Abstract
The migratory properties of memory T cells provide a model vector system for site-specific delivery of therapeutic transgene factors to autoimmune inflammatory lesions. Lymph node cells from (SWR×SJL)F1 mice immunized with the p139–151 determinant of myelin proteolipid protein (PLP) were transfected with a DNA construct that placed the anti-inflammatory cytokine interleukin-10 (IL-10) cDNA under control of an antigen-inducible IL-2 promoter region. Isolated T cell clones demonstrated antigen-inducible expression of transgene IL-10 and expressed cell surface markers consistent with the phenotype of normal memory T cells. Upon adoptive transfer, transfected T cell clones were able to inhibit onset of experimental autoimmune encephalomyelitis (EAE) and to treat EAE animals therapeutically after onset of neurologic signs. Semiquantitative immunocytochemistry showed a significant correlation between decreased demyelination and treatment with the transfected T cells. Taken together, these data indicate the autoreactive T cells can be genetically designed to produce therapeutic factors in an antigen-inducible manner resulting in a decreased severity of clinical and histological autoimmune demyelinating disease.
Experimental autoimmune encephalomyelitis (EAE) is an inflammatory neurologic disorder widely used as an animal model for multiple sclerosis (MS) (1–4). EAE is mediated by CD4+ T cells of the Th1 phenotype (IL-2, IFN-γ, TNF-β) in response to encephalitogenic peptides of central nervous system (CNS) myelin proteins. Recent studies have indicated that CD4+ Th2 (IL-4, IL-5, IL-10) play an immunoregulatory role in inhibiting the disease process (5). Thus, distinct native T cell subpopulations facilitate delivery of either proinflammatory or therapeutic factors to sites of inflammation.
We hypothesized that the antigen specificity and migratory properties of T cells may serve as an endogenous model system for site-specific delivery of therapeutic transgene factors during autoimmune disease. To test this hypothesis, primed LN cells from (SWR×SJL)F1 (SWXJ) mice immunized with the immunodominant p139–151 determinant of myelin proteolipid protein (PLP) were transfected with a transgene designed to provide expression of the anti-inflammatory cytokine IL-10 cDNA gene under control of the antigen-inducible IL-2 promoter (IL-2 Prom→ IL-10cDNA). IL-10 was chosen in the design of the transgene because increased expression of IL-10 mRNA occurs in the CNS of mice recovering from EAE and because of the ability of IL-10 to inhibit macrophage-dependent stimulation of T cells and production of proinflammatory cytokines (6–9). In addition, treatment with IL-10 has been shown to inhibit induction of EAE in rats (10). The IL-2 promoter region was selected for its ability to drive relatively high levels of expression of a reporter gene in Jurkat and EL4.E1 lymphoma cell lines (11). Thus, our strategy was to modify T cells genetically for delivering therapeutic factors in a nonconstitutive, antigen-inducible manner during an autoimmune disease.
Materials and Methods
Transgene Construction and Transfection.
