Abstract
The T cell receptor (TCR) repertoires of cytotoxic responses to the immunodominant and subdominant HLA A11–restricted epitopes in the Epstein-Barr virus (EBV) nuclear antigen-4 were investigated in four healthy virus carriers. The response to the subdominant epitope (EBNA4 399-408, designated AVF) was highly restricted with conserved Vβ usage and identical length and amino acid motifs in the third complementarity-determining regions (CDR3), while a broad repertoire using different combinations of TCR-α/β V and J segments and CDR3 regions was selected by the immunodominant epitope (EBNA4 416-424, designated IVT). Distinct patterns of interaction with the A11–peptide complex were revealed for each AVF- or IVT-specific TCR clonotype by alanine scanning mutagenesis analysis. Blocking of cytotoxic function by antibodies specific for the CD8 coreceptor indicated that, while AVF-specific TCRs are of high affinity, the oligoclonal response to the IVT epitope includes both low- and high-affinity TCRs. Thus, comparison of the memory response to two epitopes derived from the same viral antigen and presented through the same MHC class I allele suggests that immunodominance may correlate with the capacity to maintain a broad TCR repertoire.
Recognition of MHC–peptide complexes by TCRs is an essential step in the establishment of protective immunity (1). The TCR is a heterodimer composed of two polypeptide chains, α and β or γ and δ, which contain a variable domain, involved in antigen recognition, and a constant domain which is important for membrane attachment and T cell activation (2). The variable domain is encoded by multiple variable (V), diversity (D), and joining (J) gene segments. Somatic DNA rearrangement during lymphocyte differentiation in the thymus juxtapose V-J or V-D-J segments that code for the α/γ and β/δ chains, respectively. This combinatorial capacity creates an array of unique TCRs capable of recognizing a large variety of epitopes. The diversity is further increased by several possible α/β or γ/δ pairings and by nucleotide additions and/or trimming at the V(D)J junctions. The size of the repertoire that may potentially interact with a given antigen is narrowed by positive and negative selection in the thymus (3, 4). Further restrictions are imposed on the peripheral repertoire by the nature of the antigenic stimulus where the antigenic load and the persistence of the stimulus over a long period of time are likely to be important parameters (5).
CTLs expressing the TCR-α/β–heterodimer and CD8 coreceptor play a critical role in controlling infection by EBV, a widespread human herpes virus that persists in healthy carriers as a latent infection of B cells (6, 7). In spite of the large complexity of the virus, which encodes for at least 9 proteins expressed in latently infected B lymphocytes, the CTL response detected during primary EBV infection appears to be preferentially focused on only few epitopes that are mainly derived from the high molecular weight Epstein-Barr virus nuclear antigens (EBNA)1 3, 4, and 6 (also known as EBNA 3A, 3B, and 3C) (8, 9). The reason for this strong focusing is presently unknown, but it is remarkable that a very similar hierarchy of epitope choice is maintained in the memory CTL responses that can be reactivated by in vitro stimulation of lymphocytes from healthy virus carriers. We have previously reported that EBV-specific CTL responses of HLA A11+ Caucasians are frequently dominated by A11-restricted CTLs that are directed to several epitopes derived from EBNA4 (10–12). The cognate peptides of two epitopes have been mapped within EBNA4 residues 416-424 (IVTDFSVIK, designated IVT) and 399-408 (AVFDRKSDAK, designated AVF) (11, 12). IVT-specific effectors may account for as much as 80% of the EBV-specific CTL clones isolated from these donors indicating that this is the immunodominant epitope (13). The immunodominance of the IVT epitope has recently been confirmed in a study that compared the frequency of specific CTL precursors in primary and memory response (14). Mutations affecting the anchor residues of the IVT peptide were shown to abrogate CTL recognition in EBV isolates from Southeast Asia, where HLA A11 is expressed in >50% of the population. Only half of the Southeast Asian isolates carried concomitant mutations within the AVF peptide, suggesting that CTL responses to IVT may exert a stronger selective pressure in vivo (12, 15). We have exploited these features to examine to what extent immunogenicity may affect the diversity of TCR repertoires specific for different viral epitopes during long-term persistent infection, where the opportunity for selection of T cell clones with maximal affinity might be optimal.
Materials and Methods
Generation of CTL Cultures and Clones.
EBV-specific CTL cultures were obtained by stimulation of lymphocytes from the EBV seropositive donors BK (HLA A2, A11, B7, B62), CA (HLA A11, A32, B18, B35), ZA (HLA A3, A11, B35), and EA (HLA A10, A11, B35.3, B51) with the autologous B95.8 virus transformed LCLs. After two or three consecutive restimulations the cultures were expanded in complete medium supplemented with 10 U/ml recombinant IL-2 and 30% (vol/vol) culture supernatant from the gibbon lymphoma line MLA144 (16). Single cell cloning was done by limiting dilution in IL-2–supplemented medium containing 105 irradiated (3,000 Rads) allogeneic PHA-pulsed PBLs as feeder. The EBV specificity and HLA class I restriction of the clones was assessed in 51Cr-release assays against a panel of EBV-positive and -negative targets including the autologous lymphoblastoid cell lines (LCLs), allogeneic LCLs matched through single class I alleles, at least two cell lines for each allele, PHA activated blasts, HLA mismatched LCLs, and the prototype NK-sensitive target K562. PHA blasts were preincubated with 10-9 M of the relevant synthetic peptide during 51Cr labeling followed by extensive washing before the cytotoxicity tests. Sensitivity to CD8 blocking was assessed by preincubating the targets in the assay wells with the indicated amounts of purified anti-human CD8 mouse mAbs (OKT8 ATCC CRL8014) for 30 min at room temperature before addition of the effectors.
