Abstract
Bacterial superantigens induce peripheral unresponsiveness in CD4+ T cell populations that express appropriate Vβ chains. We have used Vβ3/Vα11 T cell receptor transgenic (Tg) mice and the Vβ3-specific superantigen staphylococcal enterotoxin A (SEA) to further investigate the mechanisms that contribute to such unresponsiveness. As in other models, in vivo exposure to SEA rendered the Tg CD4+ cells unresponsive to subsequent restimulation in vitro with antigen or mitogens. However, when the SEA-treated CD4+ cells were completely purified away from all other contaminating cells, they regained the ability to proliferate and secrete cytokines. Moreover, enriched CD4−CD8− cells from the SEA-treated mice suppressed the responses of fresh control CD4+ cells in mixed cultures indicating that the apparent “anergy” was both transferable and reversible. Further analysis demonstrated that interferon γ, but not the Fas receptor, played a critical role in the suppression.
Under optimum conditions, immune responses are tightly regulated to prevent prolonged inflammation and indiscriminate damage to healthy tissue. Multiple mechanisms are known to contribute to the maintenance of immune tolerance (1) including: deletion of self-reactive T cells during thymic selection (2) and in the periphery (3–5) and the induction of unresponsiveness or anergy among specific B (6) and T cell subsets (7–10). However, the relative contributions of these mechanisms to the in situ downregulation of specific immune responses remain unclear in most instances.
Many experimental models have been developed to identify mechanisms that participate in the induction of tolerance, including injection of (11–13) and oral exposure to (14) soluble proteins. Other models use natural superantigens (SAg1; 15), such as those encoded by the mouse mammary tumor virus genes (5). The fact that both bacteria and viruses have independently evolved similar strategies to subvert host immune responses suggests that SAg often play an important role in host–pathogen interactions. It is therefore important to understand the mechanisms by which SAg induce T cell anergy, both for the purpose of counteracting their effects and because similar strategies may participate in other processes that the host uses to promote self tolerance.
In contrast to some other polyclonal T cell activators, SAg interact specifically with class II MHC molecules (16), stimulating T cells through Vβ-restricted T cell–APC interactions (17) in a way that closely mimics recognition of peptide–MHC complexes. Thus, it is likely that SAg stimulate T cells with similar avidities and via the same signal transduction pathways as conventional antigens (18). In vitro, bacterial SAg induce efficient IL-2 secretion and effector cell generation from purified CD4+ cells (19). However, in vivo, SAg induced proliferative responses are surprisingly short lived with a short burst of T cell expansion and massive cytokine production that can result in shock or death (20, 21). The numbers of responding CD4+ cells then fall rapidly as they undergo apoptosis (22, 23). Remaining cells, with appropriate Vβ expression, become unresponsive or anergic (20, 21). Some reports suggest that anergy can be overcome by the introduction of IL-2 (24, 25) or removal of antigen (26), although this is not always the case.
We have used the staphylococcal SAg, staphylococcal enterotoxin A (SEA), injected into transgenic (Tg) AND mice, to further investigate the mechanisms that downregulate CD4+ cells in vivo. AND mice express a Vα11+Vβ3+ TCR specific for a peptide fragment of pigeon cytochrome C (PCCF) residues 88–104 and I-Ek class II MHC molecules (27). More than 95% of peripheral CD4+ cells express both chains of the Tg TCR and are naive in phenotype (28, 29). In vitro, they generate active effector populations, giving excellent proliferative responses and secreting high titers of IL-2 in response to either PCCF (30, 31) or SEA (Schuenke, K., G. Huston, and S. Swain, manuscript submitted). However, after SEA treatment, the Tg CD4+ cells become anergic as defined by their inability to proliferate or secrete cytokines upon restimulation. “Anergy” reaches a maximum 5–7 d after injection, when a majority of the Tg CD4+ cells shift from a naive phenotype to an activated memory phenotype, and persist for several weeks (Schuenke, K., G. Huston, and S. Swain, manuscript submitted).
By analyzing the response of a uniform population of Tg CD4+ cells to in vivo SEA exposure, we have identified a novel mechanism of T cell tolerance that depends on IFN-γ, but does not require Fas expression. We show that the anergic CD4+ cells from the SEA-treated mice could be rescued from their unresponsive state either by high level purification such as FACS® (Becton Dickinson, San Jose, CA) sorting, or by the addition of neutralizing anti–IFN-γ Ab to in vitro cultures. Moreover, enriched CD4−CD8− cells from the spleens of the SEA-treated mice were able to suppress control CD4+ cells in mixed cultures, suggesting that the SAg-induced anergy was the result of an active process of suppression, rather than an irreversible loss of autonomous T cell function.
Materials and Methods
Mice.
C57BL/6 (B6) × SJL founder AND Tg mice (27) were backcrossed to B10.BR mice for six to eight generations. Mice between 3–7 mo of age received a single injection (20 μg) of purified SEA (Toxin Technologies, Sarasota, FL) in HBSS by intravenous injection in the tail. Fas− MRL-lpr/lpr and syngeneic C3H mice were obtained from Jackson Laboratories (Bar Harbor, ME).
Cell Lines.
Murine fibroblasts transfected with I-Ek class II MHC molecules and intercellular adhesion meolcule 1 (DCEK intercellular adhesion molecules), which also express high levels of B7.1 (32), were used as APC.
T Cell Enrichment and Effector Cell Generation.
CD4+ cells were enriched by Ab and complement (C′)-mediated depletion, using anti–Thy 1.2 (HO.13.14), anti-heat stable antigen (J11D), anti– class II MHC (D3.137, M5114, and CA4), and anti-CD8 (3.155) Ab. Cell debris were removed with lympholyte separation gradients spun at 2.2 rpm for 20 min at room temperature. These CD4+ cells, referred to as “enriched”, were cultured in RPMI 1,640 medium supplemented with penicillin (200 μg/ml), streptomycin (200 μg/ml), 4 mM l-glutamine, 10 mM Hepes, 50 μM β-mercaptoethanol, and 10% FCS at 3 × 105/ml, with 105 APC/ml, 5 μM PCCF, and 20 U/ml rIL-2. Th1 and Th2 effector cells were generated as previously reported (33) using rIL-2 at 20 U/ ml, rIL-12 (5 ng/ml), and anti–IL-4 (11B11) at 10 μg/ml for Th1 cells and rIL-4 at 10 μg/ml with anti–IFN-γ (XMG1.2) at 10 μg/ml for Th2 cells.
