Abstract
Adoptive cell transfer therapy has developed into a potent and effective treatment for patients with metastatic melanoma. Current application of this therapy relies on the ex vivo generation of highly active, highly avid tumor-reactive lymphocyte cultures from endogenous tumor infiltrating lymphocytes or on the genetic engineering of cells using antigen receptor genes to express de novo tumor antigen recognition. When anti-tumor lymphocyte cultures are administered to autologous patients with high dose interleukin-2 following a lymphodepleting conditioning regimen, the cells can expand in vivo, traffic to tumor, and mediate tumor regression and durable objective clinical responses. Current investigation seeks to improve the methods for generating and administering the lymphocyte cultures, and future clinical trials aim to improve durable response rates and extend the patient populations that are candidates for treatment.
The cellular arm of the immune system is responsible for tumor rejection in animal models of transplanted tumors, and is thought to mediate human tumor immunotherapy as well. Adoptive cell transfer therapies seek to enhance the activity of these immune cells by isolating them from the endogenous environment of the tumor-bearing host. The anti-tumor cells are cultured ex vivo to activate and numerically expand them prior to infusion to the autologous tumor-bearing host for therapy. In the past two decades, the Surgery Branch has undertaken a series of Phase I and Phase I/II clinical trials to investigate specific improvements to the clinical application of cell transfer therapy. In many cases the impetus for a clinical trial derived directly from basic principles elucidated in animal models or inferred from retrospective studies of prior clinical trials. This review summarizes the lessons that have been learned from these clinical trials, describes the efforts that are currently underway in the Surgery Branch to improve cell transfer therapy for patients with melanoma and other metastatic cancers, and suggests some areas of scientific and clinical research that will lead to progress in future clinical trials.
LAK and early TIL studies
Initial clinical studies with cell transfer therapy can be traced to the availability of recombinant human cytokines such as interleukin (IL)-2 for in vitro use. The availability of this T cell growth factor enabled the large scale culture of human lymphocytes for extended periods, and promoted investigations into the use of lymphokine activated killer (LAK) cells for the treatment of patients with metastatic cancers1. LAK cell cultures consisted of a mixture of multiple lymphocyte types including NK cell precursors and T lymphocytes, and they exhibited HLA-independent lysis of tumor cells in vitro. Many of the foundations for large scale human lymphocyte cultures were established in these trials, including the formulation of serum free media, methods for sterilely handling and processing large volumes of cells, and safety and efficacy tests for human lymphocyte infusions for clinical trials2. However, LAK cells with high dose IL-2 were not shown to be effective in a randomized clinical trial when compared to IL-2 alone3.
In contrast to the non-specific activity of LAK cells, the cultures derived from some tumor infiltrating lymphocytes (TIL) that were expanded in vitro in IL-2 demonstrated marked tumor specificity. This phenomenon was initially described with lymphocytes from immunogenic transplantable tumors of mice4, and then characterized in TIL derived from resected human cancers of several histologies5–7. Although TIL cultures could sometimes be expanded from tumors of common epithelial origin, these cultures rarely demonstrated specific tumor activity. In contrast, melanoma TIL have been reliably generated using two related methods in the Surgery Branch, and tumor specific activity was detected by lysis or cytokine release in about 70% of cultures in two separate large series of samples7,8. The study of the antigen reactive lymphocytes that infiltrate melanoma metastases has informed and driven many of the Surgery Branch clinical cell transfer efforts.
Initial clinical efforts with TIL were summarized in a study using autologous TIL plus IL-2 in the treatment of 86 patients with metastatic melanoma9. In that study TIL and high dose IL-2 were administered in two cycles separated by approximately 2 weeks, constituting one treatment course. Six weeks after treatment, all known sites of disease were evaluated. The overall objective response rate in those patients was 34%, although many of the responses were of short duration. There was no significant difference in the objective response rate in patients whose therapy with high-dose IL-2 had failed (32%) compared with patients not previously treated with IL-2 (34%). Since patients who recurred after initially responding to IL-2 do not respond to additional cycles of IL-210 the response of patients who received TIL plus IL-2 strongly implied that the anti-tumor response was mediated by the TIL cells. These results illustrated the potential value of immune lymphocytes for the treatment of patients with melanoma, and laid the foundation for many of the cell transfer studies that followed in the Surgery Branch and at other institutions.
