Abstract
The pathophysiological relevance of the complement split product C3a as a proinflammatory mediator is still ill defined. The expression pattern of the human C3a receptor (C3aR) can provide important clues for the role of this anaphylatoxin in inflammation. There is strong evidence for C3aR expression on basophils, and eosinophils, but additionally, only on tumor cell lines of leukemic or hepatic origin. It is unclear whether neutrophils also express the C3aR, but need a costimulus provided by eosinophils for certain biological responses, or whether neutrophils lack the C3aR and respond to C3a via a secondary stimulus generated by eosinophils, i.e., by an indirect mode. In the present study, polyclonal antiserum raised against the second extracellular loop of the C3aR was used to characterize C3aR expression on peripheral blood leukocytes. For high degree purification of neutrophils, a negative selection method was established that decreased the contamination with CD9bright+ eosinophils down to <0.2%. Flow cytometric analyses, functional assays, and binding assays on highly purified neutrophils confirmed C3aR expression and coupling. Monocytes were identified as an additional C3aR-positive cell population of the peripheral blood. The expression of the C3aR on eosinophils could be confirmed. In contrast, the receptor could not be detected on unchallenged B or T lymphocytes (or lymphocyte-derived Raji cells).
The anaphylatoxic peptides C3a, C4a, C5a, and C5a– desArg are generated during complement activation. They are considered proinflammatory mediators with some immunomodulatory properties (1–3). There is detailed knowledge about C5a and its specific receptor, including bacterial infection models in C5a receptor knockout mice (4–6). In contrast, studies on C3a and its receptor (C3aR)1 have been almost neglected in the human system for a long time. Only recently has attention been focused on this anaphylatoxin, culminating in the recent cloning of the C3aR by us and others (7–9). There is an ongoing discussion about the expression pattern of the human C3aR, in particular on blood cells, and the thereby implied physiological importance of C3a. It is now broadly accepted that the human C3aR is expressed on basophils (10, 11), and mast cells (12), and there are functional data strongly implying its expression on eosinophils (13, 14). Until now, there was no direct proof for C3aR expression on human monocytes. However, there is some evidence. The expression of the C3aR can be induced on U937 cells by dibutyryl cAMP or IFN-γ (15, 16), a treatment that drives these monomyeloblastic cells closer to the mature monocyte–macrophage phenotype. Additionally, C3a modulates lipopolysaccharide-induced gene expression and protein synthesis on PBMCs (17). The publications on neutrophils are contradictionary. It is unclear whether neutrophils actually express the C3aR (15, 18–20), but need a costimulus provided by eosinophils for certain biological responses, or whether neutrophils lack this receptor (21) and respond to C3a by an indirect mode, via a secondary stimulus generated by eosinophils (13). Similarly, the presence in standard density gradient PMN preparations (e.g., Polymorphprep™) of up to 4% of eosinophils could account for the specific binding of [125I]–C3a to PMNs (15), and the cloning of the C3aR from cDNA of lipopolysaccharide-activated neutrophils (7). In parallel, the C3aR has been cloned from cDNA of induced U937 cells (8), and had also been published as an orphan receptor from a differentiated HL-60 cell cDNA library (9). Flow cytometric analysis suggested C3aR expression on human Burkitt lymphoma–derived Raji cells, on neutrophils, and on monocytes (9). The goal of the present study was to clarify the expression pattern of the human C3aR on subpopulations of peripheral blood leukocytes. To this aim, flow cytometry using a polyclonal Ab specific for the C3aR was combined with functional assays, binding studies, and Northern blots on highly purified neutrophils and on monocytes with a minimized contamination with C3aR-bearing granulocytes. Our results indicate that the C3aR is expressed on neutrophils, monocytes, and eosinophils. In contrast, the C3aR neither seems to be expressed on B or T lymphocytes, nor on Raji cells.
Materials and Methods
Reagents.
Human C3a was purchased from Advanced Research Technologies (San Diego, CA). The C3a analogue synthetic peptide P117 (LRRQAWRASALGLAR) (8) (amino acids 63–77 of hC3a), and the control peptide P252 (YTTDDYGHYDD) were prepared by solid-phase synthesis. Anti-CD16–PE (clone 3G8) and FITC-labeled goat anti–rabbit IgG1 were obtained from Dianova (Hamburg, Germany). Anti-CD14–PE, PE- and FITC-coupled mouse control IgG1, anti-CD9 (clone ALB6), and Optilyse B™ were supplied by Immunotech (Hamburg, Germany). Anti-CD3–Cychrome™ was purchased from PharMingen (Hamburg, Germany), whereas anti-CD19–PE was from Biozol (Eching, Germany). All marker mAbs were mouse IgG1. All other chemicals were purchased from Sigma Chemicals (Deisenhofen, Germany).
Cell Lines, Cell Culture Conditions.
The rat basophilic leukemia cell line RBL-2H3 (American Type Culture Collection [ATCC], Rockville, MD) stably transfected with C3aR cDNA was grown as described elsewhere (7). The cell culture conditions of U937 cells (ATCC) and the C3aR induction by dibutyrylic cAMP have been earlier described (15). For the culture of Raji cells (ATCC) RPMI-1640 (LIFE Technologies, Eggenstein, Germany) supplemented with 10% heat-inactivated FCS was used.
The Preparation of Polyclonal Rabbit Serum Directed Against the Human C3aR.
The cDNA of the second extracellular loop of the C3aR (amino acids 170–315) was amplified by PCR and subcloned into the pGEX5X-1 expression plasmid (Pharmacia Biotech, Inc., Piscataway, NJ). Expression and purification of the resulting glutathione–S transferase fusion protein were performed according to the instructions of the manufacturer. To raise polyclonal Abs against the human C3aR, rabbits were immunized twice with 250 μg of the fusion protein, followed by four inoculations of 125 μg each (HRP, Inc., Denver, CO).
Flow Cytometric Analyses of PBLs and Raji Cells with Polyclonal Rabbit Anti-C3aR Serum.