Positive Selection of CD4+ Cells.
“Pure” populations of CD4+ cells were isolated by positive selection using magnetic columns (>95% CD4+) or by sterile sorting (>99% CD4+) using a FACStar Plus® FACS®.
Before positive selection on Magnetic Cell Separation magnetic columns (Miltenyi, Sunnyvale, CA), nonadherent cells were enriched on nylon wool columns for 1 h at 37°C. The nonadherent cells were stained with Ab to CD4 coupled to magnetic beads, on ice for 30 min and passed over Magnetic Cell Separation columns to retain the bead-coated CD4+ cells. The bound cells were eluted by removing the magnet. The purity of the CD4+ cells was verified by FACS® analysis.
For sterile FACS® sorting, splenic lymphocytes were enriched using T cell enrichment columns (R&D Sys. Inc., Minneapolis, MN). Eluted cells were stained with PE-conjugated anti-CD4 (PE-GK1.5) and further purified by sterile sorting on a FACSTAR Plus®. The purified cells were incubated at 37°C for 1–2 h, before stimulation with immobilized anti-CD3 Ab (2C11 at 10 μg/ml) and 1 μg/ml soluble anti-CD28.
TUNEL Assay to Detect Apoptotic Cells.
Spleen cells were stained with PE-conjugated anti-CD4, and then washed and fixed with 1% paraformaldehyde at 4°C for 15 min and permeabilized with 0.5 ml cold 70% ethanol at 4°C for 30 min. Fragmented DNA was detected with exogenous terminal deoxynucleotidyl transferase (TdT; 34). End labeling was carried out in cacodylate buffer (Boehringer Mannheim, Indianapolis, IN) with biotinylated deoxy-uridine-triphosphate for 45 min at 37°C. The cells were then stained with fluoresceinated strepavidin in 4× SSC buffer, 5% nonfat milk, and 0.1% Triton X-100 for 30 min at room temperature in the dark and analyzed by two-color FACS® analysis.
Propidium Iodide Staining Used to Detect Dead Cells.
Splenocytes were stained with FITC-conjugated anti-CD4 and washed with PBS. 50 μl of fluorescent propidium iodide (PI) dye (at 100 μg/ ml) was added to each sample (106 cells) immediately before two-color FACS® analysis (35). Gated cell populations were used to identify dead and dying CD4+ cells as indicated in individual figures.
Detection of DNA Synthesis in CD4+ T Cells.
APC were treated with 100 μg/ml mitomycin C for 30–40 min at 37°C and plated at 105/well with either 5 μM PCCF or 250 ng/ml SEA. Triplicate cultures of CD4+ cells incubated at 37°C for 72 h, followed by a 6-h pulse with TdR (28). TdR incorporation was determined by harvesting the lysed cells onto glass filters and counting in a Wallace β counter.
Reverse Transcriptase for Detection of Cytokine Messenger RNA.
T cells were stimulated at 106/ml using plate-bound anti-CD3 Ab (2C11 at 10 μg/ml) and anti-CD28 (1 μg/ml). Total messenger RNA (mRNA) was isolated from 1 or 2 × 106 cells as described (36), quantitated by optical density, and visualized on denaturing formaldehyde agarose gels. Total cellular RNA was analyzed for cytokine mRNA content by competitive, semiquantitative reverse transcriptase (RT)-PCR, using linearized PQRS plasmid as competitor DNA as described (37). PQRS plasmid was linearized with Not1 enzyme and extracted with phenol/chloroform. The cut plasmid was reprecipitated and quantitated by absorbance spectroscopy. Total cellular RNA (1 μg) was used in each 20 μl reverse transcription reaction, carried out according to the method of the manufacturer (GeneAmp RT-PCR kit; Perkin-Elmer Corp., Foster City, CA). The cDNA products of reverse transcription were amplified by 50 PCR amplification cycles (37), with 2 μl of linearized competitor plasmid, diluted through a half-log dilution series, in each sample.
Cytokine ELISA and Bioassays.
An IL-2–dependent indicator cell line (NK.3) was used to quantitate the IL-2 content of culture supernatants as previously described (38). NK.3 cells (104 cells/well) were cultured with anti–IL-4 (11B11) at 10 μg/ml, with rIL-2 for standard curves. After 24 h, the cells were pulsed with TdR for 16 h and harvested on glass filters. The data are expressed as U/ml, where 1 U equals 50% of the maximum response detected by the standard curve. One unit of IL-2 corresponds to 14 pg of protein as determined by comparison to a standard curve of murine rIL-2 in this assay. IL-4, IL-5, and IFN-γ concentrations were determined by sandwich ELISAs as described (28, 38). 1 U of IFN-γ equals the quantity of cytokine required to neutralize the viral destruction of L-929 cells by 50%, as defined by Amgen Biologicals (Thousand Oaks, CA).
Results
SEA Treatment Induces Unresponsiveness of Tg CD4+ Cells.
To verify that SEA induces anergy in Tg CD4+ cells, AND mice were injected with 20 μg of SEA (intravenously). 6–8 d later, splenic CD4+ cells were enriched using Ab to CD8, class II MHC, and heat stable antigen to deplete CD8+ T and B cells. DNA synthesis and cytokine production were used to assess the effect of the SAg treatment. In a typical experiment, the enriched CD4+ cells from SEA-treated, but not control, mice failed to synthesize DNA over a wide range of cell numbers (Fig. 1 a) and PCCF doses (Fig. 1 b). Even ionomycin (Ca2+ ionophore) and PMA, which together circumvent the need for TCR signaling, elicited little response from the SEA-treated cells as compared to the untreated control cells (Fig. 1 c).
Figure 1.
Anergy of enriched CD4+ T cells from SEA-treated transgenic mice. Tg CD4+ cells were broadly unresponsive to antigenic restimulation in vitro after in vivo exposure to SEA. [3H]thymidine incorporation by CD4+ T cells stimulated with (a) 5 μM PCCF and APCs, (b) increasing concentrations of PCCF and APCs, and (c) other T cell mitogens. (d) Cytokine production by CD4+ T cells was also decreased by the in vivo SEA treatment. Similar results were seen in at least three repeated experiments.
