Abstract
Transgenic mice carrying the env-pX region of human T lymphocyte virus type I (HTLV-I) develop autoimmune arthropathy in high incidence. Adopting the approach that Fas-mediated apoptosis has a critical function in the elimination of self-reactive T cells, we examined the involvement of this apoptosis in the induction of autoimmunity in HTLV-I transgenic mice. Splenic T cells derived from the transgenic mice were more resistant to apoptosis induced by anti-Fas mAb than those of the nontransgenic mice, whereas no appreciable difference in apoptosis was detected for thymocytes from either mouse's type. The resistance of transgenic T cells may be due to Tax coded in the pX region, since Tax mediates the inhibition of anti-Fas– induced apoptosis in mature T cell line, Jurkat. Among the transgenic mice, the extent of the resistance to Fas-mediated apoptosis was further enhanced in transgenic T cells with disease. These results suggest that the escape of self-reactive T cells from Fas-mediated apoptosis in the periphery, is critical for the development of autoimmune arthropathy in HTLV-I transgenic mice.
Autoimmune diseases are characterized by tissue destruction and functional impairments caused by self-reactive cells that escape self-tolerance (1–3). The diseases may be initiated by viral infections, and the analyses of these viruses have advanced the understanding of the molecular mechanisms involved in autoimmune diseases (1). There are several proposed mechanisms for the role of the viruses in the induction of autoimmunity (1). The molecular mimicry between self-antigens and the viral peptides may induce the survival of self-reactive cells. In addition, the production of inflammatory cytokines after viral infection may stimulate the expansion of self-reactive cells. But, what determines the initial loss of tolerance to a self-antigen still remains to be elucidated.
Human T lymphocyte virus type I (HTLV-I)1 is an etiologic agent of adult T cell leukemia (4–6). HTLV-I may also be involved in several chronic inflammatory diseases of presumed autoimmune etiology, such as HTLV-I–associated myelopathy/tropical spastic paraparesis (HAM/TSP), HTLV-I–associated uveitis, and HTLV-I–associated arthropathy (HAAP) (7–9). Tax is a 40-kD nuclear protein encoded by the pX region of HTLV-I, which acts as a transcriptional activator of the viral gene as well as a number of cellular genes (5, 10–19). The aberrant expression of cellular genes by Tax is proposed to be essential for the transformation of T cells (4–6). Tax may also play a role in the pathogenesis of HTLV-I–associated diseases, presumed as autoimmune conditions, because it induces such diseases (Sjögren's syndrome-like exocrinopathy, inflammatory arthropathy resembling rheumatoid arthritis) in the transgenic mice (20, 21).
Self-reactive T cells in peripheral lymphoid tissues are eliminated by apoptosis mediated by the cell surface receptor Fas/Apo-1/CD95 (22). Accumulating evidence shows that Fas-mediated apoptosis is a crucial guardian in the maintenance of self-tolerance in vivo. For instance, the lack of Fas-mediated apoptosis in lpr mice (a mutant strain for fas gene) leads to the development of various autoimmune diseases including arthropathy (23). In this study, we show that peripheral T cells of HTLV-I transgenic mice are resistant to Fas-mediated apoptosis, and that the extent of the resistance correlates with the development of arthropathy in these mice. This resistance may be mediated by the viral protein Tax because the overexpression of Tax in Jurkat T cells resulted in anti-Fas–resistant characteristics. Thus, Fas-mediated apoptosis impaired by Tax may be a determinant in the initial loss of self-tolerance in the transgenic mice, resulting in the development of autoimmune arthropathy. These findings will be discussed in the context of the virus-induced autoimmune diseases including HTLV-I.
Materials and Methods
Animals.
Transgenic mice with HTLV-I env-pX region of the viral genome with its own long terminal repeat promoter were employed (21). Original transgenic mice with C3H/HeN background were back-crossed with BALB/c mice. Female mice of 8–11 generations after back-crossing, were used for the experiments. These mice were kept under the SPF conditions in a clean room of Animal Research Center (Institute of Medical Science, University of Tokyo).
Clinical Evaluation.
Joints of the mice paws were macroscopically examined for inflammation (swelling and redness) once a week. The mice with arthritis (RA+) show obvious swelling of their joints, and those without the disease (RA−) do not have these manifestations, respectively.
Cells and Culture Condition.
Primary splenocytes and thymocytes prepared from the mice were cultured in DMEM (NIKKEN BIO MED. LAB., Kyoto, Japan) supplemented with 10% FCS and 2-mercaptoethanol (5.5 × 10−5 M). JPX-9 and JPX/M cells are derivatives of a human T cell line Jurkat, and have a stably integrated tax and tax mutant gene under the control of a methallothionein promoter, respectively (15). JPX-9 and JPX/M were cultured in RPMI 1640 (NIKKEN BIO MED. LAB.) supplemented with 10% FCS. For the expression of Tax protein, the cells were cultured in the presence of CdCl2 (10 μM) at 37°C for 24–48 h.
Flow Cytometric Analysis and Cell Survival Assay.
For in vivo activation of T cells, staphylococcal enterotoxin B (SEB, 50 μg/ mouse) was intravenously injected into the mice. 3 d after the injection, spleen cells were isolated from the primed mice. The cells were then treated with agonistic anti-Fas mAb (RK-8; 1 μg/ml) at 37°C for 12 h, and viable cells were analyzed by the three-color flow cytometric analysis (EPICS Elite™). The Abs used were anti-CD3 (2C11) labeled with FITC (PharMingen, San Diego, CA) and anti-Vβ6 or anti-Vβ8 labeled with PE (PharMingen). Biotinylated anti-Fas mAb (RK-8) was prepared and used with streptavidin labeled with APC (Becton Dickinson, San Jose, CA). For in vitro activation of peripheral T cells, splenocytes were incubated in the presence of plate-coated anti-CD3 mAb (2C11; 5 μg/ml) at 37°C for 4 d. Activated peripheral T cells or thymocytes (4 × 104 cells/well) in 96-well flat-bottom microplate were treated with anti-Fas mAb (RK-8) at 37°C for 20 h. Then, the cells were cultured in the presence of 3-[4,5-dimethylethiazol-2-yl]-2,5-diphenyl tetrazorium bromide for 4 h (MTT assay), and the absorbance at 595 nm of each culture were measured. Cell viability was calculated as the ratio of the MTT activity of the cells treated with anti-Fas mAb relative to those without the treatment. For the flow cytometric analysis of in vitro activated splenocytes, FITC-labeled anti-CD4, PE-labeled anti-CD8, and APC-labeled anti–Thy-1 mAbs (PharMingen) were used.
