Abstract
Class I–restricted presentation is usually associated with cytoplasmic degradation of cellular proteins and is often considered inaccessible to exogenous antigens. Nonetheless, certain exogenous elements can gain entry into this so-called endogenous pathway by a mechanism termed cross-presentation. This is known to be effective for class I–restricted cytotoxic T lymphocyte (CTL) cross-priming directed against a variety of exogenous tumor, viral, and minor transplantation antigens. The related effect of cross-tolerance can also effectively eliminate responses to selected self components. In both cases, this presentation appears to require the active involvement of a bone marrow–derived antigen presenting cell (APC). Here, we show that CTL induction by cross-priming with cell-associated ovalbumin requires the active involvement of CD4+ helper T cells. Importantly, this CD4+ population is only effective when both the helper and CTL determinants are recognized on the same APC. Moreover, we would argue that the cognitive nature of this event suggests that the CD4+ T cell actively modifies the APC, converting it into an effective stimulator for the successful priming of the CTL precursor.
A large body of evidence suggests that there is a clear demarcation between class I– and class II–restricted antigen processing pathways. Class II–restricted antigens are largely derived from exogenous proteins that enter APCs via the endocytic pathway and are processed in the endosomal compartment. By contrast, antigens recognized by class I–restricted effector CTL are usually derived from endogenously synthesized proteins. Thus, exogenous proteins cannot provide antigenic determinants for class I–restricted effector CTL unless they are introduced directly into the cytoplasm of target cells (1). Presumably, this limitation ensures that only cells that are infected by virus, and not those that simply ingest viral proteins, are killed by effector CTL.
The view that class I–restricted antigens must be endogenously derived has primarily been formed by examining killing of target cells by effector CTL. When priming of CTL is examined, however, there is convincing evidence that exogenous proteins can provide a source of class I–restricted antigens (2, 3). This was first implicated in studies examining the induction of CTL to minor histocompatibility antigens and was referred to as cross-priming (2). Cross-priming has subsequently been shown to occur for viral proteins (3), protein-coated spleen cells (4), and tumor antigens (5). This has led to the proposal that cross-priming may provide the immune system with a mechanism by which it can detect and respond to tissue-tropic viruses that do not infect professional APC (6). In the absence of such a mechanism, viruses could escape immunosurveillance by avoiding professional APC. This mechanism also provides the immune system with a means to survey neoantigens expressed by newly arising tumor cells (5).
Like exogenous foreign antigens (2–4), exogenous self-antigens can enter the class I–presentation pathway (7, 8). In contrast to foreign antigens, however, CTL immunity is not induced (7). This means that in addition to class I–restricted cross-presentation, other factors are required for CTL cross-priming. In this report, we examine these requirements. OVA-specific CTL can be induced by intravenous injection of irradiated spleen cells loaded intracytoplasmically with whole OVA by osmotic shock (4). Despite introduction of OVA into the cytoplasm, CTL priming did not occur by direct presentation, but required cross-priming on a bone marrow–derived host APC. This response was found to require CD4+ T cell help, which had to be delivered to the same APCs as those seen by the CD8+ T cell population. The implications for these findings with respect to class I–restricted immunity are discussed.
Materials and Methods
Mice.
All mice were bred and maintained at the Walter and Eliza Hall Institute for Medical Research. For all experiments, mice between 8 and 16 wk of age were used. Class II–deficient mice were a gift of Dr. Dianne Matthias (Institut National de la Sante et de la Recherche Medicale, Strasbourg, France; reference 9).
CTL Priming.
Two protocols were used to generate OVA-specific CTL. In the first protocol, spleen cells were loaded with OVA by osmotic shock as previously described (1). In brief, 2 × 108 spleen cells were incubated for 10 min at 37°C in 1 ml of RPMI containing 10 mg of whole OVA (grade V, catalog No. A-5503; Sigma Chemical Co., St. Louis, MO), 25 mM Hepes, 0.5 M sucrose, and 10% polyethylene glycol 1,000. These cells were then diluted in 14 ml of 60:40 RPMI/water for 2 min at 37°C before being centrifuged at 400 g for 7 min. After being washed once in Hepes Eagle's medium (HEM)1, the cells were then irradiated for 1,500 centiGrey (cGy), washed twice more, filtered through nylon mesh to remove aggregates, counted, and finally, injected into C57BL/6 (B6) mice at 20–25 × 106/mouse intravenously in 0.5 ml of HEM.