The mouse IL-2 promoter region (−1890 to +50) (a gift from Dr. E. Rothenberg) was subcloned into a derivative of the pSI expression vector (Promega, Madison, WI). The mouse IL-10 cDNA is from pcD(SRα)-F115 (ATCC no. 68027; American Type Culture Collection, Rockville, MD) (7). 7–10 d after immunization of SWXJ mice with PLP 139–151, primed LN cells were reactivated in vitro with PLP 139–151 (25 μg/ml). After 96 h, activated blast cells were enriched by Ficoll centrifugation and transfected using polybrene/DMSO-assisted gene transfer (12, 13). Cells were suspended in flat-bottomed 24-well plates at 3 × 106 cells/ml in prewarmed transfection media consisting of 10 μg/ml IL-2Prom→ IL-10cDNA transgene, 1.0 μg/ml of pSV2neo plasmid (ATCC no. 37149), and 20 μg/ml polybrene (Sigma, St. Louis, MO) in DMEM (GIBCO BRL, Gaithersburg, MD). After 6 h, the cells were shocked with prewarmed 30% DMSO in DMEM, washed, and cultured at 1 × 106 cells/ml in 24-well plates in a total volume of 2.0 ml/well with 50 IU/ml mouse IL-2 (PharMingen, San Diego, CA) and 5 × 106 x-irradiated (2 × 103 rads) syngeneic splenocytes/well. After 48 h, cultures were treated with 1.0 mg/ml geneticin (Sigma Chem. Co., St. Louis, MO; 700 μg/ml active substance) and 50 IU/ml mouse IL-2. Cells were harvested at 6 d, reactivated with peptide plus feeders in 24-well plates at 5 × 105 cells/well, and expanded conventionally by alternate activation/ rest cycles with antigen/IL-2. Cells were cloned at 0.3–1.0 cell/ well by limiting dilution and selection with antigen and IL-2. Proliferation assays were performed in flat-bottomed 96-well microtiter plates with 5.0 × 104 T10.11 cells/well and 5.0 × 105 x-irradiated SWXJ splenocytes/well. ELISAs were performed with purified anti-mouse cytokine capture–detection antibody pairs (PharMingen, San Diego, CA) on 48-h supernatants from bulk cultures. The capture–detection antibody pairs included the following: anti-mouse IFN-γ (R4-6A2 and biotin–XMG1.2), anti-mouse IL-4 (BVD4-1D11 and biotin–BVD6-24G2), anti-mouse TNF-α (MP6-XT22 and biotin–MP6-XT3), and anti-mouse IL-10 (JES5-2A5 and biotin–SXC-1). Standard values were plotted as absorbance at 405 nm OD versus concentrations of recombinant cytokine standards (PharMingen, San Diego, CA). Unknown cytokine concentrations were determined as values within the linear part of the standard curve.
RNase Protection Assay (RPA).
RNA was isolated using either guanidine isothiocyanate and CsCl as described previously or with the TRIZOL™ reagent (GIBCO BRL, Gaithersburg, MD) following the instructions of the manufacturer (14–16). An Asp 700 fragment was subcloned from the IL-2Prom→ IL-10cDNA transgene that included the 3′ end of the IL-2 promoter region, intronic sequences, vector sequences, and the 5′ end of the mouse IL-10 cDNA. This construct was linearized with EcoRI and α-[32P]UTP-labeled RNA probe synthesized using the Bluescript T3 promoter. RPA was performed using the RNase Protection Assay (RPA) II system (Ambion, Austin, TX). The resulting digestion products were separated on 5% Hydrolink P600 gels (J.T. Baker, Phillipsburg, NJ), dried, and exposed to Biomax MR film (Eastman Kodak, Rochester, NY). The RNA expression levels were quantified using a PhosphoImager (Molecular Dynamics, Sunnyvale, CA).
Active EAE Induction.
EAE was induced as previously described (17) by subcutaneous immunization of SWXJ mice with the immunodominant PLP 139–151 peptide in an emulsion of equal volumes of water and CFA (Difco, Detroit, MI). On days 0 and 3, each mouse also received intravenously 0.6 × 1010 Bordetella pertussis bacilli (Michigan Department of Public Health). All mice were weighed and examined daily for neurologic signs in a blinded manner.
Histologic Analysis and Quantification.
Spinal cords were fixed in 10% phosphate-buffered formalin, and paraffin-embedded tissue sections were cut (10 μm each) for immunostaining. Sections were pretreated with 0.04% OsO4 and 1% H2O2 in 10% Triton (Electron Microscopy Sciences, Fort Washington, PA) and blocked with 5% normal goat serum (Vector, Burlingame, CA) and 5% nonfat dehydrated milk for 60 min. Sections were treated sequentially with PLP monoclonal IgG2a antibody (Harlan) at a 1:200 dilution for 14 h at 4°C, biotinylated goat anti–mouse IgG2a (Southern Biotechnology, Birmingham, AL) at a 1:500 dilution for 30 min at 22°C, and avidin–peroxidase complex (Vector, Burlingame, CA) for 1 h at 1:1,000 dilution. Sections were then treated with diaminobenzidine and 0.01% H2O2 for 8 min, 0.04% OsO4 for 30 s and washed in PBS. Images were digitized using the Oncor Imaging System (Gaithersburg, MD) at 640 × 480 pixel resolution. All images were normalized by adjusting background gray matter stain to the same mean intensity value using Adobe Photoshop (Adobe Systems, Mountain View, CA). At least 12 images per animal were analyzed. The dorsal columns were outlined and the percentage of pixels representing the darkest 25% of stain was determined using NIH Image software.
