Abstract
Although much is known about the activation, proliferation, and function of CD4+ T cells, little is known about how they survive as resting T cells in animals. Resting T cells have a half-life in animals of more than a week; however, when they are removed from animals and placed in tissue culture their half-life falls to ∼24 h. In this paper, we show that the survival of resting T cells in vitro is promoted by two cytokines, interleukins 4 and 7 (IL-4, IL-7). They may do this in part by maintaining levels of survival-promoting proteins such as Bcl-2 in the cells, because the levels of Bcl-2 and Bcl-Xl in resting T cells fall rapidly after the cells are isolated from animals, and are maintained by culture in IL-4. Because the IL-4 receptor is known to signal through the JAK1 and JAK3/Stat6 pathway, we tested whether Stat6 was required for IL-4– dependent T cell survival. Surprisingly, we found that IL-4 rescued T cells from apoptosis in what appeared to be a Stat6-independent manner. These results demonstrate that the survival of resting T cells is an active process that can be affected by signals delivered by cytokines and also suggest that the IL-4 receptor on resting T cells may use a novel signaling pathway to facilitate T cell viability.
Many investigators have studied the life expectancy of B and T cells (1–5). Some aspects of this subject are very controversial; for example, argument continues to rage over the matter of whether immunological memory depends upon lymphocyte proliferation in response to antigen stored in the animal versus a very long half-life for memory cells (5). Even for virgin cells, the question of half-life is not settled. Early studies suggested that a population of T cells in unprimed mice might have a half-life of only ∼2 wk (6); however, later studies have suggested that some virgin T cells may have life expectancies that are longer than this (3–5). In general, most students of this subject would agree that virgin, nondividing T cells have half-lives in vivo that are at least several weeks in length.
Although a good deal is known about what keeps activated T cells alive, surprisingly little is known about what is needed to maintain resting T cells in animals. Of course, it is possible that these cells receive no external signals, and that their half-lives in vivo are simply a reflection of their own internal clocks. Two results suggest that this simple hypothesis may not be correct. First, the half-life of peripheral T cells in animals is shortened if the cells do not express the γ chain (γc; reference 7), which is common to the receptors for a number of cytokines including IL-2, IL-4, IL-7, IL-9, and IL-15 (8–10). Second, the life expectancy of isolated virgin T cells is much shorter in tissue culture than it is in animals. In vitro, isolated T cells have a half-life of only 1–2 d, rather than the half-life of 2 wk or more found in vivo. Although this latter result could be due to inadequate tissue culture conditions, it is also possible that isolated T cells in vitro are deprived of some factor(s) that is available to them in vivo and that is essential for their existence.
To test the idea that the animal provides factors which keep virgin T cells alive and which are absent in tissue culture, we studied the effects of various cytokines on the life expectancy in vitro of T cells expressing a transgenic TCR. We reasoned that most of the T cells in these animals would not have been previously exposed to antigen and therefore would be virgin, resting cells. Experiments showed that these cells died readily in vitro but that their death could be prevented by addition of IL-4 or IL-7. Levels of the survival-related proteins, Bcl-2 and Bcl-Xl (11–15), dropped rapidly in T cells after they were isolated from animals and placed in tissue culture. Culture with IL-4 prevented this drop, suggesting that the effects of IL-4 on T cell survival might be due to its ability to maintain Bcl-2 levels in T cells. IL-4 prevented T cell death even if the cells came from a Stat6-deficient animal, although slightly higher amounts of IL-4 were required to achieve the rescue. Stat6 is a signaling protein often associated with IL-4 driven effects. Thus, IL-4 and IL-7, which share the γc in their receptors (9), may affect T cell survival via some common signaling pathway that does not involve Stat6.
Materials and Methods
Mice.
The AD10 TCR reacts with a peptide from pigeon cytochrome c bound to I-Ek (16). Mice transgenic for this TCR were the gift of Dr. S. Hedrick. They were bred in the Animal Care Facility at the National Jewish Medical and Research Center (Denver, CO). Normal C57BL/10 animals were purchased from The Jackson Laboratory (Bar Harbor, ME). Stat6−/− mice (17, 18) were bred at the Animals Care Facility, St. Jude Children's Hospital (Memphis, TN). All mice were maintained under specific pathogen-free conditions.