Supernatants were also analyzed for cytokine content, revealing substantial IL-2 production by the untreated control cells, in response to both SEA and PCCF. However, after SEA treatment, only 1–200 U of IFN-γ and small quantities of IL-2 were detected in a representative experiment (Fig. 1 d), further indicating that the CD4+ cells were broadly anergic after their in vivo exposure to the SAg.
Cells from SEA-treated Mice Suppress the Responses of Control CD4+ Cells In Vitro.
In non-Tg models, only a fraction of the T cells respond to SAg and the response is heterogeneous even for a single Vβ. Consequently, anergic cells have rarely been isolated and the possibility that unresponsiveness is maintained by a suppresser mechanism has not been adequately addressed. To test for such a suppresser mechanism in the AND mouse model, we added enriched CD4+ cells from the SEA-treated mice to control CD4+ cells in mixed cultures (Fig. 2). Since DNA synthesis is a precursor of cell division, but may not always correlate with proliferation if cells undergo apoptosis, live cell recoveries were used to measure the extent of the CD4+ cell response.
Figure 2.
Cells from SEA-treated mice suppress control CD4+ T cell responses. (a) Purity of cells after purification with Ab and C′. (b) Anergic cells from SEA-treated mice inhibited effector generation by normal CD4+ T cells in mixed wells. (c) Non-CD4+ cells from SEA-treated mice were sufficient for the suppression to be transferred between in vitro cultures. Live CD4+ T cell recovery on day 4 is shown.
Control CD4+ cells were stimulated with mitomycin C-treated APC, PCCF, and rIL-2, with increasing numbers of the SEA-treated cells (65% CD4+). After 4 d, live CD4+ recovery was determined by trypan blue staining and FACS® analysis. All live cell recoveries have been corrected for CD4+ content.
After enrichment, the control CD4+ cells (Fig. 2 a, left) contained small numbers of contaminating CD4−CD8− (double negative; DN) Vβ3+ T cells (∼8%). As expected, these cells proliferated extensively in the presence of the PCCF and APC, resulting in ∼fivefold expansions after 4 d. After enrichment, the SEA-treated cells were 65% CD4+ with 26% DN cells (Fig. 2 a, middle). The addition of these SEA-treated cells (65% CD4+) to control cultures led to dramatically reduced CD4+ recoveries, suggesting that the anergy could be transferred. To determine if the anergic CD4+ cells were directly responsible for the transferred anergy, we treated an enriched population of SEA-treated CD4+ cells with anti-CD4 Ab. When the residual cells (59% DN; Fig. 2 a, right) were mixed with control cells (Fig. 2 c), the potency of the suppression was increased, indicating that anergic CD4+ cells were not required, but that non-CD4+CD8+ cells and/or factors they produced were responsible for the suppression observed.
Extensive Purification of CD4+ Cells from SEA-treated Mice Restores In Vitro Proliferation and Cytokine Production.
Extensive purification procedures were used to determine whether the anergic CD4+ cells were intrinsically unresponsive or, alternatively, were being influenced by other cells or factors from the injected mice such as those responsible for the trans suppression. Positive selection using magnetic bead columns was used to generate pure populations of CD4+ cells with <1–5% contaminating non-CD4+ cells from both control and SEA treated mice (Fig. 3 a).
Figure 3.
(a) Purity of CD4+ cells after enrichment with Ab and C′ (left) and positive selection on magnetic columns (right). (b) Number of live CD4+ cells recovered on day 4.
CD4+ cells were also “enriched” with Ab and C′ treatment (not shown) to verify that the SEA-mediated anergy had occurred in the injected mice as expected. After 4 d in culture with APC and PCCF (Fig. 3 b), very few live CD4+ cells were recovered from the SEA-treated samples, confirming that anergy had been induced as expected. After positive selection, the proliferative capacity of these cells was significantly restored, resulting in 3.5–5-fold increases in the size of the effector cell populations generated. Furthermore, when anergic CD4+ cells from the SEA-treated mice were mixed 1:1 with the positively selected cells, live CD4+ cell recoveries were again reduced, demonstrating that the rescued cells were still sensitive to the transferable anergy.
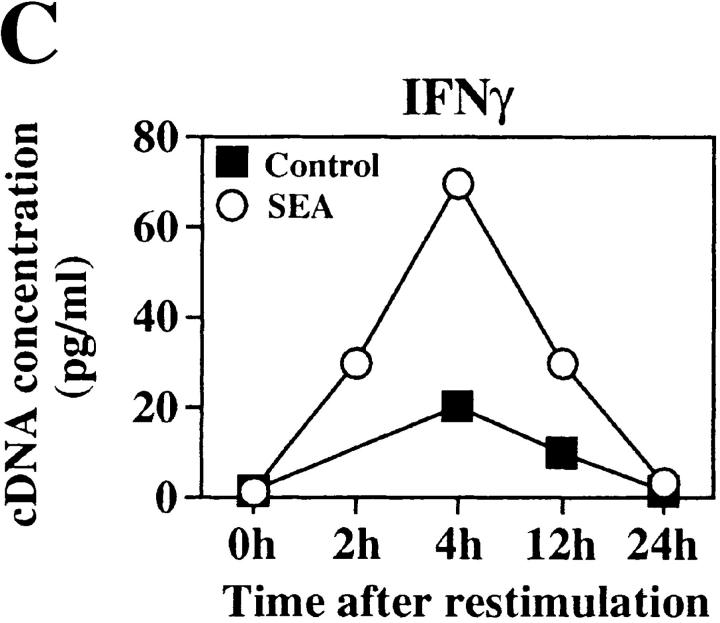
We used sterile FACS® sorting to determine whether cytokine production was restored by purification. Sorted CD4+ cells were stimulated and analyzed for the ability to synthesize cytokine mRNA (Fig. 4). 24 h supernatants were also analyzed for cytokine content (Fig. 5).
Figure 4.
Extensively purified CD4+ cells from SEA-treated mice can synthesize cytokine mRNA. The SEA-mediated T cell anergy was reversed by FACS® sorting the CD4+ cells. (A) PCR analysis of cytokine mRNAs from SEA-treated and control CD4+ T cells after high level purification by positive selection and restimulation with 2C11 and anti-CD28 Ab. PCR products were electrophoresed on 1.5% agarose gels stained with ethidium bromide. Competitor DNA is not shown. (B) PCR products were quantitated using linearized PQRS plasmid as competitor DNA. Upper band is the modified plasmid product and the lower band represents cytokine mRNA product. Two bands indicates the equivalence point of cDNA and plasmid DNA PCR products. RNA from CD4+ cells analyzed 4 h after restimulation is shown. PQRS plasmid concentration is shown in pg/ml. For IL-2 analysis, PQRS plasmid is shown at 100 pg/ml for induced samples, and 0.1 pg/ml for uninduced samples to show equivalence points before normalization to hypoxanthine phosphoribosyl transferase. (C) Kinetic analysis of mRNA synthesized by SEA-treated and control CD4+ cells is shown. Data has been normalized to hypoxanthine phosphoribosyl transferase.