RNA Extraction, First Strand cDNA Synthesis, PCR Amplification, and Sequencing.
Total RNA was extracted from 2–10 × 106 cells by the single-step acid guanidinium thiocyanate-phenol-chloroform method (17). For first-strand cDNA synthesis, 1–2 μg of total RNA were incubated at 42°C with 200 U of moloney murine leukemia virus reverse transcriptase (Seikagaku America, Inc., Rockville, MD), 30 pmol random hexamer primers (Clontech, UK), and 0.5 mM dNTP (Pharmacia, Uppsala, Sweden) in a buffer containing 50 mM Tris-HCl, pH 8.3, 75 mM KCl, and 3 mM MgCl2 in a total volume of 30 μl. The reaction was run for 60 min and stopped by heating at 94°C for 5 min. PCR primers specific for the variable and constant domains (Clontech, Palo Alto, CA) were used to amplify 22 α chain and 24 β chain TCR families. Each PCR reaction contained 0.5 μM of primers, 2% of the product of the cDNA synthesis, 0.2 mM dNTP, 2U Taq polymerase (Amersham International, Amersham, UK), 10 mM Tris-HCl, pH 8, 50 mM KCl, 1.5 mM MgCl2, and 0.01% gelatin in a final volume of 50 μl. The reaction was overlaid with 50 μl of mineral oil and amplification was run for 30 cycles of 1 min denaturation at 95°C, 1 min annealing at 55°C, and 1-min extension at 72°C. 10% of the PCR product was analyzed in a 1.8% agarose gel containing 0.5 μg/ml ethidium bromide. The amplified fragments were then cloned into the EcoRV site of the pGEM plasmid (Promega Corp., Madison, WI). 10 μg of the recombinant plasmid DNA was denatured for 10 min with NaOH, neutralized with sodium acetate, pH 4.8, ethanol precipitated, and used for sequencing. The M13 universal (CGACGTTGTAAAACGACGGCCAGT) and M13 reverse oligonucleotides (CAGGAAACAGCTATGAC) were fluorescein labeled and used to prime upstream and downstream of the cloning site. 5 pmol of the fluorescent primer was annealed to denatured plasmid DNA for 5 min at 65°C followed by 10 min at 37°C and 10 min at room temperature in a 14 μl buffer containing 140 mM Tris-HCl, pH 7.6, and 14 mM MgCl2. 8 U T7 DNA polymerase, 3 μl of DMSO and 1 μl of a mix containing 300 mM citric acid, 325 mM DTT, and 40 mM MnCl2 were added and the reaction was split in four aliquots. Each aliquot was incubated with one of the four dideoxynucleotides (Pharmacia) for 5 min at 37°C. The reactions were terminated by adding an equal volume of deionized formamide and 5 μg/μl blue dextran. Samples were loaded onto a 0.5-mm-thick 6% polyacrylamide gel containing 7 M urea and run in an A.L.F. DNA sequencer (Pharmacia). Alternatively, recombinant plasmid DNA was used as template for PCR amplification using primers located downstream and upstream of the cloning site in the pGEM vector (coordinates 2874-2892 and 222-241). One of the primers was biotinylated at the 5′ end. PCR amplified material was separated with Dynabeads M280-streptavidin (Dynal, Norway) and the immobilized double strand DNA was denatured with 0.1 N NaOH at room temperature for 6 min. The biotinylated single-strand template DNA was primed and sequenced as described with the only modification that annealing was performed at 60°C for 10 min. From each CTL clone, between three and six independent TCR α and β chain clones were sequenced.
Results and Discussion
Memory CTL responses were reactivated in vitro by stimulation of lymphocytes from four A11+ EBV carriers with autologous EBV transformed LCL and EBV-specific T cell lines were cloned by limiting dilution. In accordance with previous observations in polyclonal cultures (11), the A11-restricted response was strongly dominated by CTL clones specific for the IVT epitope in two of the donors, BK and CA (Table 1). Two donors that yield cultures with comparable IVT and AVF-specific activities (ZA and EA) were included to avoid biases due to unequal representation of the two specificities.
Table 1.
