Abstract
Interleukin 5 (IL-5) is the key cytokine involved in regulating the production and many of the specialized functions of mature eosinophils including priming, adhesion, and survival. We have generated a point mutant of human IL-5, IL-5 (E12K), which is devoid of agonist activity in both a TF-1 cell proliferation assay and a human eosinophil adhesion assay. However, IL-5 (E12K) is a potent and specific antagonist of both these IL-5–dependent functional responses. In both receptor binding and cross-linking studies the wild-type and IL-5 (E12K) mutant exhibit virtually identical properties. This mutant protein was unable to stimulate tyrosine phosphorylation in human eosinophils, and blocked the phosphorylation stimulated by IL-5. In contrast, IL-5 (E12K) is a full agonist in a human eosinophil survival assay, although with reduced potency compared to the wild-type protein. This IL-5 mutant enables us to clearly distinguish between two IL-5–dependent functional responses and reveals distinct mechanisms of receptor/cellular activation.
IL-5 is a cytokine secreted predominantly by T cells, but also by mast cells and eosinophils (1–3), that has multiple effects on cells of the eosinophil and basophil lineage. IL-5 induces the differentiation and expansion of eosinophil precursors in the bone marrow (4) and plays a key role in regulating many of the specialized functions of mature, terminally differentiated eosinophils, including adhesion (5), priming of both degranulation and chemotaxis (6, 7), and the promotion of cell survival (8).
While eosinophils appear to play a protective role during the host defense response to parasitic infection, accumulating clinical and experimental evidence has also implicated the eosinophil in the pathophysiology of a number of allergic diseases such as bronchial asthma, allergic rhinitis, and atopic dermatitis (9–11). Administration of neutralizing anti– IL-5 monoclonal antibodies in mouse, guinea pig, or primate models of allergic asthma inhibits the development of airway eosinophilia and bronchial hyperreactivity (12–16). This highlights the eosinophil, and more specifically IL-5, as an attractive target for therapeutic intervention in allergic diseases such as asthma.
The effects of IL-5 are mediated through a heterodimeric receptor complex composed of a specific ligand binding α subunit and a β subunit that is shared by the specific receptor α chains for IL-3 and GM-CSF (17). Although this common β chain (βc) alone has no inherent ligand binding capacity, it confers high-affinity ligand binding to the receptor α/β chain complex. This phenomenon of increased binding affinity in the presence of the βc chain has been termed affinity conversion, and is most profound for IL-3 (1,000-fold), with smaller effects on GM-CSF binding (20–100-fold) and IL-5 binding (2–4-fold) (18–20). The roles of the respective α and βc chains in signal transduction are unclear. Analysis of chimeric receptors composed of the extracellular domain of the IL-5 receptor α chain and the intracellular domain of the βc chain suggests that ligand-induced dimerization of the cytoplasmic domains of βc may be sufficient for receptor activation and the transduction of a proliferative signal (21). In contrast, deletion and mutational analysis has defined specific regions within the cytoplasmic domains of both the IL-5 receptor α and βc chains that are essential for the coupling of IL-5 receptors to a proliferative signal (21–24). Thus, although βc is indispensable for the signals leading to proliferation, it is possible that the receptor α chain may contribute to cytokine signal transduction either directly, or by promoting receptor oligomerization. Furthermore, the relative contributions of the two receptor chains to signal transduction leading to functional responses other than proliferation are uncharacterized. Although the precise molecular mechanisms by which the active IL-5 receptor complex directs these diverse cellular responses in the eosinophil are also poorly understood, one of the earliest measurable signaling events is the phosphorylation of a variety of cellular proteins on tyrosine residues (25).
IL-5 is a disulphide-linked homodimeric glycoprotein with 115 amino acids in each chain (26). Elucidation of the IL-5 crystal structure revealed a novel two domain structure in which each domain adopts a four α-helix bundle structure similar to the cytokine fold in IL-2, IL-4, growth hormone, and GM-CSF (27). Among these related structures, IL-5 is unique in that each bundle is composed of three helices from one monomer and a fourth helix which is contributed by the second monomer. Recently, the receptor binding sites on IL-5 have been defined by site-directed mutagenesis. Residues E88, R90, and E109, within the carboxy terminus of IL-5, define the IL-5 receptor α chain binding site (28, 29). In addition, the IL-5 mutant E12A, IL-5 (E12A), exhibits partial agonist activity in a TF-1 cell proliferation assay, suggesting that the highly conserved E12 in the amino-terminal helix defines a point of contact with the receptor βc chain which is important for βc chain activation and subsequent signal transduction (28). Furthermore, in a TF-1 proliferation assay the IL-5 mutant E13Q (Due to a single amino acid difference at the amino terminus, IL-5 [E12] as described here is equivalent to IL-5 [E13] as described by Tavernier [29].), IL-5 (E13Q), was completely inactive and exhibited antagonist properties (29).
In this study we have produced an IL-5 mutant containing a charge reversal at position 12, IL-5 (E12K). It has been characterized in a variety of functional and receptor binding assays, and we have used this protein as a tool to reveal distinct mechanisms of receptor/cellular activation in human eosinophils.
Materials and Methods
Reagents.
Recombinant IL-3, TNF-α, and GM-CSF were obtained from R&D systems (Abingdon, UK). BS3 (bis[sulfosuccinimidyl] suberate)1 cross-linking reagent was from Pierce and Warriner (Chester, UK). Na125I for protein iodination, and 125I-labeled interleukin 5 (125I-IL-5) were obtained from Amersham International (Amersham, Buckinghamshire, UK). Anti-phosphotyrosine antibody (4G10) was obtained from TCS Biologicals (Buckingham, UK). All other chemicals were obtained from Sigma (Poole, Dorset, UK).
Site-directed Mutagenesis and Protein Expression.
Recombinant human IL-5 was expressed in E. coli and purified to homogeneity as previously described (30). IL-5 (E12K) was generated by site-directed mutagenesis, expressed in E. coli and purified as previously described (28) with the following modifications. Renaturation was carried out by dropwise dilution of the protein in 6 M urea, into 0.1 M ethanolamine, pH 9.8, containing 2.5 M urea, 10% ammonium sulphate, 1 mM reduced glutathione, and 0.1 mM oxidized glutathione to a final protein concentration of 10 μg/ml, and stirred overnight at 4°C. The solution was dialyzed against three changes of 0.1 M ethanolamine, and concentrated 10-fold by ultrafiltration before gel filtration. Circular dichroism spectral analysis confirmed that the secondary and tertiary structure was identical to wild-type IL-5 (data not shown).
Receptor Binding Assays.
In competition binding studies IL-5 and IL-5 (E12K) were assayed for their relative ability to bind to recombinant IL-5 receptor α chain alone, or the receptor α/β chain complex as expressed on TF-1 cells, as previously described (31).
In brief, for low-affinity binding studies the extracellular domain of the IL-5 receptor α chain, fused with the IgG binding domain of protein A (IL-5Rα-ZZ), was incubated with rabbit IgG and anti-rabbit–coated fluoromicrosphere beads (Amersham International, UK) for 2 h at 4°C. The resultant complex was incubated with 100 pM 125I-IL-5 in the presence of increasing concentrations of unlabeled cytokines as indicated. After incubation for 4 h at room temperature, bound radioligand was measured in a Wallac 1450 Microbeta scintillation counter set up in scintillation proximity assay (SPA) mode. Nonspecific binding was measured in the presence of a 500-fold molar excess of unlabeled IL-5.