The second approach for priming OVA-specific CTL was by subcutaneous injection of the H-2K (K)b-restricted, immunodominant OVA257-264 peptide emulsified in CFA. OVA257-264 peptide at 200 μg/ml in HEM was emulsified in an equal volume of CFA. Mice were then injected subcutaneously with 100–200 μl of this emulsion containing 10–20 μg of peptide.
In Vitro Generation of OVA-specific CTL.
In most experiments, spleens were removed from primed mice 7 d after priming, and CTL were generated as previously described (10). In brief, the spleen cells from one spleen were washed, counted, and cultured for 6 d in vitro in 30 ml of RPMI containing 10% FCS, 50 μM 2-mercaptoethanol, 2 mM l-glutamine, and 107 E.G7 cells that had been irradiated for 20,000 cGy. In some cases, E.G7 cells were replaced with 108 OVA-loaded spleen cells irradiated for 1,500 cGy; this did not affect the response outcome. Their cytotoxicity was then assessed in a 51Cr–release assay using E.G7 cells, EL4 cells alone, or EL4 cells coated with 1 μg/ml OVA257-264 during 51Cr labeling.
Depletion of CD4+T Cells.
For in vivo depletion of CD4+ T cells, B6 mice were thymectomized at 4 wk of age, and 2 wk later were injected intraperitoneally with 100 μl of GK1.5 ascites twice 3 d apart. One week after the first injection, mice were primed to examine CTL induction.
For in vitro depletion of CD4+ T cells, spleen cells were exposed to a 1:3 dilution of RL172 supernatant in HEM with 2.5% FCS for 30 min at 4°C, washed once, and then exposed to a 1:8 mixture of rabbit complement (catalog No. 6004C; C-six Diagnostics Inc., Mequon, WI) in HEM with 10% FCS for 30 min at 37°C. Cells were then washed twice with HEM before in vitro culture.
Generation of Bone Marrow Chimeras.
To generate bone marrow chimeras, adult mice were lethally irradiated for 900 cGy and reconstituted with 5 × 106 T–cell depleted bone marrow cells of the appropriate background. The next day, mice were injected with 100 μl of T24 (anti-Thy-1) ascites to deplete radioresistant T cells. These mice were then left at least 8 wk before immunization.
Flow Cytometry.
Cells from the blood were stained as described (11).
Results
CTL Priming by OVA-loaded Spleen Cells Occurs by Cross-priming.
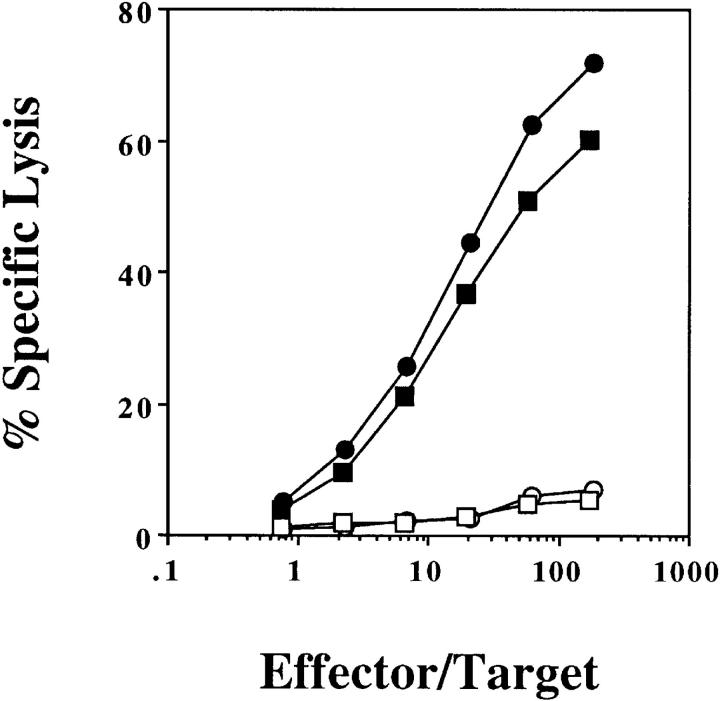
OVA-specific CTL can be generated by injecting B6 mice intravenously with irradiated, syngeneic spleen cells that have been loaded intracytoplasmically with OVA (OVA-loaded spleen cells; 4). Intracytoplasmic loading is achieved by exposing spleen cells to a hypertonic solution of OVA. Those pinosomes that were generated during this exposure burst due to osmotic shock when the cells were exposed to hypotonic conditions. This releases OVA into the cytoplasm where it can be processed into the class I pathway (1). To determine whether priming with OVA-loaded spleen cells was due to direct presentation of OVA by these cells, we took advantage of the fact that CTL can be generated in B6 mice, but are not detectable in bm1 mice, which differ only at the K locus. CTL cannot be induced in bm1 mice because Kbm1, unlike Kb, is unable to present OVA to CD8+ T cells (12). (B6 × bm1)F1 mice were primed with either B6 or bm1 OVA-loaded spleen cells, and then examined for CTL induction (Fig. 1). This showed that spleen cells expressing either haplotype could be used to prime for CTL induction, implying that host, rather than donor, cells were responsible for antigen presentation. Thus, it appeared that host APCs somehow captured OVA antigen associated with the OVA-loaded spleen cells and presented this to host T cells.