Recovery of IVT- and AVF-specific CTL Clones from Polyclonal Cultures of HLA A11–positive Individuals
| Donor | Independent Stimulations | Clones analyzed | Specificity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IVT | AVF | Other | Noncytotoxic | |||||||||
| BK | 4 | 173 | 109 | 26 | 12 | 26 | ||||||
| CA | 1 | 66 | 48 | 8 | 3 | 7 | ||||||
| ZA | 1 | 115 | 20 | 16 | 4 | 75 | ||||||
| EA | 1 | 75 | 31 | 42 | 0 | 2 | ||||||
| Total % | 429 | 208 (49) | 92 (21) | 19 (4) | 110 (26) | |||||||
CTL cultures were obtained by repeated stimulation of blood lymphocytes with the autologous B95.8 virus transformed LCL as described in Materials and Methods. The presence of IVT- and AVF-specific activity was confirmed by the capacity of the polyclonal culture to lyse HLA A11+ PHA blasts pulsed with 10−9 M of the corresponding synthetic peptides. Cultures from two donors that consistently gave a predominantly IVT-specific response and two donors with equal IVT- and AVF-specific reactivities were cloned by limiting dilution. The EBV specificity and class I restriction of the clones were confirmed by their capacity to lyse appropriate panels of EBV+ and EBV− targets matched through single class I alleles. A11-restricted clones were screened for lysis of peptide pulsed A11+ PHA blasts. Clones with other specificities were whether EBV-specific and restricted through other class I alleles, or exhibited LAK-type cytotoxicity. Synthetic peptides were synthesized by the Merrifield solid phase method, dissolved in DMSO at the concentration of 10−2 M, and further diluted in PBS to obtain the indicated concentrations before the assays. The protein concentration of the DMSO stock solutions was determined by a Biuret assay.
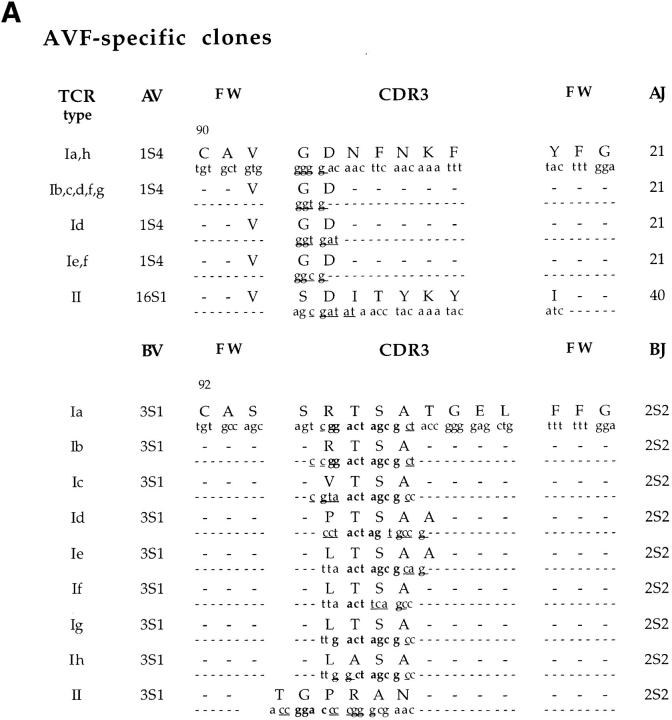
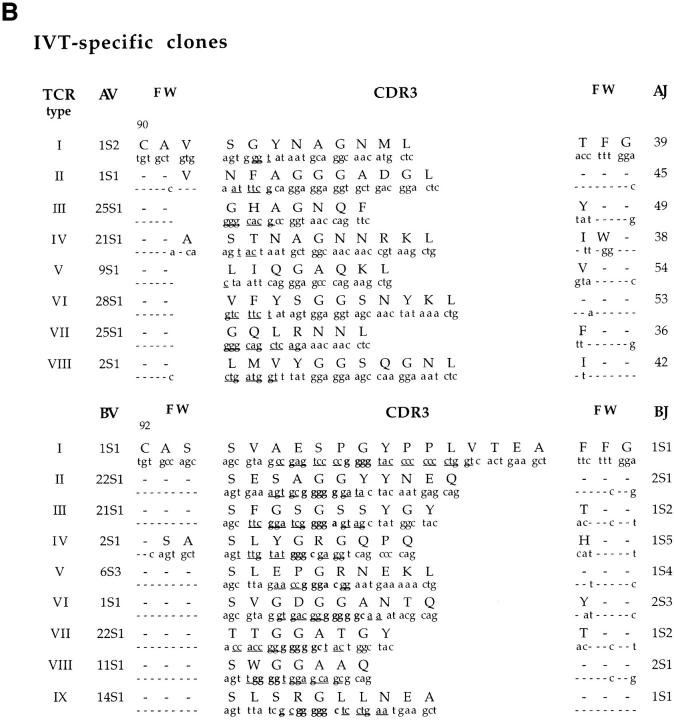
The TCR α and β chain usage of a representative panel of 19 AVF-specific clones from the four donors and 24 IVT-specific clones from three of the donors was determined by polymerase chain reaction (PCR)-assisted cDNA amplification. All AVF-specific clones expressed TCRBV3S1 (Table 2). This was paired to TCRAV1S4 in clones derived from three of the donors (BK, CA, and ZA; AVF type I), while all clones from EA expressed the closely related TCRAV16S1 (AVF type II). Sequencing of the PCR products demonstrated the regular rearrangement of BV3S1 to BJ2S2, AV1S4 to AJ21, and AV16S1 to AJ40. Comparison of the predicted amino acid sequences of the 19 Vα chains revealed CDR3 regions of equal length and identical protein sequence in all clones expressing a given VJ combination, with variations in codon usage restricted to the N regions (Fig. 1 A). The codon for Asp at position 94 was generated by N additions and was consistently preceded by the codons for Gly in clones expressing AV1S4J21, or Ser in the AV16S1J40 clones. The Vβ sequences of clones expressing the AVF type I receptor also showed a remarkable similarity. A conserved Thr-Ser-Ala motif was generated in the NDN region of all but one sequence that carried a Thr to Ala substitution. This motif was not present in the junctional region of the AVF type II TCRs, although a strict requirement for length maintenance in the CDR3 loop was suggested by the replacement of two germ line encoded Ser residues at positions 94 and 95 with ND encoded Thr and Gly. A remarkable conservation of TCR Vβ usage was previously reported in EBV-specific CTLs that recognize the EBNA3 325-333 epitope. Identical amino acid and nucleotide sequences were detected in the Vβ CDR3 regions of the EBNA3 325-333-specific TCRs isolated from four individuals (18). However, the usage of germ line sequences