Abstract
Ligation of CCR5 by the CC chemokines RANTES, MIP-1α or MIP-1β, and of CXCR4 by the CXC chemokine SDF-1α, profoundly inhibits the replication of HIV strains that use these coreceptors for entry into CD4+ T lymphocytes. The mechanism of entry inhibition is not known. We found a rapid and extensive downregulation of CXCR4 by SDF-1α and of CCR5 by RANTES or the antagonist RANTES(9-68). Confocal laser scanning microscopy showed that CCR5 and CXCR4, after binding to their ligands, are internalized into vesicles that qualify as early endosomes as indicated by colocalization with transferrin receptors. Internalization was not affected by treatment with Bordetella pertussis toxin, showing that it is independent of signaling via Gi-proteins. Removal of SDF-1α led to rapid, but incomplete surface reexpression of CXCR4, a process that was not inhibited by cycloheximide, suggesting that the coreceptor is recycling from the internalization pool. Deletion of the COOH-terminal, cytoplasmic domain of CXCR4 did not affect HIV entry, but prevented SDF-1α–induced receptor downregulation and decreased the potency of SDF-1α as inhibitor of HIV replication. Our results indicate that the ability of the coreceptor to internalize is not required for HIV entry, but contributes to the HIV suppressive effect of CXC and CC chemokines.
Expression of CD4 is necessary but not sufficient for productive infection of human cells with HIV (1, 2). The existence of an additional recognition site was postulated several years ago (3–4), and it was recently shown that some chemokine receptors fulfill such a function (5–10). CXCR4 and CCR5 are the major HIV coreceptors (5–7, 10), although similar functions were also reported for CCR2b and CCR3 (8, 9). It has been shown that the CC chemokines, RANTES, MIP-1α, and MIP-1β, which are agonists for CCR5, inhibit entry of primary, non-syncytium–inducing (NSI) strains that are preferentially isolated at early stages of the infection (11). The CXC chemokine, SDF-1α, the ligand of CXCR4, inhibits cell fusion and infection by HIV strains of the syncytium-inducing (SI) phenotype that are usually isolated at late, symptomatic stages of the disease (12, 13).
Chemokines act via seven-transmembrane domain receptors that couple to heterotrimeric Gi-proteins. Their antiviral activity is thought to depend on competition for the binding of the HIV envelope (Env) glycoprotein gp120 to chemokines receptors (14, 15). It has been reported that mere occupancy of HIV coreceptors by chemokines, in the absence of Gi protein–mediated signaling, is sufficient for inhibition of HIV infection. In fact, RANTES inhibits HIV infection of cells treated with B. pertussis toxin, and a CCR5 antagonist, RANTES(9-68), was shown to prevent infection by primary NSI isolates (14, 16). The chemokines, however, could also inhibit viral entry by downregulating the expression of their receptors which may be endocytosed upon ligand binding, as previously shown for the IL-8 and MCP-1 receptors (17, 18). Therefore, we have studied this process and the effect of receptor uptake on the HIV suppressive activity of chemokines. In this paper we describe a rapid, profound downregulation of CXCR4 by SDF-1α and of CCR5 by RANTES and RANTES(9-68) in different cells, and show that the HIV suppressive effect of chemokines is markedly reduced when receptor endocytosis does not occur.
Materials and Methods
DNA Expression Vectors, Cells, and Chemokines.