Retrospective analyses of treatment characteristics of the infused cells and the patients’ clinical outcomes revealed several strong correlations. The frequency of response to treatment was greater in patients who were treated with TIL from younger cultures (P = .0001) and TIL with shorter doubling times (P = .03). Another strong correlation was noted between response and TIL that exhibited higher lysis against autologous tumor targets (P = .0008). Probably related to this issue of tumor specific recognition, patients who received TIL generated from subcutaneous tumor deposits had higher response rates (49%) compared with those receiving TIL from lymph nodes (17%; P = .006). Some subsequent research efforts and clinical trials in the Surgery Branch have focused on the issues defined by these correlations: improved anti-tumor specificity of the treatment TIL, or improved growth and expansion conditions to yield optimized early growth of TIL that can engraft, persist, and mediate anti-tumor effects in vivo.
TIL cells from responding patients were used for molecular identification of tumor rejection antigens by cDNA expression cloning. One of the first antigens cloned was the melanoma antigen recognized by T cells (MART)-1 gene, which was cloned using both transient and stable expression systems11. MART-1 is a transmembrane protein whose expression is restricted to melanoma cells and normal melanin producing cells, but no other normal tissues or tumor histologies. MART-1 is thus a melanocyte lineage-specific protein and a widely shared melanoma antigen. In one study, the presence of gp100 reactive cells was significantly correlated with patient response (p=0.005) suggesting that gp100 may be useful for the development of immunotherapies for patients with melanoma12. Currently, many genes expressed by tumors have been identified whose normally transcribed or mutated gene products are recognized by T cells, and which are candidates for immune targetting13.
The identification of the gp100 gene product and its HLA-A2 restricted epitopes as targets for the immunotherapy opened new approaches to cell transfer therapy. The gp100:209–217 epitope was shown to be a relatively poor immunogen in humans, but its immunogenicity was improved by the modification of the nine amino acid peptide sequence to enhance its HLA-A2 binding affinity without substantially impacting the T-cell contact surface of the HLA-peptide-T cell receptor interface14. Using a nine amino acid peptide with a methionine substitution for a threonine at the second position, a T cell epitope with a better in vitro and in vivo immunizing profile was identified. Patients were immunized as part of clinical trials, both in the adjuvant setting and for metastatic disease, using the modified 209-2M peptide vaccine15. Most patients developed high levels of gp100-specific, tumor reactive CTL precursors in their peripheral circulation after vaccination. However, patients with metastatic disease rarely experienced clinical remission due to vaccination alone, and highly immunized patients recurred with disease in adjuvant vaccine studies16. However, the presence of large numbers of highly active, highly avid anti-tumor T cell precursors in the PBL enabled the design and implementation of new clinical trials to evaluate their efficacy in adoptive cell transfer experiments. Several methods were devised for the isolation, expansion, and activation of highly avid tumor-specific lymphocytes from PBL precursors, and their administration at high numbers to autologous patients.
Cloned PBL and IVS PBL
A phase I study of the adoptive transfer of cloned melanoma antigen-specific T lymphocytes for therapy of patients with advanced melanoma was undertaken in the Surgery Branch, NCI17. In that study, clones were derived from PBL or TIL of patients who had received prior immunization with 209-2M. In response to their cognate antigens, clones used for treatment secreted large amounts of interferon-gamma (IFNg) and GM-CSF, lesser amounts of IL-2 and tumor necrosis factor alpha (TNFa), and little or no IL-4 and IL-10. Clones also demonstrated recognition of HLA-matched melanomas using cytokine secretion and lysis assays. Eleven patients received 2 cycles of cells alone and then were randomized between two schedules of IL-2: low dose (125,000 IU/kg subcutaneously daily for 12 days) or high dose (720,000 IU/kg intravenously every 8hr for 4 days); one additional patient received two cycles of cloned cells alone and was discharged without finishing the treatment course. An average of 1 × 1010 cells was administered per cycle. For some patients, peripheral blood samples were analyzed for persistence of transferred cells by T-cell receptor-specific PCR. Although the transferred cells were detectable at 1hr after transfer, they rapidly declined to undetectable levels by 2 weeks. One minor response and one mixed response were observed (both in the high-dose IL-2 arm), but no patient achieved an objective clinical response by RECIST criteria.
A similar study was undertaken by Yee et al.18 with similar results. T cell clones targeting the tumor-associated antigens, MART1 and gp100, were selected and expanded in vitro for the treatment of patients with metastatic melanoma. Four infusions of autologous T cell clones were administered to 10 patients, the first without IL-2 and subsequent infusions with low- dose IL-2. The transferred T cell clones persisted while the low dose IL-2 administration was ongoing (approximately 15 days) but rapidly disappeared from the peripheral circulation thereafter. Overall, the regression of individual metastases, and minor, mixed or stable responses in individual patients were reported, but no objective clinical responses were seen by RECIST criteria.