Figure 5.
Cytokine production was restored by purification. SEA-treated and control CD4+ T cells were purified by sterile FACS® sorting or enriched with Ab and C′ and stimulated at 106 cells/ml with anti-CD3 and -CD28. Supernatants were analyzed 24 h after restimulation using ELISA assays and proliferation assays with NK.3 cells.
Sorted CD4+ cells from both control and SEA-treated mice synthesized detectable quantities of IL-2 and some IFN-γ mRNA in response to anti-CD3 and anti-CD28. Very little IL-4 mRNA was detected (Fig. 4 A). The cytokine mRNAs were quantitated by competitive RT-PCR, using linearized PQRS plasmid as competitor DNA (37). Live APC were not used for PCR experiments to avoid RNA contamination. An example of this quantitative analysis is shown for IFN-γ and IL-2 mRNAs analyzed 4 h after stimulation (Fig. 4 B). A broad range of competitor DNA (pg/ml) concentrations were analyzed, and those shown were chosen from the range that best reveals the amount of cytokine mRNA detected in each sample. The concentrations of IL-2 and IFN-γ cDNA extrapolated from several analyses are shown in Fig. 4 C. Unstimulated CD4+ cells from the SEA mice gave <1 pg/ml of cDNA for either cytokine. Upon stimulation, these mRNA levels increased 30–100-fold, reaching a peak of 70 pg/ml for IFN-γ, and 100 pg/ml for IL-2. As expected (Fig. 4 C), naive CD4+ cells from untreated mice also synthesized IL-2 mRNA (70 pg/ml) and some IFN-γ (20 pg/ml). The RNA levels in the SEA-treated group, however, were higher at an early time (4 h after stimulation) and fell off more rapidly, indicating that the kinetics of the response were more accelerated than in the control animals, but were similar to those expected for effector cells (39). Since effector cells (Th1 polarized), but not naive CD4+ cells, are very sensitive to Fas-mediated activation-induced cell death (AICD; 40, 41), it is possible that rapid apoptosis of the SEA-treated cells contributed to the decline in cytokine mRNA detected.
As expected, the unsorted (enriched) CD4+ cells from SEA-treated mice produced very little IL-2 and only small quantities of IFN-γ. However, after sorting, the CD4+ cells from protein SEA-treated and control mice produced comparable levels of IFN-γ and IL-2 protein (Fig. 5), but neither IL-4 or IL-5 were detected. Thus, in the absence of other contaminating cells, the CD4+ cells from SEA-treated mice were able to proliferate and secrete cytokines upon restimulation, demonstrating that the anergy was not an intrinsic property of the CD4+ cells.
IFN-γ Plays an Essential Role in the Suppression of CD4+ T Cell Responses.
Bacterial SAg induce massive cytokine production in vivo, including large quantities of IFN-γ (9, 42). Since small quantities of IFN-γ were detected in the anergic cultures from SEA-treated mice (Fig. 1 d), we tested this cytokine for a role in the anergy using a neutralizing Ab to IFN-γ.
Enriched populations of CD4+ cells from SEA-treated and control mice were stimulated in vitro with or without anti–IFN-γ (XMG1.2 at 10 μg/ml). After 48 and 96 h, the recovery of live CD4+ cells was determined (Fig. 6 a). In this representative experiment, treatment with anti–IFN-γ increased the live CD4+ recoveries in the SEA-treated groups to levels that were comparable with the controls, whereas in the absence of added Ab, no CD4+ cell growth occurred. Interestingly, anti–IFN-γ had comparatively little effect on the survival of control CD4+ cells from untreated animals.
Figure 6.

Anti–IFN-γ reverses SEA-induced anergy. Treatment with anti–IFN-γ Ab increased CD4+ T cell recovery and viability after SEA treatment. (a) CD4+ T cells from SEA-treated and control AND mice were cultured with neutralizing concentration of anti–IFN-γ Ab (XMG1.2). Live cell recovery on day 4 is shown. Cells recovered from above cultures were analyzed for live cell content. (b) Percentage of live cells as determined by F/S. (c) Percentage of CD4+ cells that stained positively with PI. Gated populations of CD4+ cells are shown.
The 4-d cultures were also analyzed for cell death using a combination of forward and side light scatter (F/S) and PI staining (35). The control cells were 49% live by the criteria of F/S (Fig. 6 b), with 54% of the CD4+ cells staining positively with PI (Fig. 6 c). Treatment with anti–IFN-γ (right) only modestly increased these numbers, giving 57% live cell recovery by F/S, and slightly decreased the percentage of PI+ CD4+ cells to 44%. In contrast to the controls, the SEA-treated samples contained large numbers of PI+ CD4+ cells (83%) on day 4. Treatment with anti–IFN-γ increased the viability of these cells dramatically, giving 10-fold expansions from the plating numbers by day 4 (Fig. 6 a) and increased the live cell content of the culture from 16 to 62% by F/S (Fig. 6 b), whereas the percentage of PI+ CD4+ cells decreased from 83 to only 15%.
To investigate whether IFN-γ played a similar role in the transferable suppression, we added anti–IFN-γ to mixed cultures of control CD4+ cells with enriched CD4−CD8− cells from SEA-treated mice (Fig. 7 a). As expected, the cells from the SEA-treated mice suppressed the recovery of fresh CD4+ cells by 20-fold when mixed at a ratio of 1:1 (Fig. 2). The addition of anti–IFN-γ to the mixed cultures restored the recovery of these cells to the levels seen when no SEA-treated cells were added.
Figure 7.
Anti–IFN-γ reverses the transferable suppression by cells from SEA-treated mice. (a) Mixed cultures of anergic and control CD4+ T cells were stimulated with APCs and PCCF in the presence and absence of the neutralizing XMG1.2 Ab. Live CD4+ cell recovery on day 4 is shown. (b) CD4+ effector cells generated in the presence of the XMG1.2 Ab develop the ability to secrete cytokines after antigenic restimulation. Effector cells were further purified on magnetic bead columns and restimulated with PCCF and APC. (c) 24-h supernatants were analyzed for cytokine content.
