Abstract
Leukocyte migration in response to cell attractant gradients or chemotaxis is a key phenomenon both in cell movement and in the inflammatory response. Chemokines are quite likely to be the key molecules directing migration of leukocytes that involve cell polarization with generation of specialized cell compartments. The precise mechanism of leukocyte chemoattraction is not known, however. In this study, we demonstrate that the CC chemokine receptors CCR2 and CCR5, but not cytokine receptors such as interleukin (IL)-2Rα, IL-2Rβ, tumor necrosis factor receptor 1, or transforming growth factor βR, are redistributed to a pole in T cells that are migrating in response to chemokines. Immunofluorescence and confocal microscopy studies show that the chemokine receptors concentrate at the leading edge of the cell on the flattened cell-substratum contact area, induced specifically by the signals that trigger cell polarization. The redistribution of chemokine receptors is blocked by pertussis toxin and is dependent on cell adhesion through integrin receptors, which mediate cell migration. Chemokine receptor expression on the leading edge of migrating polarized lymphocytes appears to act as a sensor mechanism for the directed migration of leukocytes through a chemoattractant gradient.
Chemokines exert their effects by interacting with seven transmembrane glycoprotein receptors coupled to a G protein signaling pathway (1, 2). Several chemokine receptors have now been cloned and classified into two groups, CC chemokine receptor (CCR) and CXC chemokine receptor (CXCR) (1). Some of these receptors, including CCR5, CCR3, and CXCR4 or fusin, have recently been described to function as coreceptors for HIV infection (see review in reference 1). Chemokine receptor expression on T cells seems to be tightly regulated at the transcriptional level. The upregulated mRNA expression of several chemokine receptors on activated and memory T cells has been described, a phenomenon that appears to be dependent on exogenous cytokines such as IL-2 (2, 3). Consequently, memory T cells represent the lymphocyte subset showing a higher transendothelial chemotactic potential (4).
Cell locomotion is extremely important, especially in the case of leukocyte migration. Certain aspects of the chemotaxis mechanism have been studied in various cell types and experimental systems. It is now becoming clear that cell motility involves cell polarization, with the formation of lamellipodia at the leading edge (5), where several unrelated reports suggest that an exocytosis process occurs (6). This promotes the redistribution of specific molecules during cell motion, such as αvβ3 (7), β actin (8), or the urokinase type plasminogen receptor (9).
The chemotactic response is fundamental in leukocyte physiology and implies recognition of an external stimulus gradient. Several mechanisms responsible for the chemotactic response have been suggested, including those dependent on receptor occupancy. Chemotactic receptor enrichment at the anterior cell pole has been difficult to demonstrate, due to the lack of specific antibodies. Preliminary evidence was shown in early studies describing the asymmetric binding of labeled N-formylmethionyl-leucylphenylalanine on neutrophils (10). These studies suggested that phenomena taking place at the leading edge might direct cell motility, but a more definitive demonstration was necessary. In this paper, we demonstrate that CCR2 and CCR5 receptors polarize to the leading edge of migrating lymphocytes, a finding that helps to understand the chemotactic response mechanism.
Materials and Methods
Cytokines and Reagents.
The anti-CCR2 mAb (monocyte chemotactic protein [MCP]-1R03) was obtained and characterized as described elsewhere. Mouse anti-CCR5 antiserum was obtained by immunization with a KLH-coupled synthetic peptide corresponding to amino acids 6–20 of the human CCR5 chemokine receptor. Recombinant human (rh) regulated on activation, normal T cell expressed and secreted (RANTES) (R&D Sys. Inc., Minneapolis, MN), and rhIL-8, and rhMCP-1 (PeproTech EC, Ltd., London, U.K.) were purchased. Recombinant IL-15 derived from a simian kidney epithelial cell line was provided by Immunex Corp. (Seattle, WA). IL-2 was provided by Hoffman-LaRoche (Nutley, NJ). PHA, poly-l-lysine (poly-l-Lys) and PMA were purchased from Sigma Chemical Co. (St. Louis, MO). The anti–intercellular adhesion molecule (ICAM)-3 mAb HP2/19 (11), the anti-CD25 mAb TP1/6 (12), and the soluble recombinant proteins recombinant soluble (rs)ICAM-1 and rs vascular cell adhesion molecule (VCAM)-1 have been previously described (11). The tryptic 38- and 80-kD fibronectin fragments (FN-40 and FN-80) were a gift of Dr. A. García-Pardo (Centro de Investigaciones Biológicas, Madrid, Spain).
