris RL. Tear osmolarity—a new gold standard? Adv Exp Med Biol. 1994b;350:495–503. doi: 10.1007/978-1-4615-2417-5_83. [DOI] [PubMed] [Google Scholar]
- Finch A, Davis W, Carter WG, Saklatvala J. Analysis of mitogen-activated protein kinase pathways used by interleukin 1 in tissues in vivo: activation of hepatic c-Jun N-terminal kinases 1 and 2, and mitogen-activated protein kinase kinases 4 and 7. Biochem J. 2001;353:275–281. doi: 10.1042/0264-6021:3530275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galcheva-Gargova Z, Derijard B, Wu IH, Davis RJ. An osmosensing signal transduction pathway in mammalian cells. Science. 1994;265:806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- Gilbard JP, Farris RL, Santamaria J. Osmolarity of tear microvolumes in keratoconjunctivitis sicca. Arch Ophthalmol. 1978;96:677–681. doi: 10.1001/archopht.1978.03910050373015. [DOI] [PubMed] [Google Scholar]
- Gilbard JP, Carter JB, Sang DN, Refojo MF, Hanninen LA, Kenyon KR. Morphologic effect of hyperosmolarity on rabbit corneal epithelium. Ophthalmology. 1984;91:1205–1212. doi: 10.1016/s0161-6420(84)34163-x. [DOI] [PubMed] [Google Scholar]
- Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B, Davis RJ. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S, Matsumoto K, Gon Y, Nakayama T, Takeshita I, Horie T. Hyperosmolarity-induced interleukin-8 expression in human bronchial epithelial cells through p38 mitogen-activated protein kinase. Am J Respir Crit Care Med. 1999;159:634–640. doi: 10.1164/ajrccm.159.2.9712090. [DOI] [PubMed] [Google Scholar]
- Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J. 2003;17:1576–1578. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- Katsuyama I, Arakawa T. A convenient rabbit model of ocular epithelium damage induced by osmotic dehydration. J Ocul Pharmacol Ther. 2003;19:281–289. doi: 10.1089/108076803321908400. [DOI] [PubMed] [Google Scholar]
- Kim HS, Jun SX, de Paiva CS, Chen Z, Pflugfelder SC, Li DQ. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp Eye Res. 2004;79:41–49. doi: 10.1016/j.exer.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Luo L, Pflugfelder SC, Li DQ. Doxycycline inhibits TGF-β1-induced MMP-9 via smad and MAPK pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:840–848. doi: 10.1167/iovs.04-0929. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Woodgett JR, Avruch J. The stress-activated protein kinases. A novel ERK subfamily responsive to cellular stress and inflammatory cytokines. Ann NY Acad Sci. 1995;766:303–319. doi: 10.1111/j.1749-6632.1995.tb26683.x. [DOI] [PubMed] [Google Scholar]
- Li DQ, Tseng SC. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol. 1995;163:61–79. doi: 10.1002/jcp.1041630108. [DOI] [PubMed] [Google Scholar]
- Li DQ, Lokeshwar BL, Solomon A, Monroy D, Ji Z, Pflugfelder SC. Regulation of MMP-9 production by human corneal epithelial cells. Exp Eye Res. 2001;73:449–459. doi: 10.1006/exer.2001.1054. [DOI] [PubMed] [Google Scholar]
- Li DQ, Shang TY, Kim HS, Solomon A, Lokeshwar BL, Pflugfelder SC. Regulated expression of collagenases MMP-1, -8, and -13 and stromelysins MMP-3, -10, and -11 by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:2928–2936. doi: 10.1167/iovs.02-0874. [DOI] [PubMed] [Google Scholar]
- Li DQ, Chen Z, Song XJ, Luo L, Pflugfelder SC. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:4302–4311. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- Liacini A, Sylvester J, Li WQ, Zafarullah M. Inhibition of interleukin-1-stimulated MAP kinases, activating protein- 1 (AP-1) and nuclear factor kappa B (NF-kappaB) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 2002;21:251–262. doi: 10.1016/s0945-053x(02)00007-0. [DOI] [PubMed] [Google Scholar]