Statistical Analysis.
The two-sided Student's t test was used for determining differences in mean clinical scores of treated and control EAE mice as well as differences in percent immunostaining between control and experimental spinal cords.
Results and Discussion
A transgene construct (IL-2Prom→ IL-10cDNA) was designed by fusing a mouse IL-2 promoter region (−1890 to +50) to the mouse IL-10 cDNA (Fig. 1 a). The transgene construct also contained intron splices sites and the SV40 late polyadenylation signal region to ensure high levels of IL-10 expression. In this way, T cells were designed so that an IL-2 promoter region would regulate synthesis of IL-10 in an antigen-inducible, nonconstitutive manner.
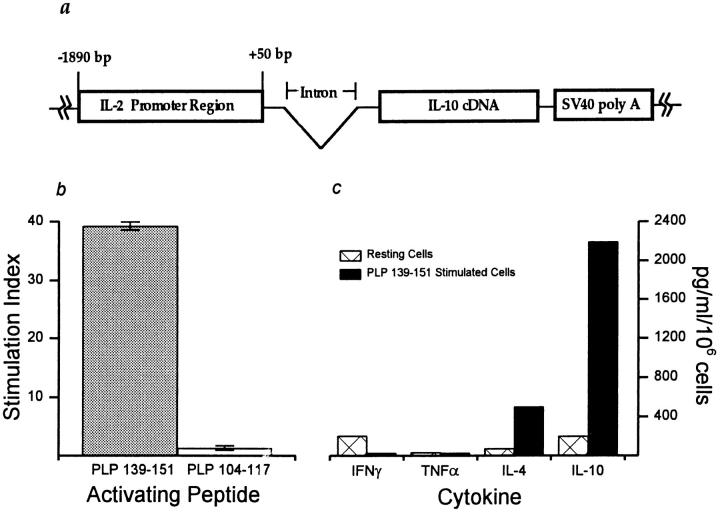
Figure 1.
The IL-2Prom→ IL-10cDNA transgene and characterization of transfected T cells. (a) Schematic representation of the transgene IL-2Prom→ IL-10cDNA showing IL-2 promoter region, IL-10 cDNA, and SV40 polyadenylation signals. Intron sequences are labeled and vector sequences are indicated by solid lines. (b) Proliferative responses of T cell clone T10.11 after peptide activation. 5 × 105 clone cells were stimulated (25 μg/ml) with the immunodominant PLP 139–151 HSLGKWLGHPDKF, and with PLP 104–117 KTTICGKGLSATVT, a noncross-reacting control encephalitogen for SWXJ mice (17, 26). The data show the stimulation index (cpm test/cpm background) of [3H]thymidine incorporation by T10.11 clone cells 48 h after activation with peptide. Error bars show ± SD. (c) Cytokine levels were measured on 48-h supernatants from clone T10.11 cells cultured without antigen (resting cells) and after activation with PLP 139–151. Cytokine concentrations were normalized to total cell numbers.
T cells were prepared by in vitro activation of primed LN cells from SWXJ mice immunized with the PLP 139– 151 peptide. In our hands, this method consistently produces encephalitogenic T cells capable of passively transferring EAE into naive animals.
