Abstract
Despite the development of an efficient specific immune response during measles virus (MV) infection, an immunosuppression occurs contributing to secondary infections. To study the role of nucleocapsid protein (NP) in MV-induced immunosuppression, we produced recombinant MV NP. Purified recombinant NP exhibited biochemical, antigenic, and tridimensional structure similar to viral NP. By flow cytometry, we showed that viral or recombinant NP bound to human and murine B lymphocytes, but not to T lymphocytes. This binding was specific, independent of MHC class II expression, and dependent of the B lymphocyte activation state. The murine IIA1.6 B cell line, deficient in the Fc receptor for IgG (FcγRII) expression, did not bind NP efficiently. Transfected IIA1.6 cells expressing either murine FcγRIIb1 or b2, or human FcγRIIa, b1*, or b2 isoforms efficiently bound NP. Furthermore, this binding was inhibited up to 90% by monoclonal antibodies 2.4G2 or KB61 specific for murine and human FcγRII, respectively. Finally, the in vitro Ig synthesis of CD40- or Ig-activated human B lymphocytes in the presence of interleukin (IL)-2 and IL-10 was reduced by 50% in the presence of recombinant NP. These data demonstrate that MV NP binds to human and murine FcγRII and inhibits in vitro antibody production, and therefore suggests a role for NP in MV-induced immunosuppression.
Measles virus (MV)1 is responsible for an acute childhood disease that is benign in industrialized countries, but is among the primary causes of infant death in developing countries. MV belongs to the Morbillivirus genus of the Paramyxoviridae family and its genome is a nonsegmented negative strand RNA encoding six structural proteins: nucleocapsid protein (NP; 60 kD), phosphoprotein (70 kD), matrix protein (37 kD), fusion (F) protein (with subunits F1, 40 kD, and F2, 20 kD), hemagglutinin (H) protein (80 kD), and large protein (250 kD). The minimal infectious unit is the ribonucleoprotein complex composed of the RNA tightly associated with the NP, phosphoprotein, and large protein.
MV infection is initiated by interaction between a viral protein, the glycoprotein H, and a cellular receptor, the human CD46 molecule (1, 2). The release of ribonucleoprotein complex into the cytosol leads to genome transcription, viral protein synthesis, and MV replication. The humoral immune response is detected at the onset of the rash, and the most abundant and rapidly produced antibodies are specific for NP (3, 4). The cellular component of the immune response against MV involves MHC class I–restricted CD8+ T cells and MHC class II–restricted CD4+ T cells. MV-specific MHC class II–restricted CD4+ T cells clones have been isolated from PBL of healthy donors with a history of MV infection. Interestingly, the CD4+ T cell clones were specific for the H, F, matrix, and NP proteins (5–7) and most of them displayed cytotoxic activity. The anti-NP T cells constitute an important component of the cellular response against MV as NP-specific CD4+ T lymphocytes can protect rats against MV-induced encephalitic disease (8).
In spite of the fact that NP is synthesized as a cytosolic protein, the dual humoral and cellular CD4+ responses against NP indicate that NP is both accessible to the B cell receptor (BCR) after its release in the extracellular compartment and to the peptide-loading compartment after its uptake by the APC. In this context, NP could be internalized by APC either by fluid-phase endocytosis or by receptor-mediated endocytosis. Targeting a soluble exogenous antigen to antigen-specific B cells via their BCR (9) or to macrophages and dendritic cells via their FcR after its opsonization with specific antibodies (10, 11) results in an enhancement of MHC class II–restricted antigen presentation to CD4+ T cells. However, it remains unknown whether receptor-mediated endocytosis via BCR, FcR, or an NP-specific cellular receptor could play a role in the induction of the MHC class II–restricted NP presentation to CD4+ T helper cells and subsequently in the high anti-NP antibody synthesis.
MV infection gives rise to a paradoxical situation: despite the development of an efficient immune response establishing long-term immunity and virus elimination, an immunosuppression occurs that contributes to secondary infections and mortality. This immunosuppression was first described by Von Pirquet (12) who observed a suppression of tuberculin skin test reactivity during the acute phase of MV infection and for several weeks thereafter. During the acute phase of measles, lymphocytes from infected individuals respond poorly to mitogens like PHA or PWM (13). Moreover, the production of cytokines from both lymphocytes and monocytes is dysregulated (14) and antibody production to the antigens of Salmonella typhi vaccine is impaired (15, 16). Finally, a suppression of IgG synthesis was recently reported in MV-infected human SCID mice (17). The respective role of viral proteins in this immunosuppression remains unclear.
After vaccination, both immune response and immune suppression are observed. In the majority of cases, children immunized with live MV vaccine develop antibodies against NP (18). Recent data reported that mitogen-induced lymphoproliferation was decreased at 3 mo after vaccination with live attenuated MV, and was more pronounced in children with the highest antibody response and immune activation (19). MV vaccination may alter the T cell repertoire. Vaccination of infants with live attenuated MV vaccine induce changes in the circulating TCR Vβ repertoire since the Vβ4 subset was decreased and the Vβ2 subset was increased (20). Such an alteration in the T cell repertoire could be due to the presence of a viral superantigen as reported for the rabies virus nucleocapsid that binds to Vβ8 and HLA class II α chain (21). In the present study, we show that measles virus NP binds to human and murine Fc receptor for IgG (FcγRII) and that NP inhibits the in vitro antibody synthesis from activated human B lymphocytes.
Materials and Methods
Cells and Antibodies.