- Loitsch SM, von Mallinckrodt C, Kippenberger S, Steinhilber D, Wagner TO, Bargon J. Reactive oxygen intermediates are involved in IL-8 production induced by hyperosmotic stress in human bronchial epithelial cells. Biochem Biophys Res Commun. 2000;276:571–578. doi: 10.1006/bbrc.2000.3504. [DOI] [PubMed] [Google Scholar]
- Luo L, Li DQ, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens. 2005;31:186–193. doi: 10.1097/01.icl.0000162759.79740.46. [DOI] [PubMed] [Google Scholar]
- Mathers WD, Lane JA, Sutphin JE, Zimmerman MB. Model for ocular tear film function. Cornea. 1996;15:110–119. doi: 10.1097/00003226-199603000-00002. [DOI] [PubMed] [Google Scholar]
- McDermott AM, Redfern RL, Zhang B, Pei Y, Huang L, Proske RJ. Defensin expression by the cornea: multiple signalling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:1859–1865. doi: 10.1167/iovs.02-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming XF, Kaiser M, Moroni C. c-jun N-terminal kinase is involved in AUUUA-mediated interleukin-3 mRNA turnover in mast cells. EMBO J. 1998;17:6039–6048. doi: 10.1093/emboj/17.20.6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages G, Berra E, Milanini J, Levy AP, Pouyssegur J. Stress-activated protein kinases (JNK and p38/HOG) are essential for vascular endothelial growth factor mRNA stability. J Biol Chem. 2000;275:26484–26491. doi: 10.1074/jbc.M002104200. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Roberts ML, Cowsert LM. Interleukin-1 beta and reactive oxygen species mediate activation of c-Jun NH2-terminal kinases, in human epithelial cells, by two independent pathways. Biochem Biophys Res Commun. 1998;251:166–172. doi: 10.1006/bbrc.1998.9434. [DOI] [PubMed] [Google Scholar]
- Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Dinarello CA. Hyperosmotic stress as a stimulant for proinflammatory cytokine production. Exp Cell Res. 1997;231:354–362. doi: 10.1006/excr.1997.3476. [DOI] [PubMed] [Google Scholar]
- Sobrin L, Liu Z, Monroy DC, Solomon A, Selzer MG, Lokeshwar BL, Pflugfelder SC. Regulation of MMP-9 activity in human tear fluid and corneal epithelial culture supernatant. Invest Ophthalmol Vis Sci. 2000;41:1703–1709. [PubMed] [Google Scholar]
- Stewart P, Chen Z, Farley W, Olmos LC, Pflugfelder SC. Effect of EDE on tear sodium [Na] in the mouse. Eye Contact Lens. 2005;31:175–178. doi: 10.1097/01.icl.0000161705.19602.c9. [DOI] [PubMed] [Google Scholar]
- Suk K. Minocycline suppresses hypoxic activation of rodent microglia in culture. Neurosci Lett. 2004;366:167–171. doi: 10.1016/j.neulet.2004.05.038. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Hino M, Kutsuna H, Hato F, Sakamoto C, Takahashi T, Tatsumi N, Kitagawa S. Selective activation of p38 mitogen-activated protein kinase cascade in human neutrophils stimulated by IL-1beta. J Immunol. 2001;167:5940–5947. doi: 10.4049/jimmunol.167.10.5940. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Wu DC, Huang FP, Yang GY. Inhibition of MEK/ERK 1/2 pathway reduces pro-inflammatory cytokine interleukin-1 expression in focal cerebral ischemia. Brain Res. 2004;996:55–66. doi: 10.1016/j.brainres.2003.09.074. [DOI] [PubMed] [Google Scholar]
- Zeigler ME, Chi Y, Schmidt T, Varani J. Role of ERK and JNK pathways in regulating cell motility and matrix metalloproteinase 9 production in growth factor-stimulated human epidermal keratinocytes. J Cell Physiol. 1999;180:271–284. doi: 10.1002/(SICI)1097-4652(199908)180:2<271::AID-JCP15>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]