The CXCR4 WT expression vector contains the LESTR cDNA (19) cloned in a pcDNA3 plasmid (InVitrogen, The Netherlands). The CXCR4 ΔCyt vector was prepared by a PCR-based strategy, by deleting the last 41 amino acids that correspond to the COOH-terminal intracytoplasmic domain of CXCR4. A PCR-synthesized CCR5 DNA insert deleted of the stop codon was fused to a red-shifted variant of the wild-type Green Fluorescent Protein (GFP) in a pEGFP plasmid (Clontech, CA). All the PCR products were sequenced by the dideoxy method. HeLa is a human epithelial cell line. U373-CD4 LTR lacZ cell clone derived from the human glioblastoma cell line U373-MG (20), was transduced with human CD4 and carries an integrated E. coli β-galactosidase reporter gene driven by a HIV-1 LTR. U373-MG cells, contrary to CEM and HeLa cells, do not express CXCR4 constitutively (21). HeLa-CCR5-GFP cells (clone P4-C5) were derived from HeLa-CD4 LTR lacZ (clone P4-2) (22) cotransfected with the CCR5-GFP vector and a plasmid carrying a hygromycin-resistance cassette. CHO is a chinese hamster epithelial cell line. The CHO-CCR5-GFP cell clone was established by transfecting the CCR5-GFP vector that encodes a neomycin resistance gene. The CCR5-GFP receptor proved to be fully competent to support viral entry and Env-mediated cell fusion by CCR5-tropic isolates (HIVJR-CSF, HIVYU2, HIVADA), both in HeLa-CCR5-GFP cells or in CHO-CCR5-GFP transiently expressing CD4 (not shown). CEM is a human lymphoblastoid CD4+ T cell line. Human peripheral blood mononuclear cells (PBMC) were isolated by Ficoll gradient centrifugation and depleted of monocytes by plastic adherence at 37°C. SDF-1α, RANTES, and the antagonist RANTES(9-68) were chemically synthesized by Dr. I. Clark-Lewis (University of British Columbia, Vancouver, Canada) (23).
Indirect Immunofluorescence Staining.
CHO- and HeLa-CCR5-GFP cells were cultured on glass coverslips in 24-well plates. CEM cells were seeded on coverslips coated with poly-l-lysine. The cells were treated for 30 min at 37°C with 200 nM SDF-1α, RANTES, or RANTES(9-68). After incubation with ligands, the cells were washed and fixed for 20 min in 3.7% paraformaldehyde-PBS, washed again in PBS, mounted in 133 mg/ml Mowiol (HOECHST), 33% glycerol, 133 mM Tris-HCl, pH 8.5, and analyzed by confocal microscopy. After fixation, CEM cells were incubated for 15 min in PBS and 0.1 M glycine to quench free aldehydes, and permeabilized by incubation with 0.05% saponin in PBS supplemented with 0.2% BSA for 15 min. The cells were then incubated for 45 min at room temperature with the anti-CXCR4 monoclonal antibody 12G5 (24), a kind gift of Dr. J. Hoxie (University of Pennsylvania Medical Center, Philadelphia, PA), and labeled with a secondary, fluorescein-conjugated (FITC) goat anti–mouse IgG antibody. For staining with iron-loaded human transferrin coupled to rhodamine (Tf-RITC), cells were deprived of transferrin for 30 min at 37°C in serum-free medium, and then incubated for 30 min at 37°C with Tf-RITC along with the appropriate chemokine or antagonist. Confocal laser scanning microscopy and double-fluorescence analysis were performed with a TCS4D confocal microscope (Leica, Nussloch, Germany) interfaced with Argon/Krypton lasers. Simultaneous double-fluorescence acquisitions were performed using the 488- and the 568-nm laser lines to excite FITC and RITC dyes using a 100× oil immersion Plan APO objective (NA = 1.4). The fluorescence was selected with the appropriate double-fluorescence dichroic mirror and band pass filters, and measured with blue-green and red side sensitive one photomultipliers.
Cell Transfection, Flow Cytometry Analysis and Recording of Intracellular Ca2+ ([Ca2+]i) Changes.
Cells (8 × 106) were resuspended in Dulbecco's modified Eagle's medium, supplemented with 10% FCS, 1–4 μg of the appropriate DNA expression vectors, and 12 μg of a non-coding carrier DNA plasmid. Electroporation was performed in 4-mm cuvettes at 220 V, 960 μF in a Bio-Rad Gene Pulser. For flow cytometry, cells (5 × 105) were incubated for 1 h with anti-CXCR4 and subsequently labeled with a secondary, PE-conjugated goat anti–mouse IgG antibody (Southern Biotechnology, Birmingham, AL). After staining, cells were fixed in 1% paraformaldehyde-PBS containing 0.2% BSA and CXCR4 expression was analyzed with a FACScan® cytofluorometer (Becton Dickinson, Mountain View, CA). CXCR4 expression in monocyte-depleted PBMC was studied on gated CD4+ cells. The cells were incubated with anti-CXCR4 and labeled with the PE-conjugated secondary antibody followed by a FITC-conjugated anti-CD4 antibody (Leu3a; Becton Dickinson). Real-time recordings of [Ca2+]i were performed with an IMSTAR imaging system in Fura-2 loaded cells as described previously (25). To remove cell-bound SDF-1α, the cells were exposed for 1 min at 37°C to an acid buffer, pH 3.0, consisting of 50 mM glycine and 100 mM NaCl buffer (106 cells per ml), and then centrifuged and resuspended into the appropriate medium (17).