Together, these reports on the transfer of cloned antigen specific T cells demonstrated the safety and feasibility of this approach for patients with cancer. However, the lack of clinical effectiveness of the cloned T lymphocytes suggested that transfer of different or additional cell types or that modulation of the recipient host environment was required for successful therapy. The need to grow cloned lymphocytes in vitro for extended times, and the single specificity of the antigen receptor were both potential reasons for the lack of effectiveness of these cloned cell protocols.
One of the particularly impressive deficits of the cloned lymphocytes in vivo was their rapid disappearance from the peripheral circulation after transfer, despite the transfer of large numbers of cells. We hypothesized that competition for homeostatic cytokines such as IL-7 or IL-15 could be limiting the persistence of the transferred tumor reactive clones, and that elimination of the endogenous host repertoire might prolong persistence of the transferred cells and enhance anti-tumor efficacy. To investigate this hypothesis, we initiated a clinical trial using a non-myeloablative, lymphodepleting chemotherapy in combination with adoptive immunotherapy in patients with metastatic melanoma19. Initially, a dose escalation phase was undertaken to evaluate the tolerance to the non-myeloablative chemotherapy in combination with IL-2. The chemotherapy conditioning schedule induced transient lymphopenia and consisted of cyclophosphamide (30 or 60 mg/kg per day for 2 days) followed by fludarabine (25 mg/m2 per day for 5 days). Immunotherapy for all patients consisted of in vitro expanded, tumor-reactive, autologous T-cell clones selected for highly avid recognition of melanoma antigens. Cohorts of three to six patients received either no interleukin (IL)-2, low-dose IL-2 (72,000 IU/kg intravenously three times a day to a maximum of 15 doses), or high-dose IL-2 (720,000 IU/kg intravenously three times a day for a maximum of 12 doses). The toxicities associated with this treatment were transient and included neutropenia and thrombocytopenia that resolved in all patients. The toxicities associated with this dose escalation chemotherapy regimen are shown in Table 1. No patient exhibited an objective clinical response to treatment, although five patients demonstrated mixed responses or transient shrinkage of some metastatic deposits. Surprisingly, high dose intravenous IL-2 was better tolerated by patients after chemotherapy than during previous immunotherapy cycles without chemotherapy, suggesting that some toxicities of IL-2 were mediated by cytokines secreted by lymphocytes secondary to activation by IL-2.
Table 1.
Grade 3-4 non-hematologic toxicities, transfusion requirements, and episodes of febrile neutopenia during the first cycle of treatment during a Phase I dose escalation clinical trial with cloned T cells.
| Number of occurrences1 |
|||||
|---|---|---|---|---|---|
| Study group2 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Total |
| Cy | 30 | 60 | 60 | 60 | |
| Flu | 25 | 25 | 25 | 25 | |
| IL-2 | - | 72,000 | 720,000 | ||
| Total treated Toxicity | 3 | 3 | 3 | 6 | 15 |
| Hypotension | 0 | 0 | 0 | 1 | 1 |
| Malaise | 0 | 0 | 0 | 0 | 0 |
| Nausea | 2 | 0 | 0 | 2 | 4 |
| Neurologic | 0 | 0 | 0 | 0 | 0 |
| Febrile neutropenia | 0 | 2 | 1 | 3 | 6 |
| RBC transfusion | 1 | 2 | 3 | 6 | 12 |
| Platelet transfusion | 1 | 3 | 1 | 6 | 11 |
Counted once for each patient at the highest grade
Cy: Cyclophosphamide (mg/kg for two days); Flu: fludarabine (mg/M2 for five days); IL-2: interleukin-2 (IU/kg every 8hr to tolerance, 15 doses maximum)
Adapted from Dudley et al., 2002 (Reference 19).
Powell et al.20 and Mackensen et al.21 used different methods to overcome the limitations of cloning techniques to generate large numbers of tumor antigen reactive lymphocytes from PBL for adoptive cell transfer protocols. Powell et al. assessed the capacity of vaccine-induced PBMC to mediate tumor regression after transfer to patients receiving the maximum dose of non-myeloablative preparative chemotherapy discussed above along with high dose IL-2. Autologous PBMC from nine gp100-vaccinated patients with metastatic melanoma were stimulated ex vivo with the 209-2M peptide and transferred in combination with high-dose IL-2 and a peptide cancer vaccine. Transferred PBMC contained highly avid, gp100:209-217 peptide-reactive CD8+ T cells. Unlike the transferred cloned cells, many of the bulk PBL populations caused a significant lymphocytosis in the patients, with peak counts approximately one week after transfer. Tumor-antigen reactive CD8+ T cells persisted at high levels in the blood of all patients and demonstrated IFN-gamma secretion when restimulated in vitro. Two of the nine patients demonstrated some evidence of biological activity and melanocyte-directed autoimmunity; however, no patient experienced an objective clinical response.