Induction of Activation-induced Cell Death.
Splenocytes (4 × 104 cells/ml) were treated with Con A (5 μg/ml) for 3 d, and they were then cultured in the presence of recombinant human IL-2 (100 IU/ml) for 7 d. These preactivated splenocytes were washed with culture medium and cultured in 96-well flat-bottom microplate coated with anti-CD3 mAb at 5 μg/ml for 48 h. Cell viability was calculated as the ratio of the MTT activity of the cells cultured with anti-CD3 mAb relative to those without the treatment.
Northern Blotting.
Cytoplasmic RNA was extracted from JPX-9 or JPX/M cells by ISOGEN™ according to the instruction of a supplier (NipponGene, Tokyo, Japan). Cytoplasmic RNA (20 μg) was then applied to 1% agarose gel and size-fractionated by the electrophoresis in the presence of 2.2 M formaldehyde, transferred to a nylon membrane and hybridized with radiolabeled tax cDNA. After stringent washing, the membrane was exposed to x-ray film.
Gel-Mobility Shift Assay.
For the preparation of nuclear extract, cells (1 × 107) were washed with phosphate-buffered saline containing 1 mM Na3VO4, 5 mM NaF. The cells were then treated with 0.2% NP-40 in lysis buffer (20 mM Hepes [pH 7.9], 20 mM NaF, 1 mM Na3VO4, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin). After centrifugation, the pellets were further treated with lysis buffer supplemented with 420 mM NaCl, 20% glycerol for 4°C for 30 min and centrifugation was repeated. The resulting supernatant was used as nuclear extract in a gel-mobility shift assay. 10 μg of nuclear extract was preincubated with 1 μg of poly(dI:dC) in 20 μl of binding buffer consisting of 13 mM Hepes (pH 7.9), 65 mM NaCl, 0.15 mM EDTA, 8% glycerol, and 1 mM DTT for 15 min on ice. Approximately 1 ng of labeled oligonucleotide was added to the reaction mixture, and further incubated for 15 min at 25°C. Complexes formed were separated from the unbound probe by the electrophoresis in 5% polyacrylamide gel containing 0.5× TBE, and 2.5% glycerol, dried and then the gel was exposed to x-ray film. A double-stranded synthetic oligonucleotide corresponding to the NF-κB binding site (top strand: AGCTTTGGGAAATTCCTCGGGTGGTAC) from the interferon gene, was labeled with γ-[32P]ATP by polynucleotide kinase, and employed as the κB-site probe.
Results
Peripheral T Cells in HTLV-I env-pX Transgenic Mice Are Resistant to Agonistic Anti-Fas mAb.
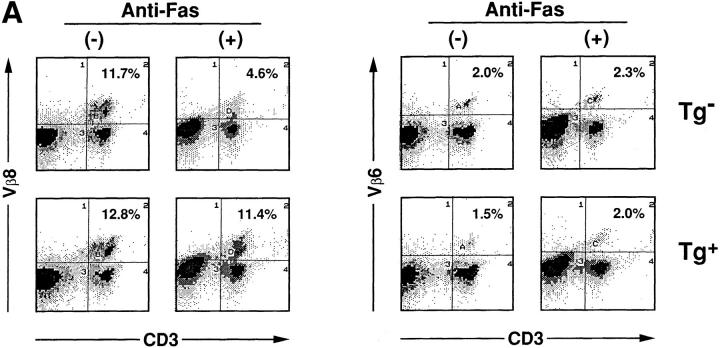
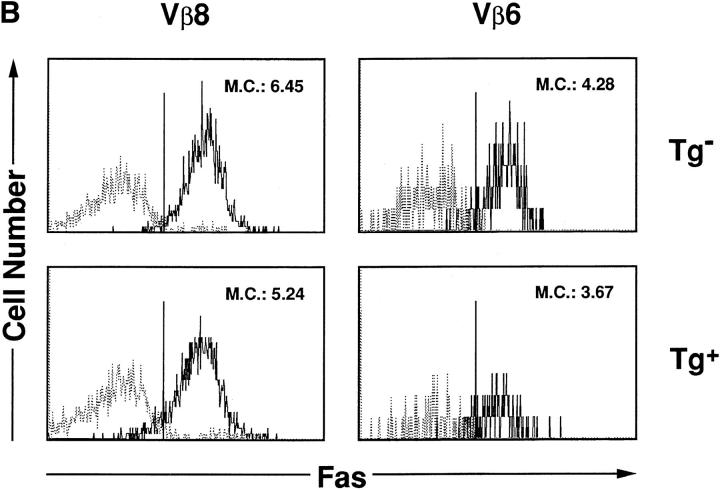
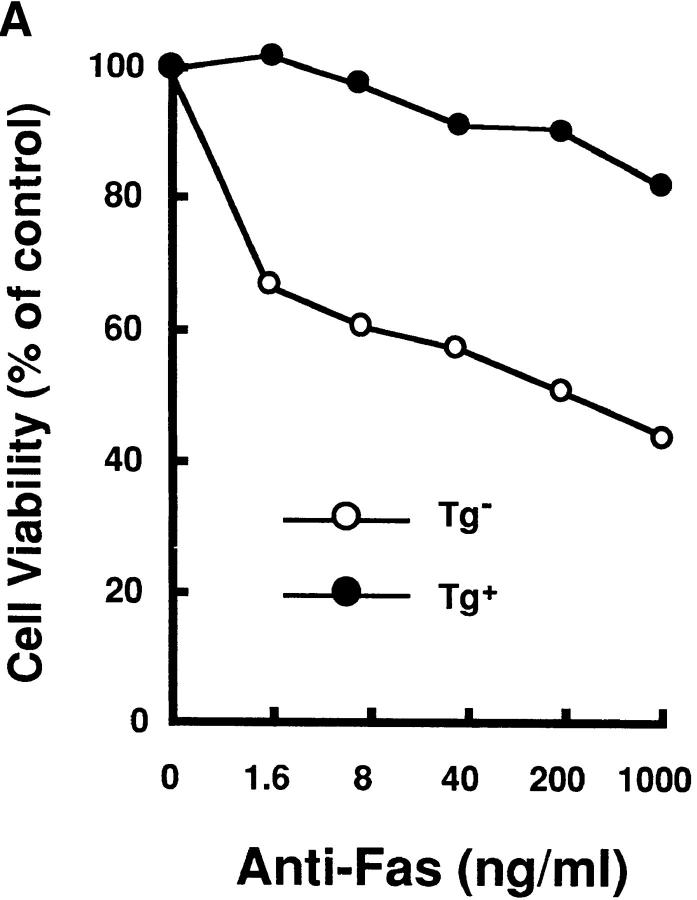
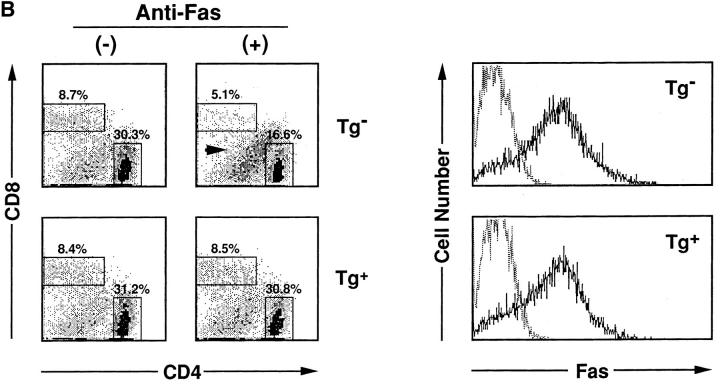
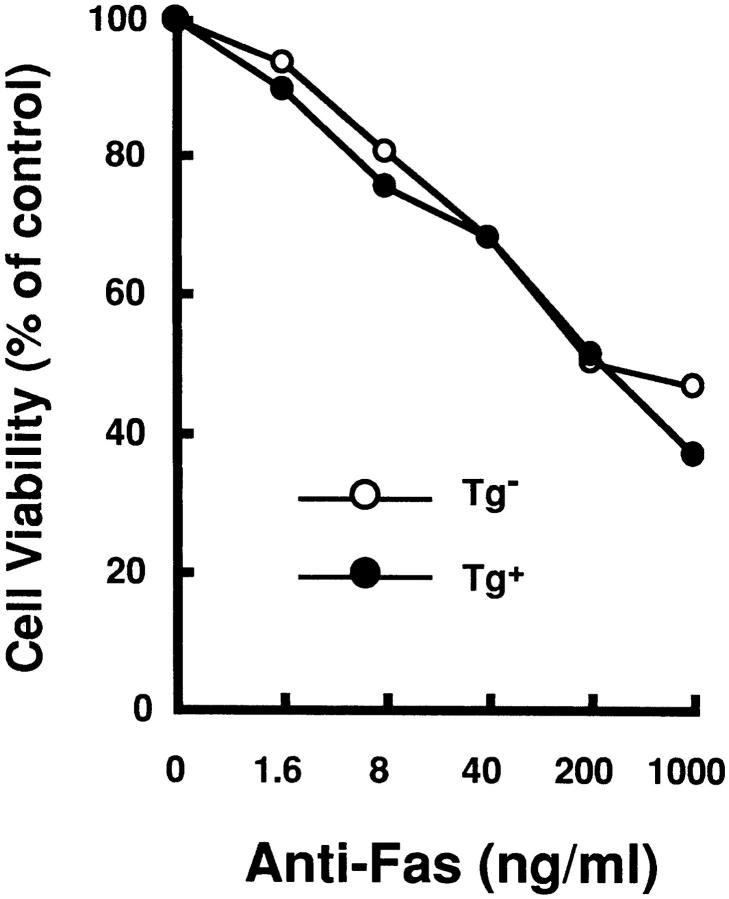
HTLV-I transgenic mice that carry the env-pX gene develop inflammatory arthropathy in high incidence (21). We first examined the sensitivity of peripheral T cells prepared from these transgenic mice to Fas-mediated apoptosis. Since fresh primary T cells from periphery are refractory to anti-Fas treatment, SEB which activates T cells carrying the TCR Vβ8 by the superantigen mechanism, was injected into the transgenic mice with arthritis. SEB-activated Vβ8+ T cells