PBLs were prepared from EDTA blood of healthy donors. Erythrocytes were lysed by NH4Cl. The remaining PBLs were washed twice (PBS at 4°C) and resuspended at a density of 1 × 107 cells/ml. Raji cells grow in suspension and tend to clump. Therefore, after harvesting and two washes in ice-cold PBS, these cells were filtered through a 30-μm nylon mesh. 5 × 105 cells in a total volume of 100 μl were incubated for 30 min with the polyclonal rabbit serum (1:4,000) raised against the C3aR. In parallel, the cells were always incubated with 1:4,000 diluted rabbit preimmune serum (of the same animal) as a negative control for C3aR-independent binding of rabbit IgG, and PBS providing the negative control for nonspecific binding of the secondary antibody. After two washes, the cell pellet was resuspended and incubated for 30 min in PBS containing FITC-labeled goat anti–rabbit IgG (1:200) and PE- or Cychrome™-labeled pretitrated anti-CD mAb's. Gating for the different leukocyte subpopulations was based on a combination of forward scatter, side scatter, and these CD markers. Anti-CD16 was used for the subsequent differentiation between neutrophils (CD16bright+) and eosinophils (CD16dim+) (22). Anti-CD14 was used for the identification of monocytes, anti-CD3 for T lymphocytes, and anti-CD19 for B lymphocytes (23). After two additional washes, the PBLs were resuspended in 100 μl of ice-cold buffer. Finally, the cells were assessed in the flow cytometer FACScan® and analyzed by CELLQuest™ Software (Becton Dickinson, Heidelberg, Germany).
Isolation of Human PMNs.
Highly purified neutrophils were prepared from citrate-anticoagulated blood of healthy human donors. PMNs were prepared by density centrifugation using Polymorphprep™ (Nycomed, Oslo, Norway), according to the instructions of the manufacturer. For the preparation of highly purified neutrophils that are only minimally contaminated with eosinophils, it was important to collect only the clearly defined upper granulocyte layer. The harvested PMNs were washed twice with PBS. The cells were counted and resuspended in PBS to 1 × 107 cells/ml. The cells were either immediately further purified (see CD9 depletion of eosinophils), or kept on ice until functional analyses.
Purification of Neutrophils by Depletion of CD9bright+ Eosinophils.
For anti-CD9 depletion of eosinophils, the granulocytes obtained by Polymorphprep™ were incubated for 30 min in a solution of an anti-CD9 mAb (10 μg/ml) in PBS at 4°C. The cells were washed twice, and incubated for 20 min in a solution of goat anti–mouse IgG coupled to microsphere magnetic beads at a concentration of 312.5 μg/ml (Dianova). The CD9bright+ cells were selected from the CD9dim+ neutrophils by the use of a magnetic separator (Dynal, Hamburg, Germany) (three times). The resulting highly purified neutrophils were resuspended at a density of 1 × 107 cells/ml. The purity of the neutrophil preparation was always checked by microscopic examination with Kimura staining (24) and Diff-Quick™ staining (Baxter Dade, Düdingen, Switzerland), as well as by flow cytometric analysis using anti-CD16–PE.
Preparation of Monocytes for Northern Blot Analyses.
First, PBMCs were isolated from blood of healthy human donors through centrifugation on a standard Ficoll gradient (Ficoll-Paque™; Pharmacia Biotech, Inc., Uppsala, Sweden). Then, the monocytes were enriched within the fraction of mononuclear cells using their adherence to cell culture dishes (25) (incubation for 2 h at 37°C in RPMI 1640 medium, supplemented with 10% heat-inactivated FCS). After washing off the nonadherent cells, the monocyte-enriched PBMCs were detached from the plastic surface with cell dissociation solution (Sigma). 1,000 stained cells (Diff-Quick™) were counted. This procedure yielded monocytes of ⩾85% purity as additionally confirmed by flow cytometry (anti-CD14). All contaminating cells were lymphocytes, whereas no granulocytes were found.
Fura-2 Assay of Neutrophils (in Suspension).
The measurement of free cytosolic Ca2+ ([Ca2+]i) on neutrophils in suspension was performed using Fura-2/AM and the Luminescence Spectrometer LS 50B (Perkin Elmer, Beaconsfield, UK). The Fura-2/ AM–loading (Calbiochem, Bad Soden/Taunus, Germany) and measurement (final concentration: 2 × 105 cells/ml) were performed exactly as described earlier (15).
Flow Cytometric [Ca2+]i Measurements of Monocytes.
The PBMCs from a standard Ficoll gradient (see above) were washed in PBS. Then, the cells, at a density of 1 × 107/ml, were loaded in a polypropylene tube with 5 μM Fluo3-AM (ICN Biomedicals GmbH, Eschwege, Germany). The fluorescence indicator was applied in RPMI-1640 medium, containing 10% FCS, and 0.2% Pluronic® F-127 (Sigma). After 25 min in a standard CO2 cell incubator at 37°C the PBMCs were washed twice. Finally, the Fluo3-loaded cells were resuspended in RPMI 1640 medium supplemented with 10% FCS, to a density of 1 × 107 cells/ml. To a 50-μl aliquot of this cell suspension 150 μl of RPMI medium with or without stimulus was added. Exactly 10 s after the addition of the stimulus, the flow cytometric determination of 10,000 cells, lasting ∼5 s, was started.
Preparation of Monocytes for Competitive Binding Studies.
PBMCs were prepared on a Ficoll gradient as described above. The mononuclear cells were further purified through positive selection with CD14-coupled MACS™ (Miltenyi Biotec, Bergisch-Gladbach, Germany) (26) exactly following the manufacturer's protocols. The purity was checked by flow cytometric analyses (CD14 staining), and microscopic control of Diff-Quick™ stained cells. Monocytes accounted for more than 90% of the resulting cell preparation. The remaining 10% were predominantly lymphocytes. No granulocytes were found within 1,000 stained cells.
Northern Blot Analyses of Human Monocytes, Raji, and U937 Cells.
Preparation of mRNA, Northern blotting, preparation of riboprobes, hybridization, and chemoluminescence detection of C3aR mRNA and mRNA of the rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (27) were performed as described previously (8, 16). All samples, which are shown in the Northern blot, were processed simultaneously.
Competitive [125I]–hC3a Binding Studies
Radiolabeling of C3a was performed with Iodogen as described for C5a and C3a (16). The purified neutrophils or monocytes were washed twice and resuspended in PBS. In the case of monocytes, 0.1% of sodium azide was added. The cells were stored on ice before use. For competitive [125I]–C3a binding assays different concentrations of unlabeled C3a in PBS as indicated were mixed in quadruplicates with a constant concentration of a [125I]–C3a solution (about 40,000 cpm for neutrophils and 50,000 cpm for monocytes per well) in a microtiter plate (Greiner, Frickenhausen, Germany). The binding reaction was started by addition of 25 μl prewarmed (see below) cell suspension of 2 × 107 cells/ml (total reaction volume, 60 μl). After 30 min of incubation at 37°C for neutrophils, or at room temperature for monocytes, cell-bound and free [125-I]– rhC3a were separated by filtration of 55 μl through a premoistened (PBS) microtiter membrane plate (Multiscreen™ HV, 0.45 μm, MAHV N45; Millipore, Molsheim, France) using a vacuum manifold (Millipore). The wells were washed twice, dried by a heat lamp, and punched out with a multiple punch assembly (Millipore). Punched-out membranes were counted on a γ-counter (Canberra Packard, Dreieich, Germany). The data were used to calculate dissociation constant (K d) and number of receptors per cell via iterative curve fitting using a commercial software (Ligand, Biosoft, Cambridge, UK).