Viral Infections.
U373-CD4 LTRlacZ cells transfected with either the CXCR4 WT or CXR4 ΔCyt vectors were seeded in microtiter plates (104 cells per well) in a final volume of 200 μl. 24 h after transfection the cells were incubated with infectious supernatants obtained from MT4 cell cultures infected with the HIV-1 molecular clone NL4-3 (HIV-1NL4-3) (40 ng of HIV-1p24 per well). SDF-1α or RANTES were added to the cells 20 min before infection and were present during the whole culture period. Cultures were carried out in triplicate. Infected cells were identified by staining for β-galactosidase. HIV-1(ΔEnv)G is a pseudotyped HIV-1NL4-3 variant with a replacement of the Env protein with the Env (G) glycoprotein of the vesicular stomatitis virus (VSV). To generate infectious supernatants of HIV-1(ΔEnv)G, plasmids encoding the HIV-1NL4-3 proviral DNA deleted of the env gene and the Env (G) glycoprotein of VSV were cotransfected in HeLa cells. U373-CD4 LTRlacZ cells were infected with HIV-1(ΔEnv)G infectious supernatants (4 ng of HIV-1 p24 per well).
Results and Discussion
Chemokine Receptor Internalization.
After incubation with SDF-1α, surface expression of CXCR4 in CEM cells (Fig. 1 a), monocyte-depleted PBMC (Fig. 1 b), or HeLa cells (Fig. 1 c), was analyzed by flow cytometry using anti-CXCR4. Before analysis, SDF-1α–treated cells were washed in an acidic glycine buffer, a procedure previously adopted to dissociate IL-8 from its receptors (17). The washing removed receptor-bound SDF-1α that could interfere with the binding of anti-CXCR4 (data not shown). As shown in Fig. 1, a–c SDF-1α induced a profound decrease of cell surface receptors in all three cell types. The downregulation of CXCR4 was specific for SDF-1α and was not observed when the cells were treated with RANTES, which does not bind CXCR4 (Fig. 1, a–c). Upon treatment with RANTES CXCR4 expression was comparable to that of control cells that were not exposed to a chemokine but washed with the glycine buffer. This indicates that the washing with the acidic buffer did not modify the expression of CXCR4 or the ability of anti-CXCR4 to recognize surface receptors.
Figure 1.
Effect of SDF-1α stimulation on CXCR4 surface expression. CEM lymphoblastoid T cells (a), monocyte-depleted PBMC (b), or HeLa cells (c) were treated for 40 min at 37°C with 200 nM SDF-1α, 200 nM RANTES, or medium alone (UNTREATED). The cells were then washed with acidic glycine buffer, labeled at 4°C with anti-CXCR4 and a PE-conjugated secondary antibody, and analyzed by flow cytometry. In b, analysis was performed on gated CD4+ cells (FITC-conjugated anti-CD4). Control cells (CTRL) were labeled with the secondary antibody only. (d) Dependence of CXCR4 downregulation on SDF-1α concentration. CEM cells were incubated for 40 min at 37°C with increasing concentrations of SDF-1α, and surface expression of CXCR4 was determined. PTX: before incubation with SDF-1α, the cells were treated with 5 μg/ml pertussis toxin for 90 min. (e) Time course of CXCR4 downregulation. Cells were pre-incubated at 4°C for 60 min with 200 nM SDF-1α or RANTES. After washing, cells were cultured at 37°C for the indicated times in the absence of chemokines. ( f ) Re-expression of CXCR4. The cells were incubated at 37°C for 40 min with 200 nM SDF-1α in the presence or absence of 100 μg/ml cycloheximide, washed in acidic glycine buffer and further cultured for up to 60 min at 37 °C with or without cycloheximide in the absence of SDF-1α. (d–f ) Relative surface expression of CXCR4 was analyzed by flow cytometry as described for a.