Preparation and Culture of T Cells.
T cells were isolated by nylon wool purification as previously reported (19), and resuspended at 5 × 106 cells/ml in DMEM supplemented with 10% of a batch of fetal bovine serum previously tested for its ability to maintain hybridoma cells at excellent viability. The medium also contained essential and nonessential amino acids (GIBCO BRL, Gaithersburg, MD), 5 × 10−5 M 2-mercaptoethanol, sodium pyruvate, glutamine, and antibiotics (20). Cultures were set up in 96-well tissue culture plates at 100 μl/well. Cytokines were added at the beginning of the culture period at the indicated concentrations. IL-4 was obtained from Genzyme (Cambridge, MA). The other cytokines were purchased from R&D Systems, (Minneapolis, MN).
Analysis of Cell Death.
At the time that the T cells were isolated and 24 and 48 h thereafter, samples of the cells were stained to measure their DNA content as previously described (21). In brief, the 96-well plates were spun, and supernatant removed from the wells by gentle dumping. A DNA staining mixture, comprised of 5 μg/ml propidium iodide (PI)1, 0.3% saponin (both from Sigma Chem. Co., St. Louis, MO), 5 mM EDTA and 50 μg/ml RNAse (Boehringer Mannheim, Indianapolis, IN) was then added as 180 μl to each well. The plates were left at room temperature for 30 min and then kept on ice. These last two procedures were carried out in the dark. The intensity of PI staining, a measure of DNA content, was assayed using a Becton Dickinson FACScan® instrument (Beckton Dickinson, Immunocytometry Systems, San Jose, CA).
Immunoprecipitation and Western Blots.
Lymph node T cells were isolated and cultured at 5 × 106 cells/ml as described above. At 0, 4, 24, and/or 48 h T cells were lysed in 0.5% NP-40, 20 mM Hepes, pH 7.2, 100 mM NaCl, 2 mM DTT, 1 mM EDTA, 1.0 mM PMSF, 1 μg/ml aprotinin (all reagents purchased from Sigma). The nuclei were pelleted for 10 min at 14,000 rpm in the cold in a microfuge. Supernatants were removed and 2 × 106 cell equivalents were fractionated by SDS-PAGE, using 10% acrylamide, and blotted onto Immobilon PVDF membranes (Millipore, Bedford, MA) using a Trans-Blot apparatus (BioRad, Freemont, CA) for 30 min at 15 V. Blots were blocked with 5% powdered milk in Tris-buffered saline pH 7.5, Bcl-2 and Bcl-Xl were detected with rabbit polyclonal antibodies purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A goat anti–rabbit Ig peroxidase-conjugated antibody (Boehringer Mannheim) was used as the secondary reagent. Bands were detected using Amersham ECL reagents (Arlington Heights, IL).
For analysis of Stat6 phosphorylation, T cells were cultured with and without IL-4 as described above. Nuclear lysates were generated by resuspending T cells in 20 mM Hepes, pH 7.9, 300 mM sucrose, 10 mM KCl, 1.5 mM MgCl2 with protease inhibitors. The cells were allowed to swell for 5 min and then spun. Cells were Dounce homogenized and spun. The nuclei were resuspended in 20 mM Hepes, pH 7.9, 300 mM sucrose, 420 mM NaCl, 1.5 mM MgCl2 with protease inhibitors. After a 30-min incubation on ice, exuded nuclear proteins were immunprecipated with anti-phosphotyrosine antibody (Santa Cruz Biotechnology), followed by precipitation with protein G PLUS–agarose (Santa Cruz Biotechnology). Immunoprecipitates were fractionated by SDS-PAGE and immunoblotted for the presence of Stat6 using rabbit polyclonal anti-Stat6 (Santa Cruz Biotechnology). Bands bound to the rabbit antibody were detected as described above.