A similar phase I study was conducted by Mackensen et al. using multiply restimulated PBL cultures that reacted with the MART-1/MELAN-A antigen in eleven HLA-A2+ patients with metastatic melanoma21. Patients in this study received at least three infusions of MART-1 reactive CTL cultures that were generated by repetitive stimulation with autologous dendritic cells pulsed with MART-1 derived peptide. Patients also received a 6-day course of low-dose IL-2 after each cell infusion. There were no serious adverse events reported. This study group included one patient with an objective partial response and one patient with a complete response, anb the transferred cells persisted at low levels in patients’ circulating blood for up to 2 weeks. Overall, these studies demonstrated the feasibility and safety of using peripheral blood derived lymphocytes for adoptive cell transfer, but suggested that the cultures induced after multiple in vitro restimulations used by Mackensen’s group, or the vaccine induced populations of cells used by Powell et al, lacked sufficient potency in adoptive immunotherapy even when transferred into lymphodepleted hosts.
TIL with a lymphodepleting preparative regimen
Several methods had been established for the generation of tumor antigen-reactive lymphocyte cultures and evaluated in clinical trials. However, highly cultured, highly expanded cloned lymphocytes lacked potency in vivo. Although bulk TIL could mediate objective clinical responses, they were often of short duration and the bulk TIL were logistically difficult to grow9. Because it had been established that a non-myeloablative conditioning regimen could be safely administered in conjunction with adoptive T-cell transfer and IL-2 in patients with metastatic melanoma, we next evaluated whether highly selected, tumor antigen reactive, bulk TIL populations that were rapidly expanded could mediate anti-tumor responses. In an initial cohort of 13 patients22, our results were markedly different than those seen after administration of cloned lymphocyte cultures. This approach resulted in the persistent repopulation of anti-tumor T cells in the cancer patients, with proliferation of functionally active transferred cells in vivo and traffic to tumor sites. This led to regression of the metastatic melanoma in six of 13 patients as well as the onset of autoimmune melanocyte destruction in some patients.
This initial cohort of patients was followed with accrual of additional patients. We reported the combination of lymphodepleting chemotherapy followed by the adoptive transfer of autologous tumor reactive lymphocytes for the treatment of 35 patients with refractory metastatic melanoma23. All but one of these patients had disease that was refractory to treatment with high-dose IL -2 and 18 had progressive disease after chemotherapy. Patients underwent non-myeloablative lymphodepleting conditioning with cyclophosphamide and fludarabine, followed by cell infusion with autologous tumor-reactive, rapidly expanded TIL cultures and high-dose IL-2 therapy. Eighteen treated patients (51%) experienced objective clinical responses by RECIST criteria, including three complete responses that are ongoing more than three years after treatment. Sites of regression included metastases to lung, liver, lymph nodes, brain, and cutaneous and subcutaneous tissues. Although the toxicities of treatment were acute and severe, including the expected toxicities of high-dose IL-2 therapy and the hematologic suppression of chemotherapy, most toxicities were transient and resolved within two weeks. These results suggested that lymphodepleting chemotherapy followed by the transfer of highly avid anti-tumor lymphocytes could mediate significant tumor regression in heavily pretreated patients with IL-2 refractory metastatic melanoma. Table 2 shows the patients who responded to this treatment, along with the duration of their response as of May, 2007, and the initial sites of disease.
Table 2.
Clinical Responses to treatment
| Patient1 | Sites of Treated Disease | Response | Duration (months)2 |
|---|---|---|---|
| 1 | Axillary, Mesenteric, and Pelvic Lymph Nodes | PR | 29 |
| 2 | Subcutaneous and Skin | PR | 8 |
| 4 | Iliac and Inguinal Lymph Nodes, Skin | PR | 2 |
| 6 | Intraperitoneal Lymph Nodes, Lungs, Subcutaneous | PR | 23 |
| 9 | Subcutaneous and Skin | PR | 11 |
| 10 | Inguinal Lymph Nodes, Subcutaneous and Skin | PR | 14 |
| 16 | Subcutaneous | CR | 58+ |
| 17 | Bone, Liver, Lung, Subcutaneous | CR | 57+ |
| 19 | Intramuscular | PR | 13 |
| 21 | Lung, Subcutaneous | CR | 51+ |
| 25 | Lung, Subcutaneous | PR | 2 |
| 26 | Inguinal Lymph Nodes, Liver, Subcutaneous | PR | 8 |
| 28 | Axillary Lymph Nodes, Brain | PR | 4 |
| 30 | Axillary and Inguinal Lymph Nodes, Intramuscular, Subcutaneous | PR | 7 |
| 31 | Liver, Lung | CR | 41+ |
| 32 | Skin | CR | 40+ |
| 33 | Lung, Subcutaneous | PR | 2 |
| 34 | Intramuscular, Pelvis | PR | 3 |
Patients 2, 6, and 17 had minor (Patient 6) or mixed (Patient 2 and Patient 17) responses after a first course of treatment, and were treated with a second course of treatment consisting of ablation, cell transfer and high dose IL-2 therapy prior to achieving an objective clinic response.