in normal mice are reportedly sensitive to Fas-mediated apoptosis (24). Splenic T cells isolated 3 d after the SEB injection were treated with anti-Fas mAb in vitro. The expression of Vβ8 and CD3 in these cells was analyzed by the flow cytometry. The injection of SEB increased the population of Vβ8+ cells among CD3+ cells in both the control and transgenic mice (data not shown), consistent with the previous observation (25). The treatment with anti-Fas mAb reduced the population of Vβ8+ T cells in the control mice from 11.7 to 4.6% probably due to apoptosis, whereas there was little effect on cells from the transgenic mice (Fig. 1 A). Apoptosis was specific to the activated T cells, since Vβ6+ cells, another T cell subpopulation which was not activated by SEB, were unaffected by anti-Fas treatment. The resistance of Vβ8+ T cells prepared from the transgenic mice was not due to the inefficient expression of Fas on these cells, since equivalent amounts of Fas were detected on Vβ8+ T cells from both the nontransgenic and the transgenic mice (Fig. 1 B). To examine the sensitivity of T cell subsets other than Vβ8+, splenocytes from the transgenic mice were preactivated with immobilized anti-CD3 mAb which can activate T cells in the spleen, followed by the treatment with anti-Fas mAb. Subsequently, ∼90% of the cells expressed Thy-1 4 d after anti-CD3 treatment (data not shown), indicating that the majority of cells were the T cell population. More than 80% of splenic cells derived from the transgenic mice survived after the treatment with anti-Fas mAb at the concentration of 1 μg/ml in comparison with less than 50% from nontransgenic mice (Fig. 2 A). Low doses of the anti-Fas mAb (1.6 ng/ml, 8 ng/ml) was still effective on T cells from the nontransgenic mice, but had little effect on the viability of T cells from the transgenic mice. Like SEB-responsive Vβ8+ T cells, anti-CD3– activated T cells derived from these two type of mice expressed similar amounts of Fas, indicating that the variation in the sensitivity to anti-Fas mAb is regulated by the factor(s) downstream from Fas in HTLV-I transgenic mice. This is completely different from lpr mice that have little Fas expression level because of fas gene mutation. There are mainly two subsets of T cells (CD4+CD8−, CD4−CD8+ T cells) in the spleen. The population of anti-CD3 activated CD4+CD8− and CD4−CD8+ cells (for 4 d) from the control mice were reduced by the treatment with anti-Fas mAb from 30.3 to 16.6% and 8.7 to 5.1 %, respectively, while those from the transgenic mice were unaffected (Fig. 2 B). Thus, both CD4+CD8− and CD4−CD8+ T cell subsets classified as mature T cells in the transgenic mice, are less sensitive to Fas-mediated apoptosis than those from the nontransgenic mice. We always observed that the number of CD4lowCD8− (with a lower CD4 intensity than CD4+ CD8− cells) increase 12 h after anti-Fas treatment. Since this population increases proportionally with the extent of apoptosis of CD4+CD8− cells, it probably represents apoptotic CD4+CD8− cells.
