Abstract
Immunity to Mycobacterium tuberculosis infection is associated with the emergence of protective CD4 T cells that secrete cytokines, resulting in activation of macrophages and the recruitment of monocytes to initiate granuloma formation. The cytokine-mediating macrophage activation is interferon-γ (IFN-γ), which is largely dependent on interleukin-12 (IL-12) for its induction. To address the role of IL-12 in immunity to tuberculosis, IL-12 p40−/− mice were infected with M. tuberculosis and their capacity to control bacterial growth and other characteristics of their immune response were determined. The IL-12 p40−/− mice were unable to control bacterial growth and this appeared to be linked to the absence of both innate and acquired sources of IFN-γ. T cell activation as measured by delayed type hypersensitivity and lymphocyte accumulation at the site of infection were both markedly reduced in the IL-12 p40−/− mice. Therefore, IL-12 is essential to the generation of a protective immune response to M. tuberculosis, with its main functions being the induction of the expression of IFN-γ and the activation of antigen-specific lymphocytes capable of creating a protective granuloma.
Acquired cellular immunity to Mycobacterium tuberculosis infection is characterized by the emergence of a population of protective CD4 T cells that secrete cytokines, resulting in local activation of macrophages and the recruitment of monocytes to initiate granuloma formation (1, 2). The kinetics of this protective immunity, which leads to the control and containment of the infection, and the onset of bacterial clearance, is closely associated with the kinetics of emergence and loss of CD4 T cells that secrete large amounts of the cytokine IFN-γ (2–4). This cytokine has been shown to be pivotal in protective immunity, as illustrated by the severe, disseminated form of tuberculosis seen in IFN-γ gene-disrupted mice (5, 6).
In vivo and in vitro infection with M. tuberculosis results in the secretion of numerous cytokines (2). As has been demonstrated previously (7), this response includes the secretion of IL-12, a cytokine that appears to have powerful immunopotentiating effects. Among these are the ability to initiate the development of Th1 phenotype in naive T cells (8, 9) and the ability to potentiate IFN-γ production in antigen-activated Th1 cells (10). Hence, the role of this cytokine in the protective immune response to M. tuberculosis, which is dependent upon the Th1-type pathway, is of interest.
Previous studies have relied upon the treatment of infected mice with exogenous IL-12, which led to increased survival (11) and lowered bacterial numbers (11, 12). The effect was not dramatic, suggesting that endogenous IL-12 was already present at sufficient levels. Depletion of IL-12 by antibodies did diminish the level of protection (12), but was not sufficient to halt immunity. This may have been due to incomplete neutralization of IL-12 activity by the antibody. Therefore, we assessed the level of protection in mice lacking endogenous IL-12. These mice having recently been generated using homologous recombination to disrupt the IL-12 p40 gene (13).
Using this mouse in a systemic model of infection, we demonstrate that IL-12 is crucial for the development of protective immunity as measured by IFN-γ production, the development of activated T cells, and the control of bacterial growth. Of particular interest was the observation that IL-12 p40−/− mice were unable to generate a delayed type hypersensitivity (DTH)1 type of reaction, or for that matter recruit organized mantles of lymphocytes into their granulomas, indicating that the presence of IL-12 is crucial to cellular accumulation in these processes.
Materials and Methods
Mice.
C57BL/6J control mice were purchased from the Jackson Laboratories (Bar Harbor, Maine). Male and female gene-disrupted mice were generated using homologous recombination in embryonic stem (ES) cells as previously described (13). In brief, chimeric animals derived from the targeted 129/Sv ES cells that were heterozygous for the IL-12 p40 mutation were mated to C57BL/6 mice. Progeny from this cross that were determined to be heterozygous for the IL-12 p40 (IL-12 p40+/−) mutation were further backcrossed to the C57BL/6 strain for a total of five backcrosses. The backcrossed IL-12 p40+/− mice were then intercrossed and screened to obtain mice homozygous for the IL-12 p40 mutation (IL-12 p40−/−). Homozyogotes were crossed to each other in order to expand the colony and their progeny used in these experiments.
Experimental Infections.
A virulent laboratory strain of M. tuberculosis (Erdman) was grown from a low passage seed lot in Proskauer-Beck liquid media to midlog phase, aliquoted, and frozen at −70°C. Mice were infected with 105 bacteria via the lateral tail vein as described previously (1). The numbers of viable bacteria in target organs was followed against time by plating serial dilutions of whole organ homogenates on nutrient Middlebrook 7H11 agar and counting bacterial colony formation after 20-d incubation at 37°C. The data are expressed as the log10 value of the mean number of bacteria recovered per organ (n = 4 animals).
Isolation of mRNA and Detection of Cytokine-specific Message by Reverse Transcriptase Polymerase Chain Reaction.