Measured from treatment date to time of first recurrence. Patients 16, 17, 21, 31 and 32 had ongoing responses as of May, 2007.
Adapted from Dudley et al., 2005 (Reference 23).
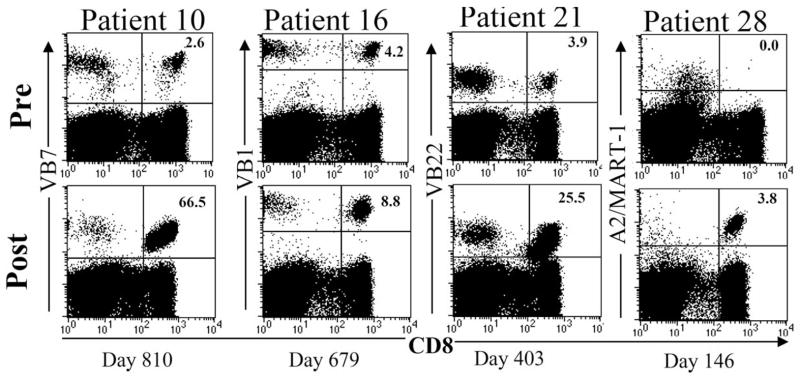
A notable immunologic correlate of successful treatment in many patients was the presence of the transferred cells in the peripheral blood for extended durations. Figure 1 shows a FACS analysis of the skewed profile of peripheral blood of several patients who responded to treatment, and the persistence of a dominant anti-tumor clonotype that was visualized by its T cell receptor V beta or tetramer profile. To quantify this relationship more exactly, TCR beta-chain V region gene product expressions was analyzed from samples obtained from 25 patients treated with TIL24. Sequence analysis demonstrated that there was a significant correlation between tumor regression and the degree of persistence in peripheral blood of adoptively transferred T cell clones, suggesting that inadequate T cell persistence might have represented a major factor limiting responses to adoptive immunotherapy in that protocol. To investigate this idea, Powell et al studied the transition from activated, cultured T cells to memory cells after transfer in serial blood draws from responding patients25. This study showed that the melanoma antigen-specific CD8+ T cells rapidly up-regulated their IL-7R-alpha in vivo. Furthermore, although the total number of tumor antigen-specific T-cells decreased two weeks after transfer, stable numbers of CD27+ CD28+ cells were maintained, and these cells formed the long-lived memory pool.
Figure 1.
Persistence of tumor reactive lymphocytes after TIL transfer is evident by FACS analysis of dominant T cell receptor V beta usage (Patients 10, 16 and 21) or tetramer staining (Patient 28). Reprinted from Dudley et al. 2005 (Reference 23).
These findings, which suggested that the proliferative potential of transferred T cells may play a role in clinical responses, led to studies investigating the role of telomere length as well as phenotypic markers expressed on the administered TIL26. After evaluating all available samples from patients who were treated with rapidly expanded TIL following non-myeloablative chemotherapy, it was found that TIL administered to patients who experienced objective clinical responses possessed a mean telomere length that was significantly longer than TIL given to non-responding patients (p < 0.01). Furthermore, individual TIL-derived T cell clonotypes that persisted in vivo following adoptive cell transfer possessed telomeres that were longer than telomeres of T cell clonotypes that failed to persist (p < 0.001). These results were supported by studies suggesting that higher CD28 expression was also associated with TIL that caused objective responses. Together these results suggest that TIL with longer telomeres, higher expression of the costimulatory molecule CD28, and greater proliferative potential will have a greater likelihood of persisting in vivo and mediating an objective clinical response.