Figure 1.
SEB-responsive splenic T cells of HTLV-I transgenic mice are resistant to Fas-mediated apoptosis. (A) SEB (50 μg/mouse) was injected intravenously into the transgenic (Tg+) and the nontransgenic mice (Tg−). Spleen cells prepared from these mice were treated with anti-Fas mAb (RK-8; 1 μg/ml) in vitro for 12 h, and then analyzed for the expression of CD3, Vβ8, and Vβ6 by the flow cytometry. The proportions of Vβ8+CD3+ or Vβ6+CD3+ T cells population are indicated. (B) SEB (50 μg/mouse) was injected intravenously into Tg+ and the Tg+ mice. The expression of Fas (M.C., mean channel) on T cells prepared from the primed mice were analyzed by the flow cytometry using anti-Fas mAb.
Figure 2.
Anti-CD3–activated mature T cells from the transgenic mice were resistant to Fas-mediated apoptosis. (A) Splenocytes from Tg− mice (open circle) and Tg+ mice with arthritis (closed circle) were activated with immobilized anti-CD3 mAb for 4 d, and then treated with anti-Fas at the indicated concentrations for 20 h. Cell viabilities were measured by the MTT assay, and Cell Viability (% of control) indicates the MTT activity in cells treated with the Ab relative to that without the treatment. data are means of triplicate determinations and are the representative of five independent reproducible studies. (B) Splenocytes from Tg− mice and Tg+ mice were activated with immobilized anti-CD3 mAb for 4 d, and then treated with anti-Fas for 12 h. The activated splenocytes prepared from these mice were treated with anti-Fas mAb (RK-8; 1 μg/ml) in vitro, and then analyzed for the expression of CD4 and CD8 by the flow cytometry. The proportions of CD4+ and CD8+ T cells population are indicated. The population indicated by an arrow was CD4lowCD8+ cells discussed in the text. The expression of Fas on the cells was also analyzed by flow cytometry.
Since Fas-mediated apoptosis is said to occur not only in peripheral T cells but also in thymocytes (22), we next examined the sensitivity of thymocytes to anti-Fas mAb. Unlike splenic T cells, preactivation was not required to sensitize thymocytes to anti-Fas mAb (Fig. 3). Thymocytes from the transgenic mice showed similar sensitivity to anti-Fas mAb as those from the nontransgenic mice (Fig. 3). This was in contrast to the resistance of splenic T cells from the transgenic mice to Fas-dependent apoptosis (Figs. 1 A and 2). Since thymocytes and splenocytes from the transgenic mice expressed equivalent amounts of the tax/env mRNA (26) (data not shown), the difference in the sensitivity to anti-Fas mAb was not due to their altered transgene expression.
Figure 3.
Thymocytes from the transgenic mice are sensitive to Fas-mediated apoptosis. Thymocytes were prepared from Tg− (open circle) and Tg+ mice (closed circle). They were then treated with the indicated concentration of anti-Fas mAb for 20 h. Cell viabilities were measured by the MTT assay. Cell Viability (% of control) indicates the MTT activity in thymocytes treated with the Ab relative to that without the treatment. Data are means of triplicate determinations and are the representative of five independent reproducible studies.
Tax inhibits Fas-mediated Cell Death in Jurkat T Cell Line.
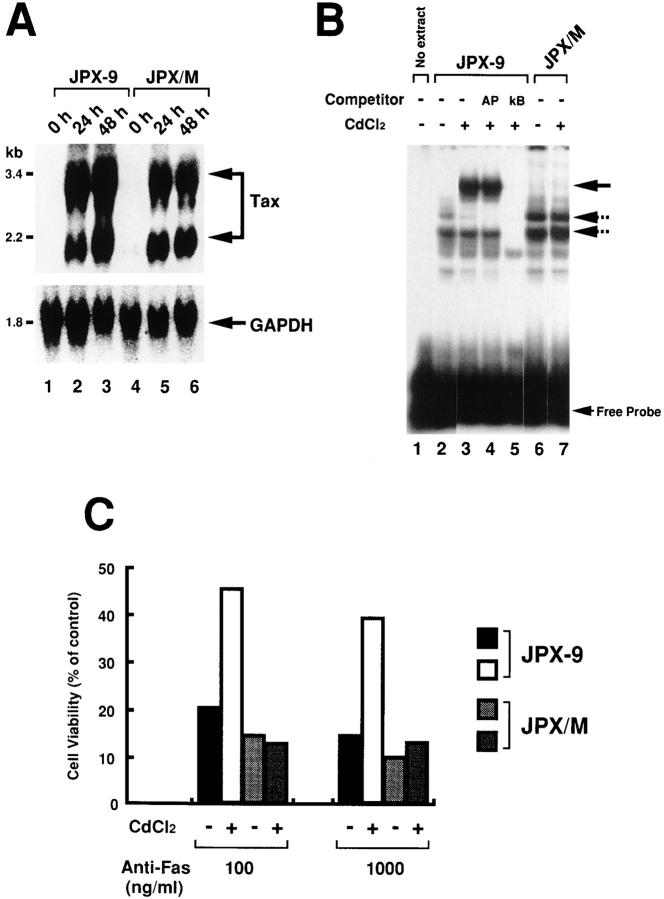
Copeland et al. (27) showed that Tax in the pX region inhibits apoptosis mediated by Fas using immortalized T cell lines. To confirm their results in our own assay system, we used JPX-9, a stable transfectant of Jurkat T cell line that has the inducible tax gene under the control of a methallothionein promoter (15). Addition of CdCl2 to culture medium induced the expression of the tax mRNA in JPX-9 cells as well as in JPX/M cells that carry the mutant tax gene (Fig. 4 A). Tax reportedly induces a NF-κB transcription factor complex in T cells. The gel-mobility shift assay showed that CdCl2 treatment for 24 h induced a complex specific to the κB sequence in JPX-9 cells, but not in JPX/M cells (Fig. 4 B), indicating that functional Tax protein was expressed only in JPX-9 cells by CdCl2 treatment. The binding specificity was confirmed by the selective inhibition of the induced complex by the homologous κB oligonucleotide, but not by unrelated ones (Fig. 4 B). CdCl2 treatment for 24 h reduced cell death mediated by anti-Fas mAb (CH-11) in JPX-9 cells at two different concentrations of the Ab (Fig. 4 C). This was due to the expression of Tax, but not by CdCl2 treatment, since the reduction was not observed in JPX/M with the mutant tax gene even after the treatment with CdCl2. These results indicate that Tax possesses the inhibitory activity to Fas-mediated apoptosis.