Results and Discussion
Two Cytokines, IL-4 and IL-7, Prevent the Death In Vitro of Resting T Cells.
T cells bearing the transgenic TCR, AD10, were purified from the lymph node cells of young mice and set up in culture. Most of these cells were resting cells that had not been exposed to antigen, as judged by their small size, low frequency (<10%) of CD69 expression, and maintenance of a TCR, AD10, not expected to engage environmental antigens. At 0 time and 24 or 48 h later, viability of the cells was assessed by measuring the amount of DNA they contained, using PI (21). Cells that have died by apoptosis, the primary mechanism of death in these cultures, degrade their DNA into small fragments, consequently the nuclei of such cells contain less than 2 N DNA.
Sample data from such an experiment are shown in Fig. 1. Before culture less than 5% of the cells were dead. However, 24 and 48 h later 50 and 77%, of the cells had died respectively.
Figure 1.
Resting T cells die in culture. This death is inhibited by culture with IL-4 or IL-7. Resting lymph node T cells from AD10 TCR transgenic mice were cultured at 2 × 105 cells/well with or without 100 U/ml of IL-4 or IL-7 for 24 and 48 h. Before culture, <5% of the cells contained <2 N DNA. After culture, cells were stained with propidum iodide and analyzed by flow cytometry to determine DNA content. The number given in each histogram is the percent of T cells that were apoptotic as defined by DNA content. Results shown are typical of three independent experiments.
Using this assay, we measured the effects of various cytokines on the appearance of dead cells in vitro. To do this, T cells were cultured for 24 or 48 h in the presence or absence of the cytokines, used at concentrations above those known to be active in vitro as judged by publications on the subject and the manufacturer's recommendations. Many cytokines had no effect on the death of resting T cells in culture. Such cytokines included IL-1β, IL-3, IL-5, huIL-8, IL-9, IL-10, IL-12, IFN-γ, TNF-α, TNF-β, RANTES, MIP-1α, MIP-1β, MIP-2, vascular endothelial growth factor (VEGF), platelet-derived endothelial cell growth factor (PEGF), and TGF-β. As we have previously reported, IL-6 proved to be an excellent survival factor (22; data not shown). However, we were surprised to find that two other cytokines, IL-4 and IL-7, also rescued resting T cells from death in culture very effectively. For example, as shown in Fig. 1 a, addition of IL-4 or IL-7 reduced the percent of dead cells after 24-h culture from 50% to 15 or 20%, respectively.
Titrations were performed to compare the relative efficacy of the two cytokines (Fig. 2). On a nanograms per milliliter basis, IL-4 was somewhat more effective than IL-7, with a half-maximal effect at about 2 ng/ml (∼10−10 M) versus 50 ng/ml (∼3 × 10−9 M) for IL-7. The effective concentration of IL-4 matched well with the dissociation constant of its high affinity receptor, 10−10 M (23). However, the dissociation constant of the high affinity IL-7 receptor is reported to be only slightly greater, at 2 × 10−10 M (24). Collectively, these data suggest that IL-4 may be acting via its high affinity receptor, whereas IL-7 may act via some combination of high and low affinity receptors (the latter have K ds of 10−8 M).
Figure 2.
Related cytokines, IL-4 and IL-7, rescue resting T cells from apoptosis. T cells were purified from the lymph nodes of AD10 TCR transgenic mice and 2 × 105 cells were cultured in triplicate for 24 h with IL-2 (□), IL-4 (▪), IL-7 (•), IL-9 (⋄), IL-13 (○), and IL-15 (▵) at the concentrations shown. At 24 h, the T cells were stained with PI and the mean percent ± SD of apoptotic cells was determined by flow cytometry.
Two other cytokines, IL-2 and IL-15, also had, at very high concentrations, some effect on the viability of the cultured cells (Fig. 2). Their effects were small but reproducible in several assays. We believe that these small effects may have been due to engagement of these cytokines by low affinity forms of their receptors. Resting T cells are thought not to bear high affinity receptors for IL-2 and perhaps also IL-15.