Human cells were cultured in RPMI 1640 (GIBCO BRL, Life Technologies SARL, Cergy Pontoise, France) supplemented with 10% FCS (TechGen, International, Les Ulis, France), 10 mM Hepes, 2 mM glutamine, and 50 μg/ ml gentamicin (GIBCO BRL). Human B cells were purified from tonsils by two successive rounds of T cells depletion by rosetting with sheep red blood cells (Biomérieux, Lyon, France). Tonsils were finely minced, the cell suspension (107 cells/ml) was mixed with 0.02 vol of packed sheep red blood cells, and 30 ml were layered over 15 ml of Ficoll (Eurobio, Les Ulis, France). Cells were centrifuged at 50 g for 15 min and at 800 g for 30 min. B cells were recovered from the interface between medium and Ficoll and were washed before another round of depletion. T cells were purified from tonsils by two successive passages on nylon wool columns. B and T cell preparations were routinely >98% and >90% pure, respectively, as revealed by their pattern of expression of CD20 and CD3 (Dako, Trappes, France). Rabbit anti-human Igs coupled to polyacrylamide beads were purchased from Bio-Rad S.A. (Ivry Sur Seine, France) and used at a final dilution of 1:600 to stimulate B cells during 2 d. PHA (Sigma-Aldrich Chimie SARL, Saint Quentin Fallavier, France) was used at 5 μg/ml to stimulate T cells during 3 d.
The following human cell lines have been used in the course of this study: HUT78 (T lymphoma), JY (lymphoblastoid cell line), cell line clone 13 (Cl.13) mutant derived from JiJoye (22), Raji (B Burkitt lymphoma), and the MHC class II–deficient cell lines RM2, RM3, and RJ2.2.5 (23, 24) derived from Raji.
The IIA1.6 B cell line is a FcγRII-negative variant of the mouse A20/2J B lymphoma. IIA1.6 cells transfected with murine FcγRII cDNA B1 and B2 were a gift from C. Bonnerot and S. Amigorena (Institut National de la Santé et de la Recherche Médicale CJF9501, Institut Curie, Paris, France; reference 10) and IIA1.6 cells expressing human FcγRIIa, b1*, and b2 isoforms were provided by J.G.J. Van de Winkel (University Hospital, Utrecht, The Netherlands; reference 25). FcγRIIb1* is a variant of FcγRIIb1 that contains a single amino acid difference in the cytoplasmic tail.
The murine cell lines M12 (B lymphoma) and 408 (T hybridoma), and the simian fibroblast cell line Vero were grown in DME supplemented with 6% FCS, 10 mM Hepes, 2 mM glutamine, 50 μM 2-mercaptoethanol, and 50 μg/ml gentamicin (GIBCO BRL). The anti-NP mAb 25αNP was purified from culture of hybridoma anti-NP clone 25 (26). The rat mAb 2.4G2 recognizing murine FcγRII (27) and mouse mAb KB61 recognizing human FcγRII (CD32), provided by D.Y. Mason (John Radcliffe Hospital, Oxford, U.K.; reference 28), were used for inhibition experiments.
Production of Recombinant NP.
AcNPV (Autographa californica nuclear polyedrosis virus) and recombinant virus stocks were grown and assayed in confluent monolayers of Sf (Sporodoptera frugiperda)-9 cells in TGV 3 (TC 100 modified medium) containing 10% FCS. Sf 9 cells and AcNPV virus were obtained from G. Devauchelle (Institut National de la Recherche Agronomique–Centre National de Recherche Scientifique, St. Christol lez Alès, France).
The cDNA for the NP of measles virus (Hallé strain) was described by R. Buckland et al. (29) and was inserted in the transfer vector pGmAc 34 (G. Devauchelle) at the SmaI site. The recombinant transfer vector pGmacNP contained the cDNA of NP under the control of the polyhedrin promoter. Sf9 cells were transfected with a mixture of plasmid pGmacNP and wild-type AcNPV DNA. Recombinant baculovirus AcNPVNP were plaque purified.
Purification of Recombinant and Viral NP.
Recombinant NP was purified from confluent monolayers of Sf9 cells infected with virus AcNPVNP at a multiplicity of 1 PFU/cell. 2 × 108 infected cells were harvested 3 d after infection by centrifugation at 200 g and washed 3 times in PBS. Purified NP were prepared from these cells using modifications of the previously described method for preparation of intracellular measles virus nucleocapsids (30). Cell pellets were resuspended in hypotonic buffer (10 mM Tris-HCl, pH 7.5, 10 mM NaCl) at 2 × 107 cells/ml in the presence of proteinase inhibitors (0.1 mM 3,4-dichloroisocoumarin, 0.02 mM E-64, 0.1 mM 1,10-Phenantroline; Sigma-Aldrich Chimie SARL). Cells were lysed by the addition of the nonionic detergent Nonidet P-40 to a final concentration of 1% (vol/vol) and left on ice for 30 min. Nuclei were removed by centrifugation at 1,000 g at 4°C for 5 min, and the supernatant was adjusted to contain 10 mM EDTA. This fraction was then clarified of particulate material by centrifugation at 10,000 g at 4°C for 10 min. This cytoplasmic extract was centrifuged through a discontinuous density gradient in SW 41 centrifuge tubes (Beckman Instrs., France SA, Gagny, France). The step gradient was comprised of 2 ml of 40% (wt/vol) CsCl, 2 ml of 30% CsCl, 2 ml of 25% CsCl, and 2 ml of 5% (wt/vol) saccharose each containing 25 mM Tris-HCl, pH 7.5, 50 mM NaCl, 2 mM EDTA, and 0.2% (wt/vol) sodium lauroyl sarcosinate. Centrifugation was performed at 12°C for 2 h at 36,000 rpm. The dense visible band observed in the 30% CsCl step was removed by needle aspiration, diluted in PBS, and centrifuged in a rotor (SW 41; Beckman Instrs. France SA) at 12°C for 2 h at 36,000 rpm. The NP pellet was solubilized in 1 ml of PBS, aliquoted, and stored at −80°C.