Infected liver tissue was excised, placed in Ultraspec (Cinna/Biotecx, Friendswood, TX), homogenized, and RNA was extracted as described previously (12, 14). 1 μg of total RNA was reverse transcribed, diluted, and underwent PCR expansion of cytokine-specific cDNA. The amount of cytokine-specific product was determined by the hybridization of fluorescein-labeled cytokine-specific probe. The fluorescein was detected by the enhanced chemiluminescence kit (ECL; Amersham Corp., Arlington Heights, IL) and the resultant light signal was detected on Hyper-film (Amersham). For the IL-12R β2 chain analysis, the RNA underwent a similar RT-PCR reaction using the primers AGC CCT GAT TTA GCT GAA TCC AG and GCT CTT CCT CTG GTG TTC GTG TTC. The amount of specific product was determined by hybridization of the amplicon to a 32P-labeled IL-12R β2-specific oligonucleotide probe, GGC AAG TGG TAC TCA ATC AAC TCA G. The hybridized filters were exposed and quantitated by integrating the volume in individual amplicons using a phosphoimager (ImageQuant version 3.3; Molecular Dynamics, Sunnyvale, CA). The amplicon also underwent HPRT-specific hybridization as a control. The linearity of the PCR reaction was determined empirically (15). Data is expressed as the mean pixel value of four samples from four separate mice. The significance of the difference between groups was determined by an unpaired Student's t test comparing the means of the signals from control versus infected tissue (n = 4 samples).
Secretion of IFN-γ In Vitro.
Spleens were harvested from control and IL-12 p40−/− mice both before and during infection. A single cell suspension was prepared from pooled spleens, cells were treated for 5 min with a 0.155 M ammonium chloride/0.010 M potassium bicarbonate solution in order to lyse the red blood cells, washed, and resuspended in complete DMEM (10% FCS, buffered with Hepes). Cell suspensions were plated at 5 × 105 cells per well in 96 well plates and incubated for 5 d at 37°C in 5% CO2. The cells were stimulated with either medium alone, culture filtrate protein antigens of M. tuberculosis, or whole live M. tuberculosis (strain Erdman). Culture filtrate proteins were received from Dr. J.T. Belisle under the NIH contract AI25147. The concentrations of IFN-γ in cell supernatants was determined by a two-site sandwich ELISA using antibodies R4.6A2 and XMG1.2 as previously described (2). The concentration of other cytokines were determined using the same procedure but the antibody pairs were as follows: TNF-α, MP6-XT3, and a polyclonal rabbit anti– TNF-α, 18352D; IL-10, JES5-2A5 and SXC-1; IL-4, BVD4-1D11, and BVD6-24G2; IL-6, MP5-20F3, and MP5-32C11. All ELISAs used gave detectable signals with concanavilin A–treated splenocyte cultures. All tissue culture reagents were obtained from Sigma Chem. Co. (St. Louis, MO) and all ELISA reagents were obtained from PharMingen (San Diego, CA).
Determination of DTH.
Infected mice were challenged in one footpad with 10 μg of purified protein derivative (PPD) of M. tuberculosis (Connaught Labs, Canada) and in the other with vehicle control. The swelling in each footpad was measured using vernier calipers and the difference taken as the amount of antigen-specific swelling. The PPD preparation did not induce swelling in noninfected animals.
Histological Analysis.
The lower right lobe of each mouse was inflated with 10% formal saline and blocked with the lobes from the other mice within the experimental group. Blocks were sectioned to allow the maximum area of each lobe to be seen and sections were stained with hematoxylin and eosin. Slides were examined blind and analyzed for differences in the size of granuloma formation and the characteristics of cells within the granulomas.
Results
IL-12 Is Crucial for the Control of Bacterial Replication.
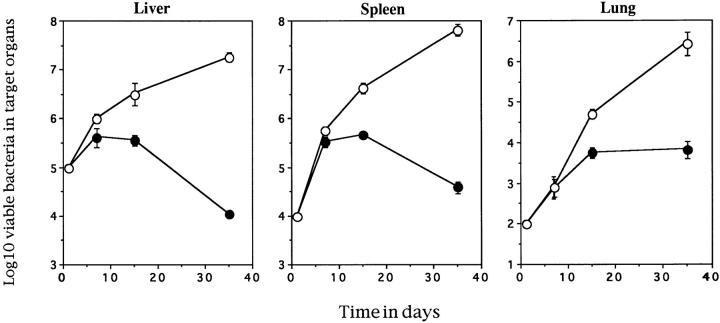
A role for IL-12 in the expression of protective immunity to M. tuberculosis infection has recently been demonstrated (2, 11, 14, 15). However, the advent of the mouse lacking the gene for the IL-12 p40 subunit has allowed a more complete determination of the role of this molecule. Therefore, the IL-12 p40−/− mice were infected with virulent M. tuberculosis and the growth of bacteria over time followed. As can be seen in Fig. 1, the absence of bioactive IL-12 results in the inability of the mice to control bacterial growth in the three major organs. In a second experiment, the mice were also unable to control growth and succumbed to infection between days 40 and 45 of infection (intact mice control bacteria and survive to old age before dying of recrudescent disease) (16).
Figure 1.
IL-12 p40−/− mice cannot control M. tuberculosis infection. Control (solid circles) and IL-12 p40−/− (open circles) mice were infected via the lateral tail vein with 105 M. tuberculosis bacteria and the number of viable bacteria present in the target organs was determined over time. The data points represent the mean and standard error of the bacterial number in the organs of four individual mice. This is a representative figure from one of two similar experiments.