Peptide-activated T cells were transfected with both the IL-2Prom→ IL-10cDNA transgene and the selectable marker plasmid, pSV2neo. Neo-resistant T cells were expanded, and T cell clones were isolated and analyzed. Clone T10.11 was selected for further study because of its marked antigen specificity (Fig. 1 b) and its ability to generate a substantial increase in IL-10 cytokine production upon activation with antigen (Fig. 1 c).
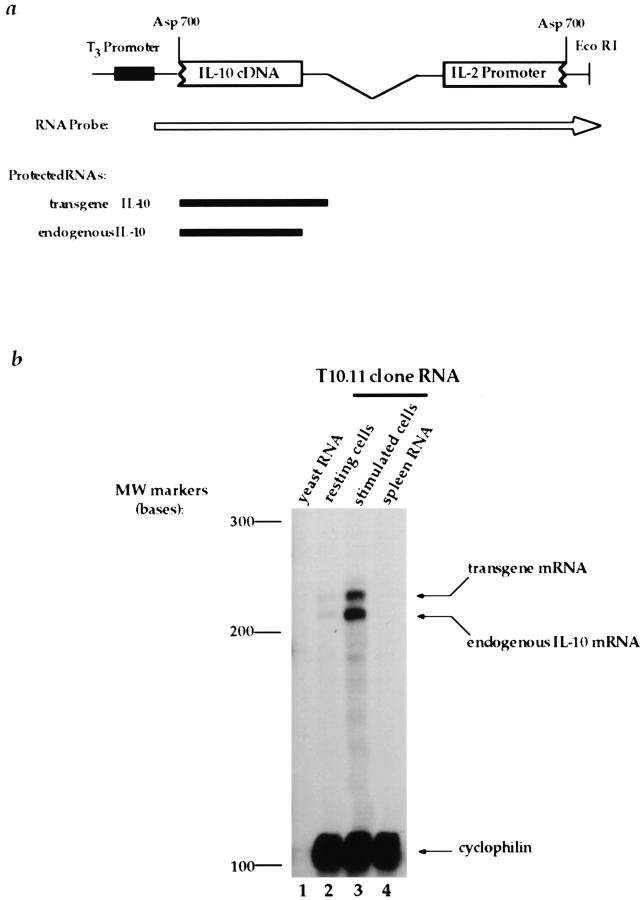
An RPA was used to differentiate between IL-2Prom→ IL-10cDNA transgene and endogenous IL-10 gene expression. RNA from resting and antigen-activated T10.11 cells was hybridized to an RNA probe prepared from the 5′ end of the IL-10 cDNA that incorporated transcribed-transgene vector sequences (Fig. 2 a). Two RNA transcripts were protected from activated T10.11 cells, indicating that both endogenous and transgene IL-10 expression were induced after stimulation with antigen (Fig. 2 b). Transgene expression represented 40% of the total IL-10 mRNA, and increased 7.5-fold after activation with PLP 139–151 compared with a 6.1-fold antigen-induced increase in endogenous IL-10 mRNA. Thus, antigen-inducible expression of transgene IL-10 mRNA occurred concomitant with endogenous IL-10 gene expression.
Figure 2.
Detection of transgene IL-10 mRNA in T10.11 clone cells. An RNase protection assay (RPA) was used for distinguishing endogenous and transgene mRNA. (a) The DNA construct used to generate the RNA probe for measuring IL-10 expression. The RNA probe is represented by an arrow and protected RNase digestion products are shown. (b) RNase-digestion products after hybridization to 32P-labeled RNA probe. Lane 1, yeast RNA; lane 2, RNA from rested T10.11 clone cell 11 d after activation with PLP 139–151; lane 3, RNA from activated T10.11 clone cells 48 h after activation with PLP 139–151; lane 4, spleen RNA.
Flow cytometry analysis showed that transfected T10.11 cells expressed a cell surface phenotype consistent with normal memory T cells. After antigen stimulation, T10.11 cells were CD3+, CD4+, CD8dim, TCRαβ+, Vβ14+ T cells with high level expression of the activation antigens CD44 (Pgp-1), CD49d (VLA-4), and CD25 (IL-2R) and low level expression of CD62L (l-selectin), a marker for native T cells (Table 1).