In Fig. 7 b, enriched cells from both control and SEA-treated mice generated large populations of CD4+ effector cells in the presence of anti–IFN-γ. However, significant numbers of CD4−CD8− cells also grew out (up to 30% in some experiments not shown). Magnetic bead columns were used to further purify the CD4+ cells in this population (>95% CD4+ not shown) for cytokine analysis (Fig. 7 c). The CD4+ cells were then incubated overnight in the presence of rIL-2, to allow the beads to fall off, and then restimulated with APC and PCCF. Cytokine analysis revealed high levels of IL-2 and IFN-γ production by the control cells, and high levels of IL-4 after SEA treatment, with only low levels of IL-2 and IFN-γ. Thus, the anti– IFN-γ treatment reversed the anergy and restored cytokine production, resulting in a somewhat Th2-polarized cytokine profile. Similar results were seen in a repeat experiment.
CD4+ Cells from SEA-treated Mice Are Suppressed by a Fas-independent Mechanism.
Since much peripheral T cell death is mediated by Fas–FasL interactions (43), we investigated the potential contribution of Fas-mediated suicide to the anergy of the CD4+ cells in our model. To do this we directly analyzed enriched CD4+ cells from SEA-treated AND mice for evidence of intracellular DNA damage (44) by TUNEL staining. CD4+ cells were isolated from the spleens of untreated control (no SEA) and injected mice, 2 and 4 d after SEA treatment (two mice each). The enriched cells were either restimulated in vitro with APC, PCCF, and rIL-2 for 12 h, or analyzed immediately (ex vivo) for DNA damage (Fig. 8). Gated populations of CD4+ cells are shown.
Figure 8.

Apoptosis of CD4+ T cells from SEA-treated Tg mice. TdT staining revealed DNA fragmentation in the CD4+ T cells from SEA-treated mice. SEA-treated animals were analyzed 2 and 4 d after SEA injection (two animals each), immediately ex vivo, and 12 h after antigenic restimulation with APCs and PCCF. TdT staining of gated CD4+ T cell populations are shown.
Less than 25% of the CD4+ cells from untreated AND mice were TdT positive when analyzed either immediately ex vivo or 12 h after in vitro stimulation. However, 4 d after SEA injection, relatively large numbers of CD4+ cells had damaged DNA. Intermediate numbers of apoptotic cells were found 2 d after SEA injection. Thus, large numbers of cells had undergone apoptosis as a result of the SEA treatment.
To determine whether Fas–FasL interactions were mediating cell death in the suppressed cultures, we compared Fas-deficient CD4+ cells from MRL-lpr/lpr mice (45) with cells from syngeneic wild-type mice, as targets of the SEA-mediated suppression in the transferable anergy model. Enriched CD4−CD8− cells from SEA-treated AND mice were mixed with Fas-deficient CD4+ cells from untreated MRL-lpr/lpr mice (Fas−) or Fas-bearing (Fas+) cells from wild-type controls (Fig. 9) and stimulated with APC, Con A, and rIL-2. In both cases, the SEA-treated lymphocytes suppressed the recovery of live CD4+ cells (Fas+ and Fas−) from untreated animals (Fig. 9, a and b, respectively). Both cell recoveries were also substantially increased by the addition of anti–IFN-γ Ab to the mixed wells. Thus, CD4+ cells that lacked a functional Fas antigen could be suppressed by the SEA-treated cells from AND mice by an IFN-γ–dependent mechanism, demonstrating that at least some of the anergy was not mediated via Fas–FasL.
Figure 9.

Fas-mediated death is not required for transferable anergy. Effector cell generation by Fas-deficient CD4+ T cells from C3H.MRL-lpr/lpr mice (b) and syngeneic controls (a) was suppressed by splenocytes from SEA-treated AND mice in mixed cultures and could be restored by anti–IFN-γ Ab. The number of live CD4+ T cells recovered on day 4 is shown.
IFN-γ has been suggested to downregulate cloned CD4+ cells in vitro (46). To investigate the possibility that IFN-γ could sensitize normal CD4+ cells to undergo apoptosis, we generated polarized Th1 and Th2 effector cells from naive CD4+ Tg cells in vitro (33). After 4 d, the recovery of these effector cells was ∼ sevenfold higher than the initial numbers plated, with only very small numbers of dead cells (10–15% PI+). The effector cells were then washed, and duplicate cultures were restimulated in the presence or absence of anti–IFN-γ. When the cultures were analyzed for dead cell content 24 h later by PI staining and FACS® analysis (Table 1), dead or dying CD4+ cells were detected in both populations, with the Th1 cells showing considerably greater sensitivity to AICD (up to 60% cell death) than Th2 cells (40, 41). However, no significant effect of the neutralizing anti–IFN-γ Ab was detected in either sample (Th1 or Th2), as determined by the percentage of dead cells or DNA synthesis.
Table 1.
AICD of Normal Effectors Was Not Reversed by Anti–IFN-γ
| Antibodies | 3H incorporation | PI+ | ||||
|---|---|---|---|---|---|---|
| cpm | % | |||||
| Th1 | None | 175,855 ± 12,000 | 65 | |||
| Th1 | XMG1.2 | 160,860 ± 9,717 | 58 | |||
| Th2 | None | 186,166 ± 8,074 | 26 | |||
| Th2 | XMG1.2 | 188,711 ± 8,045 | 38 |
We also investigated whether rIFN-γ would suppress effector cell generation by naive CD4+ cells. However, even additions of >1,000 U/ml of exogenous rIFN-γ, a high dose compared with the small amounts (∼200 U/ml) detected in the suppressed cultures (Fig. 1), did not cause any significant reduction in the size of the live cell population generated over 4 d (Fig. 10). Thus, although IFN-γ was clearly required for the SEA-induced anergy, it was not likely to be entirely responsible for the loss of CD4+ cell function.
Figure 10.
rIFN-γ does not suppress CD4+ cells from untreated AND mice. Naive CD4+ T cells were stimulated with APCs and PCCF in the presence of increasing concentrations of rIFN-γ. Live CD4+ T cell recovery on day 4 is shown. Anti– IFN-γ also did not significantly alter live cell recovery (not shown).