IFN-γ Induction in Target Organs of Infected Mice Is Reduced in the Absence of IL-12 p40.
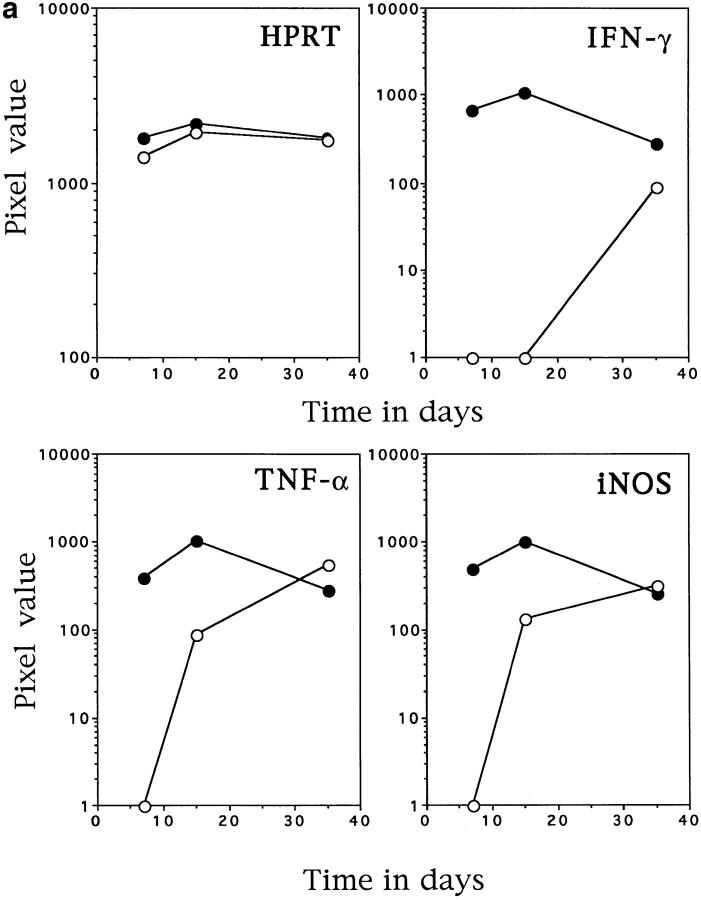
To determine the reason for the loss of control of bacterial growth the induction of mRNA for protective molecules in the livers of infected mice was examined. Fig. 2 demonstrates that, the level of IFN-γ mRNA was drastically reduced in the IL-12 p40−/− mice. In addition, the expression of mRNA for a second protective cytokine, TNF-α, was delayed in the IL-12 p40−/− mice compared with the C57BL/6 mice. The delay in expression of both of these cytokines probably resulted in the delay of the induction of macrophage activation as demonstrated in the reduced expression of mRNA for the gene for inducible nitric oxide synthase (17) (Fig. 2 a).
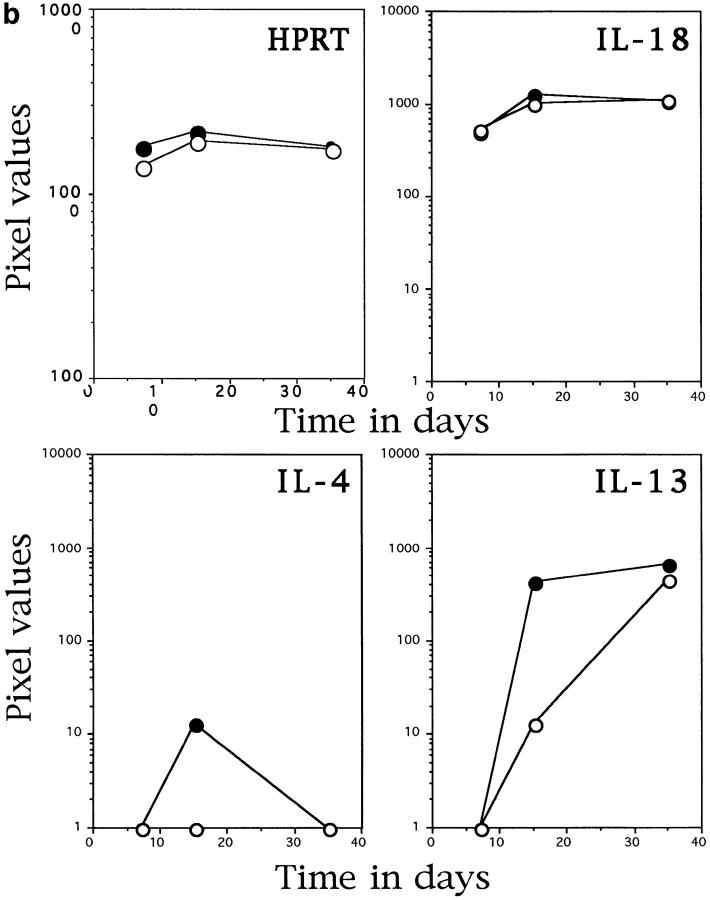
Figure 2.