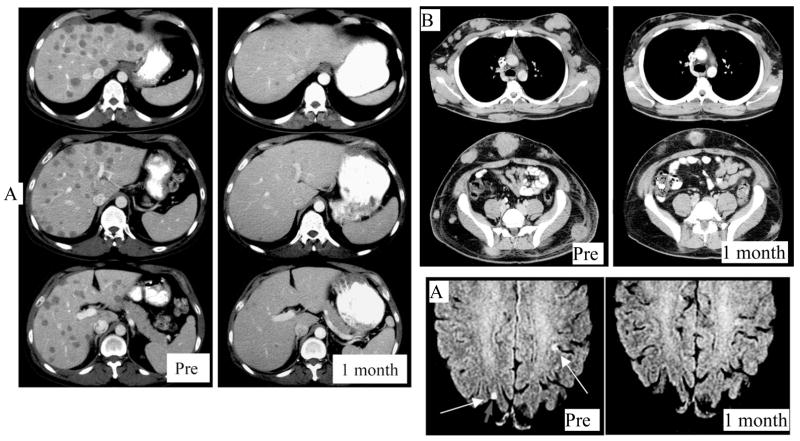
More recently we have attempted to improve on the response rate of cell transfer therapy by increasing the lymphodepleting preparative regimen. Murine models demonstrated that the intensity of lymphodepletion was directly related to the effectiveness of cell transfer therapy. Therefore, we investigated the addition of 200 cGy total body irradiation together with CD34+ hematopoeitic stem cell support to the previously reported cell transfer preparative regimen that consisted of cyclophosphamide and fludarabine. Twenty-five patients with refractory metastatic melanoma received autologous, tumor reactive lymphocytes following the increased intensity preparative regimen, and thirteen (52%) exhibited an objective response by RECIST criteria, including two complete responses. Regression of bulky tumors was observed in various sites, including cutaneous deposits, nodal disease, visceral sites, brain lesions, and bony sites. Although the toxicities of treatment were acute and often intense, included myelosuppression and the toxicities of IL-2 administration, they were typically transient and usually resolved within two weeks; however, one treatment related mortality occurred in this study. Of interest, when the TIL were administered, the circulating levels of the T cell homeostatic cytokines IL-7 and IL-15 were significantly elevated. As observed in prior cell transfer protocols, some patients exhibited prolonged persistence of the transferred lymphocytes in their peripheral blood. These results confirm the effectiveness of cell transfer immunotherapy for patients with refractory melanoma. Examples of the potency of this treatment to mediate tumor regression are shown in Figure 2.
Figure 2.
Rapid and dramatic elimination of bulky melanoma follows cell transfer therapy after lympohodepleting chemotherapy. A: bulky liver disease rapidly regressed in a patient who proceeded to have a complete response and is now disease free more than 57 months after treatment. B: Bulky cutaneous, subcutaneous and visceral metastatic deposits rapidly regressed after a patient received TIL cells following lymphodepleting chemotherapy and 200 cGy whole body irradiation with hematopoeitic stem cell support. The patient has an ongoing partial response more than 16 months after treatment. C: Several examples of brain metastases regressing after TIL administration, as seen for this patient have been observed, suggesting that the brain is not a site of immune privilege from this treatment.
TCR gene therapy with cell transfer
TIL therapy following lymphodepletion has been effective in causing tumor regression in over 50% of treated patients. However, the method for TIL production is logistically and technically demanding, and tumor-reactive TIL culture are only derived from approximately half of the resected melanoma samples intended for patient treatment. An alternate method for the production of tumor-reactive cells relies on the use of genetic engineering to confer new antigen specificity to patients’ cells. To achieve this clinically, the genes for the alpha and beta chains of a highly reactive anti-MART-1 T-cell receptor were isolated from a T-lymphocyte that mediated an objective clinical response and in vivo tumor regression in an HLA-A2+ patient with metastatic melanoma27. Retroviral-based vectors were constructed that contained both the alpha and beta TCR genes, and mediated greater than 50% gene transfer efficiency in primary T-lymphocyte cultures stimulated by an anti-CD3 antibody. The specificity and biologic activity of the TCR gene-transduced T-cells was confirmed by coculture of T-cells with MART-1 expressing tumor cells and with stimulator cells pulsed with MART-1 peptide, and the new TCR conferred the production of IFNg and GM-CSF comparable to the original TCR expressing cells. Using a similar approach, the alpha- and beta-chains of the TCR from a highly avid anti-gp100 CTL clone were isolated and used to construct retroviral vectors that also mediated high efficiency gene transfer into primary human lymphocyte cultures28. The biological activity of these transduced cells was also confirmed by cytokine production following coculture with stimulator cells pulsed with gp100 peptides and HLA-A2+ melanoma cells.