Results and Discussion
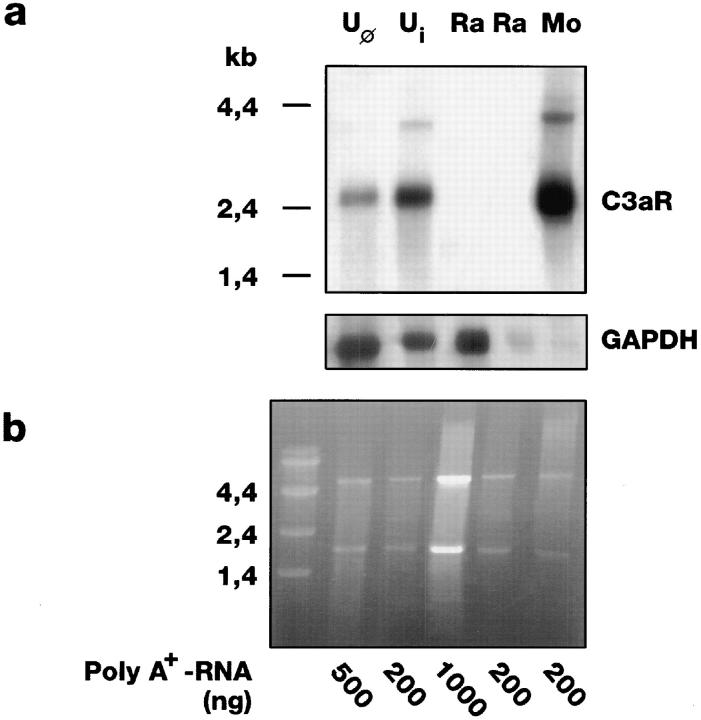
Polyclonal Anti-C3aR Serum at a Dilution of 1:4,000 Combined with a C3aR Northern Blot Can Be Used to Demonstrate the Absence of this Receptor on Raji Cells.
For flow cytometric analysis, a rabbit antiserum was raised against the second extracellular loop of the C3aR. Its optimal dilution of 1:4,000 was determined on a rat basophilic leukemia cell line (RBL-2H3 cells) stably expressing the human C3aR (7) and on nontransfected RBL cells (data not shown). Using these conditions, no difference between the fluorescence caused by immune, or preimmune sera, or the buffer control could be detected on Raji cells (data not shown). To confirm the thereby suggested absence of the C3aR on the cell surface of these cells, C3aR Northern blots with poly(A+) RNA from Raji cells were performed (Fig. 1). RNA from dibutyryl cAMP-induced (Boehringer, Mannheim, Germany) (Ui) and native U937 cells (Uφ) was applied as control. As described before (8), a prominent hybridization band at ∼2.4 kb, and a weak band at about 4 kb (16) could be detected. In contrast, even with 1 μg of poly(A+) RNA from Raji cells no C3aR transcript could be detected. The integrity of the Raji cell mRNA was affirmed by rehybridization with a probe for the housekeeping gene GAPDH (27), and the sharp rRNA bands in the corresponding gel. Consistent with these results, we could not detect any specific [125I]–hC3a binding on Raji cells (data not shown). The discrepancy to the results of Rogli ′ c et al. (9) who described C3aR expression on Raji cells antigenetically could be due to clonal differences of the analyzed cells, or the low serum dilution (1:200) used in their study. In our animals, such high concentrations result in high nonspecific binding of the preimmune serum on certain cell types.
Figure 1.
C3aR mRNA is found in human monocytes but not in Raji cells. Northern blot (a) and corresponding denaturing ethidium bromide– stained agarose gel (b) of poly(A+) RNA from nondifferentiated U937 cells (Uφ) (∼6,500 C3aR per cell; reference 16), U937 cells with induction of their C3aR expression by dibutyrylic cAMP (Ui) (∼30,000 per cell; references 15, 16), Raji cells (Ra), and monocytes (Mo), enriched from PMNCs by adherence. The intensity of the ribosomal bands indicated that similar concentrations of RNA were loaded in each lane. Additionally, the blot was rehybridized with a GAPDH probe as internal standard.
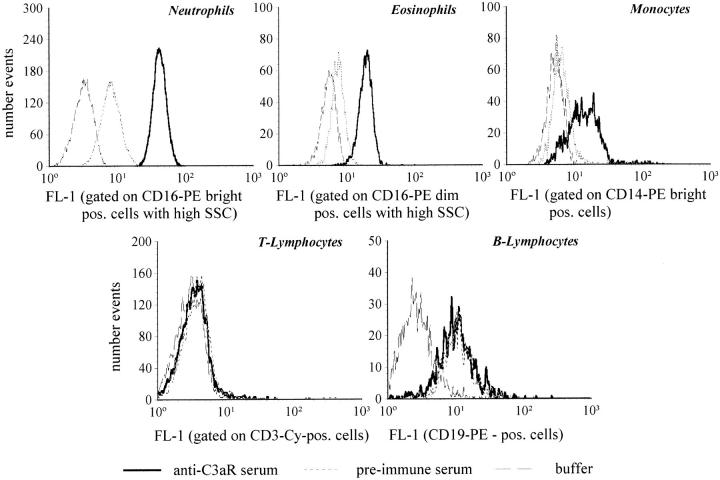
Flow Cytometric Analyses with Polyclonal Rabbit Anti-C3aR Serum Indicate C3aR Expression on Human Neutrophils, Eosinophils and Monocytes, but Not on T or B Lymphocytes.
Peripheral blood leukocytes were examined flow cytometrically for C3aR expression. The histograms for the different cell types (Fig. 2) indicate that the highest degree of C3aR expression can be found on neutrophils, whereas eosinophils showed weaker staining. The histogram of monocytes is remarkably widespread, suggesting a high diversity in the degree of C3aR expression. Possibly, monocyte subpopulations (or monocytes in different states of activation) exist that vary in their extent of C3aR expression. B and T lymphocytes did not show any C3aR-specific fluorescence as compared with the preimmune serum. The mean values of the fluorescence (FL-1) from cells obtained from five different donors confirmed for neutrophils, eosinophils, and monocytes significant differences between the fluorescence obtained by preimmune and C3aR immune serum (Table 1). The gap between the mean fluorescence obtained with the two sera on neutrophils was similar to that on RBL cells, expressing ∼32,000 C3aR per cell (data not shown).
Figure 2.