Cells and Cell Lines.
PBL and human T lymphoblasts were prepared from PBMC and cultured as described (11). T lymphoblasts cultured for 7–12 d were used in all experiments.
Flow Cytometry Analysis.
CCR2 and CCR5 cDNA transiently transfected 293 cells or human lymphoblasts were incubated with biotinylated anti-CCR2 MCP-1R03 mAb or mouse anti-CCR5 antiserum for 30 min at 4°C. Cells were then washed twice with PBS and labeled with phycoerythrin-labeled streptavidin or goat anti–mouse Ig (Southern Biotechnology Assoc., Birmingham, AL) for 20 min at 4°C. Cell-bound fluorescence was determined in a flow cytometer (Profile XL; Coulter Corp., Hialeah, FL).
Immunofluorescence Digital Confocal Microscopy and Time-lapse Videomicroscopy.
Immunofluorescence experiments were performed essentially as described (11). In brief, 2 × 106 T lymphoblasts in 500 μl complete medium were allowed to adhere to protein-coated coverslips in 24-well plates (Costar Corp., Cambridge, MA). Cytokines and chemokines (10 ng/ml) were added, and cells were incubated at 37°C in a 5% CO2 atmosphere. After 30 min, cells were fixed with 3.7% formaldehyde in PBS for 10 min at room temperature and stained with specific mAb plus an FITC-labeled rabbit F(ab′)2 anti–mouse IgG (Pierce Chem. Co., Rockford, IL). For directional migration assays, experiments were performed as above, but 1 μl of chemokine (1 μg/ml) was deposited at the lower edge of the well, and T lymphoblasts were allowed to migrate. Kinetic studies showed that chemokine gradient effects were observed from 30 min up to 2 h after chemokine application. For double-label studies, cells were incubated with HP2/19 mAb cells followed by rhodamine-labeled rabbit F(ab′)2 anti–mouse IgG (Pierce Chem. Co.). Samples were washed, saturated with 10% mouse serum, and incubated with a 1:50 dilution of the biotinylated MCP-1R03 mAb, followed by washing and labeling with FITC-avidin D (Vector Labs., Inc., Burlingame, CA). Using a photomicroscope with ×60 oil immersion objectives (Labophot-2; Nikon Inc., Melville, NY), the proportion of CCR polarization was calculated by random choice of 10 fields for each condition and direct cell counting (No. 400–500). Preparations were photographed on Ektachrome 400 film. For confocal studies, cells adhering to FN-80–coated (20 μg/ml) coverslips and stimulated with MCP-1 were stained with MCP-1R03, followed by incubation with Cy2 goat anti–mouse IgG (Amersham, Pittsburgh, PA), saturation with 10% mouse serum, and staining with biotinylated anti-ICAM-3 HP2/19 and Cy3 streptavidin (Biological Detection Systems Inc., Pittsburgh, PA). Confocal microscopy was performed (MRC-1024 Confocal Laser Scanning System; Bio Rad Labs., Richmond, CA). Videomicroscopy analysis was performed as described (13).
Results and Discussion
Chemokine Receptor Polarization in Activated Human T Lymphocytes.
