Abstract
Fas–Fas ligand interaction is thought to be a crucial mechanism in controlling lymphocyte expansion by inducing lymphocyte apoptosis. However, Fas is also broadly expressed on nonlymphoid cells, where its function in vivo remains to be determined. In this study, we describe the development of inflammatory angiogenesis induced by agonistic anti-Fas mAb Jo2 in a murine model where Matrigel is used as a vehicle for the delivery of mediators. The subcutaneous implants in mice of Matrigel containing mAb Jo2 became rapidly infiltrated by endothelial cells and by scattered monocytes and macrophages. After formation and canalization of new vessels, marked intravascular accumulation and extravasation of neutrophils were observed. Several mast cells were also detected in the inflammatory infiltrate. The phenomenon was dose and time dependent and required the presence of heparin. The dependency on activation of Fas is suggested by the observation that the inflammatory angiogenesis was restricted to the agonistic anti-Fas mAb and it was absent in lpr Fas-mutant mice. Apoptotic cells were not detectable at any time inside the implant or in the surrounding tissue, suggesting that angiogenesis and cell infiltration did not result from recruitment of phagocytes by apoptotic cells but rather by a stimulatory signal through Fas-engagement. These findings suggest a role for Fas–Fas ligand interaction in promoting local angiogenesis and inflammation.
Fas and structurally related glycoproteins form a family of surface receptors that control cell proliferation, survival, or apoptosis (1). On the other side, their ligands are homologue trimers present on soluble and/or cell-associated forms that may act as growth or death factors for several cell types. In particular, Fas ligand (FasL) is expressed on the cell surface and released by T cells (2, 3), monocytes, and neutrophils (4). So far, the biological importance of Fas–FasL interaction has been investigated in T lymphocytes, where it controls clonal deletion of autoreactive lymphocytes and mediates activation-induced suicide of T cells through a death signal transduced by Fas (1). However, several cell types, such as endothelial cells (5), monocytes (4), epithelial cells (6), and certain tumor lineages (7), are resistant to Fas-mediated apoptosis. Other cells, such as neutrophils (4) and B cells (8), rapidly acquire resistance after stimulation with growth factors or cytokines. In addition, genetic deficiency of Fas or FasL does not affect organ development with the exception of the lymphoid compartment. Therefore, the role of Fas–FasL interaction in nonlymphoid cells remains to be elucidated, and it might also involve mechanisms different from apoptosis induction. Indeed, this hypothesis is fueled by an increasing number of observations suggesting that Fas may transduce cell activation signals independently or as an alternative to cell death (9–12).
This study explores the effect of an in situ Fas stimulation by agonistic monoclonal antibodies implanted in vivo within Matrigel. In this model, anti-Fas mAb triggers neoangiogenesis and local infiltration of inflammatory cells, independently from apoptosis.
Materials and Methods
Reagents.
Hamster anti–mouse Fas Jo2 was from PharMingen (San Diego, CA) and rat anti–mouse Fas RMF6 was from MBL (Nagoya, Japan). Purified hamster IgGs (Cappel Laboratories Inc., West Chester, PA) were used as control.
Murine Angiogenesis Assay.
Wild-type and carrier of the lpr mutation in the Fas gene C57Bl/6 (13) mice were from Jackson Laboratories (Bar Harbor, Maine). Animals were used at 6 wk of age. Angiogenesis was assayed as growth of blood vessels from subcutaneous tissue into a solid gel of basement membrane containing the test sample (14). 6 mice per condition in each experiment were used. As standard procedure, Matrigel (8.13 mg/ml; Becton Dickinson Labware, Bedford, MA), in liquid form at 4°C, was mixed with heparin (64 U/ml, Sigma Chem. Co., St. Louis, MO) and the experimental substances and injected (0.25 ml) into the abdominal subcutaneous tissue of mice, along the peritoneal midline. At various times, mice were killed and gels were processed for light microscopy, detection of nonspecific esterase activity, and immunohistochemistry as previously described (15). Rabbit anti-vWF (Sigma), anti-VEGF, anti–flk-1 and anti–b-FGF (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-L3/T4 and Ly2 monoclonal antibodies (Cedarlane, Ontario, Canada), as well as control rabbit and mice IgG, were used as primary antibodies for indirect immunofluorescence. FITC-conjugated anti-rabbit IgG and anti-rat IgG were used as secondary antibodies (Cappel Laboratories). Direct immunofluorescence staining was performed with anti–MAC-1 FITC-conjugated monoclonal antibodies (Cedarlane). Mast cells were stained metachromatically with 1% Toluidine blue, pH 3.5, for 30 min. Apoptotic cells were detected in situ through the staining of DNA fragments with the TUNEL assay (ApoTag Oncor, Gaithersburg, MD) (16). Tissue from rat regressing mammary glands obtained at the fourth day after weaning was used as positive control for the technique.