For cell-based binding studies TF-1 cells (1 × 106) were incubated with 200 pM 125I-IL-5 for 2 h at room temperature in the presence of increasing concentrations of unlabeled cytokines as indicated. After separation of bound and free ligand by centrifugation through oil (16% paraffin oil, 84% silicone oil; BDH, Dorset, UK) the cell-associated radioligand was quantified in a gamma counter. Nonspecific binding was measured in the presence of a 300-fold molar excess of unlabeled IL-5.
TF-1 Cell Proliferation Assay.
Cytokine-induced proliferation of the human erythroleukemia cell line TF-1 was measured as previously described (28). In brief, assays were performed in 96-well microtiter plates containing 5 × 103 cells/well with the indicated cytokines. After incubation for 60–72 h at 37°C the induction of proliferation was measured using the Cell Titer 96™ nonradioactive cell proliferation assay (Promega, Southampton, UK), according to the manufacturer's instructions.
Eosinophil Purification.
Human peripheral blood eosinophils were isolated from healthy donors with mildly elevated eosinophil count, by a CD16-negative selection protocol as previously described (32). Eosinophil purity was always >95%.
Eosinophil Activation Assay.
Cytokine-induced activation of purified eosinophils, as measured by adhesion to immobilized IgG, was assayed as previously described (32). In brief, 5 × 103 eosinophils were incubated with the indicated cytokines for 30 min at 37°C, in a 96-well microtiter plate precoated with human IgG. After a washing step, the adherent eosinophils were lysed and the endogenous peroxidase activity measured in a colorimetric assay using o-phenylenediamine as a substrate.
Eosinophil Survival Assay.
Purified eosinophils were suspended at 106 cells/ml in DMEM containing 10% FCS, 50 U/ml penicillin, and 50 μg/ml streptomycin (GIBCO, Paisley, Scotland) in microtiter plates, in the presence or absence of wild-type IL-5 or IL-5 (E12K). After incubation at 37°C for 72 h, cell viability was assessed by trypan blue exclusion, counting a minimum of 200 cells. For antibody blocking experiments, human eosinophils were incubated with the indicated concentrations of cytokines in the presence or absence of 250 μg/ml anti–IL-5 neutralizing antibody, TRFK-5 (33). For polymyxin B experiments agonists were preincubated with 500 U/ml polymyxin B for 1 h at 37°C before addition of purified human eosinophils.
Cross-linking of Radiolabeled IL-5 or IL-5 (E12K) to IL-5 Receptor α and β Chains Expressed on COS Cells.
For cross-linking studies recombinant human IL-5 and IL-5 (E12K) were iodinated by a modified chloramine-T method essentially as described previously (28).
COS cells were plated at 10% confluence in 175-cm2 flasks and grown overnight in DMEM containing 10% FCS, 50 U/ml penicillin, and 50 μg/ml streptomycin. Cells were then transiently transfected with human IL-5 receptor α and βc chain expression plasmids (10 μg each) by a calcium phosphate precipitation method, using the Mammalian Transfection Kit (Stratagene, Cambridge, UK), according to the manufacturers instructions. Cells were harvested 48 h after transfection in PBS containing 5 mM EDTA and counted.
Transfected cells (5 × 105) were incubated for 2 h at 4°C in 200 μl binding medium (RPMI 1640 containing 0.5% wt/vol bovine serum albumin and 20 mM Hepes, pH 7.4) with 70 nM 125I-IL-5 or 125I-IL-5 (E12K) in the presence or absence of a 100-fold molar excess of unlabeled IL-5. Cells were then washed twice, resuspended in 1 ml of ice-cold PBS, and 50 mM BS3 cross-linker was added to a final concentration of 1 mM. After incubation for 30 min at 4°C, cells were washed twice in PBS and resuspended in 150 μl lysis buffer (50 mM Hepes pH 7.4, containing 2 mM EDTA, 2 mM EGTA, 1 mM 4-[2-aminoethyl] benzenesulfonyl fluoride, 5 μg/ml leupeptin, 5 μg/ml pepstatin, 5 μg/ml antipain, and 1% vol/vol Triton X-100). The cell lysate was incubated for 10 min on ice, centrifuged for 10 min at 12,000 rpm in a microfuge, and the supernatant mixed with 4× SDS sample buffer containing 10% 2-ME. Extracts were analyzed on a 4–12% NuPAGE SDS–polyacrylamide gel (Novex, San Diego, CA) run in MOPS buffer (3-[N-Morpholino] propanesulfonic acid). After electrophoresis gels were fixed and dried and the radiolabeled bands visualized using a PhosphoImager (Molecular Dynamics, Buckinghamshire, UK).
Detection of Phosphotyrosine Containing Proteins by Western Blotting.
Human eosinophils (5 × 105) were incubated in the presence of indicated concentrations of cytokines for 5 min at 37°C, in PBS containing 0.4% human serum albumin. The cells were then pelleted at 4°C, resuspended in 1× NuPAGE sample buffer containing 2.5% 2-ME and 1 mM sodium orthovanadate and boiled for 5 min. Extracts were analyzed on a 4–12% NuPAGE SDS–polyacrylamide gel run in MOPS buffer and transferred to hybond-ECL membrane (Amersham International, Amersham, UK). Blots were probed with the anti-phosphotyrosine antibody 4G10, and proteins visualized using a horseradish peroxidase-conjugated goat anti–mouse secondary antibody (Sigma, Poole, Dorset, UK) with enhanced chemiluminescence (ECL) detection (Amersham International, UK).
Data Analysis.
Receptor binding and biological data were analyzed using Grafit 3.01 as previously described (28).
Results
Relative Receptor Binding Properties of IL-5 and IL-5 (E12K).
Wild-type human IL-5 and a mutant IL-5 containing a charge reversal mutation at position 12, IL-5 (E12K), were expressed in E. coli and purified to homogeneity. As a means of comparing the receptor binding properties of wild-type IL-5 and IL-5 (E12K), both proteins were assayed for their relative ability to bind to the IL-5 receptor α chain alone, or to the high-affinity α/β receptor complex. In competition binding experiments, using the recombinant extracellular domain of the IL-5 receptor α chain in an SPA assay format, IL-5 (E12K) exhibited a 1.7 ± 0.2-fold (n = 6)-fold reduction in binding affinity to the receptor α chain relative to wild-type IL-5 (Fig. 1 A). The interaction with the high-affinity receptor complex was assessed in a cell based competition binding assay using TF-1 cells which express both the IL-5 receptor α and βc chains. In this cell based system IL-5 (E12K) exhibited a 4.5 ± 1.2-fold (n = 5) reduction in binding affinity relative to wild-type IL-5 (Fig. 1 B). The shift in relative binding of IL-5 (E12K) and wild-type IL-5 to the high-affinity α/β complex, compared to the α chain alone, was found to be statistically nonsignificant (P = 0.084), as assessed by an analysis of variance technique.
Figure 1.