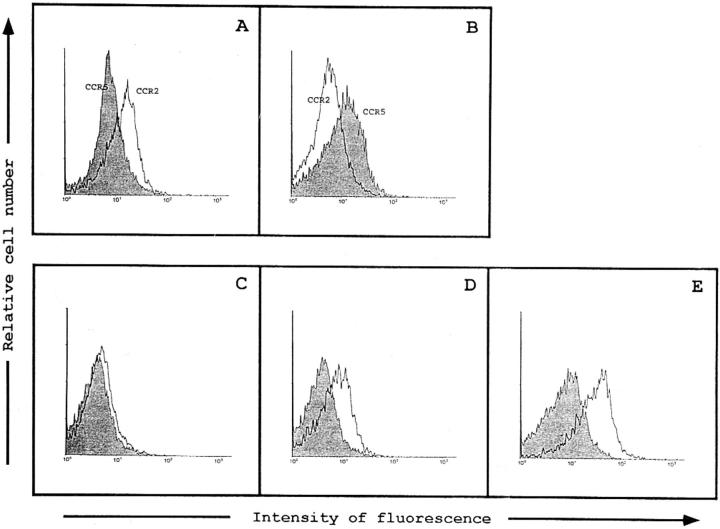
We have generated an mAb to the CCR2 receptor, a receptor that specifically binds MCP-1, -3, and -4 (14), and a mouse polyclonal antiserum to CCR5, a receptor for RANTES, macrophage inflammatory protein 1α, and macrophage inflammatory protein 1β (15). The anti-CCR2 mAb MCP-1R03 reacts with the 293 cell line transiently transfected with CCR2 cDNA (Fig. 1 A), but not with CCR5 cDNA-transfected cells (Fig. 1 B). Conversely, the CCR5-specific antiserum recognizes only CCR5, but not CCR2 cDNA-transfected cells (Fig. 1 B). Membrane expression of CCR2 and CCR5 was virtually undetectable on freshly isolated peripheral blood T lymphocytes (Fig. 1 C, and data not shown), whereas a significant fraction of IL-2– cultured, PHA-activated T lymphoblasts (>50%) express both receptors (Fig. 1, D and E).
Figure 1.
Recognition of human CCR2 and CCR5 chemokine receptors in transfected 293 cells and PBL. Flow cytometry analysis of the binding of the anti-CCR2 mAb MCP-1R03 (A) and mouse anti-CCR5 antibody (B) to the 293 cell line, transiently transfected with human CCR2 (white histogram) and CCR5 (shaded histogram) cDNA. CCR2 expression was assessed using the anti-CCR2 MCP-1R03 mAb in resting (C) or PHA-activated IL-2–cultured human PBL (D). Expression of the CCR5 in activated human PBL is shown (E). Shaded histograms correspond to the isotype-matched mAb (C and D) and irrelevant mouse antiserum (E) negative-staining controls. Cells were incubated with biotinylated anti-CCR2 mAb MCP-1R03 or mouse anti-CCR5 antiserum for 30 min at 4°C. Cell-bound fluorescence was determined in a flow cytometer (Profile XL; Coulter Corp.).
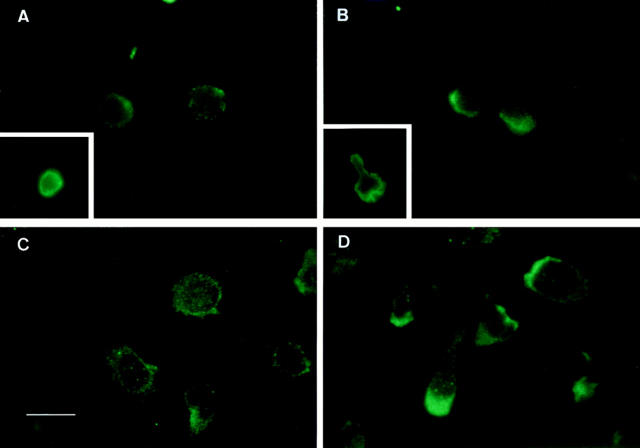
Cell migration directed by chemical attractant gradients requires cell polarization, but the fine mechanism by which this directional response is achieved remains to be described (8, 9, 16, 17). We studied the cellular distribution of chemokine receptors during T lymphocyte migration. CCR2 and CCR5 were evenly distributed throughout the T lymphoblast cell membrane (Fig. 2, A and C). Upon incubation of T lymphoblasts with MCP-1 or RANTES, a rapid cell polarization takes place that results in the redistribution of CCR2 and CCR5 receptors to the leading edge of the cell (Fig. 2, B and D). In the presence of a chemokine gradient, the cluster of CCR2 was clearly oriented towards the chemoattractant source. Parallel videomicroscopy studies showed that polarized cells correspond to migrating cells (Fig. 3). IL-2Rα, IL-2Rβ, TNFRI, and TGF-β type II receptor expression was also analyzed and found to undergo neither redistribution nor clustering during polarization (Fig. 2, A and B, insets, and data not shown).
Figure 2.