Figure 4.
Tax inhibits Fas-mediated apoptosis in Jurkat T cell line. (A) JPX-9 and JPX/M cells were cultured in the absence (lanes 1 and 4) and presence (lanes 2, 3, 5, and 6) of 10 μM CdCl2 at 37°C for 24–48 h. RNA was extracted from these cells, and the expression of the tax gene and the GAPDH gene in the extracted RNA was analyzed by the Northern blotting. (B) Nuclear extract was prepared from JPX-9 (lanes 2–5) and JPX/M cells (lanes 6 and 7) treated with (lanes 3–5) or without CdCl2 (lanes 1, 2, 6). The NF-κB activity in the nuclear extract was analyzed by the gel mobility shift assay. Binding reaction was carried out in the absence (lanes 1–3, 6, and 7) or presence of 100 ng of cold oligonucleotides of AP-1 binding sites (lane 4) or homologous NF-κB binding site (lane 5). The three complexes indicated by arrow were specific to the κB sequence, and the upper one, but not the other two, was induced by the expression of wild-type Tax. (C) JPX-9 and JPX/M treated with or without CdCl2 were incubated with the indicated concentration of anti-Fas mAb (CH-11) for 20 h. Cell viability (% of control) indicates the MTT activity in cells treated with the Ab relative to that without the treatment. Data are means of triplicate determinations and are the representative of three independent reproducible studies.
Enhanced Resistance to Fas-mediated Apoptosis of the T Cells in the Transgenic Mice with Arthritis.
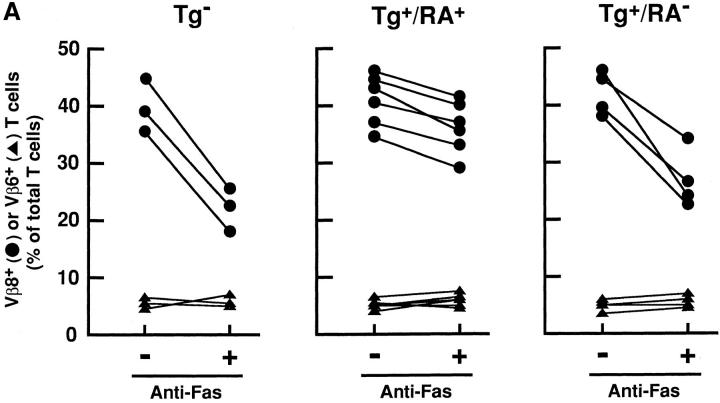
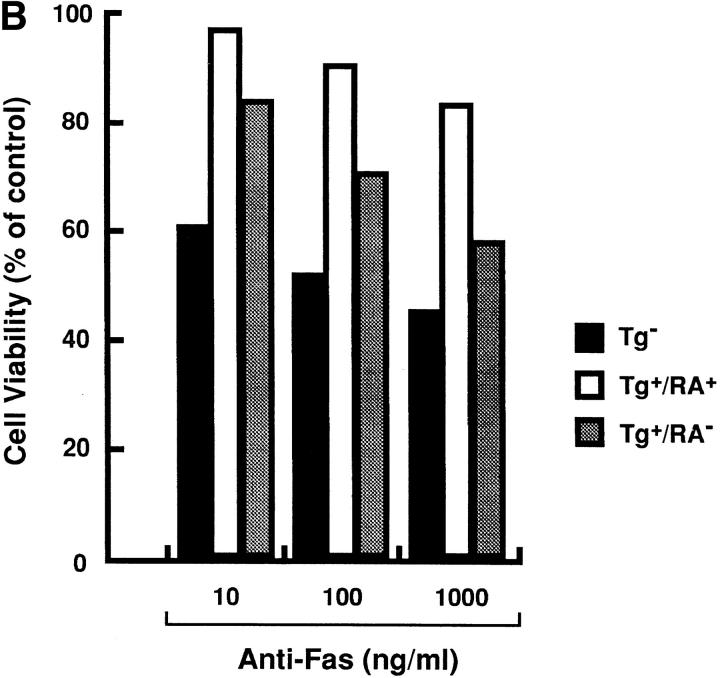
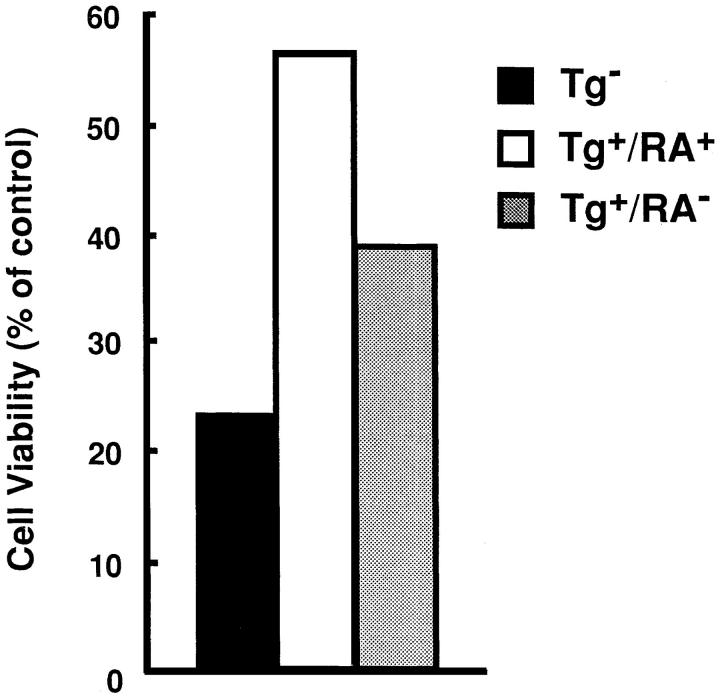
SEB was injected into the nontransgenic mice (Tg−) and the transgenic mice with arthritis (Tg+RA+) or without arthritis (Tg+RA−), and then Vβ8+ T cells prepared from these mice were examined for their sensitivity to Fas-mediated apoptosis. The Vβ8+ T cells from Tg+RA− mice were more resistant to anti-Fas mAb than those from Tg− mice, but their resistance were less than those from Tg+RA+ mice (Fig. 5 A). The difference between Tg+RA− mice and Tg+RA+ mice was not due to variation in the mice, since Vβ8+ T cells prepared from 13 animals showed a positive correlation between the resistance to the induction of apoptosis and disease development (Fig. 5 A). The treatment with anti-Fas mAb did not reduce the population of Vβ6+ T cells that are not activated by SEB, indicating that the apoptosis induction as well as the inhibition is specific to activated T cells. In addition to Vβ8+ T cells, preactivated T cells with anti-CD3 mAb, derived from the Tg+RA+ mice were more resistant to Fas-mediated apoptosis than those from the Tg+RA− mice (Fig. 5 B). These results suggest that the sensitivity of activated peripheral T cells to Fas-mediated apoptosis reduces in accompaniment with the disease development in HTLV-I transgenic mice.
Figure 5.
Enhanced resistance to Fas-mediated apoptosis in T cells derived from the transgenic mice with arthritis. (A) SEB (50 μg/mouse) was intravenously injected into Tg−, Tg+RA+, and Tg+RA− mice. The splenocytes prepared from the SEB-primed mice were treated with anti-Fas for 20 h at 37°C. The expression of CD3 and either Vβ8 or Vβ6 in the cells before and after the treatment with anti-Fas mAb, were determined by the flow cytometric analysis. Vβ8 + or Vβ6 + T cells (% of total T cells) indicates the proportion of these cells among the population of CD3+ T cells. (B) Splenocytes prepared from the mice, were activated with immobilized anti-CD3 mAb for 4 d, and then treated with anti-Fas mAb at the indicated concentrations for 20 h. Cell Viability (% of control) indicates the MTT activity in cells treated with the Ab relative to those without this treatment. Data are means of triplicate determinations and are the representative of five independent reproducible studies.