Flow cytometric analyses using polyclonal rabbit anti-C3aR serum suggests C3aR expression on human neutrophils, eosinophils, and monocytes, but not on T or B lymphocytes. The fluorescence values (FL-1) of different subpopulations of peripheral blood of one healthy human individual are depicted as histograms using anti-C3aR immune serum, preimmune serum (both sera diluted 1:4,000), and PBS. These histograms were based on the corresponding gating of neutrophils, eosinophils, monocytes, T and B lymphocytes according to their specific pattern in forward and side scatter, and cell population–specific CD markers For each cell type, one typical experiment of a total of five is depicted.
Table 1.
Statistical Evaluation of Flow Cytometric Analyses Using Polyclonal Rabbit Anti-C3aR Serum Suggests C3aR Expression on Human Neutrophils, Eosinophils, and Monocytes, but not on T or B Lymphocytes
| Neutrophils | Eosinophils | Monocytes | T lymphocytes | B lymphocytes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anti-C3aR serum | 45.5 ± 3.6* | 23.0 ± 3.9* | 24.4 ± 5.7* | 3.2 ± 1.2 | 10.5 ± 0.6 | |||||
| Negative serum | 11.3 ± 2.7 | 9.5 ± 2.1 | 10.4 ± 2.9 | 3.3 ± 1.2 | 11.1 ± 0.8 | |||||
| PBS | 3.3 ± 0.6 | 6.6 ± 1.2 | 4.9 ± 1.9 | 2.6 ± 1.0 | 2.8 ± 0.2 |
The mean fluorescence values corresponding to FL-1 in Fig. 2 using anti-C3aR immune serum, preimmune serum (serum dilutions of 1:4,000), and PBS were determined. Blood of (n = 5) different healthy donors was analyzed in independent experiments. Mean ± SD of the mean fluorescence values of neutrophils, eosinophils, monocytes, T lymphocytes, and B lymphocytes are depicted. The significance of the difference between the fluorescence obtained with the anti-C3aR serum and the control serum (P >0.999 in the Student's t test) is indicated by asterisks.
CD9 Antigen Is Highly Expressed on Human Eosinophils: It Can Be Used for High Degree Purification of Neutrophils from PMN Preparations by Negative Selection.
To show that C3a-mediated functional responses of neutrophils (including specific [125I]–C3a binding) are independent of the presence of other cells like eosinophils, a negative selection method for the purification of highly enriched neutrophils was developed. It was based on the depletion of the contaminating eosinophils from PMN preparations to minimize preactivation of the purified neutrophils, which could otherwise interfere in functional assays. A mouse anti-CD9 mAb bound to eosinophils and only a subpopulation (depending on the donor: 5–25%) of neutrophils (CD16bright+) (data not shown) confirming earlier reports (28–30). Based on this mAb, and anti-mouse IgG bound to magnetic beads, CD9dim+ neutrophils could be highly enriched by magnetic sorting. The purity of each neutrophil preparation was controlled by three independent methods: Staining by Diff-Quick™ and Kimura (counting 2,000 cells), and flow cytometry using an anti-CD16 mAb. By preselecting normal donors with a maximum of 2% of eosinophils, the corresponding highly purified neutrophil preparations were contaminated with less than 0.2% of eosinophilic granulocytes, and less than 0.2% of mononuclear cells, almost exclusively lymphocytes.
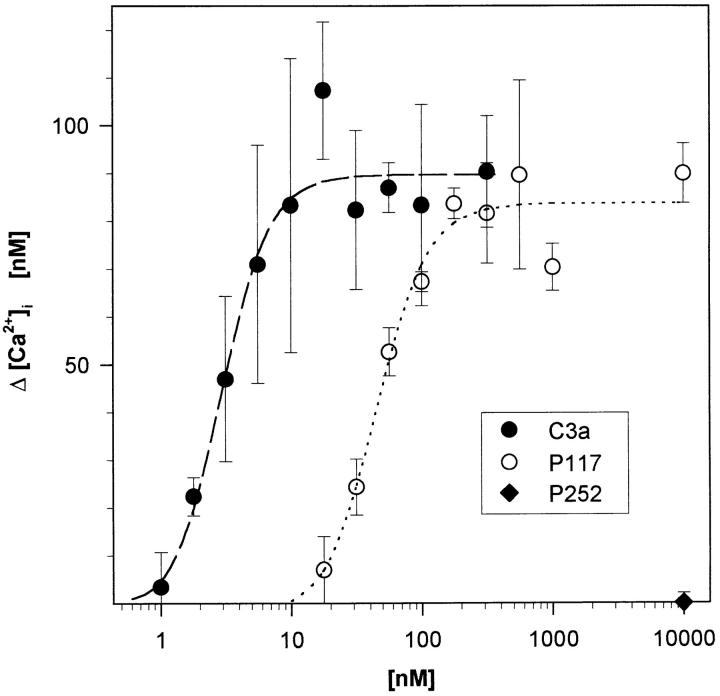
C3a and a C3a Analogue Synthetic Peptide Induce a Dose-dependent Increase of [Ca2+]i in Highly Purified Human Neutrophils.
Highly purified neutrophils isolated from three healthy donors were analyzed in the Fura-2/AM assay for their functional response to C3a, and the C3a analogue synthetic peptide P117 (Fig. 3). Highly purified neutrophils responded to C3a and P117 in a dose-dependent manner with an ED50 of 2.5 ± 1.0 nM, and 62.3 ± 2.5 nM (n = 3), respectively. Up to 10 μM of the irrelevant peptide P252 did not cause any elevation of the baseline in neutrophils (35 ± 10 nM of [Ca2+]i). Similar results (concerning ED50 and peak height) were obtained in parallel experiments using less purified granulocytes obtained from the same donors, which contained up to 5% eosinophils (data not shown). One can draw the following conclusions: (a) Being present in such a low percentage, the contaminating cells can not account for the Ca2+ signal determined in the highly purified neutrophil suspension. (b) The peak Ca2+ level of highly purified neutrophils was already reached ∼5–10 s after the addition of C3a. The short interval between C3a addition and the response of neutrophils argues additionally against an indirect C3a effect. The response of neutrophils would have to be mediated by the fast synthesis of a neutrophil-activating factor or its fast release from eosinophils. (c) This factor would then have to be already in abundance when only <0.2% of eosinophils are present. (d) Nevertheless, other C3a-dependent functional responses of neutrophils might be influenced by eosinophils, as described for neutrophil polarization (13). In contrast with the fast Ca2+ response, neutrophil polarization only slowly increases within 10–40 min after C3a addition. A minimum of ∼3% of eosinophils is needed for polarization.
Figure 3.