The CCR2 and the CCR5 receptors redistribute to the leading edge of the polarized migrating T cells. PHA-activated T lymphocytes, untreated (A and C), or treated with 10 ng/ml MCP-1 (B) or 10 ng/ml RANTES (D), were allowed to adhere to coverslips coated with 20 μg/ml FN-80 (A and B) or 10 μg/ml rsICAM-1-Fc (C and D), and then fixed and stained with the anti-CCR2 mAb MCP-1R03 (A and B) or the anti-CCR5 mouse antiserum (C and D), as described in Materials and Methods. Cells were photographed under epifluorescent conditions. Original magnification: ×1,200. Bar, 10 μm. Insets show staining of the IL-2Rα with the TP1/6 mAb on a resting (A) and a polarized (B) T lymphoblast. Original magnification: ×600.
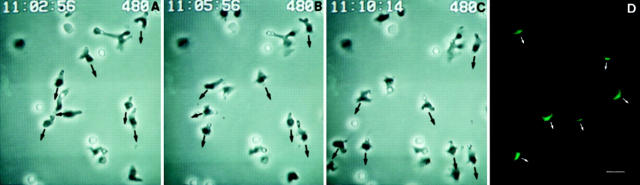
Figure 3.
Migrating T cells polarize the CCR2 receptor towards the chemoattractant gradient. (A–C) Time-lapse videomicroscopy analysis of migrating T lymphoblasts in response to MCP-1. Cells were allowed to adhere to 10 μg/ml rsICAM-1 on coverslips; MCP-1 was then deposited at one edge of the bottom of the well and cells allowed to migrate. Sequential time frames are shown. Polarized migrating lymphocytes show the phase-dark leading edge and the phase-bright uropod. (D) Parallel immunofluorescence studies show the CCR2 distribution of the migrating cells. Cells were photographed with a ×60 objective. Bar, 10 μm. The arrows indicate the direction of cell migration towards the chemokine source.
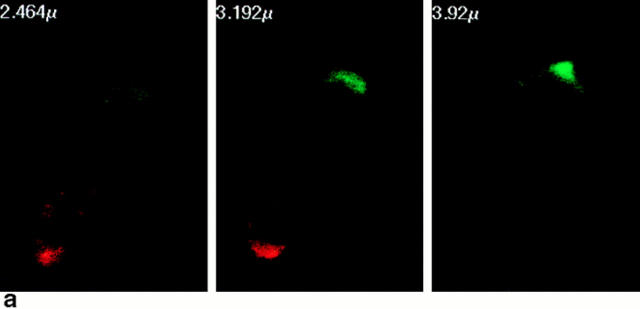
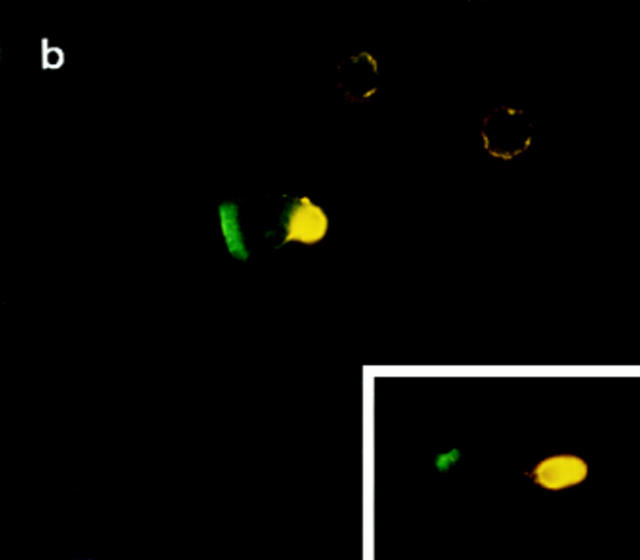
During migration, lymphocytes are polarized such that the leading edge of the cell develops cytoplasmic extensions (lamellipodia), whereas the posterior part of the cell forms an appendage (the uropod) (16, 17). Two-color fluorescence microscopy analyses allow us to conclude that the CCR2 and the CCR5 receptors are located at the advance front of migrating lymphocytes (Fig. 4, and data not shown). This is confirmed by confocal microscopy studies showing its presence on the flattened cell-substratum contact area at the leading edge. On the opposite pole of the cell, ICAM-3 was found to decorate the uropod that protrudes from the contact area of migrating T lymphocytes with endothelial or extracellular matrix substrates (Fig. 4, a and b; references 11, 12).
Figure 4.