Vessel area and the total Matrigel area were planimetrically assessed from cross-sections of Matrigel plugs observed by light microscopy as previously described (15). Results were expressed as percentage ± SE of the vessel area to the total Matrigel area.
Results
Subcutaneous injection in mice of Matrigel containing the agonistic anti-Fas mAb Jo2 caused rapid neovascularization and infiltration of inflammatory cells within the implant (Fig. 1). The phenomenon is dose and time-dependent (Figs. 2 and 3). The minimal effective dose was 100 ng/ml. Initial infiltration of cells was observed as early as 24 h after injection (Fig. 2 A) and the infiltrating cells were mainly endothelial cells (Fig. 1 A), as detected by immunofluorescence staining for vWF (Fig. 4, A and B) and MAC-1+ (Fig. 4 C) and aspecific esterase plus monocytes. Polymorphonuclear neutrophils (PMN) were seen initially at the periphery of Matrigel implant, then within the lumen of neoformed capillary sprouts (Fig. 1 D) and finally around the neoformed vessels (Fig. 1 F). Only scattered lymphocytes CD4+ or CD8+ were detected. Several mast cells (Fig. 1 F), metachromatically stained by Toluidine blue, were also detected within the Matrigel and the inflammatory infiltrate surrounding the neoformed vessels. Maximal angiogenesis was observed at day 6 with formation of canalized vessels (Fig. 1 G and 3 A). At that time, ∼50% of neoformed vessels