IL-12 p40−/− mice have altered mRNA induction in response to infection with M. tuberculosis. Liver tissue was taken from control (solid circles) and IL-12 p40−/− (open circles) which were infected as in Fig. 1. RNA was extracted from the tissue and subjected to RT-PCR with primers specific for the molecules noted in the graphics. The HPRT graph demonstrates that equivalent readable RNA was extracted for each sample. a demonstrates the amount of IFN-γ, TNF-α, and inducible nitric oxide synthase-specific mRNA in the livers of infected mice, whereas b demonstrates the amount of IL-18, IL-4, and IL-13–specific mRNA in the same tissues. The data points represent the mean pixel values resulting from the analysis of tissue from four individual mice. This data is representative of two separate experiments.
The ability of these mice to generate another IFN-γ–inducing molecule, IL-18 was also of interest. Fig. 2 b demonstrates the equivalent levels of mRNA for this molecule in the C57BL/6 mice and the IL-12 p40−/− mice. In the absence of IL-12, and therefore IFN-γ, it was possible that a strong Th2 type response could develop. Therefore, the expression of mRNA for IL-4 and IL-13, cytokines linked to this type of cellular response (18), was determined. In contrast with this reasoning, a lower induction of both IL-4 and IL-13 was observed in the IL-12 p40−/− mice (Fig. 2 b).
Absence of IL-12 Affects Both Innate and Acquired IFN-γ Protein Production.
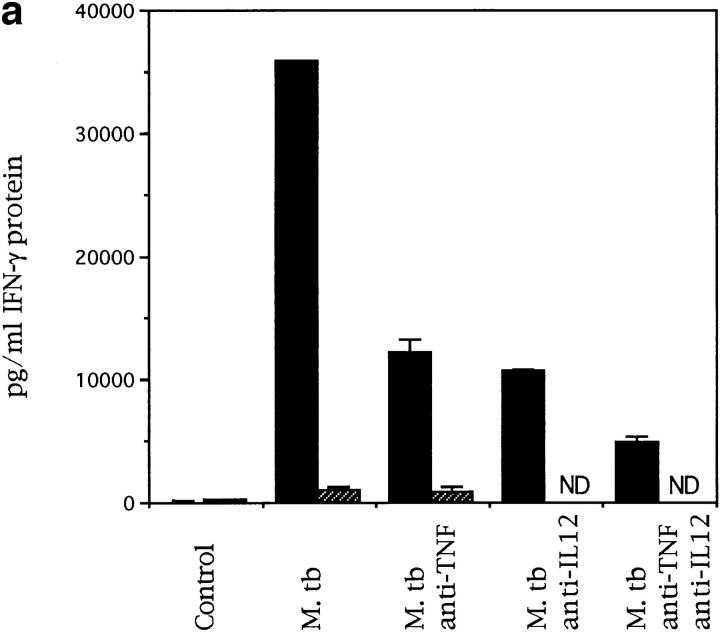
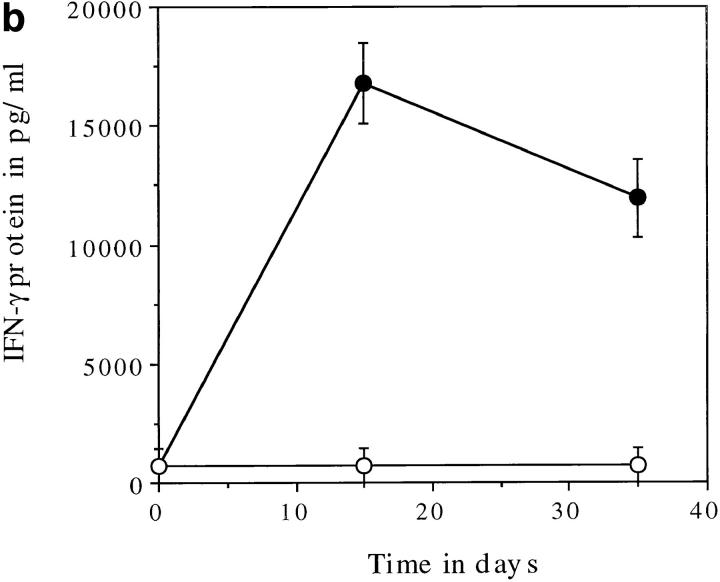
The role of IFN-γ in initiating a protective Th1 response is well documented and the requirement for this molecule in early T cell activation is established. To determine the levels of IFN-γ protein available in the initial stages of infection the ability of naive splenocytes to release IFN-γ in response to live M. tuberculosis was analyzed. IL-12 p40−/− splenocytes had a much reduced ability to secrete IFN-γ compared with the C57BL/6 splenocytes (Fig. 3 a). The addition of neutralizing antibodies to TNF-α and IL-12 demonstrates the role of both these molecules in driving this response in the C57BL/6 mice (Fig. 3 a).
Figure 3.