Highly purified human neutrophils (<0.2% of eosinophils) still respond to C3a and a C3a-analogous synthetic peptide (P117) with an increase in cytosolic Ca2+. The response was determined in a Fura-2/AM assay in suspension. The irrelevant peptide P252 served as negative control. Depicted are mean ± SD (n = 3) of one typical experiment out of three.
Taken together, the fast C3a-triggered increase in cytosolic Ca2+ of neutrophils seems to be independent of eosinophils. These data strongly suggest the expression of the C3aR on neutrophils and confirm the data obtained by FACS® demonstrating C3aR expression on these cells.
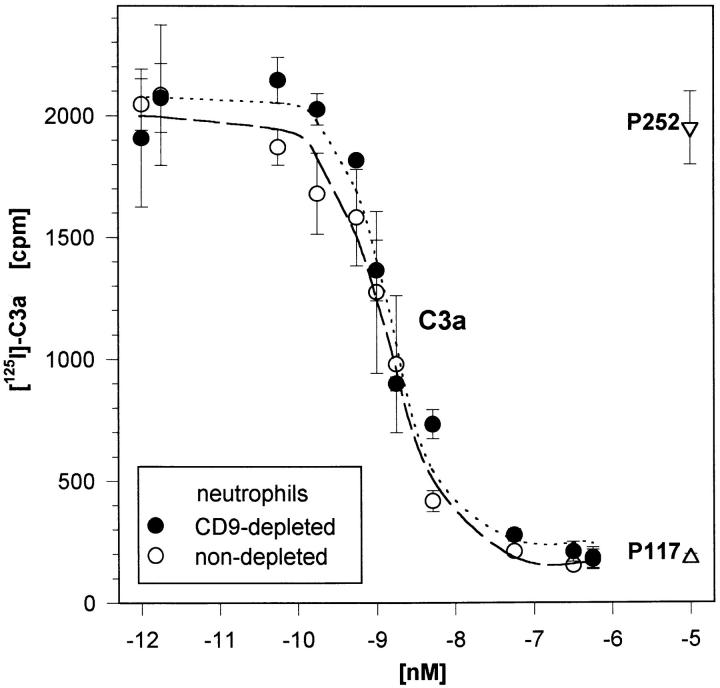
Highly Purified Neutrophils Show Essentially the Same [125I]–hC3a Binding as Less Purified Neutrophils in the Presence of Eosinophils.
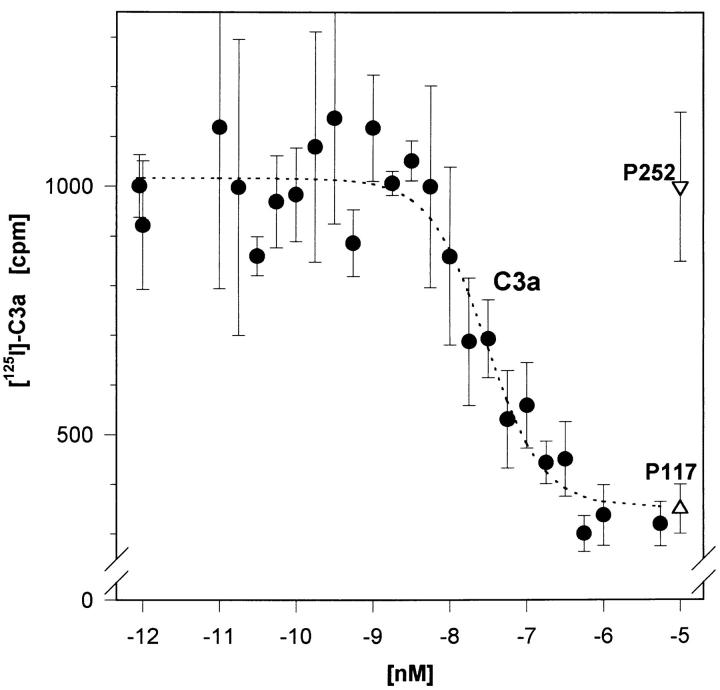
As further evidence for the expression of the C3aR on neutrophils, competitive [125I]–hC3a binding studies were performed on highly purified neutrophils (Fig. 4, closed circles). By iterative curve fitting a K d of 4.5 ± 3.4 nM and a number of 23,700 ± 14,800 C3aR per cell were calculated (n = 3 experiments). These data again strongly indicate that the C3aR is indeed present on neutrophils. If only the small percentage of eosinophils (<0.2%) accounted for the observed specific C3a binding, the few eosinophils would have to express an unlikely, extremely high number of C3aR per cell (>10,000,000). Additionally, if this was the case, the specific [125I]–hC3a binding should then depend on the percentage of C3aR-bearing contaminating eosinophils. However, we observed an essentially unchanged binding behavior on less purified neutrophils from the same donor (Fig. 4, open circles).
Figure 4.
C3aR demonstrated on highly purified human neutrophils (<0.2% of eosinophils) in competitive [125I]–hC3a binding studies. A constant concentration of [125I]–hC3a (∼40,000 cpm) was displaced by increasing concentrations of nonlabeled C3a as well as the C3a analog peptide P117, and the irrelevant peptide P252. Neutrophils from the same individual, undergoing only a mock treatment instead of an anti-CD9 depletion, were analyzed in parallel. Mean ± SD (n = 4) of one typical experiment out of three are given. (The difference in the percentage of eosinophils due to anti-CD9 depletion was factor 3–5 in these experiments).
Additional Assays to Confirm C3aR Expression on Monocytes.
Flow cytometry using 1:4,000 diluted polyclonal anti-C3aR serum had strongly suggested C3aR expression on human monocytes (see Fig. 2) Again, we wanted to confirm these data by independent methods: C3aR Northern blot, C3a-dependent [Ca2+]i response, and competitive [125I]–hC3a binding studies.
A High Steady-state Level of C3aR mRNA Is Detectable in Monocytes.
Poly(A+) RNA was prepared from monocytes for C3aR Northern blots. For this assay, monocytes (CD14+) had been enriched (>85%) from the fraction of mononuclear cells (Ficoll) by their adherence to cell culture dishes. As judged by microscopy of the stained cells, and confirmed by flow cytometry (data not shown), lymphocytic cells accounted for the residual <15%, whereas granulocytes were completely eliminated by this procedure (<0.1%). This contamination was considered to be tolerable, because on B or T lymphocytes no C3aR-specific fluorescence had been detected before with the polyclonal anti-C3aR serum (see Fig. 2). 200 ng of monocyte poly(A+) RNA was hybridized with a C3aR riboprobe in the same Northern blot as described above for mRNA of U937 and Raji cells (see Fig. 1). Strong and typical hybridization signals for C3aR mRNA could be detected for monocytes. According to the weak hybridization band obtained with the GAPDH probe, an unexpected high steady-state level of C3aR mRNA seems to be present in monocytes.