indicates that a preferentially favored rearrangement may contribute to the conservation of this response. This is clearly not the case in the AVF-specific response. The significant differences between the α and β chain nucleotide sequences and the regular occurrence of N additions confirm the origin of AVF-specific TCRs from independent recombination events even in clones derived from the same individuals (Fig. 1, donor BK). This finding stresses the importance of the conserved features of the AVF-specific TCRs for interaction with the A11–peptide complex. A different scenario was revealed by analysis of the repertoire specific for the immunodominant IVT epitope. Each of the 24 IVT-specific clones analyzed expressed one of nine distinct TCR-α/β heterodimers (Table 2). Four IVT-specific clonotypes were identified in donor BK, one in CA, and four in EA with no identical or even similar TCR isolated from more than one donor. Sequence comparison showed no preferential usage of TCRJ segments and no apparent conservation of CDR3 amino acid composition. Different sets of N or NDN additions resulted in the generation of CDR3 loops of variable length and amino acid composition (Fig. 1 B).
Table 2.
V and J Segments Used by the α and β Chain of AVF- and IVT-specific CTL Clones
| Donor | AVF specific | AVF type | IVT specific | IVT type | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of clones | AV | AJ | BV | D | BJ | No. of clones | AV | AJ | BV | D | BJ | |||||||||||||||||
| BK | 7 | 1S4 | 21 | 3S1 | 2 | 2S2 | Ic,d,e,f | 2 | 1S2 | 39 | 1S1 | 1 | 1S1 | I | ||||||||||||||
| 2 | 1S1 | 45 | 22S1 | 1 | 2S1 | II | ||||||||||||||||||||||
| 5 | 25S1 | 49 | 21S3 | 1 | 1S2 | III | ||||||||||||||||||||||
| 5 | 21S1 | 38 | 2S1 | 1 | 1S5 | IV | ||||||||||||||||||||||
| CA | 4 | 1S4 | 21 | 3S1 | 2 | 2S2 | Ia,h | 4 | 9S1 | 54 | 6S3 | 1 | 1S4 | V | ||||||||||||||
| ZA | 4 | 1S4 | 21 | 3S1 | 2 | 2S2 | Ib,g | nd | – | – | – | – | – | – | ||||||||||||||
| EA | 4 | 16S1 | 40 | 3S1 | 1 | 2S2 | II | 2 | 28S1 | 53 | 1S1 | 1 | 2S3 | VI | ||||||||||||||
| 1 | 25S1 | 36 | 22S1 | 1 | 1S2 | VII | ||||||||||||||||||||||
| 2 | 2S1 | 42 | 11S1 | 1 | 2S1 | VIII | ||||||||||||||||||||||
| 1 | nd | nd | 14S1 | 1 | 1S1 | IX | ||||||||||||||||||||||
TCR α and β chain V and J segment usage was determined by PCR-assisted cDNA amplification and sequencing as described in Materials and Methods. The TCR V gene segments are classified according to the family designation outlined by Arden et al. (30). The AJ genetic elements are assigned according to the nomenclature of Koop et al. (31) and the designation of BJ elements follows that of Toyonaga et al. (32). Subtypes of the AVF type I TCR with different BV(D)BJ junctional sequences (see Fig. 1) are indicated by low case letters. The number of clones expressing identical α and β chains are shown for each TCR type. Messages corresponding to nonproductive rearrangements of the second VA gene were detected in approximately one-sixth of the clones.
Figure 1.
Nucleotide sequence of the α and β chains V(D)J junctional regions and deduced amino acid sequence. (A) AVF-specific TCRs; (B) IVT-specific TCRs. For each TCR type, the nucleotide sequence and the deduced amino acid sequence in single letter code of the CDR3 equivalent loop, defined according to Chothia et al. (33), is shown putatively supported by two framework branches (FW). Only deviations from the consensus FW sequences are indicated. N nucleotide additions are underlined and TCRBD1 and BD2 germinal sequences are highlighted in bold. The conserved framework Cys residues in position 90 of the Vα and 92 of the Vβ chains are indicated.