Impairment of Anti-CD3–induced Apoptosis in Activated T Cells Derived from the Transgenic Mice with Arthritis.
Self-reactive T cells are eliminated by apoptosis induced by the activation through their TCR. This phenomenon was designated as activation-induced cell death (AICD), and Fas-mediated apoptosis plays a critical role in this AICD process (28). Therefore, we next examined the sensitivity of activated T cells to TCR-mediated apoptosis. Splenocytes prepared from the mice were treated with a T cell mitogen, Con A, for 3 d, and then cultured in the presence of IL-2 for 7 d. These preactivated T cells were restimulated with anti-CD3 mAb which induces AICD to the T cells. The restimulation with anti-CD3 mAb reduced the viability of the T cells from Tg+ mice, but the reduction was less than those from Tg− mice. Moreover, the resistance to anti-CD3 mAb was enhanced in the T cells from Tg+RA+ relative to those from Tg+RA− (Fig. 6). The enhancement of the resistance to AICD was consistently observed for five Tg+RA+ mice (data not shown). These results suggested that the transgenic T cells are more resistant to AICD, and the resistance is enhanced with the disease development in HTLV-I transgenic mice.
Figure 6.
Impairment of activation-induced cell death in T cell from the transgenic mice. Splenocytes prepared from the mice, were activated with Con A for 3 d, and then cultured with IL-2 for 7 d. Activated splenocytes were restimulated with immobilized anti-CD3 mAb for 48 h. Cell Viability (% of control) indicates the MTT activity in cells treated with the Ab relative to those without this treatment. Data are means of triplicate determinations and are the representative of three independent reproducible studies.
Discussion
Several lines of evidence indicate that the autoimmune mechanism may be a critical step in the development of arthropathy in the transgenic mice carrying the HTLV-I env-pX gene (26). The transgenic mice produce autoantibodies to type II collagen, IgG, and heat shock proteins. The administration of exogenous type II collagen increases the frequency of arthropathy development in the transgenic mice. The primary determinant for the survival of the self-reactive cells in these mice, however, remains to be elucidated. In this study, we show that peripheral T cells derived from HTLV-I transgenic mice are resistant to Fas-mediated apoptosis as well as AICD induced by anti-CD3 mAb, and their susceptibility to this apoptosis inversely correlated with the development of arthropathy in the mice (Figs. 1, 2, 5 and 6). The lack of Fas-mediated apoptosis observed in lpr (fas mutant) and in gld (fas ligand mutant) mice causes various autoimmune diseases by the inability to eliminate self-reactive T cells (23, 29, 30). Therefore, our present results suggest that impaired Fas-mediated apoptosis is a determinant for the survival of self-reactive T cells and thereby the development of autoimmune arthropathy in HTLV-I transgenic mice.
There are two main systems for the elimination of self-reactive T cells; one for immature T cells in thymus, and the other for mature T cells in peripheral lymphoid tissues such as spleen. Splenic T cells, but not thymocytes, derived from the transgenic mice were resistant to Fas-mediated apoptosis (Fig. 3). Indeed, single positive mature T cells carrying the transgene resisted apoptosis (Fig. 2 B). Thus, the impaired elimination of mature self-reactive T cells in peripheral lymphoid tissues may play a role in the autoimmunity induction in the transgenic mice.