Figure 1.
OVA-loaded spleen cells prime by cross-presentation on host APCs. (B6 × bm1)F1 mice were injected intravenously with 2.5 × 107 B6 (circles) or bm1 (squares) OVA-loaded spleen cells. 7 d later, their spleens were removed and stimulated in vitro with OVA-loaded, irradiated splenocytes. After 6 d, a chromium release assay using OVA257-264-pulsed EL4 (closed symbols) and EL4 (open symbols) targets was performed. This experiment was performed three times with 2–4 mice/group.
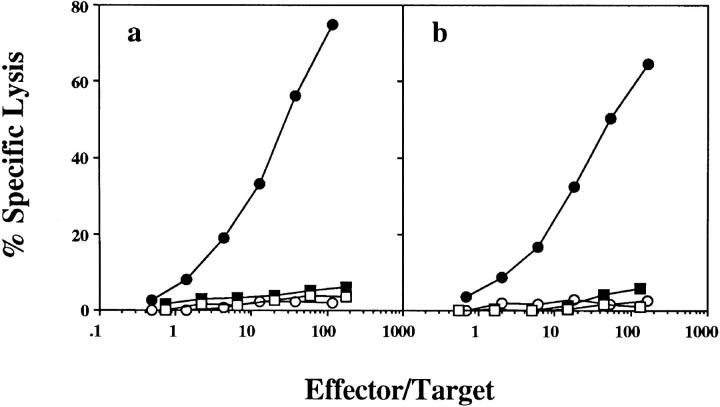
To determine whether the host APC responsible for antigen presentation was of bone marrow origin, (B6 × bm1)F1 chimeric mice were generated in which the bone marrow compartment was either from B6 or bm1 mice and, therefore, either able or unable to present OVA to CD8+ T cells, respectively. When these chimeric mice were primed with bm1 OVA-loaded spleen cells, OVA-specific CTL were only generated when the bone marrow compartment was of B6 origin (Fig. 2 a). This indicated that a bone marrow–derived APC of host origin was responsible for capturing exogenous OVA and presenting it in the class I presentation pathway for the generation of OVA-specific CTL. In support of the conclusion that presentation must proceed through a host APC, there was no CTL generation when bm1 bone marrow into (B6 × bm1)F1 chimeras were primed with B6 OVA-loaded spleen cells, even though donor spleen cells expressed the correct MHC haplotype for direct presentation of OVA (Fig. 2 b).
Figure 2.
The host APC responsible for cross-presentation is bone marrow derived. (B6 × bm1)F1 mice were lethally irradiated, reconstituted with B6 (circles) or bm1 (squares) bone marrow and, 8 wk later, were injected intravenously with 2.5 × 107 OVA-loaded bm1 (a) or B6 (b) spleen cells. 7 d later their spleens were removed and stimulated in vitro for 6 d before being tested in a chromium release assay using OVA257-264-pulsed EL4 (closed symbols) and EL4 (open symbols) targets. One of seven mice reconstituted with bm1 bone marrow and primed with B6 OVA-loaded spleen as represented in Fig. 2 b responded above background, maximizing at 26% OVA-specific lysis; no response was detected by the other six mice tested. This experiment was performed three times with 1–3 mice/group.