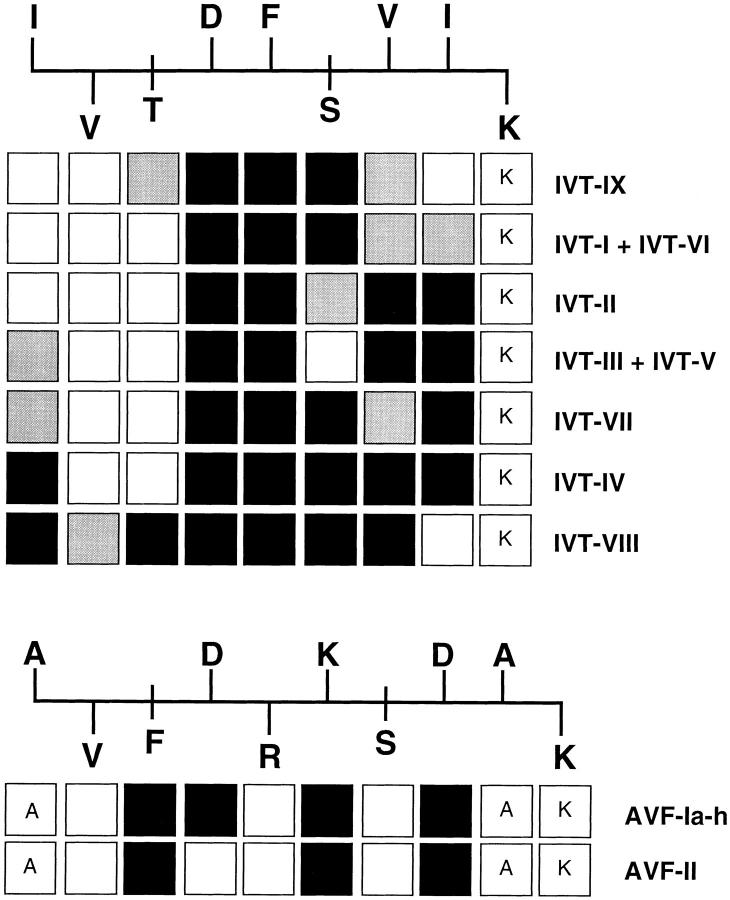
To determine how the TCR structural differences related to target recognition, the fine specificity of clones representing the two AVF-specific and nine IVT-specific TCR types was determined using peptide analogues in which each residue of IVT or AVF was sequentially substituted by Ala. Analysis of the HLA A11 binding motif (19) and molecular modeling of the IVT- and AVF-containing complexes (20) indicate that Val at position 2 (P2) and the carboxy-terminal Lys serve as the main anchors. In accordance, the IVT-A9 and AVF-A10 analogues failed to stabilize A11 expression in transporter associated with antigen presentation (TAP) mutant cells (not shown) and were not included in this study. Residues at P1, P4, P5, P7, and P8 of IVT are predicted to point away from the binding groove and be accessible to TCR while the side chains of residues in P3 and P6 are likely to face the cleft, serving as accessory anchors. Sequence variations between IVT-specific TCR types were reflected in unique fine specificity patterns (Fig. 2). Whereas all clones were affected by substitutions at P4 and P5, that may represent important TCR contact sites, the type-IX TCR interacted stringently only with the central residues of the peptide and the remaining TCRs appeared to scan the complex with preferential recognition shifting from the COOH terminus (IVT types I, VI, II) to the NH2 terminus (IVT type VIII). The full array of potential contact residues was recognized by TCR type III, V, VII, and IV, albeit with different stringency. The effect of substitutions in P2, P3, and P6 suggests that they may induce conformational changes that indirectly affect the interaction with certain TCRs. It should be noted that all clones recognized equally well the wild-type peptide with half-maximal lysis observed at concentrations between 5 and 10 pM, confirming that the different recognition of the analogues is not an artifact due to different efficiency of the CTLs (not shown). As predicted by the conserved TCR usage, AVF-specific clones showed a homogeneous pattern of interaction with the Ala replacement set. All type I TCRs were sensitive to substitution of the solvent exposed residues in P4, P6, and P8 of the AVF peptide, and were also affected by replacement of the Phe in P3, probably due to significant conformational changes. Differences were observed when clones expressing BV3S1D2J2S2 chains containing Arg, Val, Pro, or Leu at position 96 were compared for recognition of the wild-type peptide presented by other members of the HLA A11 family (A1, A3, and Aw68, not shown), suggesting a possible role of this residue in contacting the α-helix. The Thr(Ala)-Ser-Ala motif may interact with the peptide since the type II clones, that lack the motif, were not affected by substitution of the Asp in P4.
Figure 2.
Fine specificity of the various IVT and AVF-specific TCR types defined by alanine scanning mutagenesis. IVT and AVF-specific CTL clones were tested for lysis of A11+ PHA blasts pulsed with 10−9 and 10−10 M of the indicated peptide analogue. The sequence of the wild-type peptide and the predicted orientation of each residue relative to the A11 groove (20) are indicated above each Ala replacement set. Residues pointing towards the groove are shown below the peptide backbone and residues pointing towards the TCR are shown above. The putative accessory anchors are indicated crossing the backbone. Boxes below each residue indicate the corresponding Ala substituted analogue tested with clones expressing the indicated TCR type (left). When the wild-type residue is shown in the box the corresponding analogue was not tested. White boxes indicate that the analogue was recognized as efficiently as the wild-type peptide at both peptide concentrations, black boxes indicate no recognition at either peptide concentration and gray boxes correspond to at least 50% of the control lysis at a concentration of 10−9 M. Each assay was performed three times and the results obtained with individual clones were highly reproducible. All clones listed in Table 2 were tested. In several cases the screening was performed before the TCR-α/β sequences became available. Later results confirmed the absolute correlation between each TCR type and a given pattern of reactivity.