Table 1.
Flow Cytometry Analysis of IL-2Prom→ IL-10cDNA–transfected T Cells*
| Cell surface antigen | Percentage of positive staining cells | |||||
|---|---|---|---|---|---|---|
| Normal p139–151-specific T cell line | Transfected p139–151-specific T cell line | Transfected p139–151-specific T10.11 T cell clone | ||||
| CD3 | 92.3 | 91.7 | 95.1 | |||
| CD4 | 94.6 | 93.0 | 99.6 | |||
| CD8 | 0.8 | 2.9 | 0.4 | |||
| TCR αβ | 95.5 | 93.6 | 98.6 | |||
| TCR-Vβ14 | 14.9 | ND | 99.5 | |||
| CD25 (IL-2Rα) | 68.8 | 65.6 | ND | |||
| CD44 (Pgp-1) | 95.1 | 93.7 | 92.4 | |||
| CD49d (VLA-4) | 45.3 | 42.2 | 41.2 | |||
| CD62L (l-selectin) | ND | 20.6 | 19.2 | |||
Two-color flow cytometry analysis of PLP 139–151-activated fixed T cells was performed with PE-conjugated rat mAb to mouse CD3 or CD4 (GIBCO BRL) and FITC-conjugated mAb to either TCR αβ chains (TCR αβ), CD25 (IL-2R α chain), CD44 (Pgp-1, CD49d (VLA-4; α4β1 integrin), or CD62L (l-selectin) (PharMingen). Single-color analysis with FITC-conjugated antibodies (PharMingen) was used for determining TCR-Vβ utilization. Isotype-matched FITC- and PE-conjugated mAb were used as controls. Data were collected on 20,000 events with a FACScan® flow cytometer. Analysis was performed on the gated lymphoblast population using Cellquest software (Becton Dickinson).
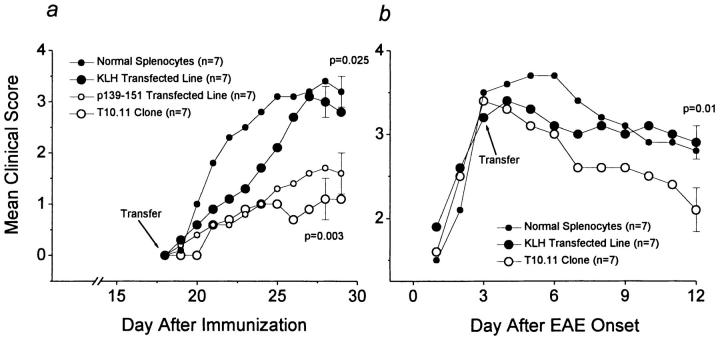
The development of EAE in SWXJ mice is characterized by acute onset of paralytic disease within 3 wk after immunization with PLP 139–151. Mice typically recover and undergo a relapsing–remitting disease course with progression to chronic disability accompanied by perivascular mononuclear infiltrates and demyelination in CNS white matter. To evaluate the therapeutic potential of IL-2Prom→ IL-10 cDNA transfected T cells, T10.11 clone cells were adoptively transferred into SWXJ mice before the anticipated onset of PLP 139–151-induced EAE as well as after onset of clinical disease. Transfer of T10.11 T cells was found to be effective in inhibiting the onset of EAE (Fig. 3 a) and in therapeutically altering the course of disease when transferred after initiation of neurologic signs (Fig. 3 b). The inhibitory effect of clone T10.11 was similarly mimicked by transfer of PLP 139–151-specific T cell lines also transfected with the IL-2Prom→ IL-10cDNA construct (Fig. 3 a). In contrast, transfer of either normal splenocytes or IL-2 Prom→ IL-10cDNA–transfected T cell lines specific for the irrelevant non-CNS antigen, KLH, produced no sustained therapeutic effect on either EAE onset or progression. KLH-specific transfected T cell lines showed antigen-inducible production of IL-10 in a manner similar to that observed in transfected autoreactive T cells (data not shown).