Unfortunately, poly(A+) RNA from highly purified neutrophils could only be prepared in a semidegraded manner, not suitable for Northern blots.
Human Monocytes Respond to C3a, or the C3a Analogue Synthetic Peptide P117, with an Increase in [Ca2+]i.
The C3a-dependent Ca2+ increase in monocytes was determined flow cytometrically starting 10 s after application of the ligand, and compared with the response of monocytes to C5a. Diff-Quick™ staining revealed that no granulocytes were present within 1,000 cells of the PMNC preparation, and that all contaminating cells (∼80%) were of lymphocytic origin. For this functional assay, monocytes were gated within the PBMCs according to forward scatter and side scatter (Fig. 5, top). Flow cytometric analyses of an aliquot of this PMNCs preparation using CD14 (LPS receptor) as marker for monocytes confirmed that the selected gating was appropriate for monocytes (data not shown). The monocytes responded with an increase in Fluo3-determined fluorescence to a C3a stimulus, as compared with the buffer control (Fig. 5, bottom). As judged by the mean fluorescence values, the maximal C3a response of monocytes is reached at concentrations of C3a near 5–10 nM, similar to neutrophils (data not shown). Intriguingly, the monocytes did not respond homogeneously to C3a. With increasing C3a concentrations progressively more cells switched from a fluorescence value corresponding to the buffer control to a distinctly higher fluorescence state. However, even at maximal C3a concentrations a portion of the gated cell population did not show any increase in Ca2+. The same effect was observed after stimulation with the C3a analogue peptide P117. P252 served again as negative control (data not shown). In kinetic studies with increased incubation times after C3a application of up to 1 min (equivalent to the approximate duration of the response), a similar proportion of monocytes seemed to be non-C3a responsive (data not shown). In contrast, almost all gated cells responded homogeneously after stimulation using a maximal concentration of recombinant C5a (20 nM). These data would most likely suggest that there is a subpopulation of monocytes lacking the ability to react to C3a. These might correspond to the heterogeneous distribution of the C3aR as determined with the polyclonal anti-C3aR serum (see Fig. 2). If this is the case, it remains to be investigated whether the inhomogeneity actually reflects different subsets within monocytes or monocytes at different states of preactivation caused by the cell preparation. Similarly, functional subpopulations within neutrophils have been described for the IL-8 receptor (31). B or T lymphocytes did not react to C3a in this assay (data not shown). There is no evidence for C3aR expression on lymphocytes. Keeping in mind that no C3aR could be detected antigenetically, and that the response reaches its maximum already 10–15 s after stimulus application, it is very unlikely that lymphocytes stimulated by C3a would influence the Ca2+ response of monocytes.
Figure 5.
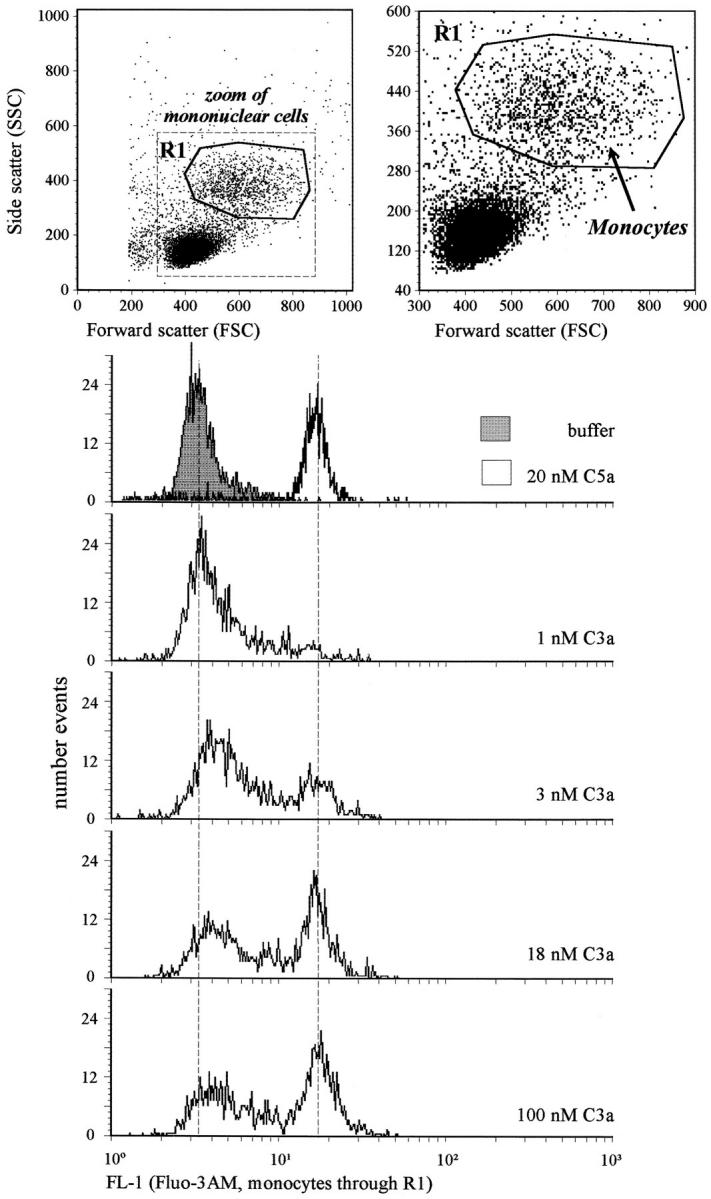
Human monocytes respond inhomogeneously to C3a. PBMCs (no granulocytes within 1,000 stained cells) were loaded with Fluo3-AM as fluorescence indicator of free cytosolic Ca2+. Monocytes were gated as indicated in the upper panels. The histograms of the fluorescence intensity (FL-1) resulting ∼10 s after stimulation with buffer or 20 nM of C5a, and increasing concentrations of C3a, respectively, are depicted. The response of monocytes to the C3a analogue peptide P117 was similar (data not shown).
Specific C3a Binding in the Nanomolar Range Can Be Detected in Purified Monocytes.
For competitive [125I]–hC3a binding studies monocytes were enriched from PBMCs through positive selection with CD14-coupled MACS™, resulting in a purity of ∼90–98%, as described earlier (26). All contaminating cells were lymphocytic cells. One representative binding study with increasing concentrations of nonlabeled C3a on these highly purified monocytes is depicted (Fig. 6). These data clearly show specific C3a binding on human monocytes, as confirmed by the binding of the control peptides. The half-maximal competitive effect on monocytes of (n = 3) different donors was reached at concentrations of C3a (10–100 nM) ten times higher than on neutrophils (see also Fig. 4), suggesting differences in the C3aR affinity (n = 3 experiments; K d, 48.2 ± 23 nM; 52,500 ± 14,000 C3aR per monocyte). One possible explanation for this difference would be the coupling of the receptor to different G proteins in the two different cell types; it is known that G protein coupling influences the receptor affinity (32) as recently demonstrated for the high affinity binding sites of the C5a receptor (33). Theoretically, one could also speculate that different subtypes of the C3aR might exist on monocytes and neutrophils. However, presently there is no other evidence for that. This phenomenon has to be clarified in future studies.