CXCR4 downregulation induced by SDF-1α was concentration dependent, and the maximal effect was reached at 200 nM as shown in CEM cells (Fig. 1 d). Pretreatment of the cells with pertussis toxin under conditions that completely inhibited Ca2+ mobilization by SDF-1α (data not shown) did not prevent the decrease of cell surface receptor expression (Fig. 1 d). This indicates that the observed downregulation of CXCR4 was not dependent on signaling by Gi-proteins. The downregulation was rapid. It was already detectable 5 min after addition of SDF-1α and progressed for ∼20 min (Fig. 1 e). Further incubation in the presence of chemokine up to 60 min did not lead to an additional decrease of the number of surface-detectable receptors.
To investigate the fate of CXCR4 after downregulation, CEM cells treated with SDF-1α and washed with the acidic buffer were further incubated at 37°C in the absence of the chemokine. Surface re-expression of CXCR4 was detected within the first 15 min (Fig. 1 f ), but no further increase in receptor density was observed for up to 60 min. Re-expression was not dependent on de novo protein synthesis since it was not affected by the presence of cycloheximide. These results suggest that after binding of SDF-1α CXCR4 is internalized and re-expressed at the cell surface by a recycling mechanism. Recycling is likely to account for the fact that the expression of CXCR4 never decreased below 10–25% even at high SDF-1α concentrations and prolonged incubation times.
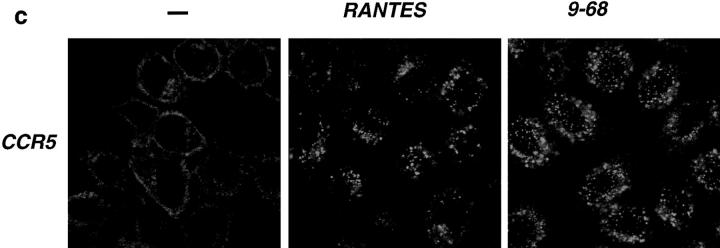
Ligand-induced endocytosis of CXCR4 was assessed in CEM cells by confocal laser scanning microscopy. In the absence of chemokine, CXCR4 was mainly detected at the cell surface. Exposure to SDF-1α induced a dramatic redistribution of the staining that is consistent with the intracellular accumulation of the receptor. This effect was specific for SDF-1α and was not observed when the cells were exposed to RANTES (Fig. 2 a, CXCR4). The subcellular distribution of internalized CXCR4 was studied by simultaneous labeling with anti-CXCR4 (green) and human transferrin coupled to rhodamine (Tf-RITC, red ) which is taken up into early endosomes. As shown in Fig. 2 a (CXCR4+ Tf-RITC), both markers were largely colocalized, as revealed by the yellow spots, indicating a significant accumulation of internalized CXCR4 in early endosomes. Similar experiments were performed to study the ligand-induced endocytosis of CCR5. Since anti-CCR5 antibodies for immunodetection were not available, we used cell clones permanently expressing CCR5 fused to the fluorescence marker GFP (CHO-CCR5-GFP and HeLa-CCR5-GFP). Addition of RANTES to either clone induced receptor endocytosis (Figs. 2, b and c, CCR5). Colocalization with Tf-RITC, in analogy to the above experiments, show that CCR5-GFP accumulates preferentially in early endosomes, as indicated by the clusters of yellow spots in the juxtanuclear region (Fig. 2 b, CCR5+Tf-RITC). The occurrence of internalized CXCR4 or CCR5 that do not colocalize with Tf-RITC (green spots) might reflect the transfer of receptors to late endosomes. The CCR5 antagonist RANTES(9-68), which was previously shown to block infection by monocytotropic HIV isolates despite its inability to elicit Ca2+ mobilization and chemotaxis (16), was as effective as RANTES as inducer of CCR5-GFP internalization (Fig. 2, b and c). This is in agreement with the observation that the SDF-1α–dependent internalization of CXCR4 was not affected by pertussis toxin and confirms that chemokine receptor endocytosis does not require signaling via Gi-proteins.
Figure 2.