Discussion
It has become clear that multiple mechanisms contribute to the downregulation of T cell responses in vivo and are likely recruited under distinct circumstances of antigen encounter. Since it is often difficult to identify rare antigen-specific T cells in normal animals so that their fate can be adequately monitored, many of the most productive studies have used bacterial SAg to analyze large polyclonal populations of T cells. The experiments reported here document the profound anergy induced in AND mice when SEA is injected. Other studies have shown that the CD4+ cells in the Tg mice decrease somewhat in number as a result of SEA injection and change to an activated/memory phenotype (Shub, F., and S.L. Swain, manuscript in preparation). Enriched CD4+ cells recovered from the SEA-treated mice are anergic in that they fail to synthesize DNA or cytokines in response to in vitro restimulation (Fig. 1). Surprisingly, our studies show that this T cell unresponsiveness is not CD4+ cell–autonomous since the anergy could be transferred between in vitro cultures and extensive purification restored the activity of the SEA-treated cells (Figs. 4 and 5).
Proteins with SAg properties are associated with growing numbers of viruses and bacteria, and are thus relevant to many natural immune responses. Studies show that T cell anergy often develops after a transient period of hyperactivity (20, 21), and that the numbers of CD4+ cells which survive in vivo SAg treatment can vary with the dose and schedule of the injections used (47). In circumstances that left large numbers of anergic CD4+ cells, many surviving cells acquired an activated phenotype (48), indicating that they had responded to the SAg in vivo, yet failed to mediate effector cell functions in vitro. However, under some circumstances, T cell anergy can be reversed, and it has been suggested that cytokines likely play a role (24, 25). Most relevantly, studies by Ramsdell et al. (26) showed that CD4 T cells recovered from mice that express endogenous mouse mammary tumor viral SAg and regain function when adoptively transferred to suitable recipients, demonstrating the reversibility of anergy in that model.
In addition to bacterial SAg, several other methods of tolerance induction lead to abortive expansions of CD4+ T cell populations in vivo (5, 13). In some instances, the subsequent unresponsiveness was attributed to incomplete T cell activation because of APC lacking necessary costimulatory molecules, such as resting B cells (11, 13, 49). The response of CD4+ cells to bacterial SAg, however, is paradoxical in that SAg often elicit massive primary responses from naive T cells before the onset of Vβ-restricted unresponsiveness (20, 21), and efficiently drive CD4+ effector cell generation in vitro (19), even though naive CD4+ cells are normally the most highly dependent upon costimulation (30, 31). This suggests that a deficiency in initial antigen exposure is an unlikely explanation for the anergy observed in the SAg-treated animals. In this study, we show that enriched CD4−CD8− splenocytes from the SEA-treated AND mice suppressed the responses of normal CD4+ in vitro (Fig. 3), suggesting that incomplete T cell activation was not responsible for the apparent anergy observed, but that other cells activated by the SEA treatment, or factors produced by them, were responsible. The anergy in the Tg model is therefore fundamentally distinct from that induced in cell lines in vitro (50). In light of these observations, alternate pathways for the development of T cell anergy to SAg need to be considered.
Some models have suggested that exhaustion (51) or deletion of specific responding cells (23) can play a role in the induction of T cell unresponsiveness, and a number of studies have focused on the role of Fas-mediated apoptosis in the downregulation of CD4+ cell responses in vivo (43, 52). The activated CD4+ cells in SAg-treated mice are similar to polarized Th1 cells (9), which are particularly sensitive to AICD (40, 41). The Tg CD4+ cells from the SEA-treated AND mice exhibited some increased sensitivity to apoptosis after SEA treatment. It is therefore possible that Fas-mediated death contributed to the downregulation of CD4+ T cell responses in vivo. However, our studies show that another mechanism which does not use the Fas antigen accompanies T cell anergy in the AND model, since the response of Fas-deficient CD4+ T cells from C3H-lpr mice (45) was suppressed by cells from SEA-treated mice (Fig. 9).
It is not clear which cells were responsible for the suppression observed. Removal of CD4+ and CD8+ T and B cells enhanced the suppressive capacity of the residual SEA-treated cells (Fig. 2), implying that CD4−CD8− cells were involved. We have found that the residual cell population contained an unusual population of CD4−CD8−CD3+ TCR+ (DN) T cells (Shub, F., and S.L. Swain, unpublished data), as well as a mixture of macrophages and other less well characterized cell populations. It has recently been reported that other TCR-αβ Tg mice can contain unusual populations of CD4−CD8− T cells that are of γ/δ lineage, but that bear the Tg TCR (53). We have not investigated the possibility that the expanded populations of DN T cells found in SEA-treated AND mice are of similar γ/δ lineage; however, our studies show that these cells can be stimulated in vitro to give rise to functional effector cells that produce high titers of IFN-γ (Shub, F., and S.L. Swain, manuscript in preparation).
Although we have shown that IFN-γ is essential to the SEA-mediated suppression in the AND model, rIFN-γ did not suppress effector cell generation by naive CD4+ cells in vitro (Fig. 10) and endogenous IFN-γ was not required for AICD of Th1 effector cells (Table 1). Furthermore, only relatively small quantities of IFN-γ were detected in the suppressed cultures (100–200 U/ml), in comparison to the large quantities produced by Th1 cells (>1,000 U/ml), without significant effect on their ability to proliferate or secrete cytokines. These results suggest that other cells or factors from the SEA-treated mice were contributing to the suppression, and raise the possibility that even states of profound unresponsiveness may be the result of cell interactions masquerading as tolerance.
Although the AND mice were profoundly unresponsive after SEA treatment, it is interesting to note that the injected animals experienced only transient discomfort as a result of the SAg treatment, with insignificant increases in mortality, even though almost all the T cells are capable of responding to SEA (Cauley, L.S., G. Huston, and S.L. Swain, unpublished data). It is possible that we have exaggerated the cytokine crisis experienced by the injected mice by using TCR Tg mice, and may have recruited a mechanism that would normally require much higher doses of antigen in non-Tg animals. However, it is also possible that the efficient downregulation of the CD4+ T cell responses in these animals directly aids in their survival from SAg-induced shock. If the DN cells in AND mice are of γ/δ lineage, their response to SAg treatment may not be entirely physiological. Our studies also suggest that the response of these cells may be reminiscent of CD8+ T cell responses to SAg treatment in non-Tg models (not shown).