Viral NP was purified from Vero cell line infected with Hallé strain of measles virus at 0.01 PFU/cell and cultured in DME 2% FCS at 33°C. The cells were harvested 4 d later when 80% of the cells formed syncytia. The cells were scraped and centrifuged at 200 g for 10 min and washed three times in PBS. Cell pellets were resuspended in hypotonic buffer, and cells were lysed as with Sf9 infected cells. NP purification was performed as described for recombinant NP purification.
Protein Analyses.
Cytoplasmic extract of Sf9-infected cells or purified NP were subjected to 10% SDS-PAGE. Gels were stained with Coomassie brilliant blue R-250 or electroblotted onto a nitrocellulose membrane using a semidry blotting system (Biometra, Göttingen, Germany). The NP was identified with mAb 25αNP and alkaline phosphatase-conjugated anti–mouse Ig (Bio-Rad S.A.). The NBT/BCIP system (Bio-Rad S.A.) was used for colorimetric detection. The protein concentration was determined using the copper-dependent BCA assay (Pierce Chem. Co., Rockford, IL) in microtiter plate with bovine serum albumin as a standard.
Flow Cytometry.
Cells (5 × 105) were incubated for 1 h at 4°C with purified NP (5 μg) in 100 μl of PBS containing 1% BSA and 0.1% NaN3. Cells were then washed three times in this solution and incubated with mAb 25αNP or biotinylated 25αNP for 30 min at 4°C. After three washes, cells were incubated with FITC-conjugated donkey anti–mouse IgG (Jackson ImmunoResearch Labs., West Grove, PA) or with streptavidin conjugated to FITC (Jackson Laboratory) for 30 min at 4°C, and then washed. Flow cytometric analyses were performed on a FACScan® (Becton Dickinson, Immunocytometry Systems, Rungis, France). The median fluorescence intensity value was chosen to quantify NP binding. For antibody inhibition experiments, the value obtained for control without NP was subtracted for each point and the value obtained without antibody was considered as 100%.
NP Biotinylation and Competition.
NP was biotinylated with succinimidyl-6-(biotinamido)hexanoate (Pierce Chem. Co.) for 2 h at 4°C and then dialyzed in PBS. Biotinylated NP binding on Cl.13 cell line was detected with FITC-conjugated streptavidin by FACS® analysis. For competition experiments, 2 μg of biotinylated NP were incubated with 0–85 μg of unlabeled NP. FITC-conjugated streptavidin was used to detect the remaining biotinylated NP binding and values of median fluorescence intensity measured. The value obtained for the control without NP was subtracted for each point to determine the percentage of NP binding. The number obtained for 2 μg of biotinylated NP was considered as 100%.
In Vitro Human Antibody Production.
Purified B cells were seeded at 2 × 105 cells/well in round-bottomed 96-well microtiter trays in a final volume of 0.25 ml and were stimulated with rabbit anti– human Igs coupled to polyacrylamide beads (1/600) or 2 × 104 mitomycin C–treated Ltk− cells stably transfected with the CD40 ligand (CD40L; gift of M. Bakkins, Vrije Universiteit, Brussels, Belgium). Purified recombinant IL-2 and IL-10, provided by A. Minty (Sanofi, Labège, France) and J. Banchereau (Schering-Plough, Dardilly, France), respectively, were used at 10 U/ml and 50 ng/ml. Purified NP was added at the onset of the culture at the optimal concentration of 30 ng/ml. Ig secretion (IgG, IgM, and IgA) was determined in 12-d culture supernatants by standard ELISA techniques, as described elsewhere (31). The percentage of inhibition in the presence of NP was calculated on the basis of maximal stimulation without NP.
Results
Recombinant and Viral NP Exhibit Similar Biochemical and Antigenic Characteristics.
As the measles nucleocapsids are difficult to purify and are often degraded in mammalian cells (32), we used the recombinant baculovirus technology to produce high level of measles NP in insect cells. We observed that the recombinant NP reached the maximal level of expression in Sf9 cells by 3 d after infection with recombinant AcNPVNP.
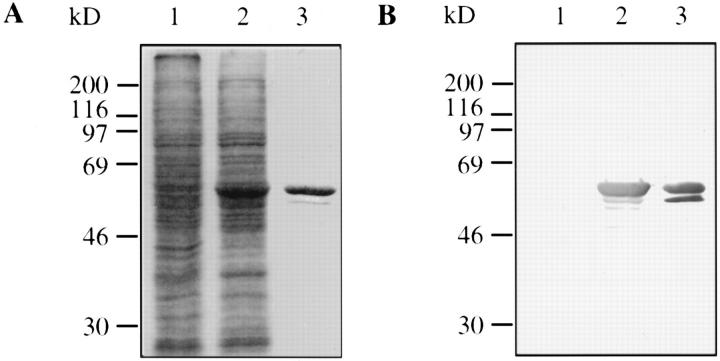
As estimated from SDS-PAGE analysis, NP represented ∼10% of total soluble proteins 3 d after infection (Fig. 1 A, lanes 1 and 2). We took advantage of the nucleocapsid formation in insect cells to purify them using centrifugation on CsCl gradients as previously described for viral nucleocapsids isolation (30). The recombinant NP formed a dense visible band in the 30% CsCl step gradient. This single-step gradient purification was sufficient to obtain homogenously purified NP that could be pelleted. Purified NP analyzed by SDS-PAGE (Fig. 1 A, lane 3) migrated as two molecular species of about 60 kD; the larger one was predominant and co-migrated with the viral NP (data not shown). Both bands were recognized by mAb 25αNP (Fig. 1 B, lane 3) that recognized no polypeptide in insect cells infected with wild-type AcNPV (Fig. 1 B, lane 1). The minor band probably represents a cleavage product of the major one as it became more abundant in cells harvested later. Under these conditions, the purification yield was ∼50% and resulted in the isolation of 1 mg of pure recombinant NP from 108 infected insect cells.