were surrounded by an intense infiltration of inflammatory cells similar to granulation-like tissue (Fig. 1 E). Control purified hamster IgG and a nonsignaling anti–mouse Fas mAb RMF6 (17) were completely ineffective (Fig. 5). mAb Jo2 was endotoxin free as tested by Limulus assay and in selected experiments preincubation for 30 min of the antibody with 5 μg/ml polymyxin B, which complexes and inactivates endotoxin, did not abrogate the inflammatory angiogenesis induced by this mAb (data not shown). All the animals that were implanted with Matrigel containing mAb Jo2 remained apparently healthy and active and the histological examination of the liver did not show macroscopic alterations.
Figure 1.
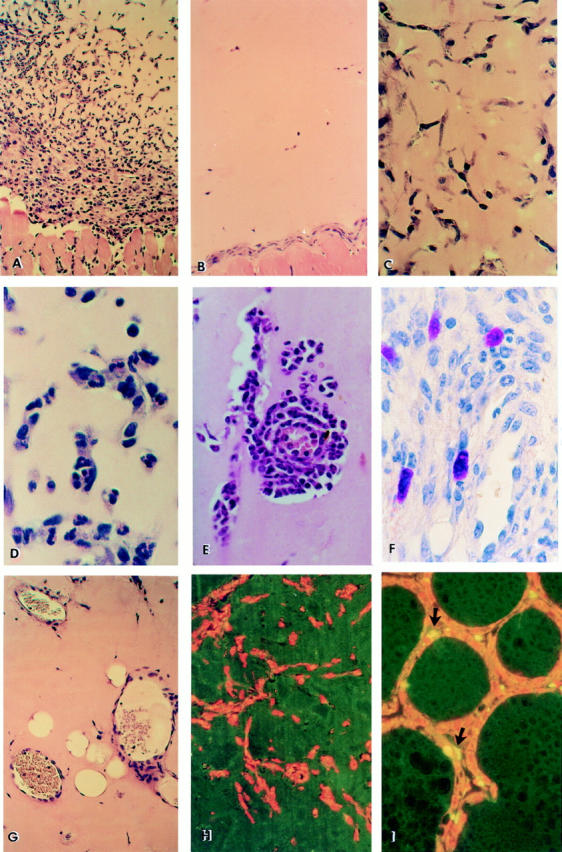
Histological and immunohistochemical analysis of Matrigel implants. Representative hematoxylin-eosin–stained section of Matrigel containing 5 μg/ml anti-Fas mAb Jo2 (A) implanted in wild-type mice and excised 6 d after injection (6 mice per condition in each experiment). Progressive invasion of cells into the Matrigel implant and formation of capillaries is observed (×200). (B) Control: mAb Jo2 was replaced by hamster IgG (×200). Similar results were obtained by using nonagonistic anti-Fas mAb RMF6 or by injecting Matrigel with mAb Jo2 into lpr Fas-mutant mice (not shown). Kinetic of anti-Fas mAb Jo2-induced inflammatory angiogenesis (×400); (C) Initial migration