In conclusion, we have shown a clear role for transferable anergy in this model of SAg-induced unresponsiveness and have identified a novel mechanism that may participate in the downregulation of CD4+ T cell responses. Our studies show that the anergy produced in AND mice, and detected in enriched populations of Tg CD4+ cells recovered from the injected animals, can be transferred between in vitro cultures or reversed by high level purification of the anergic CD4+ T cells. In light of this observation, we suggest that SAg-induced T cell anergy is not always autonomous, but in some circumstances can result from suppression mediated by non-CD4+CD8+ cells from the spleens of the SAg-treated animals. Since the end result of the anergy was a lack of cell growth and apoptosis upon restimulation, we suggest that such a mechanism may contribute to other forms of high-dose antigen-induced tolerance, and that the Tg model we have used is suitable for defining mechanisms involved in this suppression.
Acknowledgments
We thank Ron LaCourse and Debra Duso for assistance with sterile FACS® sorting experiments and Dr. R. Dutton for suggestions in writing this manuscript.
This work was supported by National Institutes of Health grants AI332204, CA56290, and AI26887.
Footnotes
1 Abbreviations used in this paper: AICD, activation-induced cell death; C′, complement; DN, CD4−CD8− double negative; F/S, forward and side light scatter; mRNA, messenger RNA; PCCF, peptide fragment of pigeon cytochrome; PI, propidium iodide; RT, reverse transcriptase; SAg, superantigen; SEA, staphylococcal enterotoxin A; TdR, [H3]thymidine; TdT, terminal deoxynucleotidyl transferase; Tg, transgenic.
References
- 1.Miller J, Morahan G. Peripheral T cell tolerance. Annu Rev Immunol. 1992;10:51–70. doi: 10.1146/annurev.iy.10.040192.000411. [DOI] [PubMed] [Google Scholar]
- 2.Kappler J, Roehm M, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 3.Rocha B, von Boehmer H. Peripheral selection to the T cell repertoire. Science (Wash DC) 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 4.Jones LA, Chin LT, Longo DL, Kruisbeek AM. Peripheral clonal elimination of functional T cells. Science (Wash DC) 1990;250:1726–1729. doi: 10.1126/science.2125368. [DOI] [PubMed] [Google Scholar]
- 5.Webb SR, Moms C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 6.Nossal GVH, Pike BL. Clonal anergy: persistence in tolerant mice of antigen-binding B lymphocytes incapable of responding to antigen or mitogen. Proc Natl Acad Sci USA. 1980;77:1602–1606. doi: 10.1073/pnas.77.3.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsdell F, Lantz T, Fowlkes B. A nondeletional mechanism of thymic self tolerance. Science (Wash DC) 1989;246:1038–1041. doi: 10.1126/science.2511629. [DOI] [PubMed] [Google Scholar]
- 8.Heeg K, Wagner H. Induction of responsiveness in superantigen-induced anergic T cells: role of ligand density and costimulatory signals. J Immunol. 1995;155:83–92. [PubMed] [Google Scholar]
- 9.Baschieri S, Lees RK, McDonald HR. Clonal anergy to staphylococcal enterotoxin B in vivo: selective effects on T cell subsets and cytokines. Eur J Immunol. 1993;23:2661–2666. doi: 10.1002/eji.1830231041. [DOI] [PubMed] [Google Scholar]
- 10.Rellahan BL, Jones LA, Kruisbeek AM, Fry AM, Matis LA. In vivo induction of anergy in peripheral Vβ8+T cells by staphylococcal enterotoxin B. J Exp Med. 1990;172:1091–1100. doi: 10.1084/jem.172.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eynon E, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liblau RS, Pearson CI, Shokat K, Tisch R, Yang X-D, McDevitt HO. High-dose soluble antigen: peripheral T-cell proliferation or apoptosis. Immunol Rev. 1994;142:193–208. doi: 10.1111/j.1600-065x.1994.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins M, Burrell E, Ashwell JD. Antigen presentation by resting B cells: effectiveness at inducing T cell proliferation is determined by costimulatory signals not T cell receptor occupancy. J Immunol. 1990;135:2953–2961. [PubMed] [Google Scholar]
- 14.Friedman A, Weiner H. Induction of anergy or active suppression following oral tolerance is determined by antigen dose. Proc Natl Acad Sci USA. 1994;91:6688–6692. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kisielow P, Swat W, Rocha B, von Boehmer H. Induction of immunological unresponsiveness in vivo and in vitroby conventional and superantigens in developing and mature T cells. Immunol Rev. 1991;122:69–85. doi: 10.1111/j.1600-065x.1991.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 16.Mollick JA, Cook RA, Rich RR. Class II MHC molecules are specific receptors for staphylococcal enterotoxin A. Science (Wash DC) 1989;244:817–820. doi: 10.1126/science.2658055. [DOI] [PubMed] [Google Scholar]
- 17.Kappler J, Kotzin B, Herron L, Gelfand EW, Bigler RD, Boylston A, Carrel S, Possnett DN, Choi Y, Marrack P. Vβ-specific stimulation of human T cells by staphylococcal toxins. Science (Wash DC) 1989;244:811–817. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- 18.Herman A, Kappler JW, Marrack P, Pullen AM. Superantigens: mechanism of T cell stimulation and role in the immune response. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 19.Gollob KJ, Coffman RL. A minority subpopulation of CD4+ T cells directs the development of naive CD4+T cells into IL-4 secreting cells. J Immunol. 1994;152:5180–5188. [PubMed] [Google Scholar]
- 20.Miethke T, Wahl C, Heeg K, Wagner H. Acquired resistance to superantigen-induced T cells shock. Vβ selective T cell unresponsiveness unfolds directly from a transient state of hyperreactivity. J Immunol. 1993;150:3776–3784. [PubMed] [Google Scholar]
- 21.MacDonald HR, Bascherieri S, Lees RK. Clonal expansion precedes anergy and death of Vβ8+ peripheral T cells responding to staphylococcal enterotoxin B in vivo. . Eur J Immunol. 1991;21:1963–1966. doi: 10.1002/eji.1830210827. [DOI] [PubMed] [Google Scholar]
- 22.Kawabe Y, Ochi A. Programmed cell death and extrathymic reduction of Vβ8+CD4+ T cells in mice tolerant to staphylococcus aureusenterotoxin B. Nature (Lond) 1991;349:245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- 23.Wahl C, Miethke T, Heeg K, Wagner H. Clonal deletion as a direct consequence of an in vivoT cell response to bacterial superantigen. Eur J Immunol. 1993;23:1197–1200. doi: 10.1002/eji.1830230536. [DOI] [PubMed] [Google Scholar]