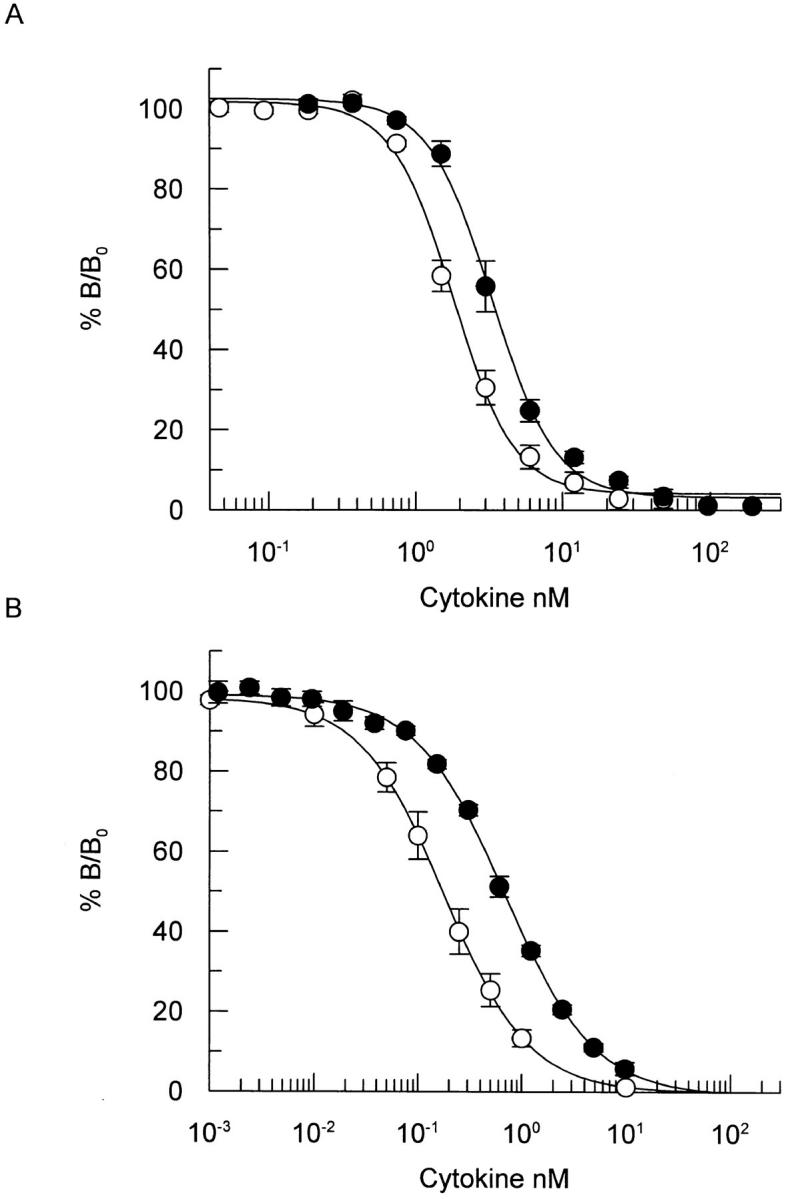
Comparative binding of wild-type IL-5 and IL-5 (E12K) to recombinant IL-5 receptor α chain (A) or to the receptor α/β complex on TF-1 cells (B). (A) Recombinant IL-5 receptor α-chain, immobilized on SPA beads, were incubated with 100 pM 125I-IL-5 in the presence of increasing concentrations of unlabeled wild-type IL-5 (open circles) or IL-5 (E12K) (closed circles). After incubation for 4 h at room temperature samples were counted in a Wallac 1450 microbeta counter set up in SPA mode. The results show competition as percentage of maximum binding (% B/Bo). Each value represents the mean ± SEM of four independent experiments for wild-type and six independent experiments for IL-5 (E12K). (B) TF-1 cells were incubated at room temperature for 2 h in the presence of 200 pM 125I-IL-5 and increasing concentrations of unlabeled wild-type (open circles) or IL-5 (E12K) (closed circles). After separation of bound and free ligand the cell-associated radioligand was quantified in a gamma counter. The results show competition as percentage of maximum binding (% B/Bo). Each value represents the mean ± SEM of five independent experiments.
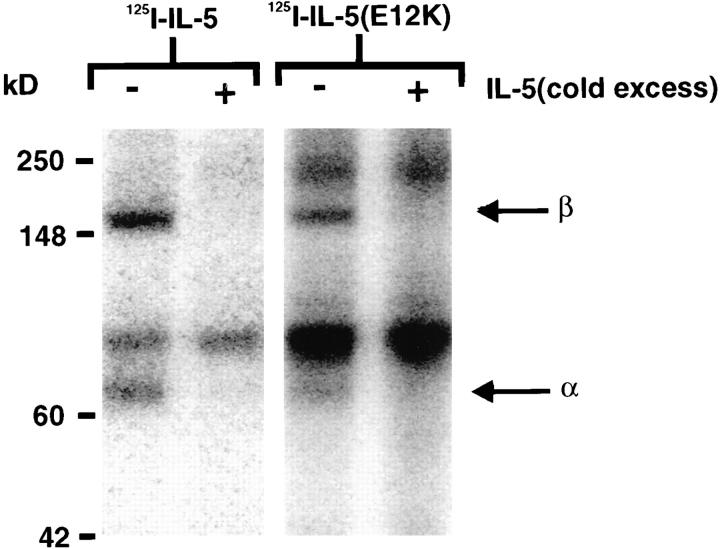
Chemical Cross-linking of Radiolabeled IL-5 and IL-5 (E12K) to IL-5 Receptors.
To determine whether IL-5 (E12K) is able to interact with the IL-5 receptor βc chain, we employed receptor cross-linking studies. The chemical cross-linking of 125I-IL-5 or 125I-IL-5 (E12K) to COS cells transfected with IL-5 receptor α and βc chains revealed identical patterns of cross-linked species of ∼70 and 150 kD under reducing conditions (Fig. 2), which correspond to the predicted molecular mass of the receptor α and βc subunits, respectively, bound to a monomer of IL-5. Furthermore, the appearance of these bands was blocked by addition of a 100-fold molar excess of unlabeled IL-5 during the incubation, confirming that both 125I-IL-5 and 125I-IL-5 (E12K) can be specifically cross-linked to both IL-5 receptor α and βc chains.
Figure 2.
Chemical cross-linking of wild-type IL-5 and IL-5 (E12K) to IL-5 receptors. COS cells transfected with human IL-5 receptor α and βc chain cDNAs were incubated with ∼70 nM 125I-IL-5 or 125I-IL-5 (E12K) for 2 h at 4°C in the presence or absence of a 100-fold molar excess of unlabeled IL-5, then cross-linked with 1 mM BS3. After detergent lysis of cells, soluble extracts were analyzed by SDS-PAGE and radiolabeled bands visualized by a PhosphoImager.
Biological Properties of IL-5 (E12K) in a TF-1 Proliferation Assay.
Wild-type IL-5 induced the proliferation of the human erythroleukemia cell line TF-1 in a concentration-dependent fashion (ED50 = 2.6 ± 0.6 pM, n = 4), while IL-5 (E12K) was unable to stimulate proliferation even at concentrations up to 400 nM, a concentration 160,000-fold higher than the concentrations of wild-type IL-5 required to elicit half maximal proliferation of TF-1 cells (Fig. 3 A).
Figure 3.