Figure 3.
Inhibition and treatment of EAE with autoreactive T cells transfected with IL-2 Prom→ IL-10cDNA. (a) Inhibition of EAE onset after adoptive transfer of 1 × 107 T10.11 clone cells or IL-2Prom→ IL-10cDNA–transfected T cell lines specific for PLP 139–151. Recipient SWXJ mice were immunized for EAE induction with PLP 139–151 18 d before tail vein injection with antigen-activated T cells. Mice showed no signs of EAE before transfer. No therapeutic effect occurred in mice receiving 1 × 107 normal splenocytes or activated IL-2Prom→ IL-10cDNA–transfected cells specific for the irrelevant control antigen, keyhole limpet hemocyanin (KLH). (b) Therapeutic treatment after EAE onset by transfer with IL-2Prom→ IL-10cDNA–transfected T10.11 clone cells. 3 d after EAE onset, mice were injected intravenously with 1 × 107 activated T cells. No therapeutic effect was observed in mice transferred with nonactivated normal splenocytes or with activated transfected T cells specific for KLH. All mice were weighed and examined daily for neurologic signs in a blinded manner according to the following criteria. Clinical scores are 0, no disease; 1, decreased tail tone or slightly clumsy gait; 2, tail atony and/or moderately clumsy gait and/or poor righting ability; 3, limb weakness; 4, limb paralysis; 5, moribund state. Error bars show ± SEM.
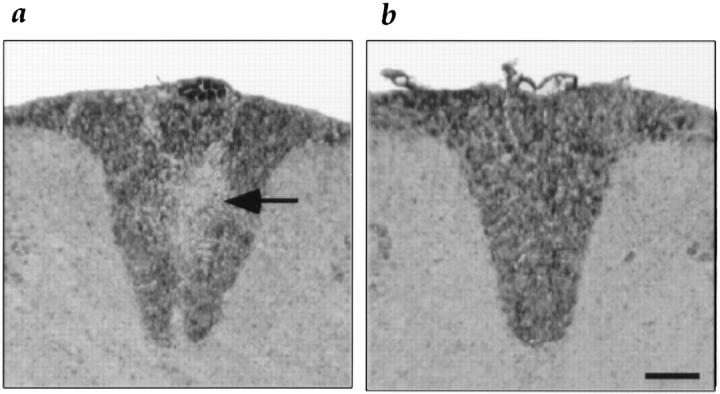
To determine the histologic effects after adoptive transfer of transfected T cells, spinal cords from mice receiving either IL-2Prom→ IL-10cDNA–transfected T cells (Fig. 4 b) or normal splenocytes (Fig. 4 a) just before EAE onset were stained immunocytochemically for PLP, and demyelination was quantified by measuring the mean intensity of dorsal column PLP staining. Adoptive transfer of IL-2Prom→ IL-10 cDNA–transfected T cells just before EAE onset resulted in a significant (P = 0.02) mean decrease of 12.2% in CNS demyelination compared with EAE mice receiving normal splenocytes.
Figure 4.
Histologic analysis of CNS tissue after adoptive transfer of transfected T cells. The extent of demyelination was measured by immunocytochemical staining for PLP. (a) Representative section showing demyelination (arrow) in the spinal cord dorsal column of SWXJ control mouse adoptively transferred with normal splenocytes before EAE onset. (b) Representative section showing uniform distribution of PLP immunostaining in the spinal cord dorsal column of SWXJ mouse adoptively transferred before EAE onset with PLP 139–151-specific T cells transfected with the IL-2Prom→ IL-10cDNA construct. Closed Bar, 50 μm.