Figure 6.
C3aR demonstrated on highly purified human monocytes in competitive [125I]–hC3a binding studies. Monocytes were highly purified by anti-CD14 MACS™ (in this particular experiment, ∼96%, 4% lymphatic cells, <0.2% granulocytes). A constant concentration of [125I]–hC3a (∼50,000 cpm) was displaced by increasing concentrations of nonlabeled C3a as well as the C3a analog peptide P117, and the irrelevant peptide P252. Mean ± SD (n = 4) of one typical experiment out of three are given.
In all experiments reported in these studies the method of purification of neutrophils or monocytes was selected and optimized in order to support the main aim of this paper, the determination of cellular subsets expressing the C3aR. One might expect a modulation of C3aR expression depending on the state of (pre-)activation of monocytes and neutrophils. Therefore, we are reluctant to judge quantitatively and to compare the data for one cell type obtained in different assays. It will be interesting to see in future studies, e.g., whether the adherence of monocytes or the binding of the anti-LPS antibody (CD14) in vitro or the migration of monocytes into inflamed tissue in vivo influences C3aR expression and/or the C3a responsiveness of this cell type.
Taken together, we have shown in the present study antigenetically, functionally, and by ligand binding the expression of the C3aR on human neutrophils and monocytes. For monocytes, we could provide further evidence by C3aR mRNA Northern blots. Additionally, the binding of the anti-C3aR serum on human eosinophils confirmed C3aR expression on this cell type. The C3aR can also be found on human basophils (10, 11), and mast cell lines (12). The emerging broad expression pattern of the C3aR on all peripheral blood cells of myeloid origin suggests an until now underestimated physiological and pathophysiological role of C3a in man.
Acknowledgments
We thank the head of the Department of Medical Microbiology in Hannover, Professor D. Bitter-Suermann for his continuous strong support, and C. Rheinheimer for her excellent technical assistance. We are thankful to all, now pale, members of the Institute, who willingly donated their blood.
This work was supported by a grant of the Deutsche Forschungsgemeinschaft (DFG, Kl 603/4-1).
Footnotes
Address corespondence to Andreas Klos, M.D., Institute of Medical Microbiology, Building, I6, Room 05-2410, Hannover Medical School, Hannover, 30625, Germany. Phone: 511-532-4342; FAX: 511-532-4366; E-mail: KLOS@MIKROBIO.MH-Hannover.de
1 Abbreviations used in this paper: C3a, an anaphylatoxic peptide generated from the complement component C3; C3aR, C3a receptor; [Ca2+]i, concentration of free cytosolic Ca2+, GAPDH, rat glyceraldehyde-3-phosphate dehydrogenase used as housekeeping gene.
References
- 1.Köhl, J., and D. Bitter-Suermann. 1993. Anaphylatoxins. In Complement in Health and Disease. K. Whaley, M. Loos, and J.M. Weiler, editors. Kluwer Academic Publishers, Dordrecht, Germany. 295–320.
- 2.Bitter-Suermann, D. 1988. The anaphylatoxins. In The Complement System. K. Rother and G.O. Till, editors. Springer-Verlag, Berlin. 367–395.
- 3.Morgan EL. Modulation of the immune response by anaphylatoxins. Complement. 1986;3:128–136. doi: 10.1159/000467890. [DOI] [PubMed] [Google Scholar]
- 4.Boulay F, Mery L, Tardif M, Brouchon L, Vignais P. Expression cloning of a receptor for C5a anaphylatoxin on differentiated HL-60 cells. Biochemistry. 1991;30:2993–2999. doi: 10.1021/bi00226a002. [DOI] [PubMed] [Google Scholar]
- 5.Gerard NP, Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature (Lond) 1991;349:614–617. doi: 10.1038/349614a0. [DOI] [PubMed] [Google Scholar]
- 6.Hopken UE, Lu B, Gerard NP, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature (Lond) 1996;383:86–89. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- 7.Ames RS, Li Y, Sarau HM, Nuthulaganti P, Foley JJ, Ellis C, Zeng Z, Su K, Jurewicz AJ, Hertzberg RP, Bergsma DJ, Kumar C. Molecular cloning and characterization of the human anaphylatoxin C3a receptor. J Biol Chem. 1996;271:20231–20234. doi: 10.1074/jbc.271.34.20231. [DOI] [PubMed] [Google Scholar]
- 8.Crass T, Raffetseder U, Martin U, Grove M, Klos A, Köhl J, Bautsch W. Expression cloning of the human C3a anaphylatoxin receptor (C3aR) from differentiated U-937 cells. Eur J Immunol. 1996;26:1944–1950. doi: 10.1002/eji.1830260840. [DOI] [PubMed] [Google Scholar]
- 9.Rogli ′ c, A., E.R. Prossnitz, S.L. Cavanagh, Z. Pan, A. Zou, and R.D. Ye. cDNA cloning of a novel G protein– coupled receptor with a large extracellular loop structure. Biochim Biophys Acta. 1996;1305:39–43. doi: 10.1016/0167-4781(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 10.Dahinden CA, Bischoff SC, Brunner T, Krieger M, Takafuji S, de Weck AL. Regulation of mediator release by human basophils: importance of the sequence and time of addition in the combined action of different agonists. Int Arch Allergy Appl Immunol. 1991;94:161–164. doi: 10.1159/000235350. [DOI] [PubMed] [Google Scholar]
- 11.Kretzschmar T, Jeromin A, Gietz C, Bautsch W, Klos A, Köhl J, Rechkemmer G, Bitter-Suermann D. Chronic myelogenous leukemia-derived basophilic granulocytes express a functional active receptor for the anaphylatoxin C3a. Eur J Immunol. 1993;23:558–561. doi: 10.1002/eji.1830230239. [DOI] [PubMed] [Google Scholar]
- 12.Legler DF, Loetscher M, Jones SA, Dahinden CA, Arock M, Moser B. Expression of high- and low-affinity receptors for C3a on the human mast cell line, HMC-1. Eur J Immunol. 1996;26:753–758. doi: 10.1002/eji.1830260405. [DOI] [PubMed] [Google Scholar]