IL-12 p40−/− mice cannot make IFN-γ protein in response to M. tuberculosis infection. Splenocytes from naive (a) or infected (b) mice were cultured, in vitro, at a concentration of 2 × 105 cells per well in 96-well plates. In a, the cells from naive control (solid bars) or IL-12 p40−/− (striped bars) mice were exposed to live bacilli in the presence or absence of anti-cytokine antibodies (ND, not determined). In b, the cells from infected control (solid circles) or IL-12 p40−/− (open circles) mice were exposed to purified culture filtrate proteins derived from M. tuberculosis. The cells were cultured for 4 d and the supernatants were then analyzed by sandwich ELISA for IFN-γ.
The amount of antigen-specific IFN-γ was determined by culturing splenocytes from infected animals with M. tuberculosis culture filtrate proteins. Fig. 3 b demonstrates that the lack of IFN-γ mRNA in the liver is reflected by an absence of IFN-γ protein in the spleens of infected animals. Some IFN-γ mRNA was seen in the livers of the IL-12 p40−/− mice at day 30 (see Fig. 2 a) although there is no antigen-specific protein at this time point (Fig. 3 b). It is possible that the IFN-γ mRNA is a response to the high levels of IL-18 mRNA seen in both C57BL/6 and IL-12 p40−/− mice throughout infection (19, 20) (Fig. 2 b).
To determine whether the absence of IFN-γ might influence the induction and control of other cytokines, the levels of several cytokines in the splenocyte cultures was determined. Table 1 shows that antigen-specific release of TNF-α, IL-10, and IL-6 was similar for both types of animal and that neither produced detectable IL-4 on day 30.
Table 1.
Antigen-specific Cytokine Release by Splenocytes from Infected Mice*
| IFN-γ | TNF-α | IL-10 | IL-4 | IL-6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 10186 ± 10 | 59 ± 0 | 207 ± 8 | 0 | 1455 ± 195 | |||||
| IL-12 p40−/− | 129 ± 11 | 35 ± 0 | 272 ± 1 | 0 | 796 ± 470 |
Cytokine release was measured by sandwich ELISA of culture supernantant from cells incubated in the presence of antigens derived from M. tuberculosis.
Values are the mean and standard error (in pg/ml) of triplicate tests on pooled cells from four animals.
The Absence of IL-12 Affects the Development of Antigen-specific Cellular Recruitment.
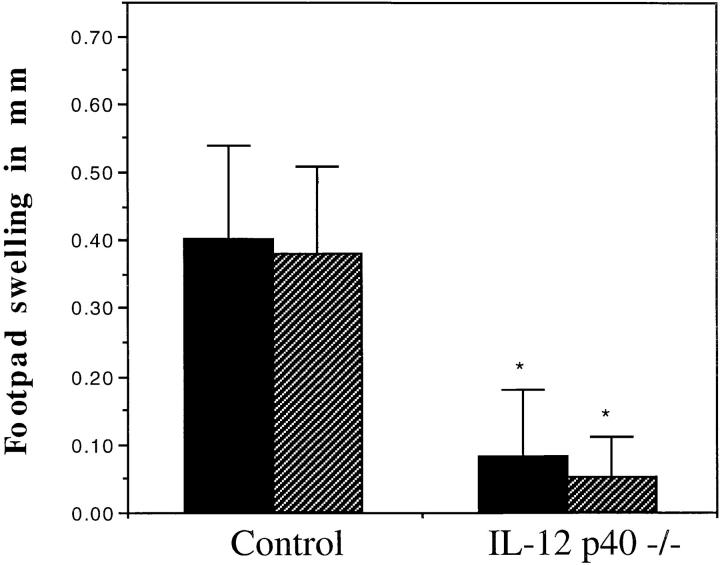
Using the footpad rechallenge model the role of IL-12 in generating a recall response to specific antigen was examined. Mice lacking IL-12 were unable to mount a DTH in response to mycobacterial antigen even after 30 d of infection (Fig. 4).
Figure 4.
IL-12 p40−/− mice fail to generate an antigen-specific recall response. Control and IL-12 p40−/− mice that were infected as in Fig. 1 were challenged in the left hind footpad with saline and the right hind footpad with 10 μg of purified protein derivative of M. tuberculosis on day 15 (solid bars) or day 30 (striped bars) of infection. The data represents the mean and standard error of the difference in footpad swelling between the saline and PPD challenged footpad for each of four mice. * P <0.05 by the Student's t test.
The ability of T cells to differentiate into IFN-γ–producing cells depends upon their ability to respond to IL-12 and this in turn depends upon the expression of a receptor for IL-12. To determine whether the absence of IL-12 could affect the expression of the IL-12 receptor (IL-12R) we measured the amount of mRNA specific for the IL-12R β2 chain in the infected liver (it was not possible to measure this molecule on the surface of the CD4 cells as there is not yet an antibody to it). At the peak of immunity on day 15 the mean ratios of IL-12R β2 mRNA compared with HPRT were 0.23 ± 0.02 for the C57BL6 mice and 0.07 ± 0.02 for the IL-12 p40−/− mice (P <0.001).