Using the retrovirus encoding the MART-1 specific T cell receptor, a clinical trial was initiated in which autologous lymphocytes from the peripheral blood of 17 HLA-A2+ patients with metastatic melanoma were transduced to confer tumor reactivity, and resulted in durable engraftment at levels exceeding ten percent of peripheral blood lymphocytes for at least two months post infusion29. Two of the patients exhibited sustained levels of circulating, engineered cells at one year post-infusion, and both demonstrated objective regression of metastatic melanoma lesions. Since the publication of that report, an additional 14 patients have been treated with MART TCR transduced PBL following non-myeloablative conditioning, and two additional patients have shown an objective tumor regression. This study suggests that genetically engineered cells may prove useful for the biologic therapy of cancer, especially when endogenous TIL are unavailable.
Future directions
Recent advances in adoptive cell transfer therapy for patients with melanoma described in this review include the use of a lymphodepleting preparative regimen prior to cell transfer, the selection of highly avid, tumor antigen-reactive cells, and the successful use of gene therapy to generate cell treatments for patients without other treatment options. The rapid progress in this field is likely to continue as more laboratories initiate clinical research efforts, and more immunological and immunotherapeutic reagents become clinically available. The Surgery Branch is pursuing several areas of clinical research based on our previous clinical studies. One main focus will be to investigate the optimal conditioning regimen for adoptive cell transfer therapy. We have already developed and initiated a clinical effort to evaluate the therapeutic efficacy of TIL transfer following a myeloablative conditioning regimen with chemotherapy and 1200 cGy TBI.
Similarly, the optimal support for the cells once they have been transferred is an area that requires more clinical research. Alternate cytokines given to support the transferred cells other than or in addition to IL-2, including IL-7, IL-15, IL-21 or IL-12, could have an impact on the efficacy of treatment. Similarly, the use of other immune stimulatory agents after cell transfer has been suggested, including co-stimulatory blockade using anti-CTLA-4, or anti-PD-1 antibody, or angiogenesis inhibitors such as anti-VEGF antibody.
Another major focus for future Surgery Branch clinical trials will be attempts to improve the quality of the administered cells. One protocol that has already been developed and is in regulatory review involves the use of TIL cells that are derived under conditions that minimized the time in cultures, thereby maximizing their telomere length and optimizing their differentiation profile prior to rapid expansion and adoptive transfer. Other preclinical research focuses on the optimization of culture conditions or the pre-selection of cell populations with a better persistence profile, and more central memory-like characteristics. Finally, gene engineering may be used to create desirable traits in the transferred cells such as over-expression of anti-apoptotic genes like bcl-2, production of autocrine cytokines like IL-230, or expression of cell surface receptors such as the CD28 costimulation receptor.
An additional direction of future Surgery Branch clinical focus is the continued pursuit of genetic engineering to retarget lymphocytes to tumors in patients without endogenous anti-tumor lymphocyte cultures. While the initial results using the MART-1 specific TCR in PBL following lymphodepleting chemotherapy were promising, the TCR used in that trial was of relatively low affinity. Protocols are being developed to genetically engineer T cells using TCR of significantly higher affinity that recognize the MART-1 or gp100 antigens31. In addition to these melanoma antigens, clinical trials are planned that will target other tumor antigens such as NY-ESO-1 or p5332,33. Finally, chimeric antibody receptors composed of an antibody-derived antigen recognition domain genetically fused to a cytoplasmic signaling domain have been constructed. Several constructs are in preclinical testing, including molecules recognizing tumor Her2/Neu, CD19, CEA, MUC1 and others. These new targeting specificities, together with potent new effector functions and the optimal host environmental conditioning may lead to potent new directions for cell based anti-tumor therapies.
Footnotes
From: Surgery Branch, National Cancer Institute, National Institutes of Health
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mark E. Dudley, Staff Scientist, Surgery Branch, National Cancer Institute, National Institutes of Health.