The results presented so far are consistent with the findings of Carbone and Bevan (4), who showed that priming with protein-coated spleen cells could generate CTL by cross-priming.
OVA-specific Cross-priming Requires CD4+ T Cell Help.
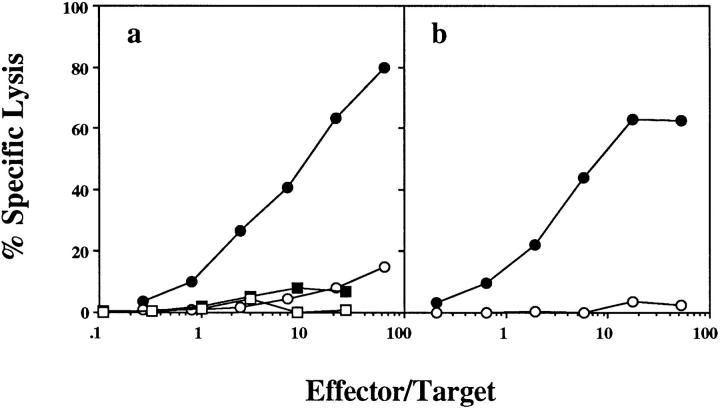
To determine whether CD4+ T cell help was required for the generation of OVA-specific CTL by cross-priming with OVA-loaded spleen cells, this response was examined in CD4+ T cell–depleted B6 mice (Fig. 3 a). The failure to generate OVA-specific CTL under these conditions indicated that CD4+ T cells were essential for CTL priming. This requirement was only apparent for in vivo priming, since depletion of CD4+ T cells during the in vitro culture period did not prevent the generation of CTL (Fig. 3 b).
Figure 3.
OVA-specific cross-priming requires CD4+ T cell help in vivo. Normal B6 mice (circles) or mice depleted of CD4+ T cells by twice weekly intraperitoneal injections of GK1.5 ascites (squares) were immunized intravenously with 2.5 × 107 OVA-loaded spleen cells. 7 d later, their spleens were removed and either left intact (a) or depleted of CD4+ T cells by treatment with RL172 antibody and complement (b). After 6 d in vitro stimulation with OVA-loaded spleen cells, a CTL assay using OVA257-264-pulsed EL4 (closed symbols) and EL4 (open symbols) targets was performed. This experiment was performed four times with 2–3 mice/ group.
It was also possible to prime Kb-restricted OVA-specific CTL by subcutaneous injection of OVA257-264 peptide emulsified in CFA. This response was, however, not dependent on CD4+ T cell help (Fig. 4).
Figure 4.
OVA-specific CTL induction by OVA257-264 in CFA is not dependent on CD4+ T cell help. Normal B6 mice (circles) or mice depleted of CD4+ T cells by twice weekly intraperitoneal injection of GK1.5 ascites (squares) were immunized subcutaneously with 20 μg OVA257-264 emulsified in 200 μl CFA. After 7 d, their spleens were removed and stimulated for 6 d in vitro before a chromium release assay using OVA257-264-pulsed EL4 (closed symbols) and EL4 (open symbols) targets was performed. This experiment was performed four times with 1–3 mice/group.
Cognate CD4+ T Cell Help Is Required for the Generation of OVA-specific CTL by OVA-loaded Spleen Cell Priming.