Ectopic expression of anti-inflammatory cytokines has produced conflicting results in the treatment of immune-mediated inflammation. Allograft survival is prolonged after retroviral-mediated transfection of murine cardiac transplants with viral IL-10 (18), and onset of collagen-induced arthritis is delayed in DBA/1 mice injected with Chinese hamster ovary (CHO) fibroblasts transfected with IL-4 or IL-13 (19). In contrast, expression of transgene IL-10 in pancreatic islet β cells actually accelerates the development of autoimmune diabetes in nonobese diabetic (NOD) mice (20). Controversy is also apparent in reports which show efficacy in treating ongoing EAE by transfer of traditional Th2 T cell clones cells (5), but only modest therapeutic effect when Th2 T cells are transferred before disease induction (21). However, it is clear that proliferation of encephalitogenic Th1 T cells can be inhibited by IL-10, but not by IL-4 secreted from Th2 T cells having identical antigen specificity (22). It is worth noting that treatment with recombinant IL-10 has also produced conflicting outcomes resulting in either exacerbation (23) or amelioration (10) of EAE.
In a recent report related to the present study, Shaw et al. (24) demonstrated a delay in EAE onset and a decrease in disease severity after transfer of myelin basic protein–specific T cell hybridomas that had been retrovirally transduced with IL-4. However, in contrast with the present study, expression of the IL-4 transgene was constitutive, and all of the mice eventually died from overgrowth of the hybridoma tumor cells.
The results of the present study show that transfected antigen-specific T cells are effective when used either to inhibit onset of EAE or to treat ongoing disease. In a broader sense, our data indicate that T cells can be genetically altered with nonviral vectors to provide antigen-inducible production of therapeutic transgene proteins while maintaining an otherwise normal memory T cell phenotype. Thus, genetic modification of T cells may provide a means for both identifying and delivering therapeutic transgene factors capable of modulating inflammation. Moreover, it may be possible to use transgene-altered T cells for delivering appropriate growth factors for tissue repair, particularly in light of recent experiments showing that T cells constitutively expressing nerve growth factor are less capable of mediating experimental autoimmune neuritis (25). Insights gained from genetic modification of T cells in the EAE animal model may provide a rational basis for treating the autoimmune demyelination widely believed to be responsible for chronic progression of MS.
Acknowledgments
We thank Dr. E. Rothenberg for the IL-2 promoter region and for her helpful advice.
This work was supported by National Multiple Sclerosis Society grants RG-2768 (V.K. Tuohy) and PP0483 (P.M. Mathisen) and by National Institutes of Health grant NS-36054 (V.K. Tuohy).
References
- 1.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 2.Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 3.Lisak RP. Multiple sclerosis: evidence for immunopathogenesis. Neurology. 1980;30:99–105. doi: 10.1212/wnl.30.7_part_2.99. [DOI] [PubMed] [Google Scholar]
- 4.McFarlin DE, McFarland HF. Multiple sclerosis (first of two parts) N Engl J Med. 1982;307:1183–1188. doi: 10.1056/NEJM198211043071905. [DOI] [PubMed] [Google Scholar]
- 5.Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: applications to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 6.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse helper T cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, Mosmann TR. Homology of cytokine synthesis inhibitory factor (IL-10) to Epstein–Barr virus gene BCRFI. Science (Wash DC) 1990;24:1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- 8.Vieira P, de Waal-Malefyt R, Dang M-N. Isolation and expression of human cytokine synthesis inhibitory factor (CSIF/IL10) cDNA clones: homology to Epstein–Barr virus open reading frame BCRFI. Proc Natl Acad Sci USA. 1991;88:1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy MK, Torrance DS, Picha KS, Mohler KM. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- 10.Rott O, Fleischer B, Cash E. Interleukin-10 prevents experimental allergic encephalomyelitis in rats. Eur J Immunol. 1994;24:1434–1440. doi: 10.1002/eji.1830240629. [DOI] [PubMed] [Google Scholar]