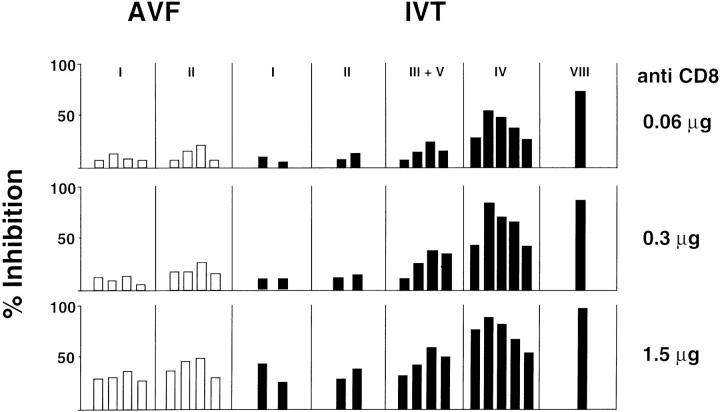
Tolerance to self antigens may diversify the peripheral repertoire specific for a given epitope by deleting cross-reactive TCRs of high affinity (21–24). We sought to determine whether a similar phenomenon may explain the discrepancy between IVT- and AVF-specific responses. In vitro studies with soluble molecules have revealed that CD8 enhances the T cell receptor interaction with its ligand (25). The triggering of cytotoxic functions is regulated by the cooperative influence of several factors where, in the presence of equal ligand density, TCR affinity correlates with resistance to blocking of the CD8 coreceptor (26, 27). The IVT and AVF peptides bind equally well to HLA A11 and similar titration curves are obtained when IVT- and AVF-specific effectors are compared for lysis of peptide-pulsed targets (13). Taking advantage of these features, we tested the effect of increasing amounts of anti-CD8 antibodies on the cytotoxic activity of clones expressing different TCRs (Fig. 3). Reproducible levels of inhibition were observed in repeated experiments performed with each clone. The AVF-specific effectors were not affected by addition of 60 ng of the purified antibody and <50% inhibition was achieved at 75-fold higher concentrations suggesting that they express TCRs of high affinity. In contrast, the IVT-specific repertoire included TCRs of both high and low affinity, with each TCR type falling in a distinct range of sensitivity. This and the demonstration that all potential TCR contact residues are recognized by at least some of the IVT-specific effectors supports the view that the diversity of this repertoire is not caused by elimination of a self-reactive TCR.
Figure 3.
TCR affinity defined by sensitivity to blocking by anti-CD8 antibodies. CTL clones representing various TCR types were tested for lysis of A11+ PHA blasts pulsed with 10−9 M of synthetic peptides with or without addition of the indicated amounts of purified anti-human CD8 mAbs. The bars represent the mean % inhibition of at least three separate tests performed with each individual clone at E/T ratio 5:1. The standard deviations were within 10% of the mean. The percent specific lysis in the absence of antibodies varied between 40–50% and was highly reproducible for each clone. Anti-CD4 antibodies and isotype matched irrelevant antibodies gave no more than 5% inhibition at the highest concentration. CD8 expression was tested in parallel by indirect immunofluorescence and FACS® analysis and showed no significant variations between clones.
We have shown that the differences in immunogenicity of the AVF and IVT epitopes correlate with the diversity of the epitope-specific T cell repertoire recruited in memory response. Antigen load is likely to play a crucial role in the maintenance of this repertoire. An interesting parallel may be drawn with the development of antibody responses. When antigen becomes scarce in the late phase of the response, clones expressing low-affinity immunoglobulin receptors develop into plasma cells, whereas clones carrying high-affinity receptors remain in the proliferating pool for additional cycles of replication (28, 29). It is tempting to speculate that antigen load may operate in a similar fashion in the peripheral selection of distinct populations of specific T cells. This would predict a direct correlation between the efficiency of presentation of a given epitope and the degree of diversity of the selected repertoire. In line with this prediction, we have recently reported that 10-fold higher amounts of the immunodominant IVT epitope can be recovered from HLA A11 positive LCLs as compared to the subdominant AVF (13). It is likely that chronic stimulation by cells presenting a low density of ligands, as in the case of the AVF epitope, would favor the generation of homogeneous responses composed of clones with high affinity TCRs. Conversely, the higher IVT load would allow the selection of both high and low affinity resulting in a broader response.
To our knowledge, this is the first demonstration that a highly restricted or a diverse repertoire may be recruited in the memory response by two epitopes derived from the same protein and restricted through the same class I allele. Our data suggest that the diversity of the peripheral repertoire is principally a function of the antigenic peptide itself and is influenced by the degree of immunogenicity. Antigen load is likely to account for our findings, although the structure of the epitopes, notably the TCR contact surface of AVF, with three charged residues in adjacent positions (Asp-P4, Lys-P6, and Asp-P8), may also play a role. Differences in the repertoire may already exist before peripheral selection as fewer naive cells may meet the TCR-CDR3 charge requirements for efficient docking to the AVF epitope. Ligand density would superimpose further selection in the periphery to generate the final profile of the memory repertoire. Further studies of TCR usage in conditions where these epitopes are not in short supply, as during primary EBV infection, are needed to assess to which extent the repertoires detected in memory responses reflect the preservation of selected T cell clones.