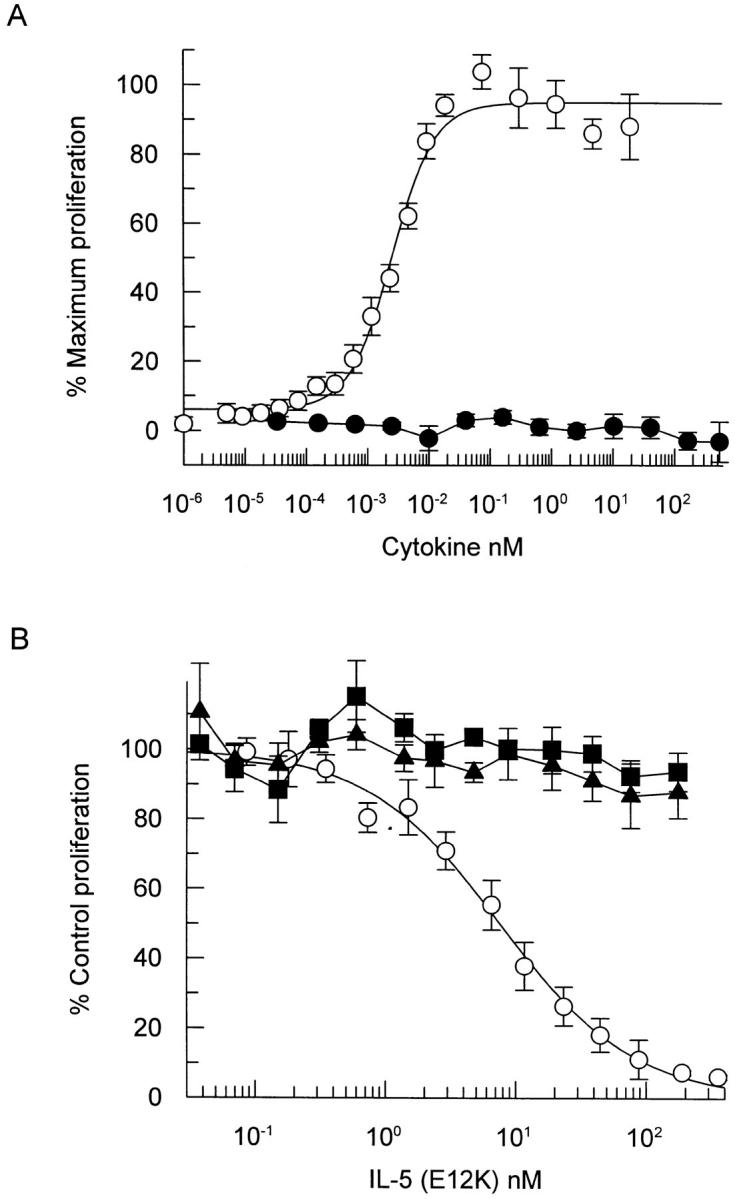
IL-5 (E12K) exhibits no agonist activity (A) and is a specific IL-5 antagonist (B) in a TF-1 cell proliferation assay. (A) TF-1 cells were incubated with increasing concentrations of either wild-type (open circles) or IL-5 (E12K) (closed circles) for 60–72 h and the induction of proliferation was measured using a nonradioactive cell proliferation assay. Each value represents the mean ± SEM of four independent experiments. (B) TF-1 cell proliferation was assayed at either 77 pM IL-5 (open circles), 133 pM IL-3 (closed squares) or 14 pM GM-CSF (closed triangles) in the presence of increasing concentrations of IL-5 (E12K). The concentrations of wild-type cytokines represent ∼ED80's (concentration of cytokine required to give 80% of the maximum biological response) for proliferation in our TF-1 cell line. Each value represents the mean ± SEM of six independent experiments. 100% values for IL-5–, IL-3–, and GM-CSF–induced proliferation were equivalent to A550 of 0.68 ± 0.02, 0.73 ± 0.18, and 0.64 ± 0.04, respectively. Unstimulated levels were 0.03 ± 0.02.
Having determined that IL-5 (E12K) was able to bind to IL-5 receptors with almost wild-type affinity, yet was unable to elicit a proliferative response in TF-1 cells, we tested this mutant for its ability to antagonize the effects of IL-5 in a TF-1 proliferation assay. As seen in Fig. 3 B, IL-5 (E12K) antagonized the effect of IL-5 in a concentration-dependent manner. Proliferation of TF-1 cells, in the presence of 77 pM wild-type IL-5, was totally inhibited by 100 nM IL-5 (E12K) and 50% inhibition was achieved at 10.3 ± 2.8 nM (n = 6), which represents a 130-fold molar excess of IL-5 (E12K) over wild-type. Furthermore, in the same functional assay IL-5 (E12K) did not significantly inhibit TF-1 proliferation stimulated by IL-3 or GM-CSF (Fig. 3 B). This demonstrates not only that IL-5 (E12K) is a specific IL-5 receptor antagonist, but also excludes the possibility of IL-5 (E12K)–dependent cellular toxicity.
Biological Properties of IL-5 (E12K) in an Eosinophil Activation Assay.
To confirm and extend our observations to additional IL-5–induced cellular responses, we switched our studies to human peripheral blood eosinophils, a terminally differentiated cell type in which IL-5 stimulates multiple functional responses. We have established a sensitive and reliable in vitro assay for analysis of cytokine-induced eosinophil activation based on adhesion to immobilized IgG. In this assay, wild-type IL-5 induced a concentration-dependent increase in eosinophil adhesion to IgG (ED50 = 1.3 ± 0.1 pM, n = 3), while the mutant E12K was incapable of inducing eosinophil adhesion even up to concentrations of 1 μM, a concentration 400,000-fold higher than the ED50 for wild-type IL-5 (Fig. 4 A). In the same functional assay IL-5 (E12K) completely antagonized the effects of IL-5 in a concentration-dependent fashion. Eosinophil adhesion in the presence of 20 pM wild-type IL-5 was completely inhibited by 50 nM IL-5 (E12K) with 50% inhibition achieved at 1.9 ± 0.1 nM (n = 3), which represents a 95-fold molar excess of IL-5 (E12K) over wild-type (Fig. 4 B). In addition, the inhibitory effect of IL-5 (E12K) was specific for IL-5 induced adhesion with no significant inhibition of IL-3, GM-CSF, or TNF-α across the range of IL-5 (E12K) concentrations found to inhibit IL-5 (Fig. 4 B).
Figure 4.