Figure 1.
Measles NP purification from Sf9 cells infected with recombinant baculovirus AcNPVNP. Sf9 cells were infected with wild-type AcNPV or recombinant AcNPVNP and the cells harvested 3 d after infection. Proteins recovered from the cell extracts or NP purified on CsCl gradient were separated by SDS-PAGE and stained with Coomassie brilliant blue (A) or analyzed by Western blot using mAb 25αNP (B). Molecular weight markers are shown on the left. Lane 1, Sf9 infected with AcNPV; lane 2, Sf9 infected with AcNPVNP; lane 3, purified NP.
Electron microscopy analysis (data not shown) confirmed the assembly of recombinant NP in nucleocapsids similar to measles nucleocapsids (33). These observations show that the insect cell expression system permits the production of large quantities of purified NP that preserve its native structure.
NP Binds to Human and Murine B Cells.
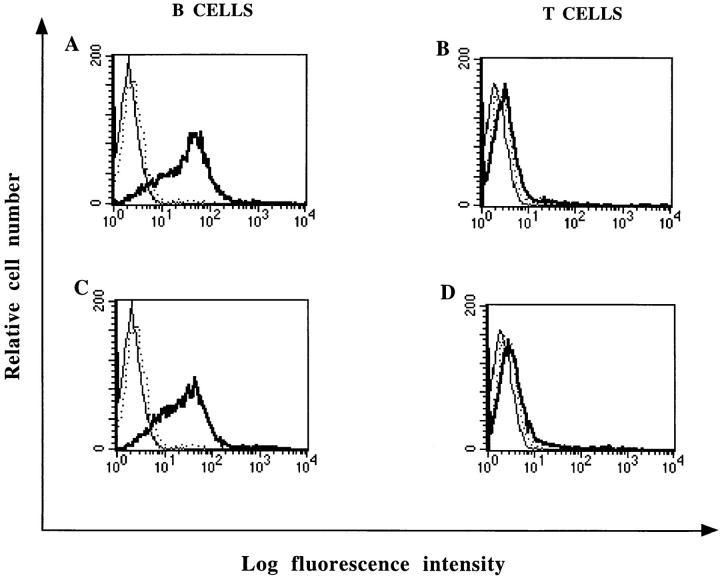
Tonsillar human B and T lymphocytes were incubated with variable amounts of recombinant or viral NP and assayed for NP binding by flow cytometry. Only B lymphocytes bound both recombinant and viral NP (Fig. 2, A and C). T lymphocytes failed to do so (Fig. 2, B and D).
Figure 2.
FACS® analysis of recombinant and viral NP binding to human B and T lymphocytes. Purified B cells (A and C) and purified T cells (B and D) were isolated from tonsils and incubated for 1 h with 5 μg of purified recombinant NP (A and B) or 5 μg of purified viral NP (C and D). NP binding was revealed with mAb 25αNP and FITC-conjugated donkey anti–mouse IgG (——). Cells were either incubated with mAb 25αNP and FITC-conjugated donkey anti–mouse IgG (...) or FITC-conjugated donkey anti–mouse alone (——) as controls.
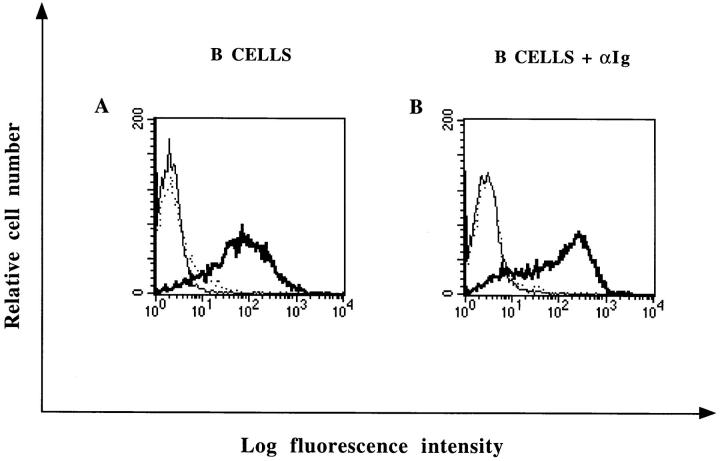
In the course of this study, we observed that the proportion of B cells susceptible to bind NP was variable and ranged between 65 and 90%. Since different tonsil specimens were used for each experiment, this observation suggested that NP binding could be modulated after B cell activation. The data shown in Fig. 3 indicate that the level of NP binding to anti–Ig-activated B lymphocytes (Fig. 3 B; median 107.5) was significantly higher than for unstimulated B lymphocytes (Fig. 3 A; median 62.6). As summarized in Table 1, a similar increase in NP binding was also observed in the human B lymphoblastoid cell line JY after anti-Ig stimulation. In contrast, PHA-activated T lymphocytes or HUT78 lymphoma cells did not bind NP (Table 1). Moreover, Burkitt lymphoma or lymphoblastoid cell lines were both able to bind NP (Table 1).