Internalization of CXCR4 (a) and CCR5 (b and c) were analyzed by confocal laser scanning microscopy in CEM (a), CHO-CCR5-GFP (b), and HeLa-CCR5-GFP (c) cells after exposure to 200 nM SDF-1α, RANTES or RANTES(9-68) for 30 min at 37°C. –, untreated cells; CXCR4, cells labeled with anti-CXCR4 and a FITC-conjugated secondary antibody; CCR5, autofluorescence of CCR5-GFP; CXCR4+, Tf-RITC and CCR5+Tf-RITC, simultaneous detection of Tf-RITC (red ) and either CXCR4 or CCR5-GFP ( green). Yellow spots indicate colocalization of chemokine receptor and Tf-RITC.
COOH-terminally Truncated Receptor.
It was previously shown that endocytosis of IL-8 receptors requires an intact COOH-terminal cytoplasmic domain (26). The role of the corresponding domain of CXCR4 was, therefore, investigated using HeLa cells that were transiently transfected with a vector expressing a CXCR4 cDNA deleted of the last 41 amino acids (CXCR4 ΔCyt). HeLa cells were chosen because they constitutively express CXCR4, and allow to perform simultaneous analysis of SDF-1α effects on endogenous and transfected CXCR4. Cotransfection of either CXCR4 WT or CXCR4 ΔCyt with a GFP reporter vector permitted to distinguish transfected from nontransfected cells. Incubation with SDF-1α induced a profound downregulation of CXCR4 WT, but did not affect the surface expression of the COOH-terminally truncated receptor, CXCR4 ΔCyt (Fig. 3, a and b). As expected the endogenous CXCR4, in nontransfected cells which are identified by the lack of GFP fluorescence, was also markedly down-regulated by SDF-1α (Fig. 3 a). PMA had a similar effect. It downregulated CXCR4 WT (Fig. 3 b) as well as the endogenous CXCR4 (not shown), but not CXCR4 ΔCyt (Fig. 3 b). These results are in agreement with a previous report showing downregulation of CXCR4 by PMA in human T lymphocytes (27), and suggest that phosphorylation of serines and threonines in the COOH-terminal region are involved in internalization. It has been shown that phosphorylation of the COOH-terminal domain is essential for arrestin-mediated uptake of seven-transmembrane domain receptors via clathrin-coated pits (28, 29).
Figure 3.
Effect of deletion of the COOH-terminal cytoplasmic domain of CXCR4. (a) HeLa cells were transiently transfected with the CXCR4 WT or the CXCR4 ΔCyt expression vector, along with a plasmid encoding the reporter gene GFP (pEGFP). 24 h later, the cells were incubated for 45 min at 37°C in the presence or absence of 300 nM SDF-1α, labeled with anti-CXCR4, and analyzed by flow cytometry. Expression of GFP allows to distinguish transfected (GFP +) and nontransfected (GFP −) cells. After transfection with CXCR4 WT or CXCR4 Δcyt, SDF-1α–dependent downregulation of the endogenous and the transfected receptor were monitored in GFP− and GFP+ cells, respectively. (–) HeLa cells were transiently transfected with the CXCR4 WT or CXCR4 ΔCyt expression vector, along with the pEGFP plasmid. 24 h later, the cells were incubated for 45 min at 37°C with 300 nM SDF-1α or with 20 ng/ml PMA, labeled with anti-CXCR4 and surface expression of CXCR4 was analyzed by flow cytometry in GFP+ cells. (c) CHO cells were transfected with either the CXCR4 WT or the CXCR4 ΔCyt expression vectors and were loaded 48 h later with Fura-2. CHO control cells were transfected with vector DNA alone (pcDNA3). Recordings of [Ca2+]i changes after stimulation with 200 nM of SDF-1α are shown.
CHO cells transiently expressing either CXCR4 WT or CXCR4 ΔCyt were used to assess receptor signaling. As shown by the changes in the cytosolic free Ca2+ concentration in response to SDF-1α, the wild-type and the COOH-terminally truncated receptor were equally capable of eliciting a response indicating that the COOH-terminal domain of CXCR4 is not required for Gi-protein coupling (Fig. 3 c). This result is in agreement with a previous report indicating that deletion of the COOH-terminal domain of the IL-8 receptors did not affect signal transduction (26).
HIV Entry.