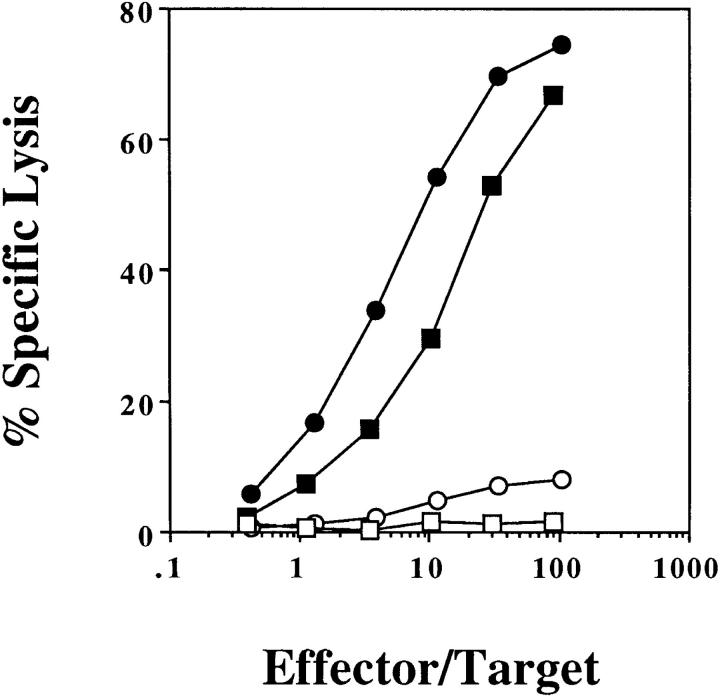
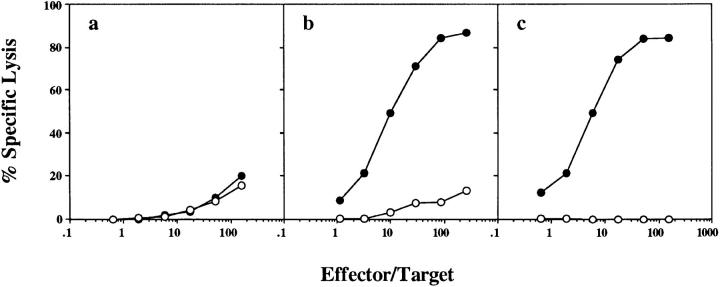
To investigate the basis of CD4+ T cell help, we asked whether CD4+ and CD8+ T cells had to see OVA on the same APC. (B6 × bm1)F1 mice were reconstituted with equal amounts of two types of bone marrow: bm1 bone marrow, and class II knockout B6 bone marrow. This generated a mixture of bm1 APCs that could present OVA to CD4+ but not CD8+ T cells, and class II–deficient APCs that could present OVA to CD8+ but not CD4+ T cells. When these mice were primed with OVA-loaded spleen cells, there was no induction of OVA-specific CTL (Fig. 5 a). This suggested that CD4+ and CD8+ T cells needed to recognize antigen on the same APC. OVA-loaded spleen cell priming used in this experiment was shown to be effective, since it primed for CTL in mice reconstituted with (B6 × bm1)F1 bone marrow, which contain APCs capable of presenting to both CD4+ and CD8+ T cells (Fig. 5 b).
Figure 5.
OVA-specific cross-presentation requires cognate CD4+ T cell help. (B6 × bm1)F1 mice were lethally irradiated and reconstituted with either equal parts of H-2A−/− (class II–deficient) and bm1 bone marrow (a and c) or (B6 × bm1)F1 bone marrow alone (b). 8 wk later, these mice were immunized with 2.0 × 107 OVA-loaded bm1 spleen cells intravenously (a and b) or 10 μg OVA257-264 in 100 μl CFA subcutaneously (c). After 1 wk, their spleen cells were stimulated in vitro for 6 d and a chromium release assay using OVA257-264-pulsed EL4 (closed symbols) and EL4 (open symbols) targets was performed. This experiment was performed twice with 2–3 mice/group.