Figure 3.
FACS® analysis of NP binding to anti-Ig activated B lymphocytes. Purified B lymphocytes were cultured for 2 d in the presence (B) or in the absence (A) of anti–human Ig coupled to beads. Cells were incubated with 2.5 μg of recombinant NP. NP binding was revealed with biotinylated mAb 25αNP and FITC-conjugated streptavidin (——). Cells were either incubated with biotinylated mAb 25αNP and FITC-conjugated streptavidin (...) or FITC-conjugated streptavidin alone (——) as controls.
Table 1.
NP Binding on Human and Murine Lymphocytes
| Cells | MHC class II | NP binding | ||
|---|---|---|---|---|
| Human lymphocytes | ||||
| B | + | ++ | ||
| B + αIg | + | +++ | ||
| T | − | − | ||
| T + PHA | + | − | ||
| Human cell lines | ||||
| JY (BEBV) | + | + | ||
| JY + αIg | + | ++ | ||
| C1.13 (Burkitt) | + | ++ | ||
| Raji (Burkitt) | + | + | ||
| RJ2.2.5 | − | + | ||
| RM2 | − | + | ||
| RM3 | − | + | ||
| HUT78 | + | − | ||
| Murine lymphocytes | ||||
| B | + | ++ | ||
| T | − | − | ||
| Murine cell lines | ||||
| M12 | + | + | ||
| IIA1.6 | + | − | ||
| hybridoma T 408 | − | − |
NP binding was detected as described in Fig. 2. The staining intensity estimated by the median fluorescence intensity is indicated by the number of minus or plus signs (−, median <6; +, median between 6 and 20; ++, median between 20 and 80; +++, median between 80 and 300). BEBV, B lymphoblastoid cell line.
Binding studies were performed with the Cl.13 cell line, a mutant cell line derived from JiJoye, that bound NP very efficiently (Table 1) and biotinylated recombinant NP. NP binding was saturable and the maximal binding capacity derived from these experiments was 8 μg/5 × 105 cells (133 pmol/5 × 105 cells; Fig. 4 A). Competition experiments indicated that a 25-fold excess of unlabeled NP was required to inhibit, by 93%, biotinylated NP binding (Fig. 4 B). Altogether, these results indicate that the Cl.13 cell line express a specific membrane receptor for NP.
Figure 4.
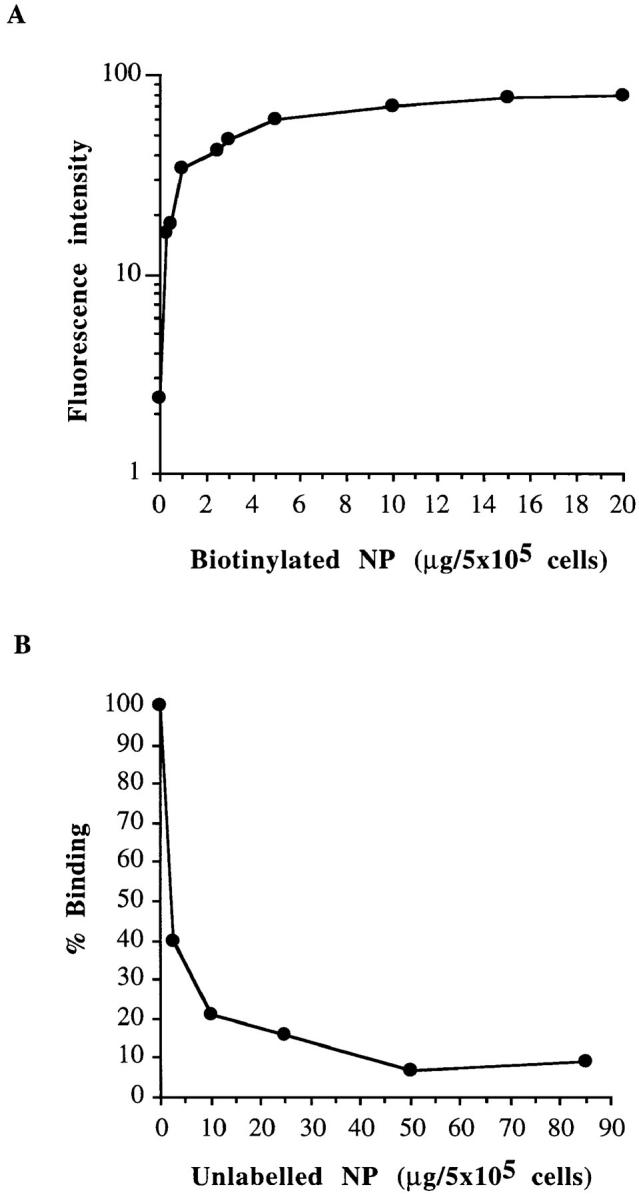
Specificity of NP fixation on Cl.13 cell line. (A) Dose-dependent binding of biotinylated NP. The median intensity value of the fluorescence histograms are indicated. FITC-conjugated streptavidin was used for the detection of NP binding. (B) Competition between unlabeled NP and biotinylated NP. The Cl.13 cell line was incubated with 2 μg of biotinylated NP and various amounts of unlabeled NP. Percentages of biotinylated NP binding detected as above was calculated as indicated in Materials and Methods.