Fas-mediated apoptosis plays a critical role in elimination of self-reactive T cells in peripheral lymphoid tissues, but it may not be the only determinant, because apoptosis induced by TNF may play a role in this process (31). Recent findings showed that the induction of NF-κB activity inhibits apoptosis induced by TNF (32–34), but not by anti-Fas mAb (34). Since Tax induces NF-κB activity (Fig. 4 B), it may also inhibit the TNF-induced apoptosis. However, it is difficult to examine the activity of Tax to TNF-induced apoptosis because TNF by itself activates NF-κB in most of cell lines including JPX-9 and Tax activates the expression of TNF gene. Therefore, further analysis is required to determine the involvement of TNF-induced apoptosis in the elimination of self-reactive T cells in HTLV-I transgenic mice.
Among the proteins coded in the pX region of HTLV-I, Tax displayed the inhibitory activity to Fas-mediated apoptosis in Jurkat T cells (Fig. 4 C) (27). Therefore, the Tax activity may explain the resistant phenotype of T cells carrying this transgene. However, this may not explain the enhanced resistance of T cells from mice with arthritis relative to those without disease (Fig. 5), since both types of mice equivalently expressed the transgene in spleen (26). There are at least two possible mechanisms to explain the enhanced resistance in Tg+RA+ mice: first, other factor(s) apart from Tax may be involved in the resistance to Fas-mediated apoptosis. Therefore, such cellular factors and Tax may collaboratively contribute to the prevention of Fas-mediated apoptosis in activated mature T cells. Second, unlike spleen, the expression of Tax is more enhanced in the mouse joints with arthritis in comparison to those without disease (26). Consequently, Tax-inducible soluble factor(s) in the affected joints may circulate to the spleen, changing the sensitivity of splenic T cells to Fas-mediated apoptosis.
In addition to the inhibition of apoptosis, Tax has various activities on cells. Therefore, we can not exclude the possibility for the contribution of other cofactors or accelerators with Tax to develop autoimmune arthritis. Most notably, Tax can affect the IL-2 autocrine loop and immortalize T cells in the presence of IL-2 in vitro (12, 35). Therefore, once self-reactive T cells escape the Fas-mediated surveillance system, they may be further expanded by the immortalization function of Tax. Furthermore, Tax activates the transcription of a number of cytokine genes such as TNF-α, IL-6, and IL-8 (5, 18, 19). These cytokines may play a role as executioners in the inflammatory response as well as the proliferation of synovial fibroblasts observed in the affected joints of the mice. Among the cytokines, TNF-α is especially noteworthy, since it induces inflammatory arthritis in the transgenic mice (36). Taken together, the cooperation of multiple Tax-inducible functions including the inhibition of peripheral T cell apoptosis via Fas may be necessary for the development of arthritis in HTLV-I transgenic mice.
In some HTLV-I–associated diseases such as HAM/TSP, anti-DNA or anti-immunoglobulin antibodies as well as increased numbers of self-reactive T cells are detected in patient sera. This indicates that the autoimmunity may play a role in the pathogenesis of these HTLV-I–associated diseases in human as well as in the transgenic mice (8, 37, 38). Accordingly, we are currently studying the involvement of Fas-mediated apoptosis in these human diseases.
A number of viruses are suggested to be involved in autoimmune diseases, but the mechanism of the autoimmunity induced by the virus infection remains to be elucidated (1). Various viruses encode anti-apoptotic proteins such as an E1B for an adenovirus (39) and BHRF1 for an Epstein-Barr virus (40). Perhaps, the viral protein-mediated inhibition of apoptosis could also be involved in autoimmune diseases induced by viruses other than HTLV-I.
Acknowledgments
We thank Y. Nishimura, Y. Yamasaki, and T. Tsukahara for technical assistance, M. Nakamura for helpful discussion and advice, and T. Imaoka for the manuscript preparation.
This work was supported in part by a grant-in aid for cancer research from the ministry of Education, Science and Culture of Japan.
Footnotes
1 Abbreviations used in this paper: AICD, activation-induced cell death; HAM/TSP, HTLV-I–associated myelopathy/tropical spastic paraparesis; HAAP, HTLV-I–associated arthropathy; SEB, staphylococcal enterotoxin.
References
- 1.Gianani R, Sarvetnick N. Viruses, cytokines, antigens, and autoimmunity. Proc Natl Acad Sci USA. 1996;93:2257–2259. doi: 10.1073/pnas.93.6.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridgway WM, Weiner HL, Fathman CG. Regulation of autoimmune response. Curr Opin Immunol. 1994;6:946–955. doi: 10.1016/0952-7915(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 3.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 4.Franchini G, Streicher H. Human T cell leukemia virus. Baillieres Clin Haematol. 1995;8:131–148. doi: 10.1016/s0950-3536(05)80235-5. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida M, Suzuki T, Fujisawa J, Hirai H. HTLV-1 oncoprotein tax and cellular transcription factors. Curr Top Microbiol Immunol. 1995;193:79–89. doi: 10.1007/978-3-642-78929-8_4. [DOI] [PubMed] [Google Scholar]
- 6.Sugamura, K., and Y. Hinuma. 1993. Human Retroviruses: HTLV-I and HTLV-II. The retroviridae. Jay A. Levy, editor. Plenum Press, New York. 2:399–435.