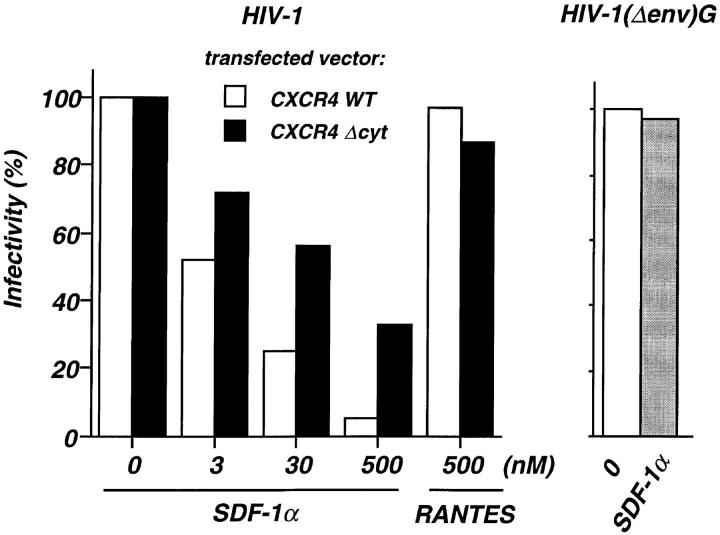
The capacity of the CXCR4 ΔCyt molecule to act as coreceptor for HIV-1 entry was studied in the human, U373-CD4 LTRlacZ astrocytoma cell clone which does not express endogenous CXCR4 and carries an integrated β-galactosidase reporter gene driven by the HIV-1 LTR. Both CXCR4 ΔCyt and the wild-type receptor could be expressed with similar efficiency, as assessed by FACS® analysis and by scoring of HIV-1 infected cells. It was, therefore, possible to study the role of receptor occupancy and receptor internalization as mechanisms for the HIV suppressive activity of SDF-1α, using U373-CD4 LTRlacZ cells that were transiently transfected with CXCR4 ΔCyt or CXCR4 WT. HIV infection was inhibited by SDF-1α in a concentration-dependent manner in cells expressing the truncated or the wild-type receptor, confirming that both bind the chemokine and can act as HIV coreceptors. However, the efficacy of SDF-1α as an inhibitor of infection was markedly lower in cells expressing CXCR4 ΔCyt (Fig. 4). In cells with the wild-type receptor SDF-1α has two effects that decrease infectability, competition for HIV binding and a drastic reduction of receptor numbers by rapid endocytosis. Both mechanisms operate at the same time suggesting that the full antiviral effects of SDF-1α is a combination of competition for receptor binding by the virus and receptor endocytosis. In cells bearing the COOH-terminally truncated receptor downregulation cannot occur, and protection by SDF-1α is less efficient because receptor density remains high. Findings similar to those in the U373-CD4 LTRlacZ glioblastoma cells were obtained in HeLa-CD4 LTRlacZ cells, where SDF-1α was less efficient as an inhibitor of HIV replication when CXCR4 ΔCyt was expressed (data not shown). The selectivity of SDF-1α as an inhibitor of CXCR4-dependent infection is indicated by two lines of evidence. RANTES was unable to block entry of the CXCR4-dependent HIVNL4-3 viral clone in U373-CD4 LTRlacZ cells, and SDF-1α did not inhibit infection by an Env-deleted HIVNL4-3 clone pseudotyped with the Env (G) protein of VSV (Fig. 4).
Figure 4.
SDF-1α–dependent inhibition of HIV infection in cells expressing wild-type or COOH-terminally truncated CXCR4. U373-CD4 LTRlacZ were transfected with either CXCR4 WT or CXCR4 ΔCyt expression vector, plated in 96-well plates (104 cells per well) and infected with the HIV-1NL4-3 strain or the pseudotyped HIV-1(Δenv)G, in the presence of the indicated concentrations of SDF-1α or RANTES. HIV-1(Δenv)G–infected cells were treated with 500 nM SDF-1α. Cell cultures were carried out in triplicates. Surface expression of CXCR4 WT or CXCR4 ΔCyt was assessed 24 h after transfection by FACS® analysis and amounted to 482 and 533 fluorescence units, respectively. HIV infection was revealed 24 h later by staining for β-galactosidase activity and scoring of positive cells. The numbers of HIV-infected cells per well in the absence of chemokines were 168 and 232 (mean of five experiments) for cultures transfected with CXCR4 WT and CXCR4 ΔCyt, respectively. In cultures infected with HIV-1(Δenv)G the average number of infected cells per well was 280. Shown are the percentages of infected cells in the presence of chemokines. Infection with HIV-1NL4-3 or HIV-1(Δenv)G in the absence of chemokines was set to 100%.