of endothelial cells aligning to form cord-like structures at day 1; (D) appearance of PMN inside the lumen of neocapillaries (day 2); (E) marked extravasation of PMN throughout the neoformed capillary into the Matrigel (day 6); (F) metachromatic staining by Toluidine blue of mast cells infiltrating Matrigel at day 6 (×600); (G) formation of canalized vessels containing erythrocytes and leukocytes (day 4–6). Immunohistochemical detection of apoptotic cells by the TUNEL method (normal nuclei are counterstained with propidium iodide in red); (H) Section of Matrigel with anti-Fas mAb Jo2 at day 6, displaying total absence of apoptotic cells among the infiltrating cells (×200). The same results were obtained on Matrigel excised 6 h, 12 h, 24 h, 5 d, and 10 d after injection (not shown); (I) Rat regressing mammary glands obtained at the fourth day after weaning showing apoptotic cells (arrows) along the acini, as positive control (×200).
Figure 2.
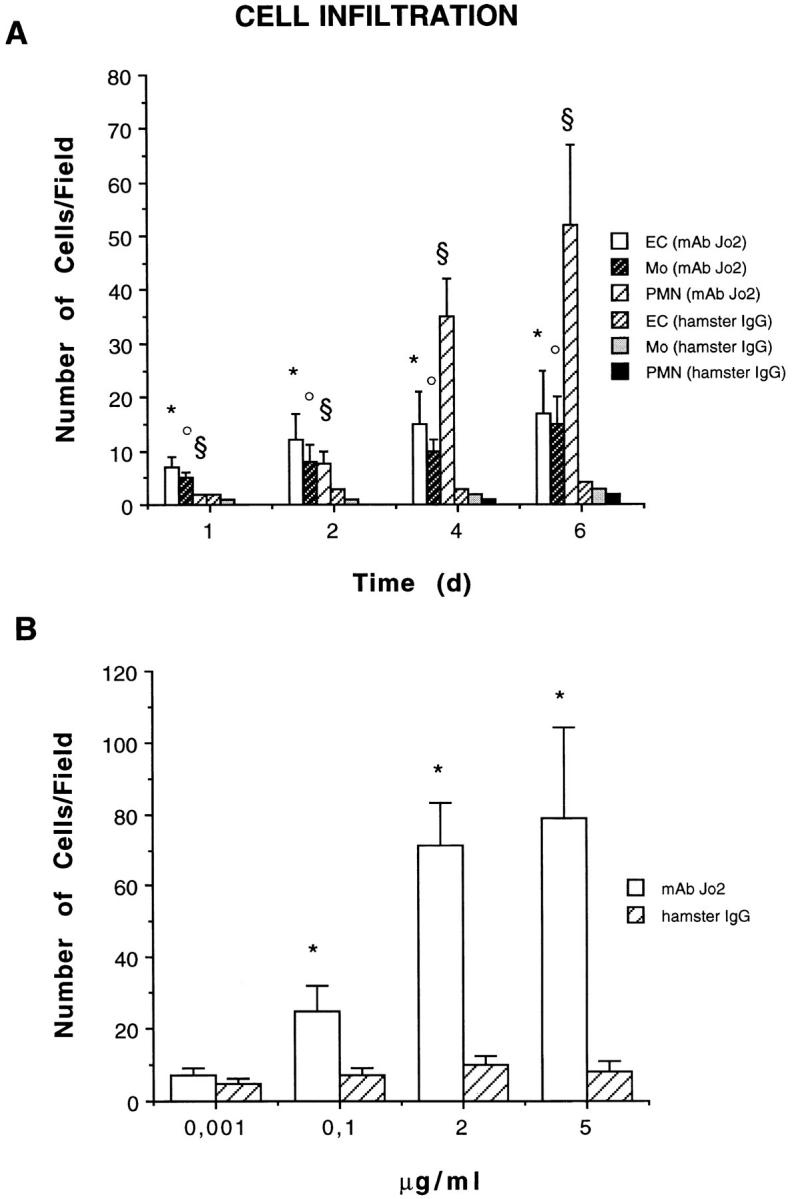
Quantitative and qualitative evaluation of cells infiltrating Matrigel. (A) Time course studies on infiltration induced by 5 μg/ml anti-Fas mAb Jo2 or control hamster IgG. Endothelial cells (EC) were counted as vWF-positive cells; monocytes (Mo) were counted as MAC-1–esterase-positive cells; and PMN were counted in section stained by hematoxylin and eosin. (B) Dose-dependent studies on Matrigel infiltration observed 6 