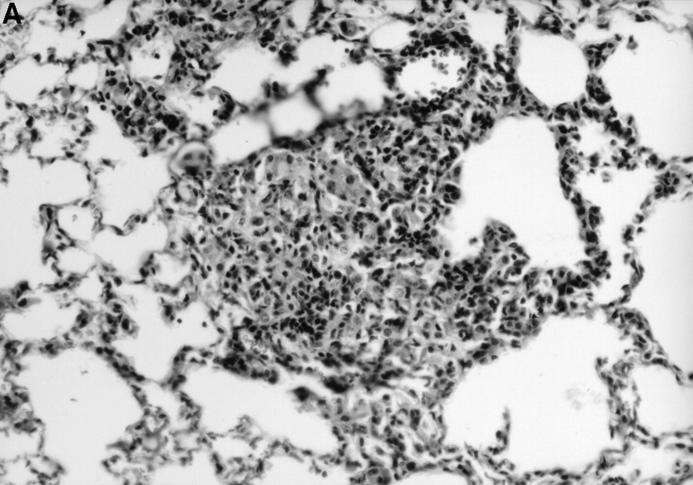
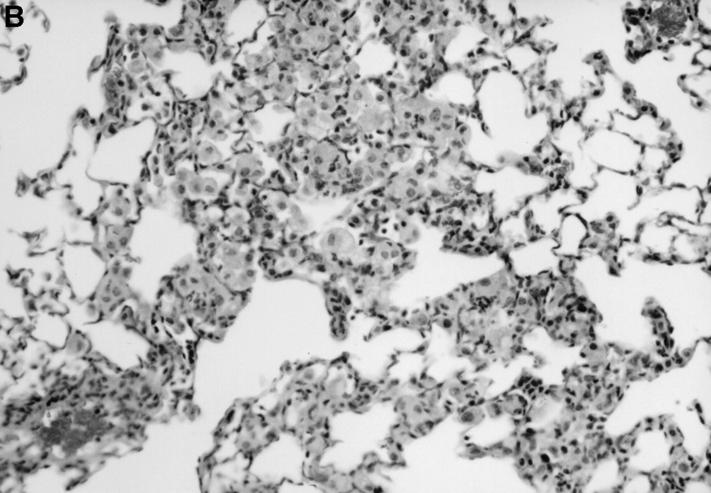
The absence of a strong DTH in the IL-12 p40−/− mice was mirrored in the lower number of lymphocytes in the lung granulomas of IL-12–deficient animals (Fig. 5). Many macrophages in the IL-12 p40−/− tissues appeared vacuolated due to the presence of large numbers of bacteria, whereas bacteria are very sparse in the tissues of C57BL/6 control mice.
Figure 5.
IL-12 p40−/− mice have defective granuloma formation. Tissue from infected control (A) and IL-12 p40−/− (B) mice was fixed in formal saline, sectioned, and stained with hematoxylin and eosin. At sites of mononuclear accumulation in the lung tissue there are more lymphocytes in the control (A) tissue compared with IL-12 p40−/− (B) tissue. Original magnification ×200.
Discussion
We demonstrate here that the absence of the bioactive IL-12 molecule results in unrestrained growth of M. tuberculosis bacteria in all target organs after a systemic infection. This growth correlates with a much reduced expression of mRNA for both IFN-γ and TNF-α and the subsequent absence of macrophage activation as evidenced by reduced mRNA for inducible nitric oxide synthase. The reduction in mRNA for IFN-γ was mirrored by a dramatic reduction of both nonspecific and antigen-specific IFN-γ protein production. Attendant to the absence of IFN-γ there was a marked delay in the expression of antigen-specific cellular accumulation both at sites of infection and at sites of antigen rechallenge. This was linked to a delayed T cell activation as manifested by the reduced induction in expression of the IL-12R β2 chain. Taken together, the lack of immunity seen in the IL-12 p40−/− mice is crucially linked to the absence of IFN-γ and to the inability of the mice to generate antigen-specific activated T cells.
This report confirms our previous conclusion, based on antibody depletion studies, that IL-12 is important in the protective IFN-γ response in tuberculosis (11, 14, 15). Previously, however, we had noted that expression of mRNA for IFN-γ preceded that for IL-12 (15), thus making it unclear which of these two molecules is the initiator of the response. The ability of IL-12–deficient mice to produce a small amount of IFN-γ has been reported both here, in response to M. tuberculosis, and previously in response to LPS (13). However, this innate reponse to both stimuli was much reduced in comparison to control mice and apparently is unable to activate macrophages to an anti-microbial state.
It was also possible that this limited IFN-γ production may have been due to the activity of the IFN-γ–inducing factor IL-18 (19, 20). This molecule was first described as a product from the livers of Proprionobacteria acnes–treated mice and it was thus plausible that it would be induced in M. tuberculosis infections (19). We show here that mRNA for IL-18 was present in the livers of infected animals but that it was not strongly modulated during infection. The recently reported observation that this molecule needs to be activated by the IL-1β converting enzyme (21) may explain the poor expression of IFN-γ in the IL-12 p40−/− mice even though IL-18 mRNA was detected. Therefore, this clearly indicates that IL-18 is not able to compensate for the lack of IL-12 production in mice infected with M. tuberculosis.