We have shown that CXCR4 and CCR5 are rapidly down-regulated by endocytosis when the cells are exposed to the appropriate chemokine ligand. The mechanism of receptor internalization was ligand dependent, but was clearly dissociable from chemokine-induced Gi-protein signaling. In fact, CXCR4 internalization was not affected by Gi-protein inactivation with pertussis toxin, and CCR5 internalization was induced by the antagonist RANTES(9-68) which binds to CCR5 but does not activate Gi-proteins. On the other hand, no internalization was observed in cells expressing CXCR4 deleted of its COOH-terminal, cytoplasmic domain, although Gi protein–dependent [Ca2+]i changes were not impaired.
Deletion of the COOH-terminal domain does not affect the ability of CXCR4 to act as a coreceptor for HIV entry. If receptor endocytosis is prevented by truncation, however, SDF-1α is clearly less efficient as an inhibitor of HIV replication. Our present results show that the ability of the receptor to internalize is not required for HIV entry, but may contribute to the HIV suppressive effect of chemokine ligands by reducing the density of coreceptors. Viral entry is a complex phenomenon in which gp120 attachment to CD4 creates a high-affinity binding site for the coreceptor, leading to membrane fusion (30, 31). Chemokines thus appear to exert two types of anti-HIV activities, competition for HIV-1 binding, and downregulation of coreceptor surface expression. Both processes are functionally linked since receptor occupancy triggers internalization, and are likely to synergize with each other.
Acknowledgments
We thank E. Perret for help with confocal laser scanning analysis, I. Clark-Lewis for the generous gift of synthetic chemokines, and B. Moser for helpful discussions. Critical reading of the manuscript by A. Trautmann is acknowledged.
This work was supported by Agence Nationale de Recherche sur le SIDA (ANRS), Fondation pour la Recherche Médicale, a Concerted Action of the European Union (program BIOMED, project ROCIO), and Grant 31-39744.93 of the Swiss National Science Foundation (to M. Baggiolini). A. Amara and S. Le Gall were supported by fellowships from Ensemble contre le SIDA and ANRS, respectively (France). M. Montes is supported by a fellowship from COLCIENCIAS (Colombia).
Footnotes
A. Amara and S. Le Gall contributed equally to this work.
References
- 1.Maddon PJ, Dalgleish AG, McDougal JS, Clapham PR, Weiss RA, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 2.Ashorn PA, Berger EA, Moss B. Human immunodeficiency virus envelope glycoprotein/CD4-mediated fusion of nonprimate cells with human cells. J Virol. 1990;64:2149–2156. doi: 10.1128/jvi.64.5.2149-2156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dragic T, Charneau P, Clavel F, Alizon M. Complementation of murine cells for human immunodeficiency virus envelope/CD4-mediated fusion in human/murine heterokaryons. J Virol. 1992;66:4794–4802. doi: 10.1128/jvi.66.8.4794-4802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broder CC, Dimitrov DS, Blumenthal R, Berger EA. The block to HIV-1 envelope glycoprotein-mediated membrane fusion in animal cells expressing human CD4 can be overcome by a human cell component(s) Virology. 1993;193:483–491. doi: 10.1006/viro.1993.1151. [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science (Wash DC) 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 6.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature (Lond) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 7.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature (Lond) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 8.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 9.Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, Parmentier M, Collman RG, Doms RW. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 10.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1αlpha, MIP-1βeta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science (Wash DC) 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 11.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science (Wash DC) 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 12.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for lestr/fusin and blocks hiv-1 entry. Nature (Lond) 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 13.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, et al. The CXC chemokine SDF-1 is the ligand for lestr/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature (Lond) 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 14.Oravecz T, Pall M, Norcross MA. Beta-chemokine inhibition of monocytotropic HIV-1 infection. Interference with a postbinding fusion step. J Immunol. 1996;157:1329–1332. [PubMed] [Google Scholar]