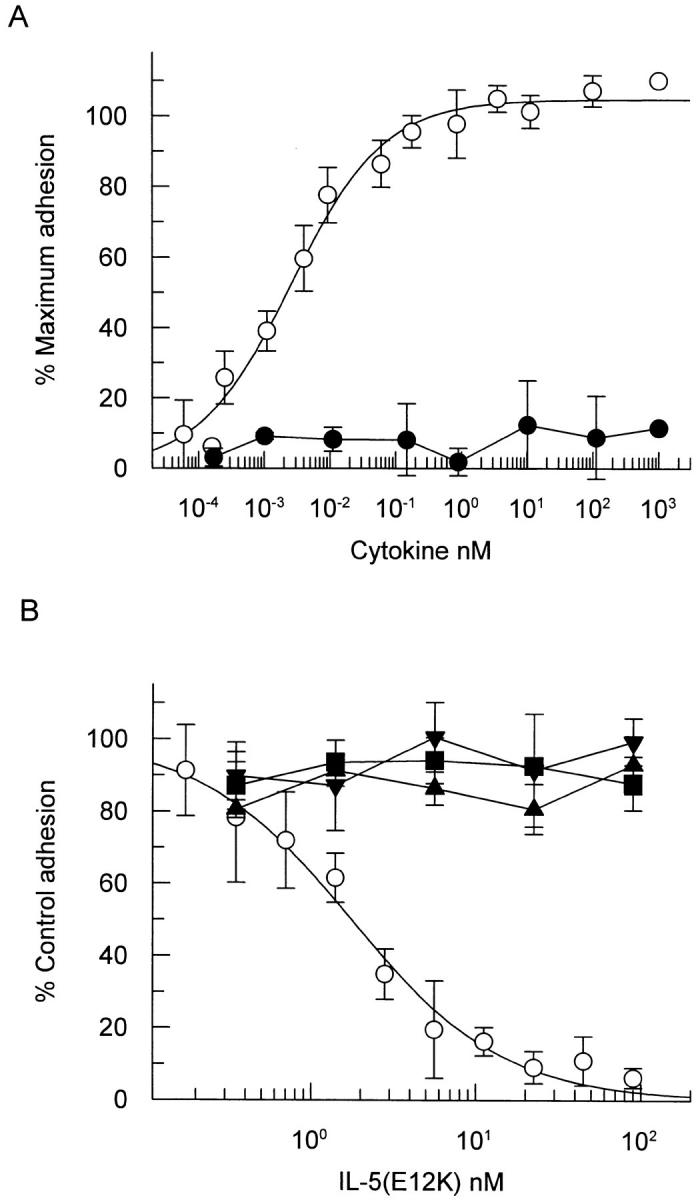
IL-5 (E12K) exhibits no agonist activity (A) and is a specific IL-5 antagonist (B) in a cytokine induced eosinophil activation assay. (A) Human eosinophils were incubated with increasing concentrations of either wild-type (open circles) or IL-5 (E12K) (closed circles) for 30 min at 37°C, in a 96-well microtiter plate precoated with human IgG. After a washing step, the adherent eosinophils were lysed and the endogenous peroxidase activity measured in a colorimetric assay. (B) In antagonist experiments eosinophils were incubated with either 20 pM IL-5 (open circles), 180 pM IL-3 (closed squares), 6 pM GM-CSF (closed triangles), or 1,000 pM TNF-α (inverted triangles) in the presence of increasing concentrations of IL-5 (E12K). The concentrations of wild-type cytokines represent ∼ED80's in the eosinophil adhesion assay. In both panels each value represents the mean ± SEM of three independent experiments using different blood donors. 100% values for IL-5–, IL-3–, GM-CSF–, and TNF-α–induced adhesion were 0.56 ± 0.07, 0.76 ± 0.05, 0.41 ± 0.07, and 0.49 ± 0.06, respectively. Unstimulated levels were 0.15 ± 0.05.
Biological Properties of IL-5 (E12K) in an Eosinophil Survival Assay.
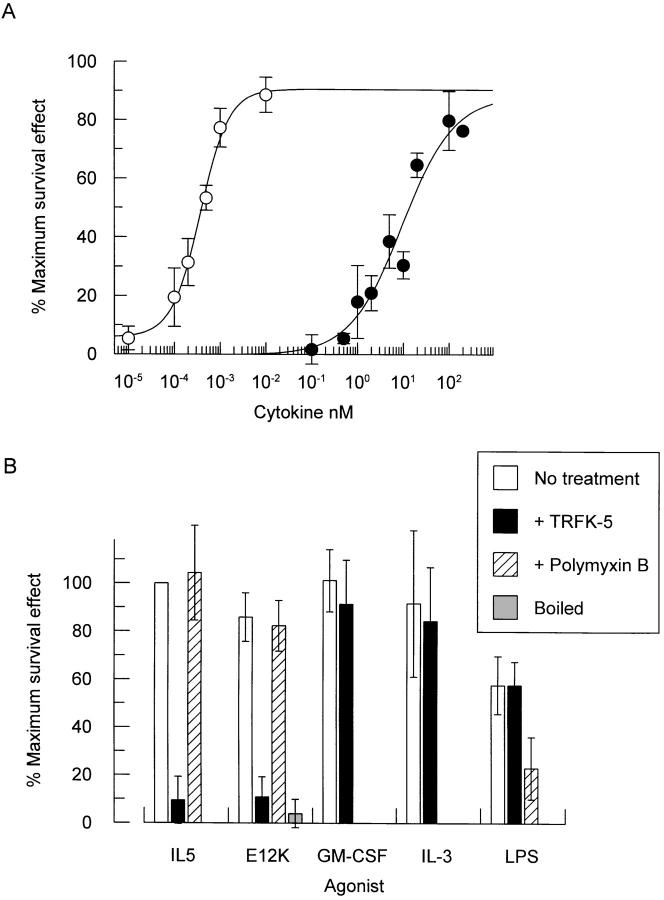
Mature eosinophils that have been separated from peripheral blood do not survive more than 4 d in vitro without the addition of cytokines. The eosinophilopoietic cytokines IL-5, IL-3, and GM-CSF have all been reported to promote eosinophil survival, and so maintain cell viability (8, 34, 35). We therefore assessed the relative ability of wild-type IL-5 and IL-5 (E12K) to promote eosinophil survival. Purified human peripheral blood eosinophils were incubated in the presence of increasing concentrations of wild-type IL-5 or IL-5 (E12K) and eosinophil viability measured by trypan blue exclusion, after a period of 72 h. Both IL-5 and IL-5 (E12K) were able to promote eosinophil survival in a concentration-dependent manner with ED50 values of 0.4 ± 0.1 pM and 20.6 ± 8.7 nM, respectively (n = 5) (Fig. 5 A). Although IL-5 (E12K) promoted eosinophil survival with a 50,000-fold reduction in biological potency with respect to wild-type IL-5, it was still a full agonist capable of eliciting a maximal biological response not significantly different from wild-type IL-5.
Figure 5.
Both wild-type IL-5 and IL-5 (E12K) promote eosinophil survival (A). The agonist activity of E12K can be abolished by boiling, or pretreatment with a neutralizing anti–IL-5 antibody but not by polymyxin B (B). (A) Increasing concentrations of either wild-type (open circles) or IL-5 (E12K) (closed circles) were incubated with purified human eosinophils for 72 h at 37°C and eosinophil viability measured by trypan blue exclusion. Data expressed as a percentage of the maximum survival effect obtained with wild-type IL-5 in each experiment (66% ± 5.6 viable cells, compared to 2.7% ± 1.7 in the absence of cytokine). Each value represents the mean ± SEM of six independent experiments using different blood donors. (B) Human eosinophils were incubated with either 1 pM IL-5, 50 nM IL-5 (E12K), 40 pM IL-3, 20 pM GM-CSF, or 1 ng/ml LPS alone, or in the presence of 250 μg/ml anti–IL-5 neutralizing antibody, TRFK-5, or after pretreatment with 500 U/ml polymyxin B for 1 h at 37°C. For boiling experiments, IL-5 (E12K) was boiled for 15 min before incubation with eosinophils. Eosinophil viability was measured after 72 h by trypan blue exclusion. The concentrations of cytokines represent ∼ED80's in the eosinophil survival assay. Data expressed as a percentage of the maximum survival effect obtained with wild-type IL-5 in each experiment (61% ± 8.0 viable cells, compared to 17% ± 3.7 in the absence of cytokine). Each value represents the mean ± SEM of at least three independent experiments.