Recent developments suggest that IL-12 receptor expression is crucial to the maturation of the Th1 IFN-γ–producing phenotype among T cells. The ability to respond to this recently described dimeric molecule (22, 23) is as important in the development of Th1 cells (24) as the presence of IFN-γ (25). Conversely, an inability to respond to IL-12 is essential to the development of a fixed Th2 phenotype (26). This inability of cells to respond to IL-12 is due to the absence of IL-12R β2 mRNA expression and the expression of this molecule is dependent upon IFN-γ–mediated signaling (27). To address the role of this molecule in the response to M. tuberculosis, we quantitated the amount of IL-12R β2 chain induced in the liver as a result of M. tuberculosis infection. Here, we report for the first time that IL-12R β2 receptor is induced in response to infection and that this induction does not occur in the absence of IL-12 p40. That this increase occurs before the maximal antigen-specific IFN-γ production and killing of bacteria (1) suggests that it is an important correlate of protection.
We have previously suggested that IL-12 plays an important role in the generation of a successful granuloma. This was based on the observations that exogenous IL-12 improves granuloma formation in old mice (14) and that anti-IL-12 disrupts granuloma formation in young mice (12). That there is very little detectable antigen-specific T cell response in terms of IFN-γ production, DTH, or lymphocyte accumulation at the site of infection suggests that the defective granuloma formation in IL-12 p40−/− mice could be linked to the importance of IFN-γ–producing Th1 cells in this mechanism. The importance of Th1 cells in the generation of DTH has been suggested previously and the absence of DTH in both this model and in the previous description of the IL-12 p40−/− mouse (13) fully supports this contention. The reason for this importance may reside in the recently described expression by Th1 cells of the receptors for both E- and P-selectin (28); molecules that have also recently been shown to be crucial to recruitment of these Th1 cells into inflamed tissue (29).
However, IL-12 may affect cellular recruitment by reducing the levels of IFN-γ–dependent chemokines such as RANTES, which is specific for activated or memory T cells and which plays a role in DTH (30). There is also evidence for IFN-γ–independent mechanisms as in the absence of IFN-γ, IL-12 can cause profound inflammation in the lungs (31) and directly induce the expression of cutaneous lymphocyte-associated antigen on activated CD4 cells (32). In addition, the lack of IL-12 in old mice may explain the high incidence of recrudescent tuberculosis in this animal model (16), and is in keeping with the clinical observation that tuberculosis in elderly humans often presents in a miliary form (33). Whatever the mechanism, it is clear the data reported here supports the contention that a function of IL-12 in the protective immune response to M. tuberculosis may be the control of dissemination by maintaining the integrity of the granulomatous response.
It is clear that IL-12 is required both for the initiation, maturation, and maintenance of protective immunity to tuberculosis. Moreover, in contrast with the original description (13) of the immune capacity of these knockout mice, we did not in the current study observe induction of Th2 type cytokines such as IL-4. This cytokine usually occurs later in the immune response to M. tuberculosis and is not considered protective but instead probably contributes to the clearance of mycobacterial antigens released after bacteria are killed by activated macrophages (2). The absence of any IL-4 in these experiments might simply reflect the time points examined, but it is nevertheless interesting to observe even in the complete absence of a strong Th1 response there is no Th2 type response.
In conclusion, it appears that IL-12 is central to the generation of antigen-specific lymphocytes that are able to produce IFN-γ. The data reported above suggest a straightforward explanation for this dependence, i.e, in the initial interaction with the host the bacteria induces IFN-γ, the maximal expression of which is dependent upon IL-12 and TNF-α; this large induction of IFN-γ is required to induce the expression of the IL-12R β2 chain on naive T cells; the expression of this receptor then allows the T cells to respond to IL-12 and, therefore, to become antigen-specific IFN-γ–producing cells.
Acknowledgments
This work was supported by National Institutes of Health grant AI-40488.
Footnotes
1 Abbreviations used in this paper: DTH, delayed type hypersensitivity; ES, embryonic stem; HPRT, hypoxanthine phosphoribosyl transferase; PPD, purified protein derivative.