(a) The CCR2 receptor patches on the cell-sustratum contact areas at the leading edge of the migrating T cell. Confocal microscopy was performed as described in Materials and Methods. Three representative optical cell sections are shown, out of the eight obtained. Serial sections are from the upper level (left, 2.464 μm), which shows staining of ICAM-3 (red fluorescence) at the uropod, to the sustratum level (right, 3.92 μm), where CCR-2 (green fluorescence) is found at the leading edge. (b) Polarized distribution of the CCR2 receptor and ICAM-3 on migrating T cells. Cells adhered to FN-80–coated coverslips were double stained with anti-ICAM-3 HP2/19 (yellow fluorescence) and anti-CCR2 mAb MCP-1R03 (green fluorescence). Original magnification: ×1,200. Bar, 10 μm. The inset shows another T lymphoblast from the same sample at equal magnification.
Redistribution of Chemokine Receptors to the Leading Edge Requires a Migrating T Cell Phenotype.
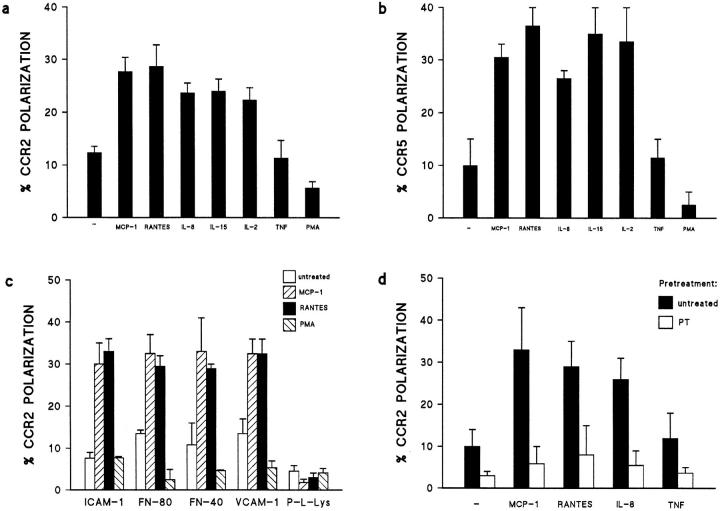
The redistribution of either CCR2 or CCR5 to the leading edge of the cell was triggered by the chemokines MCP-1, RANTES, and IL-8, as well as by the cytokines IL-2 and IL-15 (Fig. 5, a and b), known to be chemotactic factors (12, 18, 19). In contrast, the proinflammatory cytokine TNF-α, which does not induce cell polarization, exerted no effect. PMA, which promotes cell spreading and blocks cell migration, decreased the basal level of CCR polarization (Fig. 5, a and b). Other T cell polarization-inducing agents, such as mAb against ICAM-3 and CD43 (11, 20), also triggered CCR2 redistribution (data not shown). These data indicate that CCR polarization in leukocytes correlates with the acquisition of the migrating phenotype and is independent of the chemoattractant stimulus. Anti-MCP-1 or CCR2 mAb that block chemotaxis also inhibited the acquisition of the polarized morphology and the redistribution of CCR2 to the leading edge (data not shown). The sum of these results reveals the domain specialization that takes place in activated, migrating T lymphocytes. The receptor involved in detection of the chemoattractant gradients is located at one pole, whereas at the other pole, the receptor implicated in cell adhesion recruits bystander leukocytes (13).
Figure 5.
(a and b) The role of chemoattractants in the redistribution of CCR2 and CCR5 receptors on migrating T lymphocytes. T lymphoblasts stimulated with 10 ng/ml of several polarizing chemokines and cytokines were allowed to adhere to coverslips coated with 20 μg/ml FN-80 (a) or 10 μg/ml rsICAM-1-Fc (b). Cells were then stained and the percentage of cells on which the CCR2 (a) or CCR5 (b) receptors were redistributed was calculated as described. The arithmetic mean ± SEM of four (a) and three (b) independent experiments is shown. (c) The role of various substrates on CCR2 receptor polarization. T lymphoblasts were allowed to adhere to coverslips coated with 10 μg/ml rsICAM-1-Fc, 20 μg/ml FN-80, 30 μg/ml FN-40, 20 μg/ml rsVCAM-1-Fc, or 20 μg/ml poly-L-Lys, and then stimulated with chemokines or PMA (10 ng/ml). Cell samples were processed as described. The arithmetic mean ± SEM of three independent experiments is represented. (d) Chemokine-induced polarization of the CCR2 receptor is blocked by pertussis toxin. PHA-activated T lymphocytes were pretreated with pertussis toxin (1 μg/ml) for 20 min at 37°C, and then allowed to adhere to rsICAM-1 (as in the legend to Fig. 2). The effect of the chemokines on CCR2 redistribution to the leading edge of cells was quantified as in Fig. 2. The arithmetic mean ± SEM of three independent experiments is shown.