IL-4 and IL-7 did not inhibit death by inducing cell cycle progression, because at 24 and 48 h T cells cultured with these cytokines were still in G0/G1 and did not contain more than 2-N amounts of DNA (see Fig. 1; data not shown). Thus, these two cytokines acted as true survival factors in this assay.
Culture with IL-4 Maintains Bcl-2 Levels in Resting T Cells.
In many cases, lymphocyte survival is affected by proteins in the Bcl-2 family (11–15). Therefore, we tested whether culture in IL-4 had any effect on the levels of Bcl-2 and/or Bcl-Xl in the T cells. Purified resting T cells were cultured with or without IL-4 for 4, 24, and 48 h. At each timepoint, cells were lysed and lysates were electrophoresed in SDS polyacrylamide gels. The proteins were transferred onto immobilon and probed with antibodies directed to Bcl-2 or Bcl-Xl. At time 0 h, the T cell lysates contained moderate levels of Bcl-2, which began to disappear at 4 h and were even lower at 24 and 48 h (Fig. 3 a). Bcl-Xl disappeared with similar kinetics, although it was less easily detectable in the starting population (Fig. 3 b). By contrast, lysates of cells cultured with IL-4 continued to express Bcl-2 and the small amounts of Bcl-Xl at 4, 24, and even 48 h. In fact, levels of Bcl-2 appeared higher in cells cultured for 24 or 48 h in IL-4 than they were in cells before culture. These data support the idea that IL-4 acts by regulating the levels of Bcl-2–related proteins in T cells.
Figure 3.
IL-4 prevents decay of Bcl-2 and Bcl-Xl but not phosphorylated Stat6. Lymph node T cells from AD10 TCR transgenic mice were cultured in the presence or absence of 25–50 U/ml of IL-4 for 0, 4, 24, and 48 h (IL-4 was added at 0 and 24 h). At these times T cells were lysed and proteins fractionated by SDS-PAGE. After transfer onto immobilon, BCL-2 (a) and BCL-Xl (b) were detected by immunoblotting. (c) T cells were incubated with or without IL-4 for 0, 4, and 24 h. Nuclear lysates were immunoprecipitated with anti-phosphotyrosine and immunoblotted for the presence of Stat6.
It is well known that IL-4 transduces signals through a JAK/Stat pathway (17, 19, 25–28). The IL-4 receptor (IL-4R) is made up of the IL-2R common γ chain and a ligand-specific α chain (9, 23). The common γ chain associates with JAK3 and the α chain with JAK1 (25, 26). Upon ligation, Stat6 associates with the IL-4R complex and is tyrosine phosphorylated by JAK1 (27–31). Activated Stat6 forms homodimers and translocates to the nucleus (27). Because Stat6 is activated by IL-4R ligation, we hypothesized that Stat6 may be responsible for transducing the T cell survival signal delivered by IL-4.
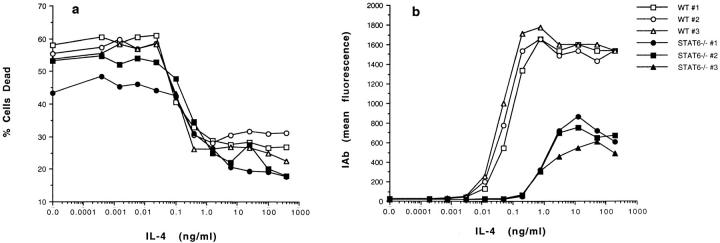
To test this, we compared the effects of IL-4 on the survival of resting T cells from Stat6-deficient (Stat6−/−) and normal mice (17, 18). The results are shown in Fig. 4 a. In the absence of added cytokines, T cells from Stat6−/− mice, like those from normal animals, died in culture. Increasing doses of IL-4 prevented the death of T cells from both types of animals. The sensitivity of the two types of cells to IL-4 was slightly different, with the Stat6−/− cells requiring between two- and fivefold more IL-4 than normal T cells for half-maximal rescue.
Figure 4.