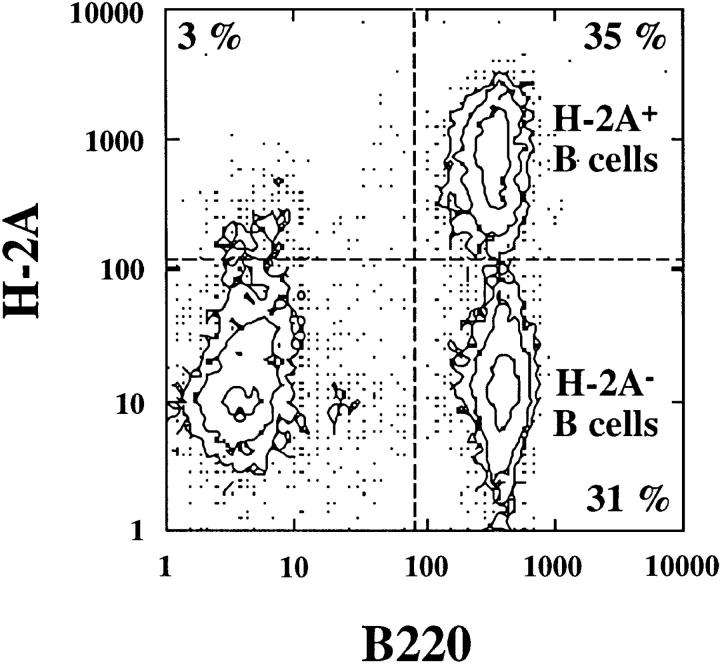
To show that the mixed bone marrow chimeras had been properly reconstituted, these mice were primed with OVA257-264 peptide in CFA and examined for CTL induction (Fig. 5 c). As shown above, this form of priming induces OVA-specific CTL in the absence of CD4+ T cell help (Fig. 4). Induction of CTL in the mixed bone marrow chimeras indicated that their T cell compartment had been successfully reconstituted. To show that class II–deficient and bm1 bone marrow–derived APCs were both present, the blood of chimeric mice was examined by flow cytometry for coexpression of class II and B220 (Fig. 6). Detection of both class II negative and positive B220+ cells indicated that both bone marrow types were present in the reconstituted animals.
Figure 6.
Both class II–deficient B6 and class II–sufficient bm1 bone marrow–derived APCs were present in the mixed bone marrow chimeras. Flow cytometry was performed on the peripheral blood of all mice before immunization for studies shown in Fig. 5.
Discussion
In this report, we show that induction of CTL by priming with OVA-loaded spleen cells occurs by cross-priming on host bone marrow–derived APCs. Indeed, we found that OVA-loaded splenocytes were unable to present OVA directly to host T cells and prime a specific CTL response. Admittedly, this absence could represent a peculiarity of the mode of antigen delivery, specifically hypertonic loading versus a more traditional mechanism such as inoculation using infectious virus. However, it has been shown that the selective depletion of macrophages could effectively eliminate influenza-specific CTL priming (13), even though macrophages do not represent the primary target of viral replication. Such a result would be generally consistent with the data presented here and suggests that cross-presentation may represent a general mechanism involved in CTL priming. Moreover, the involvement of a cross-presenting APC in viral responses would provide an effective means of CTL priming within the secondary lymphoid compartment in the absence of active synthesis of the target antigen, as would occur in cases where viruses show tropisms that do not include lymphoid tissues.
The central role for the APC in priming may explain how helper T cell responses augment the process of CTL priming. There are numerous examples of CD4+ T cell dependent or quasi-dependent CTL responses. The CTL response to minor histocompatibility antigens (14, 15) as well as responses that lead to allograft rejection (16) appear to be CD4+ T cell dependent. Even in viral-specific CTL responses, which often do not require CD4+ T cell help (17– 20), there is a reduction or progressive loss of memory CTL in the absence of helper T cells (21, 22). This suggests that even in responses that can occur in the absence of CD4+ T cells, these helper cells may be able to provide important functions. Here we show that CTL priming with OVA-loaded spleen cells is CD4+ T cell dependent and, more importantly, that these CD4+ helper T cells must recognize antigen on the same APC as those seen by the CD8+ T cell population. The possible existence of such cognate CD4+ T cell–dependent recognition has been raised in other studies (23–26). Keene and Forman (23) provided perhaps the most elegant demonstration that the helper T cell and the CTL precursor must see the same APC, but they examined the response to the alloantigen Qa1, leaving open the possibility that such cognate help was only required for this unusual antigen. Other reports either examined the response of primed T cells or failed to demonstrate a true role for each of the three cells involved in this interaction, merely showing that a helper determinant was important for CTL induction. Rock (27) suggested that class II–restricted T cell help was important for induction of CTL in response to OVA bound to latex beads, but did not address whether this must take place on the same APC as seen by the CD8+ T cells. Shirai et al. (28) demonstrated a requirement for direct linkage between helper and CTL epitopes for successful peptide vaccination. However, this determinant-linkage requirement simply implied a need for cooperation between helper and cytotoxic T cells, and left undefined the mechanism underlying this event. Therefore, our results represent the first direct evidence that CTL induction by cross-priming involves cognate recognition by CD4+ helper T cells of a central bone marrow–derived APC that must also be seen by the CD8+ T cell.