Steven A. Rosenberg, Chief, Surgery Branch, National Cancer Institute, National Institutes of Health
Reference List
- 1.Mule JJ, Shu S, Schwarz SL, Rosenberg SA. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science. 1984;225:1487–1489. doi: 10.1126/science.6332379. [DOI] [PubMed] [Google Scholar]
- 2.Muul LM, Nason-Burchenal K, Carter CS, Cullis H, Slavin D, Hyatt C, Director EP, Leitman SF, Klein HG, Rosenberg SA. Development of an automated closed system for generation of human lymphokine-activated killer (LAK) cells for use in adoptive immunotherapy. J Immunol Methods. 1987;101:171–181. doi: 10.1016/0022-1759(87)90148-7. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Lotze MT, Yang JC, Topalian SL, Chang AE, Schwartzentruber DJ, Aebersold P, Leitman S, Linehan WM, Seipp CA. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer [published erratum appears in J Natl Cancer Inst 1993 Jul 7;85(13):1091] J Natl Cancer Inst. 1993;85:622–632. doi: 10.1093/jnci/85.8.622. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor- infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 5.Schwartzentruber DJ, Topalian SL, Mancini M, Rosenberg SA. Specific release of granulocyte-macrophage colony-stimulating factor, tumor necrosis factor-alpha, and IFN-gamma by human tumor- infiltrating lymphocytes after autologous tumor stimulation. J Immunol. 1991;146:3674–3681. [PubMed] [Google Scholar]
- 6.Topalian SL, Solomon D, Rosenberg SA. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989;142:3714–3725. [PubMed] [Google Scholar]
- 7.Yannelli JR, Hyatt C, McConnell S, Hines K, Jacknin L, Parker L, Sanders M, Rosenberg SA. Growth of tumor-infiltrating lymphocytes from human solid cancers: summary of a 5-year experience. Int J Cancer. 1996;65:413–421. doi: 10.1002/(SICI)1097-0215(19960208)65:4<413::AID-IJC3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor- infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 10.Sherry RM, Rosenberg SA, Yang JC. Relapse after response to interleukin-2-based immunotherapy: patterns of progression and response to retreatment. J Immunother. 1991;10:371–375. doi: 10.1097/00002371-199110000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, Miki T, Rosenberg SA. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, Robbins PF, Sette A, Appella E, Rosenberg SA. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995;154:3961–3968. [PubMed] [Google Scholar]
- 13.Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother. 2005;54:187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parkhurst MR, Salgaller ML, Southwood S, Robbins PF, Sette A, Rosenberg SA, Kawakami Y. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- 15.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, Wunderlich JR, Parkhurst MR, Kawakami Y, Seipp CA, Einhorn JH, White DE. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg SA, Sherry RM, Morton KE, Yang JC, Topalian SL, Royal RE, Kammula US, Restifo NP, Hughes MS, Schwarz SL, Ngo LT, Mavroukakis SA, White DE. Altered CD8(+) T-cell responses when immunizing with multiepitope peptide vaccines. J Immunother (1997) 2006;29:224–231. doi: 10.1097/01.cji.0000190399.98802.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, Schwartzentruber DJ, Hwu P, Marincola FM, Sherry R, Leitman SF, Rosenberg SA. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry RM, Marincola FM, Leitman SF, Seipp CA, Rogers-Freezer L, Morton KE, Nahvi A, Mavroukakis SA, White DE, Rosenberg SA. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powell DJ, Jr, Dudley ME, Hogan KA, Wunderlich JR, Rosenberg SA. Adoptive transfer of vaccine-induced peripheral blood mononuclear cells to patients with metastatic melanoma following lymphodepletion. J Immunol. 2006;177:6527–6539. doi: 10.4049/jimmunol.177.9.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24:5060–5069. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 22.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robbins, P. F., Dudley, M. E., Wunderlich, J., el Gamil, M., Li, Y. F., Zhou, J., Huang, J., Powell, D. J., Jr., and Rosenberg, S. A. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powell DJ, Jr, Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–250. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes MS, Yu YY, Dudley ME, Zheng Z, Robbins PF, Li Y, Wunderlich J, Hawley RG, Moayeri M, Rosenberg SA, Morgan RA. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan RA, Dudley ME, Yu YY, Zheng Z, Robbins PF, Theoret MR, Wunderlich JR, Hughes MS, Restifo NP, Rosenberg SA. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171:3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer Regression in Patients After Transfer of Genetically Engineered Lymphocytes. Science. 2006 doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu K, Rosenberg SA. Transduction of an IL-2 gene into human melanoma-reactive lymphocytes results in their continued growth in the absence of exogenous IL-2 and maintenance of specific antitumor activity. J Immunol. 2001;167:6356–6365. doi: 10.4049/jimmunol.167.11.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson LA, Heemskerk B, Powell DJ, Jr, Cohen CJ, Morgan RA, Dudley ME, Robbins PF, Rosenberg SA. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177:6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol. 2005;174:4415–4423. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen CJ, Zheng Z, Bray R, Zhao Y, Sherman LA, Rosenberg SA, Morgan RA. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J Immunol. 2005;175:5799–5808. doi: 10.4049/jimmunol.175.9.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]