Footnotes
The authors are indebted to Andrew J. McMichael for helpful comments on the manuscript.
This investigation was supported by grants from the Swedish Cancer Society, the Pediatric Cancer Foundation, the Karolinska Institute, and the Magnus Bergwal Stiftelse, Stockholm, Sweden. V. Levitsky and M.P. Imreh are supported by fellowships from the Cancer Research Institute, New York and Concern Foundation, Los Angeles. P.-O. de Campos-Lima was partially supported by a fellowship from the Swedish Royal Academy of Sciences. The research in the laboratory of RG was supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC).
The first two authors have contributed equally to this work and are placed in alphabetical order.
1 Abbreviations used in this paper: EBNA, Epstein-Barr virus nuclear antigen; CDR3, third complementarity determining region; FW, framework; LCL, lymphoblastoid cell lines.
References
- 1.Davis M, Björkman P. T-cell receptor genes and T-cell recognition. Nature (Lond) 1989;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 2.Moss PAH, Rosenberg WMC, Bell JI. The human T cell receptor in health and disease. Annu Rev Immunol. 1992;10:71–96. doi: 10.1146/annurev.iy.10.040192.000443. [DOI] [PubMed] [Google Scholar]
- 3.Allen PM. Peptides in positive and negative selection: a delicate balance. Cell. 1994;76:593–596. doi: 10.1016/0092-8674(94)90497-9. [DOI] [PubMed] [Google Scholar]
- 4.Ashton-Rickardt PG, Tonegawa S. A differential-avidity model for T-cell selection. Immunol Today. 1994;15:362–366. doi: 10.1016/0167-5699(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 5.Tanchot C, Rocha B. The peripheral T cell repertoire: independent homeostatic regulation of virgin and activated CD8+T cell pools. Eur J Immunol. 1995;25:2127–2136. doi: 10.1002/eji.1830250802. [DOI] [PubMed] [Google Scholar]
- 6.Moss DJ, Rickinson AB, Pope JH. Long-term T-cell mediated immunity to Epstein-Barr virus in man: III. Activation of cytotoxic T cells in virus infected leukocyte cultures. Int J Cancer. 1978;23:618–625. doi: 10.1002/ijc.2910230506. [DOI] [PubMed] [Google Scholar]
- 7.Wallace LE, Rickinson AB, Rowe M, Epstein MA. Epstein-Barr virus-specific cytotoxic T-cell clones restricted through a single HLA antigen. Nature (Lond) 1982;297:413–415. doi: 10.1038/297413a0. [DOI] [PubMed] [Google Scholar]
- 8.Steven NM, Leese AM, Annels NE, Lee SP, Rickinson AB. Epitope focusing in the primary cytotoxic T cell response to Epstein-Barr virus and its relationship to T cell memory. J Exp Med. 1996;184:1801–1813. doi: 10.1084/jem.184.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silins SL, Cross SM, Elliott SL, Pye SJ, Burrows SR, Burrows JM, Moss DJ, Argaet VP, Misko IS. Development of Epstein-Barr virus-specific memory T cell receptor clonotypes in acute infectious mononucleosis. J Exp Med. 1996;184:1815–1824. doi: 10.1084/jem.184.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gavioli R, De Campos-Lima P-O, Kurilla MG, Kieff E, Klein G, Masucci MG. Recognition of the EBV encoded nuclear antigens EBNA-4 and EBNA-6 by HLA A11 restricted cytotoxic T-lymphocytes. Implications for the down-regulation of HLA A11 in Burkitt's lymphoma. Proc Natl Acad Sci USA. 1992;89:5862–5866. doi: 10.1073/pnas.89.13.5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavioli R, Kurilla M, de Campos-Lima P-O, Wallace LE, Dolcetti R, Murray RJ, Rickinson AB, Masucci MG. Multiple HLA A11 restricted CTL epitopes of different immunogenicity in the Epstein-Barr virus (EBV) encoded nuclear antigen-4 (EBNA4) J Virol. 1993;67:1572–1578. doi: 10.1128/jvi.67.3.1572-1578.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Campos-Lima P-O, Levitsky V, Brooks J, Lee SP, Hu LF, Rickinson AB, Masucci MG. T cell responses and virus evolution: Epstein-Barr virus isolates from highly HLA A11-positive populations carry mutations selectively involving anchor residues of A11-restricted CTL epitopes. J Exp Med. 1994;179:1297–1305. doi: 10.1084/jem.179.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitsky V, Zhang Q-J, Levitskaya J, Masucci MG. The lifespan of MHC–peptide complexes influences the efficiency of presentation and immunogenicity of two class I restricted cytotoxic T lymphocyte epitopes in the Epstein-Barr virus nuclear antigen-4. J Exp Med. 1996;183:915–926. doi: 10.1084/jem.183.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steven NM, Leese AM, Annels NE, Lee SP, Rickinson AB. Epitope focusing in the primary cytotoxic T cell response to Epstein-Barr virus and its relationship to T cell memory. J Exp Med. 1996;184:1801–1813. doi: 10.1084/jem.184.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Campos-Lima P-O, Gavioli R, Zhang Q-J, Wallace L, Rowe M, Rickinson AB, Masucci MG. HLA-A11 epitope loss isolates of Epstein-Barr virus from a highly A11+population. Science (Wash DC) 1993;260:98–100. doi: 10.1126/science.7682013. [DOI] [PubMed] [Google Scholar]