To determine whether NP could be a superantigen, we tested if the NP binding was dependent on MHC class II molecule expression. As mentioned before, PHA-activated T cells or HUT78 cells, which both express MHC class II molecules, did not bind NP. Moreover, equivalent levels of NP binding were obtained using RJ2.2.5, RM2, and RM3 MHC class II–deficient mutants of the Raji cell lines as well as the wild-type MHC class II–expressing Raji (Table 1). These results clearly indicate that NP binding is independent of MHC class II expression.
Murine B lymphocytes and various murine B cell lines were then tested for their ability to bind NP. NP binding was observed to murine splenic B cells and to the B lymphoma cell line M12 (Table 1). In contrast, no significant NP binding was detected to splenic T cells or the T cell hybridoma 408 (Table 1).
Taken together, these findings indicate that the binding of NP to lymphocytes is: (a) specific and restricted to the B cell lineage, (b) increased after B cell activation, and (c) independent of MHC class II expression. The only exception was the murine IIA1.6 cell line derived from the B cell lymphoma A20 (Table 1).
FcγRII Is a B Cell–specific Receptor for NP.
As IIA1.6 cells are deficient for FcγRII expression, we investigated whether FcγRII could be involved in NP binding. To test this hypothesis, murine splenocytes were incubated with recombinant NP in the presence or absence of mAb 2.4G2 recognizing murine FcγRII. As shown in Fig. 5 B, the NP binding was abolished by mAb 2.4G2. To further explore the nature of the NP receptor expressed by B cells, two variants of the IIA1.6 cell line expressing the murine FcγRIIb1 or b2 isoforms were used. As illustrated in Fig. 5, expression of FcγRIIb1 or b2 allowed IIA1.6 cells to efficiently bind NP. Moreover, the addition of mAb 2.4G2 inhibited NP binding to both transfectants by 86 and 88% for the FcγRIIb1 and b2, respectively. These results support the notion that murine FcγRII are specific NP receptors.
Figure 5.
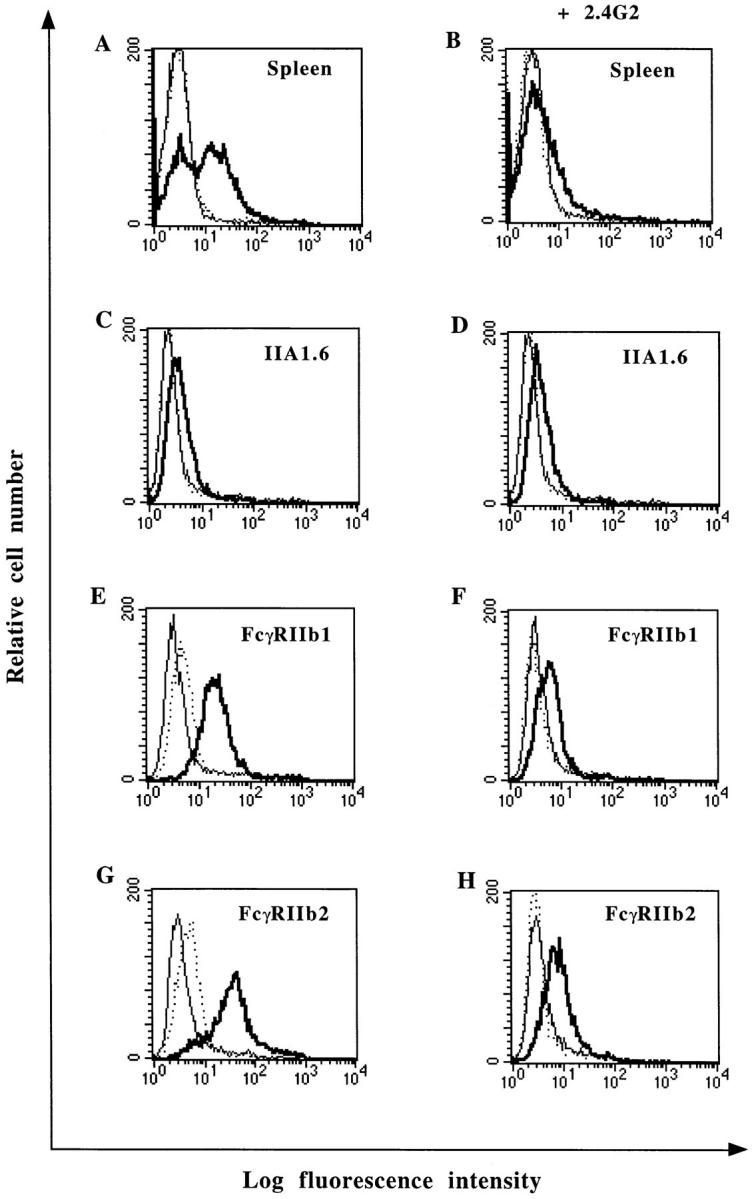
NP binding to mouse FcγRII transfectants and inhibition of NP binding by mAb 2.4G2. Spleen cells (A and B), cell lines IIA1.6 (C and D), IIA1.6 expressing murine FcγRIIb1 (E and F), or FcγRIIb2 (G and H) were incubated with 5 μg of NP in the absence (A, C, E, and G) or in the presence (B, D, F, and H) of mAb 2.4G2. NP binding was detected with biotinylated mAb 25αNP and FITC-conjugated streptavidin (——). Cells were either incubated with biotinylated mAb 25αNP and FITC-conjugated streptavidin (...) or FITC-conjugated streptavidin alone (——) as controls.