In addition to the already reported CTL cross-priming phenomenon, we have recently shown that cross-presentation is effective in terms of the constitutive presentation of self-antigens (8). However, this self presentation, which occurred in the absence of any obvious T cell help, ultimately led to CTL tolerance (28a). Together with the demonstration here that CD4+ T cell help is required for effective CTL priming, this suggests that the underlying helper T cell requirement may play a key role in immune surveillance, primarily focusing CTL responses towards nonself components and avoiding harmful antiself responses. The involvement of the APC and the essential requirement for cognate CD4+ T cell help in the induction of CTL immunity, suggests that the immune system has developed an intricate mechanism to prevent inappropriate CD8+ CTL responses. First, the antigen must be in a form accessible to the cross-presenting APC, such as cell-associated debris, or particulate in nature (29, 30). Second, CTL induction by cross-priming can only occur if a second population of T cells, the helper cells, are also able to recognize antigen.
The process by which helper T cells contribute to cross-priming remains unknown. One possibility is that they provide local lymphokines such as IL-2 for CTL expansion. However, we favor the view that they alter the APC in some way so that it becomes stimulatory for CD8+ T cells. Indeed, access to other mechanisms that modify APC function may form the basis of CD4+ T cell–independent CTL priming. For example, as noted above, many antiviral CTL responses contain a considerable component that is largely helper T cell independent. This could arise as a consequence of some inherent ability of these infectious pathogens to directly activate APC. This would also explain why CD4+ T cell–independent CTL priming by peptide antigens requires the use of strong microbial adjuvants such as CFA. Alternatively, however, cross-priming may use an APC population completely different from that responsible for activation of CD8+ T cells in CD4+ T cell–independent responses. The details of how the cross-priming APC is activated, as well as the underlying mechanism that permits normally exogenous antigen to enter the otherwise endogenous class I presentation pathway remain to be determined. Elucidating these functions may provide a better understanding of the interaction between pathogens and the immune system, and will be of benefit in the development of more effective vaccines.
Acknowledgments
We thank Dr. Dianne Mathis for the use of her class II–deficient mice and Jenny Falso and Tatiana Banjanin for technical assistance.
This work was supported by the Cooperative Research Centre for Vaccine Technology, National Institutes of Health grant AI-29385, and grants from the National Health and Medical Research Council and the Australian Research Council.
Footnotes
1 Abbreviations used in this paper: cGy, centiGrey; K, H-2K; HEM, Hepes Eagle's medium.
References
- 1.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 2.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gooding LR, Edwards CB. H-2 antigen requirements in the in vitro induction of SV40-specific cytotoxic T lymphocytes. J Immunol. 1980;124:1258–1262. [PubMed] [Google Scholar]
- 4.Carbone FR, Bevan MJ. Class I–restricted processing and presentation of exogenous cell-associated antigen in vivo. J Exp Med. 1990;171:377–387. doi: 10.1084/jem.171.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow–derived cells in presenting MHC class I–restricted tumor antigens. Science (Wash DC) 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 6.Bevan MJ. Immunology. Stimulating killer cells. Nature (Lond) 1989;342:478–479. doi: 10.1038/342478a0. [DOI] [PubMed] [Google Scholar]
- 7.von Boehmer H, Hafen K. Minor but not major histocompatibility antigens of thymus epithelium tolerize precursors of cytolytic T cells. Nature (Lond) 1986;320:626–628. doi: 10.1038/320626a0. [DOI] [PubMed] [Google Scholar]
- 8.Kurts C, Heath WR, Carbone FR, Allison J, Miller JFAP, Kosaka H. Constitutive class I–restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 10.Blanas E, Carbone FR, Allison J, Miller JFAP, Heath WR. Induction of autoimmune diabetes by oral administration of autoantigen. Science (Wash DC) 1996;274:1707–1709. doi: 10.1126/science.274.5293.1707. [DOI] [PubMed] [Google Scholar]