IL-4 blocks the death of resting T cells that lack Stat6. (a) Prevention of resting T cell death by IL-4 is only slightly affected by lack of Stat6 in the T cell. T cells from normal or Stat6−/− mice were purified and cultured at 1.2 × 105 cells/well with various concentrations of IL-4. 42 h later, cells were harvested and assayed for dead cells using propidium iodide staining. Open symbols, T cells from normal mice; closed symbols, T cells from Stat6−/− mice. (b) Induction of class II is affected by lack of Stat6 in B cells. Spleen cells from normal or Stat6−/− mice were cultured at 3 × 105 cells/well with various concentrations of IL-4. 42 h later, the cells were harvested and stained with FITC–anti-IAb α chain and phycoerythrin-labeled anti-B220. Data shown are the mean anti-IAb fluorescences after gating on the B220 positive cells. Open symbols, B cells from normal mice; closed symbols, B cells from Stat6−/− mice.
As a control we compared the sensitivity to IL-4 of B cells from the two types of animals. B cell responses to IL-4 were measured by the ability of the cytokine to increase MHC class II levels on the surfaces of the cells during 24 h of culture. As shown in Fig. 4 b, high concentrations of IL-4 did induce increased amounts of class II on Stat6−/− B cells. Even so, at optimum IL-4 concentrations less class II appeared on the knockout cells. Also, half-maximal induction of class II B cells from Stat6−/− mice required ∼20 times more IL-4 than normal B cells did. It has previously been reported that IL-4 could not induce class II expression on Stat6−/− B cells (17, 18). However, the previous experiments were done with low concentrations of IL-4, concentrations near the threshold required to observe the induction shown in Fig. 4 b.
These results suggested that the ability of IL-4 to rescue resting T cells from death did not require the presence in T cells of the transcription factor Stat6, which is usually associated with IL-4 signal transduction (27–31). However, the presence of Stat6 caused the rescuing signal to be delivered slightly more efficiently, suggesting that the Stat6 pathway might act to amplify the rescuing signal. This conclusion was supported by the effects on Stat6−/− B cells of IL-4. As in T cells, response to IL-4 occurred in Stat6−/− B cells, although the response was considerably amplified in the presence of Stat6. Alternatively, it was possible that rescue required Stat6 and that the Stat6−/− cells contained some very small residual Stat6 activity due to expression of a modified Stat6 protein from the knocked out Stat6 gene (18). If this latter explanation was correct, resting T cell responses to IL-4 were driven by lower amounts of signal than B cell responses were.
However, cells from genetically deficient animals are not always equivalent to those from normal mice. Sometimes they use intracellular signaling pathways that are not employed by normal cells. Therefore, we did a second experiment to find out whether or not Stat6 signaling was a component of IL-4 rescue of normal resting T cells from death. Nuclear lysates were prepared from resting T cells from normal mice, after various times of culture with or without IL-4. The lysates were immunoprecipitated with anti-phosphotyrosine polyclonal sera, the precipitate was electrophoresed, blotted onto immobilon, and probed with anti-Stat6. As shown in Fig. 3 c, phosphorylated Stat6 was present in resting T cells at the time they were harvested from the animals. This material rapidly disappeared from resting T cells cultured without cytokines and was not apparent after as little as 4 h in culture. Phosphorylated Stat6 increased somewhat in amount during the first 4 h of culture of resting T cells with IL-4 but then decreased to levels that were not detectable in T cells after 24 h in culture, whether or not IL-4 was present.
These data indicated that an early event in the signaling pathway whereby IL-4 rescued resting T cells from death might involve Stat6. However, activated Stat6 did not have to be in the cells permanently for rescue to occur. IL-4 prevented the death of normal cultured T cells 24 and 48 h after the beginning of the culture, at a time when levels of phosphorylated Stat6 were undetectable.
Collectively, these data suggest that Stat6 is not an essential component of the intracellular signaling pathway whereby IL-4 prevents the death of normal resting T cells after 24 and 48 h.