- 11.Novak TJ, White PM, Rothenberg EV. Regulatory anatomy of the murine interleukin-2 gene. Nucleic Acids Res. 1990;18:4523–4533. doi: 10.1093/nar/18.15.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aubin RA, Weinfeld M, Mirzayans R, Paterson MC. Polybrene/DMSO-assisted gene transfer. Mol Biotech. 1994;1:29–48. doi: 10.1007/BF02821509. [DOI] [PubMed] [Google Scholar]
- 13.Chisholm O, Symonds G. Transfection of myeloid cell lines using polybrene/DMSO. Nucleic Acids Res. 1988;16:23523. doi: 10.1093/nar/16.5.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathisen PM, Miller L. Thyroid hormone induction of keratin genes: a two-step activation of gene expression during development. Genes Dev. 1987;1:1107–1117. doi: 10.1101/gad.1.10.1107. [DOI] [PubMed] [Google Scholar]
- 15.Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active RNA from sources enriched in ribonucleases. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 16.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. BioTechniques. 1993;15:532–537. [PubMed] [Google Scholar]
- 17.Tuohy VK, Lu Z, Sobel RA, Laursen RA, Lees MB. Identification of an encephalitogenic determinant of myelin proteolipid protein for SJL mice. J Immunol. 1989;142:1523–1526. [PubMed] [Google Scholar]
- 18.Qin L, Chavin KD, Ding Y, Tahara H, Favaro JP, Woodward JE, Suzuki T, Robbins PD, Lotze MT, Bromberg JS. Retrovirus-mediated transfer of viral IL-10 gene prolongs murine cardiac allograft survival. J Immunol. 1996;156:2316–3232. [PubMed] [Google Scholar]
- 19.Bessis N, Boissier MC, Ferrera P, Blankenstein T, Fradelizi D, Fournier C. Attenuation of collagen-induced arthritis in mice by treatment with vector cells engineered to secrete interleukin-13. Eur J Immunol. 1996;26:2399–2403. doi: 10.1002/eji.1830261020. [DOI] [PubMed] [Google Scholar]
- 20.Wogensen L, Lee MS, Sarvetnick N. Production of interleukin 10 by islet cells accelerates immune-mediated destruction of β cells in nonobese diabetic mice. J Exp Med. 1994;179:1379–1384. doi: 10.1084/jem.179.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoruts A, Miller SD, Jenkins MK. Neuroantigen-specific Th2 cells are inefficient suppressors of experimental autoimmune encephalomyelitis induced effector Th1 cells. J Immunol. 1995;155:5011–5017. [PubMed] [Google Scholar]
- 22.van der Veen RC, Stohlman SA. Encephalitogenic Th1 cells are inhibited by Th2 cells with related peptide specificity: relative roles of interleukin (IL)-4 and IL-10. J Neuroimmunol. 1993;48:213–220. doi: 10.1016/0165-5728(93)90194-4. [DOI] [PubMed] [Google Scholar]
- 23.Cannella B, Gao YL, Brosman C, Raine CS. IL-10 fails to abrogate experimental autoimmune encephalomyelitis. J Neurosci Res. 1996;45:735–746. doi: 10.1002/(SICI)1097-4547(19960915)45:6<735::AID-JNR10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 24.Shaw MK, Lorens JB, Dhawan A, DalCanto R, Tse HY, Bonpane C, Eswaran SL, Brocke S, Sarvetnick N, Steinman L, Nolan GP, Fathman CG. Local delivery of interleukin-4 by retrovirus-transduced lymphocytes ameliorates experimental autoimmune encephalomyelitis. J Exp Med. 1997;185:1541–1547. doi: 10.1084/jem.185.9.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramer R, Zhang Y, Gehrmann J, Gold R, Thoenen H, Wekerle H. Gene transfer through the blood– nerve barrier: NGF-engineered neuritogenic T lymphocytes attenuate experimental autoimmune neuritis. Nature Med. 1995;1:1162–1166. doi: 10.1038/nm1195-1162. [DOI] [PubMed] [Google Scholar]
- 26.Tuohy VK, Thomas DM. Sequence of 104– 117 of myelin proteolipid protein is a cryptic encephalitogenic T cell determinant for SJL/J mice. J Neuroimmunol. 1995;56:161–170. doi: 10.1016/0165-5728(94)00143-c. [DOI] [PubMed] [Google Scholar]