- 13.Daffern PJ, Pfeifer PH, Ember JA, Hugli TE. C3a is a chemotaxin for human eosinophils but not for neutrophils. I. C3a stimulation of neutrophils is secondary to eosinophil activation. J Exp Med. 1995;181:2119–2127. doi: 10.1084/jem.181.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsner J, Oppermann M, Czech W, Dobos G, Schopf E, Norgauer J, Kapp A. C3a activates reactive oxygen radical species production and intracellular calcium transients in human eosinophils. Eur J Immunol. 1994;24:518–522. doi: 10.1002/eji.1830240304. [DOI] [PubMed] [Google Scholar]
- 15.Klos A, Bank S, Gietz C, Bautsch W, Köhl J, Burg M, Kretzschmar T. C3a receptor on dibutyryl– cAMP-differentiated U937 cells and human neutrophils: the human C3a receptor characterized by functional responses and 125I–C3a binding. Biochemistry. 1992;31:11274–11282. doi: 10.1021/bi00161a003. [DOI] [PubMed] [Google Scholar]
- 16.Burg M, Martin U, Bock D, Rheinheimer C, Köhl J, Bautsch W, Klos A. Differential regulation of the C3a and C5a receptors (CD88) by IFN-gamma and PMA in U937 cells and related myeloblastic cell lines. J Immunol. 1996;157:5574–5581. [PubMed] [Google Scholar]
- 17.Takabayashi T, Vannier E, Clark BD, Margolis NH, Dinarello CA, Burke JF, Gelfand JA. A new biologic role for C3a and C3a desArg: regulation of TNF-alpha and IL-1 beta synthesis. J Immunol. 1996;156:3455–3460. [PubMed] [Google Scholar]
- 18.Gerardy-Schahn R, Ambrosius D, Saunders D, Casaretto M, Mittler C, Karwarth G, Gorgen S, Bitter-Suermann D. Characterization of C3a receptor–proteins on guinea pig platelets and human polymorphonuclear leukocytes. Eur J Immunol. 1989;19:1095–1102. doi: 10.1002/eji.1830190620. [DOI] [PubMed] [Google Scholar]
- 19.Norgauer J, Dobos G, Kownatzki E, Dahinden C, Burger R, Kupper R, Gierschik P. Complement fragment C3a stimulates Ca2+ influx in neutrophils via a pertussis-toxin–sensitive G protein. Eur J Biochem. 1993;217:289–294. doi: 10.1111/j.1432-1033.1993.tb18245.x. [DOI] [PubMed] [Google Scholar]
- 20.Ehrengruber MU, Geiser T, Deranleau DA. Activation of human neutrophils by C3a and C5A. Comparison of the effects on shape changes, chemotaxis, secretion, and respiratory burst. FEBS Lett. 1994;346:181–184. doi: 10.1016/0014-5793(94)00463-3. [DOI] [PubMed] [Google Scholar]
- 21.Van-Epps DE, Chenoweth DE. Analysis of the binding of fluorescent C5a and C3a to human peripheral blood leukocytes. J Immunol. 1984;132:2862–2867. [PubMed] [Google Scholar]
- 22.Hansel TT, De Vries IJ, Iff T, Rihs S, Wandzilak M, Betz S, Blaser K, Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Methods. 1991;145:105–110. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- 23.Leucocyte Typing IV: White Cell Differentiation Antigens. 1989. Knapp, W., B. Dörken, W.R. Gilks, E.P. Rieber, R.E. Schmidt, H. Stein, and A.E.G.Kr. von dem Borne, editors. Oxford University Press, Oxford. 1182 pp.
- 24.Kimura I, Moritani Y, Tanizaki Y. Basophils in bronchial asthma with reference to reagin-type allergy. Clin Allergy. 1973;3:195–202. doi: 10.1111/j.1365-2222.1973.tb01321.x. [DOI] [PubMed] [Google Scholar]
- 25.Colotta F, Peri G, Villa A, Mantovani A. Rapid killing of actinomycin D–treated tumor cells by human mononuclear cells. I. Effectors belong to the monocyte–macrophage lineage. J Immunol. 1984;132:936–944. [PubMed] [Google Scholar]
- 26.Brock TG, McNish RW, Coffey MJ, Ojo TC, Phare SM, Peters M, Golden Effects of granulocyte– macrophage colony-stimulating factor on eicosanoid production by mononuclear phagocytes. J Immunol. 1996;156:2522–2527. [PubMed] [Google Scholar]
- 27.Fort, P., L. Marty, M. Piechaczyk, S. el Sabrouty, C. Dani, P. Jeanteur, and J.M. Blanchard. 1985. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 13:1431–1442. [DOI] [PMC free article] [PubMed]
- 28.Hansel TT, Braunstein JB, Walker C, Blaser K, Bruijnzeel PL, Virchow JC, Jr, Virchow CS. Sputum eosinophils from asthmatics express ICAM-1 and HLA-DR. Clin Exp Immunol. 1991;86:271–277. doi: 10.1111/j.1365-2249.1991.tb05809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forsyth KD. Anti-CD9 antibodies augment neutrophil adherence to endothelium. Immunology. 1991;72:292–296. [PMC free article] [PubMed] [Google Scholar]
- 30.Saito H, Yamada K, Breard J, Yoshie O, Mathe G. A monoclonal antibody reactive with human eosinophils. Blood. 1986;67:50–55. [PubMed] [Google Scholar]
- 31.Elsner J, Kaever V, Emmendorffer A, Breidenbach T, Lohmann ML, Matthes, Roesler J. Heterogeneity in the mobilization of cytoplasmic calcium by human polymorphonuclear leukocytes in response to fMLP, C5a and IL-8/NAP-1. J Leukocyte Biol. 1992;51:77–83. doi: 10.1002/jlb.51.1.77. [DOI] [PubMed] [Google Scholar]
- 32.Butkerait P, Zheng Y, Hallak H, Graham TE, Miller HA, Burris KD, Molinoff PB, Manning DR. Expression of the human 5-hydroxytryptamine1A receptor in Sf9 cells. Reconstitution of a coupled phenotype by co-expression of mammalian G protein subunits. J Biol Chem. 1995;270:18691–18699. doi: 10.1074/jbc.270.31.18691. [DOI] [PubMed] [Google Scholar]
- 33.Raffetseder U, Roper D, Mery L, Gietz C, Klos A, Grotzinger J, Wollmer A, Boulay F, Köhl J, Bautsch W. Site-directed mutagenesis of conserved charged residues in the helical region of the human C5a receptor. Arg2O6 determines high-affinity binding sites of C5a receptor. Eur J Biochem. 1996;235:82–90. doi: 10.1111/j.1432-1033.1996.00082.x. [DOI] [PubMed] [Google Scholar]