Do either of the cytokines studied in these experiments actually affect the life expectancy of mature resting T cells in vivo? In an uninfected animal it is unlikely that IL-4 levels are high enough to manage this. Resting B cells also bear high affinity IL-4R, yet in animals they do not have elevated class II MHC protein levels that would be expected of B cells exposed to inducing concentrations of IL-4 (32). However, during infections, IL-4 levels in lymphoid tissues are certainly high enough to bind to high affinity IL-4R and hence increase the half-lives of bystander resting T cells. Under these circumstances, IL-4 may affect the life expectancy of bystander resting T cells.
On the other hand, experiments suggest that IL-7 may be at effective concentrations in lymphoid tissues, even in uninfected animals, and IL-7 has already been reported to affect T cell turnover time (7, 33). Future experiments will determine whether IL-7 does affect T cell life expectancy in vivo.
Finally, it is worth noting that cytokines are known to increase the half-lives af activated T cells as well and that the cytokines that do this (IL-2, IL-4, IL-7; references 34, 35) are either the same as or related to the two cytokines identified here. Perhaps this is not a coincidence.
Acknowledgments
The authors thank Drs. B. Wheat and J. Hagman for their advice and assistance in preparation of the nuclear lysates and E. Kushnir and P. Mount very much for their help with animal breeding.
This work was supported by US Public Health Service grants AI-17134, AI-18785, and AI-22295.
Footnotes
1 Abbreviation used in this paper: PI, propidium iodide.
References
- 1.Osmond DG. The turnover of B-cell populations. Immunol Today. 1993;14:34–37. doi: 10.1016/0167-5699(93)90322-C. [DOI] [PubMed] [Google Scholar]
- 2.Freitas AA, Rocha BB. Lymphocyte lifespans; homeostasis, selection and completion. Immunol Today. 1993;14:25–29. doi: 10.1016/0167-5699(93)90320-K. [DOI] [PubMed] [Google Scholar]
- 3.Sprent J, Schaefer M, Hurd M, Surh C, Ron Y. Mature B and T cells transferred to SCID mice can survive indefinitely and many maintain a virgin phenotype. J Exp Med. 1991;174:717–728. doi: 10.1084/jem.174.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Boehmer H, Haven K. The lifespan of naïve α/β T cells in secondary lymphoid organs. J Exp Med. 1993;177:891–896. doi: 10.1084/jem.177.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed R, Gray D. Immunological memory and protective immunity. Science (Wash DC) 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 6.Kappler JW, Hunter PC, Jacobs D, Lord E. Functional heterogeneity among the T-derived lymphocytes of the mouse. I. Analysis by adult thymectomy. J Immunol. 1974;113:27–36. [PubMed] [Google Scholar]
- 7.Nakajima H, Shores EW, Noguchi M, Leonard WJ. The common cytokine receptor γ chain plays an essential role in regulating lymphocyte homeostasis. J Exp Med. 1997;185:189–196. doi: 10.1084/jem.185.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ. Interleukin-2 receptor γ chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 9.Leonard WJ, Noguchi M, Russell SM. Sharing of a common gamma chain, gamma c, by the IL-2, IL-4 and IL-7 receptors: implications for X-linked severe combined immunodeficiency. Adv Exp Med Biol. 1994;365:225–232. doi: 10.1007/978-1-4899-0987-9_23. [DOI] [PubMed] [Google Scholar]
- 10.Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen A, Park LS, Cosman D, Anderson D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO (Eur Mol Biol Organ) J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. Bcl-2 inhibits multiple forms of apoptosis but not negative selection inthymocytes. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 12.Strasser A, Harris AW, Cory S. Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 13.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 14.Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4:327–332. [PubMed] [Google Scholar]