- 7.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I-associated myelopathy, a new clinical entity. Lancet (N Am Ed) 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 8.Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, G. dT. Antibodies to human T-lymphotropic virus type I in patients with tropical spastic paraparesis. Lancet (N Am Ed) 1985;ii:407–409. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 9.Nishioka K, Nakajima T, Hasunuma T, Sato K. Rheumatic manifestation of human leukemia virus infection. Rheum DisClin N Am. 1993;19:489–503. [PubMed] [Google Scholar]
- 10.Sodroski JG, Rosen CA, Haseltine WA. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science (Wash DC) 1984;225:381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- 11.Inoue J, Seiki M, Taniguchi T, Tsuru S, Yoshida M. Induction of interleukin 2 receptor gene expression by p40xencoded by human T-cell leukemia virus type I. EMBO (Eur Mol Biol Organ) J. 1986;5:2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maruyama M, Shibuya H, Harada H, Hatakeyama M, Seiki M, Fujita T, Inoue J, Yoshida M, Taniguchi T. Evidence for aberrant activation of the interleukin-2 autocrine loop by HTLV-1-encoded p40xand T3/Ti complex triggering. Cell. 1987;48:343–350. doi: 10.1016/0092-8674(87)90437-5. [DOI] [PubMed] [Google Scholar]
- 13.Cross SL, Feinberg MB, Wolf JB, Holbrook NJ, Wong-Staal F, Leonard WJ. Regulation of the human interleukin-2 receptor alpha chain promoter: Activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987;49:47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- 14.Fujii M, Sassone-Corsi P, Verma IM. c-fospromoter trans-activation by the tax1 protein of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1988;85:8526–8530. doi: 10.1073/pnas.85.22.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagata K, Ohtani K, Nakamura M, Sugamura K. Activation of endogenous c-fos proto-oncogene expression by human T-cell leukemia virus type I-encoded p40taxprotein in the human T-cell line. J Virol. 1989;68:3220–3226. doi: 10.1128/jvi.63.8.3220-3226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SJ, Kehrl JH, Burton J, Tendler CL, Jeang KT, Danielpour D, Thevenin C, Kim KY, Sporn MB, Roberts AB. Transactivation of the transforming growth factor β1 (TGF-β1) gene by human T lymphotropic virus type 1 tax: a potential mechanism for the increased production of TGF-β1 in adult T cell leukemia. J Exp Med. 1990;172:121–129. doi: 10.1084/jem.172.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujii M, Niki T, Mori T, Matsuda T, Matsui M, Nomura N, Seiki M. HTLV-1 Tax induces expression of various immediate early serum responsive genes. Oncogene. 1991;6:1023–1029. [PubMed] [Google Scholar]
- 18.Miura S, Ohtani K, Numata N, Niki M, Ohbo K, Ina Y, Gojobori T, Tanaka Y, Tozawa H, Nakamura M, Sugamura K. Molecular cloning and characterization of a novel glycoprotein, gp34, that is specifically induced by the human T-cell leukemia virus type I transactivator p40tax . Mol Cell Biol. 1991;11:1313–1325. doi: 10.1128/mcb.11.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamashita I, Katamine S, Moriuchi R, Nakamura Y, Miyamoto T, Eguchi K, Nagataki S. Transactivation of the human interleukin-6 gene by human T-lymphotropic virus type 1 Tax protein. Blood. 1994;84:1573–1578. [PubMed] [Google Scholar]
- 20.Green JE, Hinrichs SH, Vogel J, Jay G. Exocrinopathy resembling Sjögren's syndrome in HTLV-1 tax transgenic mice. Nature (Lond) 1989;341:72–74. doi: 10.1038/341072a0. [DOI] [PubMed] [Google Scholar]
- 21.Iwakura Y, Tosu M, Yoshida E, Takiguchi M, Sato K, Kitajima I, Nishioka K, Yamamoto K, Takeda T, Hatanaka M, et al. Induction of inflammatory arthropathy resembling rheumatoid arthritis in mice transgenic for HTLV-I. Science (Wash DC) 1991;253:1026–1028. doi: 10.1126/science.1887217. [DOI] [PubMed] [Google Scholar]
- 22.Nagata S, Golstein P. The Fas death factor. Science (Wash DC) 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature (Lond) 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura Y, Ishii A, Kobayashi Y, Yamasaki Y, Yonehara S. Expression and function of mouse Fas antigen on immature and mature T cells. J Immunol. 1995;154:4395–4403. [PubMed] [Google Scholar]
- 25.Kawabe Y, Ochi A. Programmed cell death and extrathymic reduction of Vβ8+ CD4+ T cells in mice tolerant to Staphylococcus aureusenterotoxin B. Nature (Lond) 1991;349:245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- 26.Iwakura Y, Saijo S, Kioka Y, Nakayama-Yamada J, Itagaki K, Tosu M, Asano M, Kanai Y, Kakimoto K. Autoimmunity induction by human T cell leukemia virus type 1 in transgenic mice that develop chronic inflammatory arthropathy resembling rheumatoid arthritis in humans. J Immunol. 1995;155:1588–1598. [PubMed] [Google Scholar]
- 27.Copeland KF, Haaksma AG, Goudsmit J, Krammer PH, Heeney JL. Inhibition of apoptosis in T cells expressing human T cell leukemia virus type I Tax. AIDS Res Hum Retroviruses. 1994;10:1259–1268. doi: 10.1089/aid.1994.10.1259. [DOI] [PubMed] [Google Scholar]
- 28.Parijis LV, Ibraghimov A, Abbas AK. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity. 1996;4:321–328. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]
- 29.Nagata S, Suda T. Fas and Fas ligand: lpr and gldmutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 31.Sytwu H-K, Liblau RS, McDevitt HO. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 32.Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science (Wash DC) 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 33.Wang C-Y, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis:potentiation by inhibition of NF-κB. Science (Wash DC) 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 34.Antwerp DJV, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-α-induced apoptosis by NF-κB. Science (Wash DC) 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 35.Akagi T, Shimotohno K. Proliferative response of Tax1-transduced primary human T cells to anti-CD3 antibody stimulation by an interleukin-2-independent pathway. J Virol. 1993;67:1211–1217. doi: 10.1128/jvi.67.3.1211-1217.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias D. Transgenic mice expressing human tumor necrosis factor: a predictive genetic model of arthritis. EMBO (Eur Mol Biol Organ) J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Usuku K, Sonoda S, Osame M, Yashiki S, Takahashi K, Matsumoto M, Sawada T, Tsuji K, Tara M, Igata A. HLA haplotype-linked high immune responsiveness against HTLV-I in HTLV- I-associated myelopathy:comparison with adult T-cell leukemia/lymphoma. Ann Neurol. 1988;23:S143–S150. doi: 10.1002/ana.410230733. [DOI] [PubMed] [Google Scholar]
- 38.Osame M, Matsumoto M, Usuku K, Izumo S, Ijichi N, Amitani H, Tara M, Igata A. Chronic progressive myelopathy associated with elevated antibodies to human T-lymphotropic virus type I and adult T-cells leukemia-like cells. Ann Neurol. 1987;21:117–122. doi: 10.1002/ana.410210203. [DOI] [PubMed] [Google Scholar]
- 39.White E, Sabbatini P, Debbas M, Wold WS, Kusher DI, Gooding LR. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor alpha. Mol Cell Biol. 1992;12:2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]