d after stimulation with anti-Fas mAb Jo2 or control hamster IgG. The results were expressed as a mean ± SE of cells/field (×400). ANOVA with Dunnett multi-comparison test was performed: control hamster IgG vs. anti-Fas mAb Jo2: (A) EC* P <0.05; Mo• P <0.05; PMN§ P <0.05. (B) total cell count *P <0.05.
Figure 3.
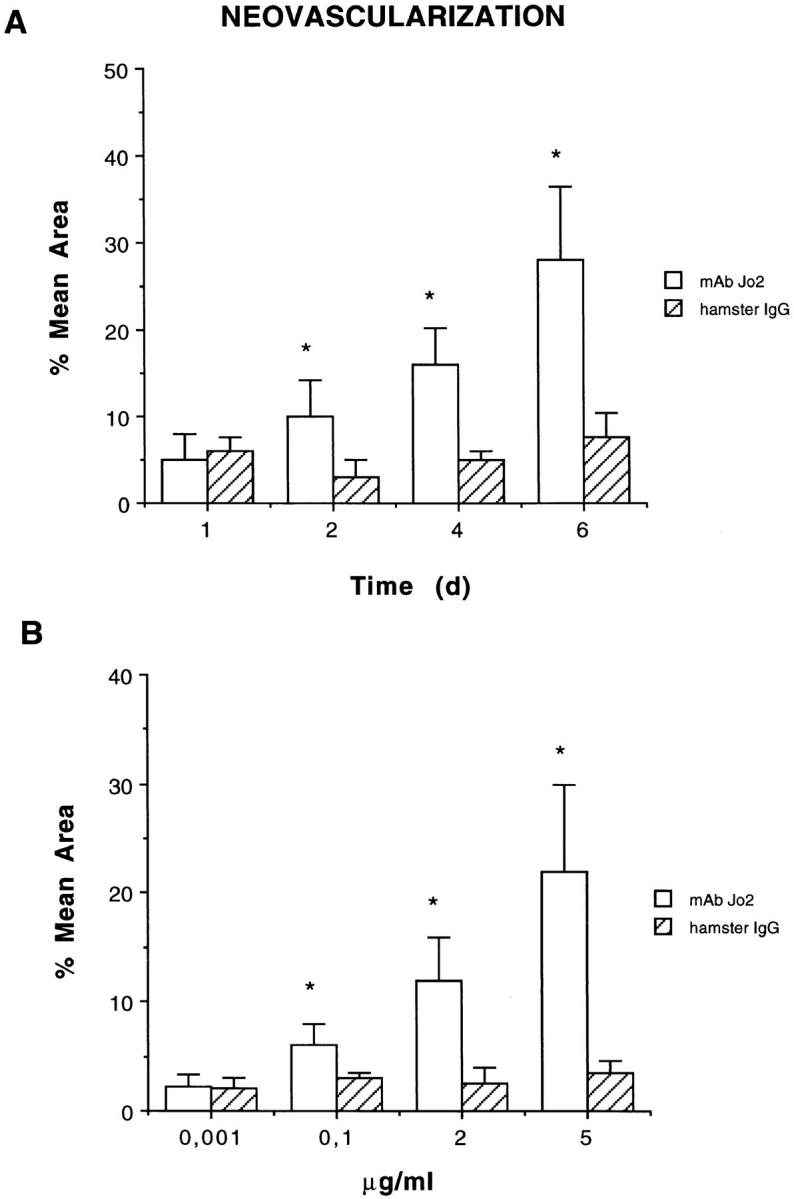
Quantitative evaluation of neoformed vessels infiltrating Matrigel. (A) Time course studies on neoangiogenesis induced by 5 μg/ml anti-Fas mAb Jo2 or control hamster IgG. (B) Dose-dependent studies on neoangiogenesis observed 6 d after implantation of Matrigel containing anti-Fas mAb Jo2 or control hamster IgG. The results were expressed as percentage ± SE of the vessel area to the total Matrigel area. ANOVA with Dunnett multi-comparison test was performed between control hamster IgG and anti-Fas mAb Jo2 (*P <0.05).
Figure 4.
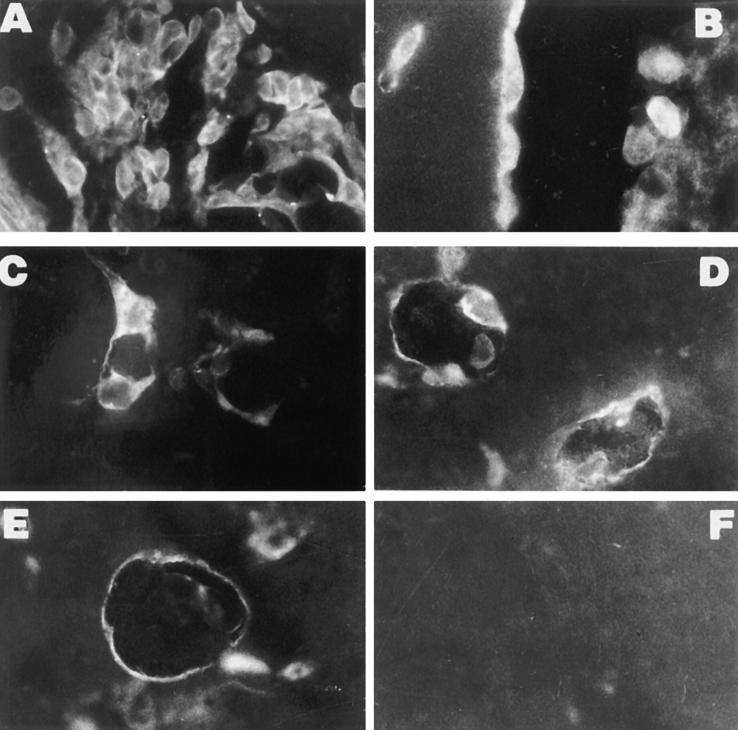
Immunofluorescence analysis of Matrigel plugs containing 5 μg/ml mAb Jo2. (A) Penetration of vWF-positive cells in Matrigel 24 h after implantation (×250). (B) vWF-staining of endothelial cells lining canalized vessels 6 d after implantation (×400). (C) Scattered MAC-1–positive monocytes detected 24 h after implantation (×400). Staining of endothelial cells of small vessels for VEGF (D) and its receptor flk-1 (E) at day 2 (×250). (F) Negative staining for b-FGF at day 2 (×250).
Figure 5.