- 15.Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ. Bcl-xL and Bcl-2 repress a common pathway of cell death. J Exp Med. 1995;182:821–828. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaye J, Hsu ML, Sauron ME, Jameson SC, Gascoigne NR, Hedrick SM. Selective development of CD4+T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature (Lond) 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwarmura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signaling. Nature (Lond) 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 18.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, et al. Lack of IL-4–induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature (Lond) 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 19.Julius M, Simpson E, Herzenberg L. A rapid method for the isolation of functional thymus-derived lymphocytes. Eur J Immunol. 1973;3:645–648. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 20.Kappler J, Skidmore B, White J, Marrack P. Antigen-inducible, H-2–restricted, interleukin-2 producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981;153:1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vella AT, McCormack JE, Linsley PS, Kappler JW, Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 1995;2:261–270. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 22.Teague, T.K., P. Marrack, J.W. Kappler, and A.T. Vella. 1997. Interleukin-6 rescues naïve T cells from apoptosis. J. Immunol. In press. [PubMed]
- 23.Russell SM, Keegan AD, Harada N, Nakamura Y, Noguchi M, Leland P, Freidmann MC, Miyajima A, Puri RK, Paul WE, et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science (Wash DC) 1993;262:1880–1883. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- 24.Kondo M, Takeshita T, Higuchi M, Nakamura M, Sudo T, Nishikawa S, Sugamura K. Functional participation of the IL-2 receptor gamma chain in IL-7 receptor complexes. Science (Wash DC) 1994;263:1453–1454. doi: 10.1126/science.8128231. [DOI] [PubMed] [Google Scholar]
- 25.Witthuhn BA, Silvennoinen O, Miura O, Lai KS, Cwik C, Liu ET, Ihle JN. Involvement of the Jak-3 Janus kinase in signaling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature (Lond) 1994;370:153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- 26.Ihle JN. The Janus protein tyrosine kinase family and its role in cytokine signaling. Adv Immunol. 1995;60:1–35. doi: 10.1016/s0065-2776(08)60582-9. [DOI] [PubMed] [Google Scholar]
- 27.Hou, J., U. Schindler, W.J. Henzel, T.C. Ho, M. Brasseur, and S.L. McKnight. 1994. An interleukin-4–induced transcription factor: IL-4 Stat. Science (Wash. DC). 1701–1706. [DOI] [PubMed]
- 28.Quelle FW, Shimoda K, Thierfelder W, Fischer C, Kim A, Ruben SM, Cleveland JL, Pierce JH, Keegan AD, Nelms K, et al. Cloning of murine Stat6 and human Stat6, Stat proteins that are tyrosine phosphorylated in responses to IL-4 and IL-3 but are not required for mitogenesis. Mol Cell Biol. 1995;1555:3336–3343. doi: 10.1128/mcb.15.6.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 30.Mikita T, Campbell D, Wu P, Williamson K, Schindler U. Requirements for interleukin-4–induced gene expression and functional characterization of Stat6. Mol Cell Biol. 1996;16:5811–5820. doi: 10.1128/mcb.16.10.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotanides H, Reich NC. Interleukin-4–induced Stat6 recognizes and activates a target site in the promoter of the interleukin-4 receptor gene. J Biol Chem. 1996;271:25555–25561. doi: 10.1074/jbc.271.41.25555. [DOI] [PubMed] [Google Scholar]
- 32.Roehm NW, Leibson HJ, Zlotnik A, Kappler J, Marrack P, Cambier JC. Interleukin induced increase in Ia expression by normal mouse B cells. J Exp Med. 1984;160:679–689. doi: 10.1084/jem.160.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maraskovsky E, Teepe M, Morrissey PJ, Braddy S, Miller RE, Lynch DH, Peschon JJ. Impaired survival and proliferation in IL-7 receptor deficient peripheral T cells. J Immunol. 1996;157:5315–5323. [PubMed] [Google Scholar]
- 34.Boise LH, Minn AJ, June CH, Lindsten T, Thompson CB. Growth factors can enhance lymphocyte survival without committing the cell to undergo cell division. Proc Natl Acad Sci USA. 1995;92:5491–5495. doi: 10.1073/pnas.92.12.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akbar AN NJ, Bothwick, Wickremasinghe RG, Panayiotidis P, Pilling D, Bofill M, Krajewski S, Reed JC, Salmon M. Interleukin-2 receptor common γ chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xl) but not proapoptotic (bax, bcl-xs) gene expression. Eur J Immunol. 1996;26:294–299. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]