We next tested the ability of NP to bind to human FcγRII using IIA1.6 cell line expressing the human FcγRIIa, b1*, or b2 isoforms. As shown in Fig. 6, all FcγRII transfectants bind NP efficiently. Furthermore, the NP binding was inhibited by the CD32-specific mAb KB61 to IIA1.6 cells expressing FcγRIIa, b1*, or b2 (92, 89, and 81% inhibition of NP binding, respectively). Similar binding experiments performed on tonsillar B lymphocytes showed that mAb KB61 also inhibits the NP binding on normal B cells by up to 76% (data not shown). Taken together, these observations demonstrate that both murine and human FcγRII are receptors for measles virus NP.
Figure 6.
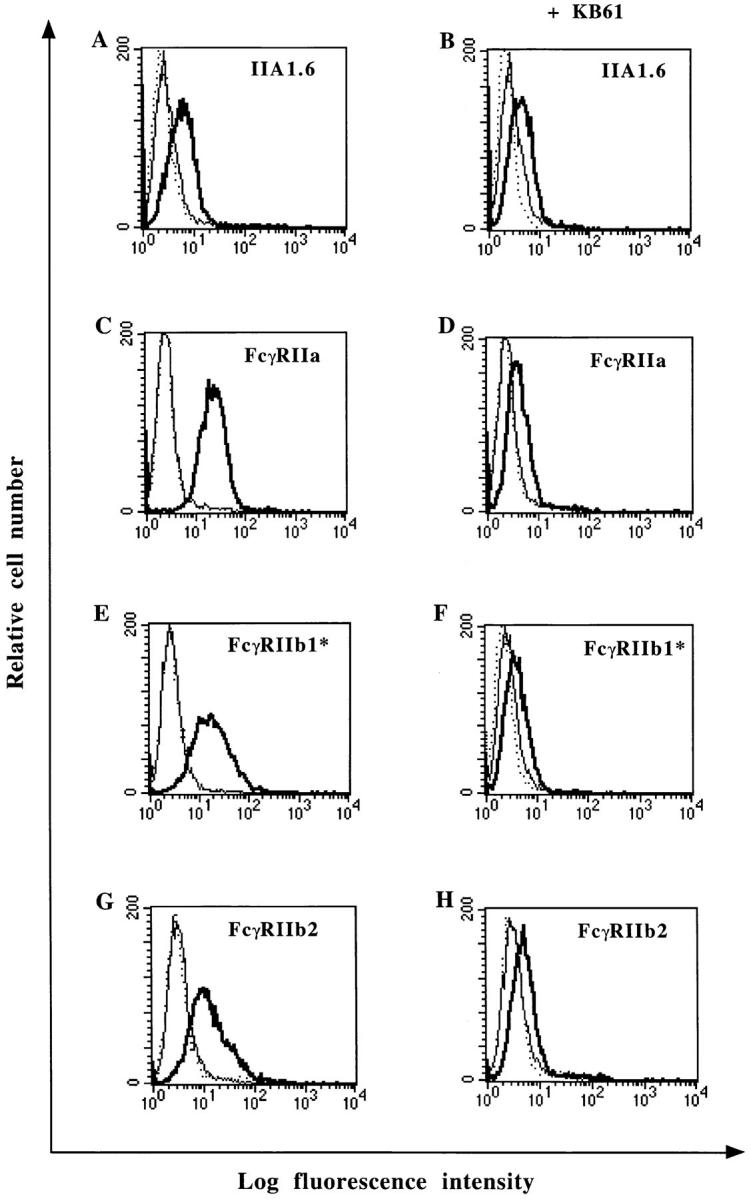
NP binding to human FcγRII transfectants and inhibition of NP binding by mAb KB61. Cell lines IIA1.6 (A and B), IIA1.6 expressing human FcγRIIa (C and D), FcγRIIb1* (E and F), or FcγRIIb2 (G and H) were incubated with 5 μg of NP in the absence (A, C, E, and G) or in the presence (B, D, F, and H) of mAb KB61. NP binding was detected with biotinylated mAb 25αNP and FITC-conjugated streptavidin (——). Cells were either incubated with biotinylated mAb 25αNP and FITC-conjugated streptavidin (...) or FITC-conjugated streptavidin alone (——) as controls.
NP Inhibits the In Vitro Ig Synthesis.
It has long been known that immune complexes can exert a negative feedback on B cell responses via FcγRII (34). The three-dimensional structure of the NP could induce cross-linking of the FcγRII expressed on B cells and mimics the antagonistic signal evoked by immune complexes. To test this possibility, purified tonsillar B cells were costimulated with IL-2, IL-10, and either immobilized anti-Ig antibodies or a CD40L transfectant, in the presence or absence of an optimal dose of recombinant NP. The combination of IL-2 and IL-10 was chosen with regard to its capacity to efficiently stimulate Ig synthesis (35). Determination of the polyclonal IgM, IgG, and IgA secretion after a 12-d culture period was chosen as the read-out system.
As shown in Table 2, IL-2 and IL-10 elicited a strong polyclonal antibody response both in the anti-Ig and in the CD40L systems. Addition of NP in the cultures resulted in a significant inhibition of the Ig response (ranging between 49 and 62%), irrespective of the isotype considered. This finding suggests that the binding of NP to the FcγRII downmodulates the capacity of B cells to differentiate into Ig-secreting cells.
Table 2.