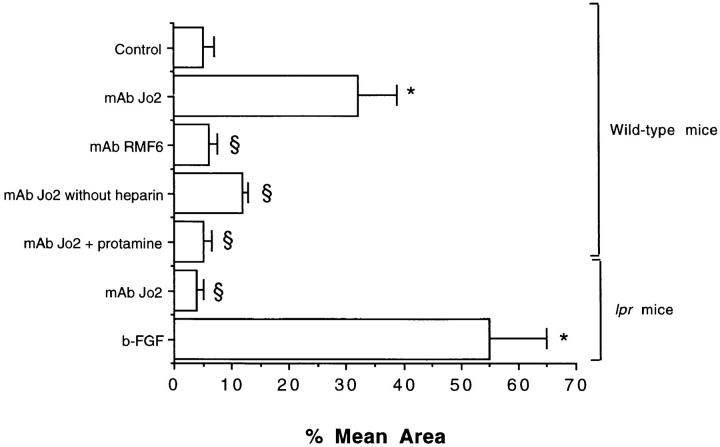
Angiogenesis in wild-type mice and in mice lpr Fas-mutant stimulated with agonistic (mAb Jo2) and nonagonistic (mAb RMF6) anti-Fas mAbs and role of heparin in Fas-induced angiogenesis. 5 μg/ml anti-Fas mAb Jo2 or RMF6 were used. As standard procedure Matrigel was supplemented with 64 U/ml heparin (see Materials and Methods). In selected experiments, heparin was omitted or 64 μg/ml protamine was added. b-FGF was used at the dose of 10 ng/ml. The results were expressed as percentage ± SE of the vessel area to the total Matrigel area. ANOVA with Newmann-Keuls multi-comparison test was performed: control vs. mAb Jo2, mAb RMF6, and b-FGF (*P <0.05); mAb Jo2 in wild-type mice vs. mAb RMF6, mAb Jo2 without heparin, mAb Jo2 plus protamine, mAb Jo2 in lpr mice (§ P <0.05).
The requirement of heparin for the development of Fas-induced angiogenesis suggests the involvement of heparin-binding angiogenic factors (Fig. 5). By immunohistochemistry, VEGF and Flk-1 (Fig. 4, D and E), but not b-FGF (Fig. 4 F), were detectable on the endothelial cells infiltrating Matrigel and lining the neoformed capillaries, suggesting that VEGF rather than b-FGF may have an autocrine role in Fas-induced neovascular formation.
To explore the relevance of Fas-stimulation in the angiogenesis induced by mAb Jo2, experiments were performed in lpr mice. mAb Jo2 did not elicit neoangiogenesis or inflammatory infiltration of Matrigel in lpr mice (Fig. 5). In contrast, b-FGF-induced angiogenesis was normally maintained in these mutants.
In situ detection of apoptotic cells by the TUNEL technique indicated the absence of significant apoptosis in the cells infiltrating Matrigel and in the surrounding tissue at any time tested (6 h, 12 h, 24 h, 4 d, and 6 d after injection) (Fig. 1 H). Tissue from rat regressing mammary glands obtained at the fourth day after weaning and used as positive control, displayed apoptotic cells along the acini (Fig. 1 I). Incubation of this tissue without TdT enzyme, as negative control, resulted in absence of staining.
Discussion
Interaction of Fas with its ligand, FasL, or agonistic antibodies may lead to induction of intracellular pathway of apoptosis. However, while this phenomenon is easily observable on activated lymphocytes (1) and certain tumor lineages (7), it does not affect several Fas-expressing cell types such as endothelial (5), monocytes (4), eosinophils (4), and epithelial cells (6), or it is prevented by a number of environmental survival signals (e.g., growth factors, extracellular matrix, cell-to-cell interaction molecules, antigens) in cells such as neutrophils (4) and B-lymphocytes (8). In addition, Fas and FasL mutant MRL mice that develop a spontaneous autoimmune disorder due to failure in deleting lymphocytes, do not show tissue-specific alterations unrelated to the autoimmune process (18). These observations seem to limit the physiologic apoptotic effect of Fas to the immune cell compartment.
Recently, an increasing amount of evidence suggest that Fas may transduce also cell-activation signals independently or in alternative to cell death (9–12). Therefore, in vivo Fas may potentially have alternative functions as observed for other members of its family including TNF-receptors (19), and CD40 (20). All these molecules may indeed trigger activation and proliferation or, conversely, apoptosis in distinct cell types or in different experimental conditions.