- 15.D'souza MP, Harden VA. Chemokines and HIV-1 second receptors—confluence of two fields generates optimism in AIDSresearch. Nat Med. 1996;2:1293–1300. doi: 10.1038/nm1296-1293. [DOI] [PubMed] [Google Scholar]
- 16.Arenzana-Seisdedos F, Virelizier JL, Rousset D, Clark-Lewis I, Loetscher P, Moser B, Baggiolini M. HIV blocked by chemokine antagonist. Nature (Lond) 1996;383:400. doi: 10.1038/383400a0. [DOI] [PubMed] [Google Scholar]
- 17.Samanta AK, Oppenheim JJ, Matsushima K. Interleukin 8 (monocyte-derived neutrophil chemotactic factor) dynamically regulates its own receptor expression on human neutrophils. J Biol Chem. 1990;265:183–189. [PubMed] [Google Scholar]
- 18.Fanci C, Gosling J, Tsou CL, Coughlin SR, Charo IF. Phosphorylation by a G protein-coupled kinase inhibits signaling and promotes internalization of the monocyte chemoattractant protein-1 receptor. J Immunol. 1996;157:5606–5612. [PubMed] [Google Scholar]
- 19.Loetscher M, Geiser T, O'Reilly T, Zwahlen R, Baggiolini M, Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem. 1994;269:232–237. [PubMed] [Google Scholar]
- 20.Harrington RD, Geballe AP. Cofactor requirement for human immunodeficiency virus type 1 entry into a CD4-expressing human cell line. J Virol. 1993;67:5939–5947. doi: 10.1128/jvi.67.10.5939-5947.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pleskoff, O., N. Sol, B. Labrosse, and M. Alizon. 1997. Human immunodeficiency virus strains differ in their ability to infect CD4+ cells expressing the rat homolog of CXCR4 (Fusin). J. Virol. In press. [DOI] [PMC free article] [PubMed]
- 22.Clavel F, Charneau P. Fusion from without directed by human immunodeficiency virus particles. J Virol. 1994;68:1179–1185. doi: 10.1128/jvi.68.2.1179-1185.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark-Lewis I, Moser B, Walz A, Baggiolini M, Scott GJ, Aebersold R. Chemical synthesis, purification, and characterization of two inflammatory proteins, neutrophil activating peptide 1 (interleukin-8) and neutrophil activating peptide. Biochemistry. 1991;30:3128–3135. doi: 10.1021/bi00226a021. [DOI] [PubMed] [Google Scholar]
- 24.Endres MJ, Clapham PR, Marsh M, Ahuja M, Turner JD, McKnight A, Thomas JF, Stoebenauhaggarty B, Choe S, Vance PJ, et al. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 25.Donnadieu E, Bismuth G, Trautmann A. Antigen recognition by helper T cells elicits a sequence of distinct changes of their shape and intracellular calcium. Curr Biol. 1994;4:584–595. doi: 10.1016/s0960-9822(00)00130-5. [DOI] [PubMed] [Google Scholar]
- 26.Prado GN, Suzuki H, Wilkinson N, Cousins B, Navarro J. Role of the C terminus of the interleukin 8 receptor in signal transduction and internalization. J Biol Chem. 1996;271:19186–19190. doi: 10.1074/jbc.271.32.19186. [DOI] [PubMed] [Google Scholar]
- 27.Lapham CK, Ouyang J, Chandrasekhar B, Nguyen NY, Dimitrov DS, Golding H. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science (Wash DC) 1996;274:602–605. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 28.Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. β-arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature (Lond) 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson SSG, Downey WE, Colapietro AM, Barak LS, Ménard L, Caron MG. Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science (Wash DC) 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 30.Wu L, Gerard NP, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso AA, Desjardin E, Newman W, et al. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR5. Nature (Lond) 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 31.Trkola A, Dragic T, Arthos J, Binley J, Olson WC, Allaway GP, Cheng-Mayer C, Robinson J, Maddon PJ, Moore JP. CD4-dependent, antibody-sensitive interaction between HIV-1 and its co-receptor CCR5. Nature (Lond) 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]