- 16.Torsteinsdottir S, Masucci MG, Ehlin-Henriksson B, Brautbar C, Ben H, Bassat, Klein G, Klein E. Differentiation dependent sensitivity of human B-cell derived lines to major histocompatibility complex-restricted T-cell cytotoxicity. Proc Natl Acad Sci USA. 1986;83:5620–5624. doi: 10.1073/pnas.83.15.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 18.Argaet VP, Schmidt CW, Burrows SR, Silins SL, Kurilla MG, Doolan DL, Suhrbier A, Moss DJ, Kieff E, Sculley TB, Misko IS. Dominant selection of an invariant T cell antigen receptor in response to persistent infection by Epstein-Barr virus. J Exp Med. 1994;180:2335–2340. doi: 10.1084/jem.180.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q-J, Gavioli R, Klein G, Masucci MG. An HLA A11-specific motif in nonamer peptides derived from viral and cellular proteins. Proc Natl Acad Sci USA. 1993;90:2217–2221. doi: 10.1073/pnas.90.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q-J, Lindquist Y, Levitsky V, Masucci MG. Solvent exposed side chains of peptides bound to HLA A11 have similar effects on the reactivity of alloantibodies and specific T cell receptors. Int Immunol. 1996;8:927–938. doi: 10.1093/intimm/8.6.927. [DOI] [PubMed] [Google Scholar]
- 21.Cibotti R, Cabaniols J-P, Pannetier C, Delarbre C, Vergnon I, Kanellopoulos JM, Kourilsky P. Public and private Vβ T cell receptor repertoires against hen egg white lysosyme (HEL) in nontransgenic versus HEL transgenic mice. J Exp Med. 1994;180:861–872. doi: 10.1084/jem.180.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabaniols J-P, Cibotti R, Kourilsky P, Kosmatopoulos K, Kanellopoulos JM. Dose-dependent T cell tolerance to an immunodominant self peptide. Eur J Immunol. 1994;24:1743–1749. doi: 10.1002/eji.1830240804. [DOI] [PubMed] [Google Scholar]
- 23.Pannetier C, Even J, Kourilsky P. T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol Today. 1995;16:176–181. doi: 10.1016/0167-5699(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 24.Burrows SR, Silins SL, Moss DJ, Khanna R, Misko IS, Argaet VP. T cell receptor repertoire for a viral epitope in humans is diversified by tolerance to a background major histocompatibility complex antigen. J Exp Med. 1995;182:1703–1715. doi: 10.1084/jem.182.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia KC, Scott CA, Brunmark A, Carbone FR, Peterson PA, Wilson IA, Teyton L. CD8 enhances formation of stable T cell receptor/MHC class I molecule complexes. Nature (Lond) 1996;384:577–581. doi: 10.1038/384577a0. [DOI] [PubMed] [Google Scholar]
- 26.Alexander MA, Damico CA, Wieties KM, Hansen TE, Connolly JM. Correlation between CD8 dependency and determinant density using peptide-induced Ld-restricted cytotoxic T lymphocytes. J Exp Med. 1991;173:849–858. doi: 10.1084/jem.173.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Ramadi BK, Jolonek MT, Boyd LF, Margulies DH, Bothwell ALM. Lack of strict correlation of functional sensitization with the apparent affinity of MHC/ peptide complexes for the TCR. J Immunol. 1995;155:662–673. [PubMed] [Google Scholar]
- 28.Berek C, Milstein C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol Rev. 1987;96:23–41. doi: 10.1111/j.1600-065x.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 29.Weinand RG. Somatic mutation, affinity maturation and the antibody repertoire: a computer model. J Theor Biol. 1990;143:343–382. doi: 10.1016/s0022-5193(05)80034-7. [DOI] [PubMed] [Google Scholar]
- 30.Arden B, Clark SP, Kabelitz D, Mak TW. Human T-cell receptor variable gene segment families. Immunogenetics. 1995;42:455–500. doi: 10.1007/BF00172176. [DOI] [PubMed] [Google Scholar]
- 31.Koop BF, Rowen L, Wang K, Kuo CL, Seto D, Lenstra JA, Howard S, Shan W, Deshpande P, Hood L. The human T-cell receptor TCRAC/TCRDC (Cα/ Cδ) region: organization, sequence, and evolution of 97.6 kb of DNA. Genomics. 1994;19:478–493. doi: 10.1006/geno.1994.1097. [DOI] [PubMed] [Google Scholar]
- 32.Toyonaga B, Yoshikai Y, Vadasz V, Chin B, Mak TW. Organization and sequence of the diversity, joining and constant region genes of the human T-cell receptor beta chain. Proc Natl Acad Sci USA. 1985;82:8624–8628. doi: 10.1073/pnas.82.24.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chothia C, Boswell DR, Lesk AM. The outline structure of the T-cell αβ receptor. EMBO (Eur Mol Biol Organ) J. 1988;7:3745–3755. doi: 10.1002/j.1460-2075.1988.tb03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]