This study provides evidence of a role of Fas in promoting angiogenesis and inflammatory cell recruitment in vivo. In mice subcutaneous implants of Matrigel containing anti-Fas mAb are rapidly neovascularized and infiltrated by inflammatory cells. Neoangiogenesis is triggered also by VEGF (21), b-FGF (14), TNF-α (15), and PAF (15), however, no inflammatory infiltration is caused by these agents. Therefore, the inflammatory angiogenesis observed in the present study is peculiar of Fas-stimulation. Indeed, a nonsignaling anti-Fas mAb (17) failed in inducing this effect, suggesting that anti-Fas mAb does not act simply by anchoring or facilitating the interaction of Fas-expressing cells with the Matrigel. In addition, the role of a functional activation of Fas is suggested by the absence of inflammatory angiogenesis in Fas-deficient mice stimulated with agonistic anti-Fas mAb.
A time course analysis of apoptosis did not reveal the presence of apoptotic cells in the surrounding tissue or inside the Matrigel containing the anti-Fas mAb, suggesting that inflammatory cell accumulation cannot be ascribed to the recruitment of phagocytes by apoptotic cells. Cell necrosis was not observed.
Interestingly, in a model of xenotransplantation, FasL expression by a graft implanted subcutaneously in nude mice elicited a rejection reaction which was mediated by neutrophil recruitment and activation (22). Our results demonstrate that in the subcutaneous site Fas-stimulation induces per se a marked angiogenic and inflammatory response. The local prevalence of cells naturally resistant to Fas-mediated apoptosis and/or the availability of survival signals, such as growth factor supply and extracellular matrix interaction, may account for the lack of apoptosis in this model. Therefore, it is conceivable that Fas stimulation by agonistic mAb may uncover in this model the cell activation rather than the apoptotic signals. Furthermore, the observation that heparin was required for Fas-induced inflammatory angiogenesis, suggests that heparin-binding growth factors may be involved as secondary mediators. The immunohistochemical study revealed the expression of VEGF and its receptor Flk-1 by the endothelial cells infiltrating Matrigel or lining the neoformed vessels. In the present experimental model, multiple cell types may act as a target for anti-Fas antibodies. However, the early infiltrating cells were endothelial cells and monocytes. The time-course studies showed that the recruitment of PMN follows that of endothelial cells. When the vessels are canalized PMN were seen adherent to the abluminal surface of endothelial cells and in the extravascular space around the neoformed vessels, suggesting a leukocyte recruitment dependent on endothelial cell activation. Neutrophils constitutively express both Fas and its ligand and in vitro they become resistant to Fas-mediated apoptosis when cultured in the presence of G-CSF, GM-CSF, IFN-γ, TNF-α, or dexamethasone (4). In addition, the deficiency of Fas in lpr mice seems to impair the inflammatory response involving polymorphonuclear leukocytes extravasation in the course of host defense against bacteria (23).
In conclusion, Fas–FasL interaction is likely to occur at the sites of inflammation because FasL may be expressed by several inflammatory cells such as T cells, monocytes and neutrophils (4). Furthermore, FasL may also be released by proteolytic cleavage as a biologically active soluble form (3, 24), which is detectable in several pathologic conditions (24), including synovial fluids of patients with rheumatoid arthritis (Biancone, L., and G. Camussi, unpublished observation). Such a proteolytic process seems to be catalyzed by metalloproteinases that are usually overproduced during inflammation (24).
Acknowledgments
This work was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), Targeted Project Angiogenesis, by MURST 40% and 60%, and by the Istituto Superiore di Sanità, Research Project “Artificial organs and organ transplantation” to G. Camussi.