- 11.Heath WR, Allison J, Hoffmann MW, Schonrich G, Hammerling G, Arnold B, Miller JFAP. Autoimmune diabetes as a consequence of locally produced interleukin-2. Nature (Lond) 1992;359:547–549. doi: 10.1038/359547a0. [DOI] [PubMed] [Google Scholar]
- 12.Nikolic-Zugic J, Bevan MJ. Role of self-peptides in positively selecting the T-cell repertoire. Nature (Lond) 1990;344:65–67. doi: 10.1038/344065a0. [DOI] [PubMed] [Google Scholar]
- 13.Debrick JE, Campbell PA, Staerz UD. Macrophages as accessory cells for class I MHC–restricted immune responses. J Immunol. 1991;147:2846–2851. [PubMed] [Google Scholar]
- 14.Husmann LA, Bevan MJ. Cooperation between helper T cells and cytotoxic T lymphocyte precursors. Ann NY Acad Sci. 1988;532:158–169. doi: 10.1111/j.1749-6632.1988.tb36335.x. [DOI] [PubMed] [Google Scholar]
- 15.Guerder S, Matzinger P. A fail-safe mechanism for maintaining self-tolerance. J Exp Med. 1992;176:553–564. doi: 10.1084/jem.176.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieger NR, Yin DP, Fathman CG. CD4+ but not CD8+cells are essential for allorejection. J Exp Med. 1996;184:2013–2018. doi: 10.1084/jem.184.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed R, Butler LD, Bhatti L. T4+T helper cell function in vivo: differential requirement for induction of antiviral cytotoxic T-cell and antibody responses. J Virol. 1988;62:2102–2106. doi: 10.1128/jvi.62.6.2102-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nash AA, Jayasuriya A, Phelan J, Cobbold SP, Waldmann H, Prospero T. Different roles for L3T4+ and Lyt 2+T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987;68:825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- 19.Buller RM, Holmes KL, Hugin A, Frederickson TN, Morse HC., III Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature (Lond) 1987;328:77–79. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Mullbacher A. The generation and activation of memory class I MHC restricted cytotoxic T cell responses to influenza A virus in vivo do not require CD4+T cells. Immunol Cell Biol. 1989;67:413–420. doi: 10.1038/icb.1989.58. [DOI] [PubMed] [Google Scholar]
- 21.von Herrath MG, Yokoyama M, Dockter J, Oldstone MBA, Whitton JL. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J Virol. 1996;70:1072–1079. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a γ-herpesvirus in the absence of CD4+T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keene JA, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cassell D, Forman J. Linked recognition of helper and cytotoxic antigenic determinants for the generation of cytotoxic T lymphocytes. Ann NY Acad Sci. 1988;532:51–60. doi: 10.1111/j.1749-6632.1988.tb36325.x. [DOI] [PubMed] [Google Scholar]
- 25.Juretic A, Malenica B, Juretic E, Klein J, Nagy ZA. Helper effects required during in vivo priming for a cytolytic response to the H-Y antigen in nonresponder mice. J Immunol. 1985;134:1408–1414. [PubMed] [Google Scholar]
- 26.Mitchison NA, O'Malley C. Three-cell-type clusters of T cells with antigen-presenting cells best explain the epitope linkage and noncognate requirements of the in vivo cytolytic response. Eur J Immunol. 1987;17:1579–1583. doi: 10.1002/eji.1830171109. [DOI] [PubMed] [Google Scholar]
- 27.Rock KL. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996;17:131–137. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 28.Shirai M, Pendleton CD, Ahlers J, Takeshita T, Newman M, Berzofsky JA. Helper-cytotoxic T lymphocyte (CTL) determinant linkage required for priming of anti-HIV CD8+CTL in vivo with peptide vaccine constructs. J Immunol. 1994;152:549–556. [PubMed] [Google Scholar]
- 28a.Kurts, C., H. Kosaka, F.R. Carbone, J.F.A.P. Miller, and W.R. Heath. 1997. Class I–restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. In press. [DOI] [PMC free article] [PubMed]
- 29.Jondel M, Schirmbeck R, Reimann J. MHC class I–restricted CTL responses to exogenous antigens. Immunity. 1996;5:295–302. doi: 10.1016/s1074-7613(00)80255-1. [DOI] [PubMed] [Google Scholar]
- 30.Carbone FR, Bevan MJ. Induction of ovalbumin-specific cytotoxic T cells by in vivo peptide immunization. J Exp Med. 1989;169:603–612. doi: 10.1084/jem.169.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]