One trivial explanation for the agonist activity of IL-5 (E12K) in the eosinophil survival assay is the contamination of this E. coli expressed cytokine with bacterial LPS, which has been reported to promote eosinophil survival through the autocrine production of GM-CSF (36). To exclude this possibility a series of control experiments were performed (Fig. 5 B). First, the agonist activity of IL-5 (E12K) was completely abolished after boiling for 15 min. Furthermore, pretreatment with polymyxin B, an LPS inhibitor (36), blocked LPS- but not IL-5 or IL-5 (E12K)–induced eosinophil survival. In addition, the anti–IL-5 neutralizing antibody TRFK-5 blocked the agonist activity of IL-5 (E12K) and wild-type IL-5, but not IL-3, GM-CSF or LPS in the eosinophil survival assay (Fig. 5 B). This confirms that the eosinophil survival activity is specifically mediated via IL-5 (E12K) and excludes the possibility of an LPS-dependent mechanism.
Relative Effects of IL-5 and IL-5 (E12K) on Tyrosine Phosphorylation in Eosinophils.
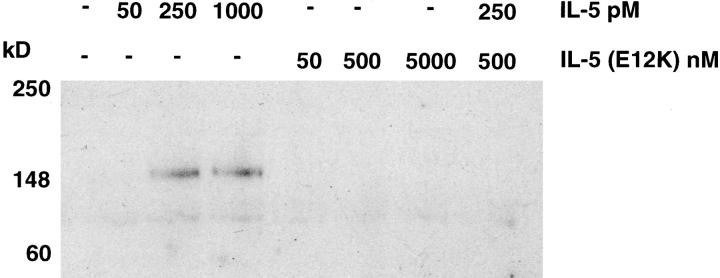
Stimulation of eosinophils with IL-5 induced an increase in the phosphotyrosine content of a number of proteins, in a concentration-dependent manner, with the predominant phosphorylated species at 150-kD (Fig. 6). In contrast to wild-type IL-5, IL-5 (E12K) did not stimulate tyrosine phosphorylation even at concentrations up to 5 μM. Furthermore, 500 nM IL-5 (E12K) completely inhibited the tyrosine phosphorylation of this 150-kD protein stimulated by 250 pM IL-5 (Fig. 6).
Figure 6.
Wild-type IL-5 but not IL-5 (E12K) stimulates tyrosine phosphorylation of a 150-kD protein in human eosinophils. Human eosinophils were stimulated with the indicated concentrations of cytokines for 5 min at 37°C. Cells were pelleted, resuspended in SDS sample buffer and lysates were electrophoresed and immunoblotted with anti-phosphotyrosine antibody.
Discussion
In a systematic program of alanine scanning mutagenesis we previously demonstrated that IL-5 (E12A) exhibited partial agonist activity in a TF-1 cell proliferation assay and proposed that E12 defined a contact point for the IL-5 receptor β chain. We based this conclusion not only on biological activity but on the reduction in affinity of E12A for the IL-5 receptor α/β chain compared to the α chain alone (28). Generating a charge reversal at this putative βc chain binding site residue, IL-5 (E12K), has allowed us to further investigate the effect of this mutation.
Glutamic acid-12 resides on the amino-terminal helix of IL-5 at an analogous position to E21 in GM-CSF and E22 in IL-3. Charge reversal mutations at these positions in IL-3 and GM-CSF resulted in proteins which were unaltered in binding their respective α chains, but demonstrated a reduction in high-affinity binding (37, 38). For IL-5 (E12K) we also see essentially wild-type binding to the IL-5 receptor α chain, but a reduction in high affinity binding of ∼2.6-fold. Although this result is entirely consistent with the reduction in high affinity we previously observed with IL-5 (E12A) (28), the apparent reduction in the affinity of IL-5 (E12K) for the α/β complex is not statistically significant (P = 0.084). Since the affinity conversion conferred by βc on the IL-5/IL-5 receptor–α chain complex is small (two- to fourfold), compared to the much larger shifts in affinity seen with both GM-CSF and IL-3, this lack of statistical significance is probably due to the difficulty of measuring such small changes. The contribution of the βc chain to IL-5 (E12K) high-affinity binding is difficult to measure clearly through competition binding studies. However we demonstrated by chemical cross-linking studies that, as with wild-type IL-5, IL-5 (E12K) is still capable of contacting not only the receptor α chain but also the βc chain. This result is consistent with the pattern of cross-linking obtained with IL-5 (E13Q), which has a similar antagonist biological profile in TF-1 cells (29). Such cross-linking experiments have not been published with human GM-CSF (E22R) or IL-3 (E21R) and it is not clear whether these mutants can similarly cross-link to the βc chain in the absence of high affinity binding. However, a biologically active murine GM-CSF mutant (E21A) has been described which binds the α/β complex with low affinity only, yet is able to cross-link to both the α and βc chains of the receptor (39). As suggested by Tavernier et al. (29), this may indicate that affinity conversion and receptor activation can be uncoupled.
In contrast to IL-5 (E12A), the IL-5 (E12K) mutant was completely devoid of agonist activity in a TF-1 cell proliferation assay and was a full and selective antagonist of IL-5 in this functional assay. While IL-3 (E21R) retains agonist activity for TF-1 proliferation at 20,000-fold reduced potency relative to wild-type IL-3 (37), GM-CSF (E22R) lacks agonist activity and is a specific GM-CSF antagonist (40). Furthermore, the antagonistic properties of IL-5 (E12K) are not restricted to inhibition of IL-5–induced cell proliferation. In human eosinophils IL-5 (E12K) was a selective antagonist of IL-5 induced eosinophil activation, as assessed by adhesion to immobilized IgG, exhibiting no effect on eosinophil activation induced by the related cytokines IL-3 and GM-CSF or the unrelated cytokine TNF-α.
Taken together these mutagenesis studies suggest that the structural conservation at both the cytokine and receptor level for IL-5, IL-3, and GM-CSF underlie a common mechanism of receptor binding and activation, and that the acidic residues within the amino terminal helix of all three cytokines form a contact point for the βc chain which contributes to receptor activation and, for the latter two cytokines at least, high-affinity binding. Somewhat surprisingly however, IL-5 (E12K) was clearly a full agonist in an eosinophil survival assay, albeit with a 50,000-fold reduction in potency relative to wild-type IL-5. This survival effect of IL-5 (E12K) was not due to the presence of contaminating LPS and was consistently seen in the same eosinophils in which IL-5 (E12K) antagonized IL-5–induced adhesion to IgG. Clearly this single point mutation in IL-5 is able to distinguish between two IL-5–dependent functional responses in the same cell type. This apparent functional dichotomy in the actions of IL-5 (E12K) implies that different functional responses within the same cell, activation versus survival, are mediated through distinct mechanisms of cellular activation.
In the context of IL-5, E12 appears to be necessary for activating the IL-5 receptor βc leading to an eosinophil activation signal. However, the fact that IL-5 (E12K) promotes eosinophil survival suggests that residues on IL-5 other than E12 are involved in receptor activation leading to a survival signal. The reduction in potency of IL-5 (E12K) with respect to wild-type IL-5 further suggests that, although not required for full agonist activity, E12 must contribute to the efficiency of receptor activation. This would indicate that multiple points of contact between IL-5 and its receptor complex are required for complete receptor activation. Since IL-5 (E12K) can be cross-linked to the receptor βc, residues other than E12 may directly contact βc contributing to its complete activation. Such a model may also account for the ability of human IL-3 (E22R) and murine GM-CSF (E21A) to exhibit biological activity despite the lack of high-affinity binding (37,39). However, a direct role for the IL-5 receptor α chain in signal transduction has been inferred from both deletion and point mutation studies in the intracellular domain of the α chain, resulting in the loss of IL-5–induced cell proliferation (21, 22). Furthermore, it has been proposed that the ligand-specific α subunits of the IL-5, IL-3, and GM-CSF receptors may mediate cytokine-specific signals leading to different cellular responses (41). Since mutations at E12 appear to be unaffected in α-chain binding, it is also possible that elements of the survival signal emanate from the α chain.