- 24.Beverly B, Kang S-M, Lenardo MJ, Schwartz RH. Reversal of in vitroclonal anergy by IL-2 stimulation. Int Immunol. 1991;4:661–671. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- 25.Vella AT, McCormack JE, Linsley PS, Kappler JW, Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 1995;2:261–270. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 26.Ramsdell F, Fowlkes BJ. Maintenance of in vivo tolerance by persistence of antigen. Science (Wash DC) 1992;257:1130–1134. doi: 10.1126/science.257.5073.1130. [DOI] [PubMed] [Google Scholar]
- 27.Kaye J, Hsu M-L, Sauron M-E, Jameson SC, Gascoigne NR, Hedrick S. Selective development of CD4+T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature (Lond) 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 28.Croft M, Duncan D, Swain S. Responses of naive antigen-specific CD4+T cells in vitro: characteristics and antigen-presenting cell requirements. J Exp Med. 1992;176:1431–1437. doi: 10.1084/jem.176.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linton P-J, Haynes L, Klinman NR, Swain SL. Antigen-independent changes in naive CD4 T cells with aging. J Exp Med. 1996;184:1891–1900. doi: 10.1084/jem.184.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubey C, Croft M, Swain S. Costimulatory requirements of naive CD4+ T cells: ICAM-1 or B7-1 can costimulate naive CD4+T cell activation but both are required for optimum response. J Immunol. 1995;155:45–57. [PubMed] [Google Scholar]
- 31.Dubey C, Croft M, Swain SL. Naive and effector CD4+T cells differ in their requirements for T cell receptor versus costimulatory signals. J Immunol. 1996;157:3280–3289. [PubMed] [Google Scholar]
- 32.Kuhlman P, Moy V, Lolle B, Brian A. The accessory function of murine intercellular adhesion molecule-1 in T lymphocyte activation. J Immunol. 1991;146:1773–1782. [PubMed] [Google Scholar]
- 33.Swain SL. Generation and in vivo persistence of polarized Th1 and Th2 memory cells. Immunity. 1994;1:543–552. doi: 10.1016/1074-7613(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 34.Gorczyca W, Gong J, Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res. 1993;53:1945–1951. [PubMed] [Google Scholar]
- 35.Nicoletti I, Migliorali G, Pagliacci MC, Gringnan F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining. J Immunol Meth. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 36.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 37.Reiner S, Zheng S, Corry D, Locksley RM. Constructing competitor cDNAs for quantitative PCR. Immunol Methods. 1993;165:37–46. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- 38.Bradley LM, Duncan D, Yoshimoto K, Swain SL. Memory effectors: a potent, IL-4–secreting helper T cell population that develops in vivo after restimulation with antigen. J Immunol. 1993;150:3119–3130. [PubMed] [Google Scholar]
- 39.Weinberg AD, English M, Swain SL. Distinct regulation of lymphokine production is found in fresh versus in vitro primed murine helper T cells. J Immunol. 1990;144:1800–1807. [PubMed] [Google Scholar]
- 40.Ramsdell F, Seaman MS, Miller RE, Picher KS, Kennedy MK, Lynch DH. Differential ability of Th1 and Th2 cells to express Fas ligand and to undergo activation–induced cell death. Int Immunol. 1994;6:1545–1553. doi: 10.1093/intimm/6.10.1545. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, X., T. Brunner, L. Carter, R.W. Dutton, P. Rogers, T. Sato, J. Reed, D. Green, and S.L. Swain. 1997. Unequal death in Th1 and Th2 effectors: Th1 but not Th2 effectors undergo rapid Fas/FasL-mediated apoptosis. J. Exp. Med. In press. [DOI] [PMC free article] [PubMed]
- 42.Carlsson R, Sjogren HO. Kinetics of IL-2 and interferon-γ production, expression of IL-2 receptors, and cell proliferation in human mononuclear cells exposed to staphylococcal enterotoxin A. Cell Immunol. 1985;96:175–183. doi: 10.1016/0008-8749(85)90349-1. [DOI] [PubMed] [Google Scholar]
- 43.Ju ST, Panka DJ, Cul H, Ettinger R, El-Khatib M, Sherr DH, Stanger SZ, Marshak-Rothstein A. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature (Lond) 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 44.Ucker DS, Ashwell JD, Nickas G. Activation driven T cell death. I. Requirements for de novo transcription and translation and association with genome fragmentation. J Immunol. 1989;143:3461–3469. [PubMed] [Google Scholar]
- 45.Watanabe-Fukunaga R, Brannan C, Copeland N, Jenkins N, Nagata S. Lymphoproliferation disorder in mice explained by defect in Fas antigen that mediates apoptosis. Nature (Lond) 1993;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Janeway C., Jr Interferon γ plays a critical role in induced cell death of effector T cells: a possible third mechanism of self-tolerance. J Exp Med. 1990;172:1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCormack JE, Callahan JE, Kappler J, Marrack PC. Profound deletion of mature T cells in vivo by chronic exposure to exogenous superantigens. J Immunol. 1993;150:3785–92. [PubMed] [Google Scholar]
- 48.Miethke T, Wahl C, Holzmann B, Heeg K, Wagner H. Bacterial superantigens induce rapid and T cell receptor Vβ-selective down-regulation of L-selectin (gp90mel-14) in vivo. . J Immunol. 1993;151:6777–6782. [PubMed] [Google Scholar]
- 49.Fuchs E, Matzinger P. B cells turn off virgin but not memory T cells. Science (Wash DC) 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 50.Jenkins MK. The ups and downs of T cell costimulation. Immunity. 1994;1:443–446. doi: 10.1016/1074-7613(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 51.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature (Lond) 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 52.Brunner T, Mogil RJ, Laface D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DN, Ware CF, Green D. Cell-autonomous Fas (CD95)/ Fas–ligand interaction mediates activation-induced apoptosis in T cell hybridomas. Nature (Lond) 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 53.Bruno L, Fehling HG, von Boehmer H. The αβ T cell receptor can replace the γδ receptor in the development of γδ lineage cells. Immunity. 1996;5:343–352. doi: 10.1016/s1074-7613(00)80260-5. [DOI] [PubMed] [Google Scholar]