It has been established that chemokines regulate integrin-mediated cell adhesion to extracellular matrix and endothelium (21). Here we show that CCR2 polarization is induced by MCP-1 and RANTES when T lymphoblasts adhere to the integrin ligands FN-80 and FN-40, to the endothelial counter-receptor ICAM-1, and to VCAM-1, but not on T cells immobilized on poly-L-Lys (Fig. 5 c). Leukocyte adhesion through integrins is required to induce cell polarization (11) and redistribution of chemokine receptors, but not of the cytokine receptors tested. These data further support the model that chemokines may act in combination with adhesion molecules to steer leukocyte traffic to tissues (22).
Leukocyte motion involves several phenomena, including changes in cell shape, integrin affinity, and integrin recycling at the cell's leading edge (7, 8). These events appear to be mediated by phosphorylation signals through chemokine receptors, although the signal transduction machinery implicated has not been fully elucidated (23). Chemokine receptors are coupled to a G protein signaling pathway, and we previously reported that chemokine-induced lymphocyte polarization is abrogated by pertussis toxin (11). We have now found that pertussis toxin treatment of T lymphocytes also abolishes chemokine-mediated CCR2 polarization (Fig 5 d), indicating a requirement for a signal triggered by the chemokine receptor.
Leukocyte migration is a rather complex phenomenon, where chemokines are very important players. The patching of chemokine receptors to the leading edge of the cell implies two remarkable consequences: first, the functional specialization of this cell domain in signal transduction, and second, the establishment of an endogenous polarity in the cell, which may be crucial for chemotaxis and other immune responses involving chemokines. Finally, it is worth insisting that the clustering of chemokine receptors to the advance front of migrating cells is induced by the polarization process itself, regardless of the polarizing or chemotactic agent involved. Hence, the redistribution of CCR2, CCR5 (described above), and CXCR3 (data not shown) to the leading edge of cells adopting the polarized migrating phenotype, may also apply to other chemokine receptors. In fact, although not yet characterized, an early report showed the asymmetric distribution of the labeled N-formyl peptide bound to polarized neutrophils, mainly in the mid-region of the cell and at a lower density on the tail (10).
Finally, lymphocyte polarization is involved in many processes such as antigen presentation, differentiation, activation, target cell recognition by cytotoxic T lymphocytes, and cell killing (24–26). Polarization of the chemokine receptors may act as a sensor mechanism to increase cell responsiveness to chemokines and to guide cell–cell interaction, as well as to marshal lymphocyte motion along a chemokine gradient during lymphoid cell trafficking in immune and inflammatory responses.
Acknowledgments
We thank Drs. A. Aragay and J. Gutierrez for the CCR2B and CCR5 transfectants, respectively, M.A. Ollacarizqueta and E. de la Rosa for technical assistance with confocal microscopy, C. Cabañas for assistance with the time-lapse videomicroscopy studies, M.A. del Pozo for excellent advice on the experimental work, both him and Dr. R. Gonzalez-Amaro for critical reading of the manuscript, and C. Mark for editorial assistance.
This work was supported by grant SAF 96/0039 from the Ministerio de Educación y Ciencia, grant 07/44/ 96 from Comunidad Autónoma de Madrid, and a grant from Asociación de la Lucha contra el Cancer (to F. Sánchez-Madrid). The Department of Immunology and Oncology was founded and is supported by the Consejo Superior de Investigaciones Cientificas and Pharmacia & Upjohn.