These properties of IL-5 (E12K) highlight its potential use as a tool for dissecting signaling pathways from the activated IL-5 receptor complex leading to distinct IL-5–dependent functional responses, in particular eosinophil survival. Initial experiments in eosinophils measuring one of the earliest detectable signaling events, tyrosine phosphorylation, highlighted opposing effects of IL-5 and IL-5 (E12K). Wild-type IL-5, but not IL-5 (E12K), was able to activate tyrosine phosphorylation of a major 150-kD protein species and furthermore, the IL-5–dependent phosphorylation of this protein was inhibited by IL-5 (E12K).
In summary, we have identified an IL-5 point mutant, IL-5 (E12K), which lacks agonist activity and is a potent antagonist in both a TF-1 cell proliferation assay and an eosinophil activation assay, yet exhibits agonist activity in promoting eosinophil survival. The dual agonist and antagonist properties of IL-5 (E12K) can be explained via distinct mechanisms of cellular/receptor activation. We are currently attempting to elucidate the precise mechanism by which this IL-5 mutant exerts its agonist effect.
Acknowledgments
The authors would like to thank Miss Gillian Amphlett for statistical analysis and Dr. Tim Mosmann (DNAX, Palo Alto, CA) for providing TRFK-5.
Footnotes
1 Abbreviations used in this paper: BS3, bis(sulfosuccinimidyl) suberate; ED80, concentration required to give 80% of the maximum biological response; IL-5R-zz, extracellular domain of the IL-5 receptor α chain, fused with the IgG binding domain of protein A; MOPS, 3-[N-morpholino] propanesulfonic acid; SPA, scintillation proximity assay.
References
- 1.Bohjanen PR, Okajima M, Hodes RJ. Differential regulation of interleukin 4 and interleukin 5 gene expression: a comparison of T-cell gene induction by anti-CD3 antibody or by exogenous lymphokines. Proc Natl Acad Sci USA. 1990;87:5283–5287. doi: 10.1073/pnas.87.14.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plaut M, Pierce JH, Watson CJ, Hanley-Hyde J, Nordan RP, Paul WE. Mast cell lines produce lymphokines in response to cross-linkage of FcεRI or to calcium ionophores. Nature (Lond) 1989;339:64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- 3.Dubucquoi S, Desreumaux P, Janin A, Klein O, Goldman M, Tavernier J, Capron A, Capron M. Interleukin 5 synthesis by eosinophils: association with granules and immunoglobulin-dependent secretion. J Exp Med. 1994;179:703–708. doi: 10.1084/jem.179.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clutterbuck EJ, Hirst EM, Sanderson CJ. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GM-CSF. Blood. 1989;73:1504–1512. [PubMed] [Google Scholar]
- 5.Walsh GM, Hartnell A, Wardlaw AJ, Kurihara K, Sanderson CJ, Kay AB. IL-5 enhances the in vitro adhesion of human eosinophils, but not neutrophils, in a leucocyte integrin (CD11/18)-dependent manner. Immunology. 1990;71:258–265. [PMC free article] [PubMed] [Google Scholar]
- 6.Fujisawa T, Abu-Ghazaleh R, Kita H, Sanderson CJ, Gleich GJ. Regulatory effect of cytokines on eosinophil degranulation. J Immunol. 1990;144:642–646. [PubMed] [Google Scholar]
- 7.Wang JM, Rambaldi A, Biondi A, Chen ZG, Sanderson CJ, Mantovani A. Recombinant human interleukin 5 is a selective eosinophil chemoattractant. Eur J Immunol. 1989;19:701–705. doi: 10.1002/eji.1830190420. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi Y, Hayashi Y, Sugama Y, Miura Y, Kasahara T, Kitamura S, Torisu M, Mita S, Tominaga A, Takatsu K, Suda T. Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival. IL-5 as an eosinophil chemotactic factor. J Exp Med. 1988;167:1737–1742. doi: 10.1084/jem.167.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–3109. [PubMed] [Google Scholar]
- 10.Kroegel C, Virchow JC, Jr, Luttmann W, Walker C, Warner JA. Pulmonary immune cells in health and disease: the eosinophil leucocyte (Part I) Eur Respir J. 1994;7:519–543. doi: 10.1183/09031936.94.07030519. [DOI] [PubMed] [Google Scholar]
- 11.Kroegel C, Warner JA, Virchow JC, Jr, Matthys H. Pulmonary immune cells in health and disease: the eosinophil leucocyte (Part II) Eur Respir J. 1994;7:743–760. doi: 10.1183/09031936.94.07040743. [DOI] [PubMed] [Google Scholar]
- 12.Kung TT, Stelts DM, Zurcher JA, Adams GK, III, Egan RW, Kreutner W, Watnick AS, Jones H, Chapman RW. Involvement of IL-5 in a murine model of allergic pulmonary inflammation: prophylactic and therapeutic effect of an anti-IL-5 antibody. Am J Respir Cell Mol Biol. 1995;13:360–365. doi: 10.1165/ajrcmb.13.3.7654390. [DOI] [PubMed] [Google Scholar]
- 13.Blyth DI, Pedrick MS, Savage TJ, Hessel EM, Fattah D. Lung Inflammation and Epithelial changes in a murine model of atopic asthma. Am J Respir Cell Mol Biol. 1996;14:425–438. doi: 10.1165/ajrcmb.14.5.8624247. [DOI] [PubMed] [Google Scholar]
- 14.Van Oosterhout AJ, Ladenius AR, Savelkoul HF, Van Ark I, Delsman KC, Nijkamp FP. Effect of anti-IL-5 and IL-5 on airway hyperreactivity and eosinophils in guinea pigs. Am Rev Respir Dis. 1993;147:548–552. doi: 10.1164/ajrccm/147.3.548. [DOI] [PubMed] [Google Scholar]
- 15.Akutsu I, Kojima T, Kariyone A, Fukuda T, Makino S, Takatsu K. Antibody against interleukin-5 prevents antigen-induced eosinophil infiltration and bronchial hyperreactivity in the guinea pig airways. Immunol Lett. 1995;45:109–116. doi: 10.1016/0165-2478(94)00241-i. [DOI] [PubMed] [Google Scholar]
- 16.Mauser PJ, Pitman AM, Fernandez X, Foran SK, Adams GK, III, Kreutner W, Egan RW, Chapman RW. Effects of an antibody to interleukin-5 in a monkey model of asthma. Am J Respir Crit Care Med. 1995;152:467–472. doi: 10.1164/ajrccm.152.2.7633694. [DOI] [PubMed] [Google Scholar]
- 17.Tavernier J, Devos R, Cornelis S, Tuypens T, Van der Heyden J, Fiers W, Plaetinck G. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific α chain and a β chain shared with the receptor for GM-CSF. Cell. 1991;66:1175–1184. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- 18.Kitamura T, Sato N, Arai K, Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared β subunit for the human IL-3 and GM-CSF receptors. Cell. 1991;66:1165–1174. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- 19.Hayashida K, Kitamura T, Gorman DM, Arai K, Yokota T, Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci USA. 1990;87:9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murata Y, Takaki S, Migita M, Kikuchi Y, Tominaga A, Takatsu K. Molecular cloning and expression of the human interleukin 5 receptor. J Exp Med. 1992;175:341–351. doi: 10.1084/jem.175.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takaki S, Kanazawa H, Shiiba M, Takatsu K. A critical cytoplasmic domain of the interleukin-5 (IL-5) receptor α-chain and its function in IL-5-mediated growth signal transduction. Mol Cell Biol. 1994;14:7404–7413. doi: 10.1128/mcb.14.11.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornelis S, Fache I, Van der Heyden J, Guisez Y, Tavernier J, Devos R, Fiers W, Plaetinck G. Characterization of critical residues in the cytoplasmic domain of the human interleukin-5 receptor α chain required for growth signal transduction. Eur J Immunol. 1995;25:1857–1864. doi: 10.1002/eji.1830250710. [DOI] [PubMed] [Google Scholar]