References
- 1.Orme IM. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138:293–298. [PubMed] [Google Scholar]
- 2.Orme IM, Roberts AD, Griffin JP, Abrams JS. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–525. [PubMed] [Google Scholar]
- 3.Orme IM, Miller ES, Roberts AD, Furney SK, Griffin JP, Dobos KM, Chi D, Rivoire B, Brennan PJ. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992;148:189–196. [PubMed] [Google Scholar]
- 4.Orme IM. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol. 1988;140:3589–3593. [PubMed] [Google Scholar]
- 5.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, Chan SH, Kobayashi M, Young D, Nickbarg E, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin-12 [IL-12]) induces T helper type 1 (Th1)–specific immune responses and inhibits the development of IL-4–producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science (Wash DC) 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 10.Murphy EE, Terres G, Macatonia SE, Hsieh CS, Mattson J, Lanier L, Wysocka M, Trinchieri G, Murphy K, O'Garra A. B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med. 1994;180:223–231. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn JL, Goldstein MM, Triebold KJ, Sypek J, Wolf S, Bloom BR. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995;155:2515–2524. [PubMed] [Google Scholar]
- 12.Cooper AM, Roberts AD, Rhoades ER, Callahan JE, Getzy DM, Orme IM. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 13.Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu C-Y, Ferrante J, Stewart C, Sarmiento U, Faherty DA, Gately MK. IL-12–deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 14.Cooper AM, Callahan JE, Griffin JP, Roberts AD, Orme IM. Old mice are able to control low-dose aerogenic infections with Mycobacterium tuberculosis. Infect Immun. 1995;63:3259–3265. doi: 10.1128/iai.63.9.3259-3265.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper AM, Flynn JL. The protective immune response to Mycobacterium tuberculosis. Curr Opin Immunol. 1995;7:512–516. doi: 10.1016/0952-7915(95)80096-4. [DOI] [PubMed] [Google Scholar]
- 16.Orme IM. A mouse model of the recrudescence of latent tuberculosis in the elderly. Am Rev Respir Dis. 1988;137:716–718. doi: 10.1164/ajrccm/137.3.716. [DOI] [PubMed] [Google Scholar]
- 17.Roach TIA, Kiderlen AF, Blackwell JM. Role of inorganic nitrogen oxides and tumor necrosis factor alpha in killing Leishmania donovaniamastigotes in gamma interferon lipopolysaccharide-activated macrophages from Lshs and Lshr congenic mouse strains. Infect Immun. 1991;59:3935–3944. doi: 10.1128/iai.59.11.3935-3944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zurawski G, de Vries JE. Interleukin 13, an interleukin 4–like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994;15:19–26. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 19.Okamura H, Nagata K, Komatsu T, Tanimoto T, Nukata Y, Tanabe F, Akita K, Torigoe K, Okura T, Fukuda S, Kurimoto M. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect Immun. 1995;63:3966–3972. doi: 10.1128/iai.63.10.3966-3972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamura H, Tsutsui H, Komatsu T, Yatsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature (Lond) 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 21.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, et al. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science (Wash DC) 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 22.Wu CY, Warrier RR, Carvajal DM, Chua AO, Minetti LJ, Chizzonite R, Mongini PK, Stern AS, Gubler U, Presky DH, Gately MK. Biological function and distribution of human interleukin-12 receptor beta chain. Eur J Immunol. 1996;26:345–350. doi: 10.1002/eji.1830260212. [DOI] [PubMed] [Google Scholar]
- 23.Presky DH, Yang H, Minetti LJ, Chua AO, Nabavi N, Wu CY, Gately MK, Gubler U. A functional interleukin 12 receptor complex is composed of two b-type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guler ML, Gorham JD, Hsieh CS, Mackay AJ, Steen RG, Dietrich WF, Murphy KM. Genetic susceptibility to Leishmania: IL-12 responsiveness in Th1 cell development. Science (Wash DC) 1996;271:984–987. doi: 10.1126/science.271.5251.984. [DOI] [PubMed] [Google Scholar]
- 25.Wenner CA, Guler ML, Macatonia SE, O'Garra A, Murphy KM. Roles of IFN-gamma and IFN-alpha in IL-12–induced T helper cell-1 development. J Immunol. 1996;156:1442–1447. [PubMed] [Google Scholar]
- 26.Szabo SJ, Jacobson NG, Dighe AS, Gubler U, Murphy KM. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity. 1995;2:665–675. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 27.Szabo SJ, Gubler U, Murphy KM. Selective expression of IL-12 receptor beta 2 subunit mRNA in Th1 cells: regulation by IFN-gamma. J Exp Med. 1997;185:817–825. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallman R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissue. Nature (Lond) 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 29.Borges E, Tietz W, Steegmaier M, Moll T, Hallmann R, Hamann A, Vestweber D. P-selectin glycoprotein ligand-1 (PSGL-1) on T helper 1 but not on T helper 2 cells binds to P-selectin and supports migration into inflamed skin. J Exp Med. 1997;185:573–578. doi: 10.1084/jem.185.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature (Lond) 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 31.Car BD, Eng VM, Schnyder B, LeHir M, Shakhov AN, Woerly G, Huang S, Aguet M, Anderson TD, Ryffel B. Role of interferon-gamma in interleukin 12–induced pathology in mice. Am J Pathol. 1995;147:1693–1707. [PMC free article] [PubMed] [Google Scholar]
- 32.Leung DY, Gately M, Trumble A, Ferguson-Darnell B, Schlievert PM, Picker LJ. Bacterial superantigens induce T cell expression of the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen, via stimulation of interleukin 12 production. J Exp Med. 1995;181:747–753. doi: 10.1084/jem.181.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagami PH, Yoshikawa TT. Tuberculosis in the geriatric patient. J Amer Ger Soc. 1983;31:356–363. doi: 10.1111/j.1532-5415.1983.tb05747.x. [DOI] [PubMed] [Google Scholar]