References
- 1.Mackay CR. Chemokine receptors and T cell chemotaxis. J Exp Med. 1996;184:799–802. doi: 10.1084/jem.184.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 3.Loetscher PM, Seitz M, Baggiolini M, Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med. 1996;184:569–577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quin S, LaRosa G, Campbell JJ, Smith-Heath H, Kassam N, Shi X, Zeng L, Butcher EC, Mackay CR. Expression of monocyte chemoattractant protein-1 and interleukin-8 receptors on subsets of T cells: correlation with transendothelial chemotactic potential. Eur J Immunol. 1996;26:640–647. doi: 10.1002/eji.1830260320. [DOI] [PubMed] [Google Scholar]
- 5.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 6.Bretscher MS. Moving membrane up to the front of migrating cells. Cell. 1996;85:465–467. doi: 10.1016/s0092-8674(00)81246-5. [DOI] [PubMed] [Google Scholar]
- 7.Lawson MA, Maxfield FR. Ca2+and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature (Lond) 1996;377:75–78. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- 8.Condeelis J. Life at the leading edge. Annu Rev Cell Biol. 1993;9:411–444. doi: 10.1146/annurev.cb.09.110193.002211. [DOI] [PubMed] [Google Scholar]
- 9.Estreicher A, Muhlhauser J, Carpenteir J-L, Orci L, Vasalli JD. The receptor for urokinase type plasminogen activator polarizes expression of the protease to the leading edge of migrating monocytes and promotes degration of enzyme inhibitor complexes. J Cell Biol. 1990;111:783–792. doi: 10.1083/jcb.111.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan SJ, Daukas G, Zigmond SH. Asymmetric distribution of the chemotactic peptide receptor on polymorphonuclear leukocytes. J Cell Biol. 1984;99:1461–1467. doi: 10.1083/jcb.99.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Pozo MA, Sánchez-Mateos P, Nieto M, Sánchez-Madrid F. Chemokines regulate cellular polarization and adhesion receptor redistribution during lymphocyte interaction with endothelium and extracellular matrix. Involvement of cAMP signaling pathway. J Cell Biol. 1995;131:495–508. doi: 10.1083/jcb.131.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nieto M, del Pozo MA, Sánchez-Madrid F. Interleukin-15 induces adhesion receptor redistribution in T lymphocytes. Eur J Immunol. 1996;26:1302–1307. doi: 10.1002/eji.1830260619. [DOI] [PubMed] [Google Scholar]
- 13.del Pozo MA, Cabañas C, Montoya MC, Ager A, Sánchez-Mateos P, Sánchez-Madrid F. ICAMs redistributed by chemokines to cellular uropods as a mechanism for recruitment of T lymphocytes. J Cell Biol. 1997;137:493–508. doi: 10.1083/jcb.137.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charo IF, Myers SJ, Herman A, Fraci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci USA. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raport CJ, Gosling J, Schweickart VL, Gray PW, Charo IF. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1β, and MIP-1α. J Biol Chem. 1996;271:17161–17166. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 16.Devreotes NP, Zigmond SH. Chemotaxis in eukaryotic cells. Annu Rev Cell Biol. 1988;4:649–686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- 17.Haston WS, Shields JM, Wilkinson PC. Lymphocyte locomotion and attachment on two-dimensional surfaces and in three-dimensional matrices. J Cell Biol. 1982;92:747–752. doi: 10.1083/jcb.92.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkinson PC, Liew FY. Chemoattraction of human blood T lymphocytes by interleukin-15. J Exp Med. 1995;181:1255–1259. doi: 10.1084/jem.181.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McInnes IB, Al-Mughales J, Field M, Leung BP, Huang F, Dixon R, Sturrock RD, Wilkinson PC, Liew FY. The role of IL-15 in T cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-Mateos P, Campanero MR, del Pozo MA, Sánchez-Madrid F. Regulatory role of CD43 leukosialin on integrin-mediated T cell adhesion to endothelial and extracellular matrix ligands and its polar redistribution to a cellular uropod. Blood. 1995;86:2228–2239. [PubMed] [Google Scholar]
- 21.Tanaka Y, Adams DH, Hubscher S, Hirano H, Siebenlist U, Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1β. Nature (Lond) 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- 22.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 23.Bokoch MG. Chemoattractant signaling and leukocyte activation. Blood. 1995;86:1649–1660. [PubMed] [Google Scholar]
- 24.Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 25.Negulescu PA, Krasieva TB, Khan A, Kerschbaum HH, Cahalan MD. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity. 1996;4:421–430. doi: 10.1016/s1074-7613(00)80409-4. [DOI] [PubMed] [Google Scholar]
- 26.Helander TS, Carpén O, Turunen O, Kovanen PE, Vaheri A, Timonen T. ICAM-2 redistributed by ezrin as a target for killer cells. Nature (Lond) 1996;382:265–268. doi: 10.1038/382265a0. [DOI] [PubMed] [Google Scholar]