- 23.Sakamaki K, Miyajima I, Kitamura T, Miyajima A. Critical cytoplasmic domains of the common β subunit of the human GM-CSF, IL-3 and IL-5 receptors for growth signal transduction and tyrosine phosphorylation. EMBO (Eur Mol Biol Organ) J. 1992;11:3541–3549. doi: 10.1002/j.1460-2075.1992.tb05437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato N, Sakamaki K, Terada N, Arai K, Miyajima A. Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common β subunit responsible for different signaling. EMBO (Eur Mol Biol Organ) J. 1993;12:4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Bruggen T, Kok PT, Raaijmakers JA, Verhoeven AJ, Kessels RG, Lammers JW, Koenderman L. Cytokine priming of the respiratory burst in human eosinophils is Ca2+independent and accompanied by induction of tyrosine kinase activity. J Leukoc Biol. 1993;53:347–353. doi: 10.1002/jlb.53.4.347. [DOI] [PubMed] [Google Scholar]
- 26.Kinashi T, Harada N, Severinson E, Tanabe T, Sideras P, Konishi M, Azuma C, Tominaga A, Bergstedt-Lindqvist S, Takahashi M, et al. Cloning of complementary DNA encoding T-cell replacing factor and identity with B-cell growth factor II. Nature (Lond) 1986;324:70–73. doi: 10.1038/324070a0. [DOI] [PubMed] [Google Scholar]
- 27.Milburn MV, Hassell AM, Lambert MH, Jordan SR, Proudfoot AE, Graber P, Wells TN. A novel dimer configuration revealed by the crystal structure at 2.4 Å resolution of human interleukin-5. Nature (Lond) 1993;363:172–176. doi: 10.1038/363172a0. [DOI] [PubMed] [Google Scholar]
- 28.Graber P, Proudfoot AE, Talabot F, Bernard A, McKinnon M, Banks M, Fattah D, Solari R, Peitsch MC, Wells TN. Identification of key charged residues of human interleukin-5 in receptor binding and cellular activation. J Biol Chem. 1995;270:15762–15769. doi: 10.1074/jbc.270.26.15762. [DOI] [PubMed] [Google Scholar]
- 29.Tavernier J, Tuypens T, Verhee A, Plaetinck G, Devos R, Van der Heyden J, Guisez Y, Oefner C. Identification of receptor-binding domains on human interleukin 5 and design of an interleukin 5-derived receptor antagonist. Proc Natl Acad Sci USA. 1995;92:5194–5198. doi: 10.1073/pnas.92.11.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proudfoot AE, Fattah D, Kawashima EH, Bernard A, Wingfield PT. Preparation and characterization of human interleukin-5 expressed in recombinant Escherichia coli. Biochem J. 1990;270:357–361. doi: 10.1042/bj2700357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banks M, Graber P, Proudfoot AE, Arod CY, Allet B, Bernard AR, Sebille E, McKinnon M, Wells TN, Solari R. Soluble interleukin-5 receptor α-chain binding assays: use for screening and analysis of interleukin-5 mutants. Anal Biochem. 1995;230:321–328. doi: 10.1006/abio.1995.1481. [DOI] [PubMed] [Google Scholar]
- 32.Fattah D, Page KR, Bezbaruah S, Preist R, Horgan CM, Solari R. A rapid activation assay for human eosinophils based on adhesion to immobilized ICAM-1, VCAM-1 and IgG. Cytokine. 1996;8:248–259. doi: 10.1006/cyto.1996.0034. [DOI] [PubMed] [Google Scholar]
- 33.Schumacher JH, O'Garra A, Shrader B, van Kimmenade A, Bond MW, Mosman TR, Coffman RL. The characterisation of four monoclonal antibodies specific for mouse interleukin (IL-)-5 and development of mouse and human enzyme-linked immunosorbent. J Immunol. 1988;141:1576–1581. [PubMed] [Google Scholar]
- 34.Yamaguchi Y, Suda T, Ohta S, Tominaga K, Miura Y, Kasahara T. Analysis of the survival of mature human eosinophils: interleukin-5 prevents apoptosis in mature human eosinophils. Blood. 1991;78:2542–2547. [PubMed] [Google Scholar]
- 35.Her E, Frazer J, Austen KF, Owen WF., Jr Eosinophil hematopoietins antagonize the programmed cell death of eosinophils. Cytokine and glucocorticoid effects on eosinophils maintained by endothelial cell-conditioned medium. J Clin Invest. 1991;88:1982–1987. doi: 10.1172/JCI115524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takanaski S, Nonaka R, Xing Z, O'Byrne P, Dolovich J, Jordana M. Interleukin 10 inhibits lipopolysaccharide-induced survival and cytokine production by human peripheral blood eosinophils. J Exp Med. 1994;180:711–715. doi: 10.1084/jem.180.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barry SC, Bagley CJ, Phillips J, Dottore M, Cambareri B, Moretti P, D'Andrea R, Goodall GJ, Shannon MF, Vadas MA, Lopez AF. Two contiguous residues in human interleukin-3, Asp21 and Glu22, selectively interact with the α- and β-chains of its receptor and participate in function. J Biol Chem. 1994;269:8488–8492. [PubMed] [Google Scholar]
- 38.Lopez AF, Shannon MF, Hercus T, Nicola NA, Cambareri B, Dottore M, Layton MJ, Eglinton L, Vadas MA. Residue 21 of human granulocyte-macrophage colony-stimulating factor is critical for biological activity and for high but not low affinity binding. EMBO (Eur Mol Biol Organ) J. 1992;11:909–916. doi: 10.1002/j.1460-2075.1992.tb05129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shanafelt AB, Kastelein RA. High affinity ligand binding is not essential for granulocyte-macrophage colony-stimulating factor receptor activation. J Biol Chem. 1992;267:25466–25472. [PubMed] [Google Scholar]
- 40.Hercus TR, Bagley CJ, Cambareri B, Dottore M, Woodcock JM, Vadas MA, Shannon MF, Lopez AF. Specific human granulocyte-macrophage colony-stimulating factor antagonists. Proc Natl Acad Sci USA. 1994;91:5838–5842. doi: 10.1073/pnas.91.13.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mire-Sluis A, Page LA, Wadhwa M, Thorpe R. Evidence for a signaling role for the α chains of granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and IL-5 receptors: divergent signaling pathways between GM-CSF/IL-3 and IL-5. Blood. 1995;86:2679–2688. [PubMed] [Google Scholar]