References
- 1.Nagata S, Golstein P. The Fas death factor. Science (Wash DC) 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 2.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO (Eur Mol Biol Organ) J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med. 1996;184:429–440. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson BC, Lalwani ND, Johnson KJ, Marks RM. Fas ligation triggers apoptosis in macrophages but not endothelial cells. Eur J Immunol. 1994;24:2640–2645. doi: 10.1002/eji.1830241111. [DOI] [PubMed] [Google Scholar]
- 6.Boonstra JC, van der Woude FJ, Laterveer JC, Wever PC, van Es LA, Daha MR, van Kooten C. Fas (CD95) is expressed on tubular epithelial cells in situ and in vitro, but Fas-mediated apoptosis is actively suppressed in cultured cells. J Am Soc Nephrol. 1996;7:1692. [Google Scholar]
- 7.Clement M-V, Stamenkovic I. Fas and tumor necrosis factor receptor-mediate cell death: similarities and distinctions. J Exp Med. 1994;180:557–567. doi: 10.1084/jem.180.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagresle C, Mondiere P, Bella C, Krammer PH, Defrance T. Concurrent engagement of CD40 and the antigen receptor protects naive and memory cells from APO-1/Fas-mediated apoptosis. J Exp Med. 1996;183:1377–1388. doi: 10.1084/jem.183.4.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alderson MR, Armitage RJ, Maraskovsky E, Tough TW, Roux E, Schooley K, Ramsdell F, Lynch DH. Fas transduces activation signals in normal human T lymphocytes. J Exp Med. 1993;178:2231–2235. doi: 10.1084/jem.178.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponton A, Clement M-V, Stamenkovic I. The CD95 (APO-1/Fas) Receptor activates NF-kB independently of its cytotoxic function. J Biol Chem. 1996;271:8991–8995. doi: 10.1074/jbc.271.15.8991. [DOI] [PubMed] [Google Scholar]
- 11.Rensing-Ehl A, Hess S, Ziegler-Heitbrock HW, Rietmuller G, Engelmann H. Fas/Apo-1 activates nuclear factor kappa B and induces interleukin-6 production. J Inflamm. 1995;45:161–174. [PubMed] [Google Scholar]
- 12.Aggarwal BB, Singh S, LaPushin R, Totpal K. Fas antigen signals proliferation of normal human diploid fibroblast and its mechanism is different from tumor necrosis factor receptor. FEBS Lett. 1995;364:5–8. doi: 10.1016/0014-5793(95)00339-b. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature (Lond) 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 14.Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR. A simple quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- 15.Montrucchio G, Lupia E, Battaglia E, Passerini G, Bussolino F, Emanuelli G, Camussi G. Tumor necrosis factor α–induced angiogenesis depends on in situ platelet-activating factor biosynthesis. J Exp Med. 1994;180:377–382. doi: 10.1084/jem.180.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura Y, Ishii A, Kobayashi Y, Yamasaki Y, Yonehara S. Expression and function of mouse Fas antigen on immature and mature T cells. J Immunol. 1995;154:4395–4403. [PubMed] [Google Scholar]
- 18.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 19.Beutler B, Van Huffel C. Unraveling function in the TNF ligand and receptor families. Science (Wash DC) 1994;264:667–668. doi: 10.1126/science.8171316. [DOI] [PubMed] [Google Scholar]
- 20.Hess S, Engelmann H. A novel function of CD40: induction of cell death in transformed. J Exp Med. 1996;183:159–167. doi: 10.1084/jem.183.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung DW, Cachianes G, Kuang W-J, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science (Wash DC) 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 22.Yagita H, Seino K-I, Kayagaki N, Okumura K. CD95 ligand in graft rejection. Nature (Lond) 1996;379:682. doi: 10.1038/379682a0. [DOI] [PubMed] [Google Scholar]
- 23.Lowrance JH, O'Sullivan FX, Caver TE, Waegell W, Gresham HD. Spontaneous elaboration of transforming growth factor β suppresses host defense against bacterial infection in autoimmune MRL/lpr mice. J Exp Med. 1994;180:1693–1703. doi: 10.1084/jem.180.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka M, Suda T, Haze K, Nakamura N, Sato K, Kimura F, Motoyoshi K, Mizuki M, Tagawa S, Ohga S, et al. Fas ligand in human serum. Nat Med. 1996;2:317–322. doi: 10.1038/nm0396-317. [DOI] [PubMed] [Google Scholar]