NP Inhibits the Ig Response of Activated B Cells
| Stimulation | NP | Ig synthesis | ||||||
|---|---|---|---|---|---|---|---|---|
| IgG | IgM | IgA | ||||||
| μg/ml | ||||||||
| − | − | 1.0 | 1.4 | <0.2 | ||||
| αIg | − | 2.9 | 0.2 | 0.3 | ||||
| αIg + IL-2 + IL-10 | − | 7.7 | 18.7 | 0.4 | ||||
| αIg + IL-2 + IL-10 | + | 2.9 (62) | 7.1 (62) | <0.2 (>50) | ||||
| CD40L | − | 2.9 | 7.3 | 0.3 | ||||
| CD40L + IL-2 + IL-10 | − | 89.5 | 73.7 | 12.7 | ||||
| CD40L + IL-2 + IL-10 | + | 43.4 (52) | 36.6 (50) | 6.5 (49) | ||||
B cells were stimulated with either an anti-Ig antibody or a CD40L transfectant in combination with IL-2 and IL-10, with or without an optimal dose (30 ng/ml) of NP. Ig synthesis was evaluated in 12-d culture supernatants. Values in brackets indicate the percentage of inhibition calculated as indicated in Materials and Methods. Standard deviations never exceeded 10% of the mean value.
Discussion
In the present study, we have shown that viral or recombinant NP can bind to the surface of B cells. This NP binding is specific, restricted to the B cell lineage both in humans and mice, increased after B cell activation, and does not require MHC class II expression. We also demonstrated that the murine and human FcγRII are receptors for measles virus NP and that the binding of NP inhibits the Ig synthesis by activated B cells.
To produce large quantities of recombinant NP, we developed a baculovirus expression system. The recombinant NP exhibits similar biochemical and antigenic characteristics of viral NP. In agreement with Fooks et al. (36), we observed that in the absence of other viral proteins, NP could self-assemble into nucleocapsid-like structures very similar to those observed for measles nucleocapsids (data not shown and reference 33). Such particles were also observed in mammalian cells infected with recombinant vaccinia virus expressing NP (37). The recombinant NP thus presents a native structure suitable for binding and functional studies.
Binding studies using biotinylated NP showed a saturation of NP binding to Cl.13 cell line and a competition with unlabeled NP indicating that NP binding on B cells is specific. Rabies N protein was previously described as a human viral superantigen that binds to the HLA class II α chain (21). However, our results show that MV NP binding is independent of MHC class II expression, suggesting that it is unlikely to behave as a superantigen. Consequently, NP is probably not directly involved in the alterations of the T cell repertoire observed after MV vaccination (20).
Among the various human or mouse B cell lines tested, only one, the IIA1.6 mutant, did not bind NP efficiently. The fact that this mutant does not express FcγRII prompted us to examine whether the low-affinity IgG receptor (FcγRII; CD32) could be a cellular receptor for NP. Human B cells preferentially express FcγRIIb and phagocytic cells FcγRIIa molecules. Two different FcγRIIb isoforms are generated by alternative splicing, IIb1 and IIb2, which are identical except for a 19– or a 47–amino acid insertion in the cytoplasmic tail of the mouse or human IIb1 isoform, respectively. On mouse B cells, only the IIb1 form is expressed, the IIb2 molecules being mainly expressed on myeloid cells. Human and mouse IIb2 isoforms mediate endocytosis of their ligands, whereas human IIa isoform is favoring phagocytosis (10, 25). FcγRIIa bears a 26–amino acid immunoreceptor tyrosine-based activation motif in its intracytoplasmic domain involved in stimulatory functions (38). The immunoreceptor tyrosine-based activation motif is replaced by a 13–amino acid immunoreceptor tyrosine-based inhibitory motif in the intracytoplasmic domain of FcγRIIb isoforms. This motif has been previously shown to be required for the inhibition of the antiimmunoglobulin-induced B cell activation by immune complexes (10, 25). When IIA1.6 cells transfected with expression vectors encoding either for murine FcγRIIb1 or b2 isoforms or human FcγRIIa, b1*, or b2 isoforms, all transfectants efficiently bound recombinant NP. This binding was specific since it was inhibited by mAb directed against human or murine FcγRII. These results demonstrate that murine and human FcγRII are specific NP receptors. However, since the inhibition of NP binding with specific anti-FcγRII antibody was never complete and a very low NP binding could be detected to the IIA1.6 cells, we can not definitively exclude the presence of another cell-surface receptor for NP.
The mAb 2.4G2 blocks the interaction between murine FcγRII and immune complexes, but also inhibits the NP binding to FcγRII, thus suggesting that immune complexes and NP share the same binding site on FcγRII. The central core (amino acids 189–373) of NP is essential for NP–NP interaction (39) and is probably not accessible for FcγRII interaction. By contrast, the NH2- or COOH-terminal regions seem to be accessible to antibodies directed against NP (40), and thus could be involved in the interaction with FcγRII. Several genotypes have been described for MV, but variations between strains of a same genotype is small. Moreover, for group A, comprising all the vaccine strains like Hallé, no more than one nucleotide difference was found in the nucleotide sequence encoding NP (41).
During the acute phase of measles infection, high amounts of NP-specific antibodies are produced suggesting an interaction between the BCR and NP released from MV-infected cells. In this context, all APCs expressing FcγRII could efficiently internalize NP before any production of specific anti-NP antibodies. This receptor-mediated endocytosis without opsonization could target NP to an endocytic compartment where formation of MHC class II–peptide occurs, and could explain the efficient CD4+ T helper cell response against NP observed during MV infection.
Cross-linking of the BCR with either human FcγRIIb1, IIb2, or murine FcγRIIb1 molecules results in inhibition of antibody production. This inhibition involves the immunoreceptor tyrosine-based inhibitory motif of FcγRIIb that recruits phosphatases (42). It was originally thought that the BCR and FcR have to be co-ligated for this mechanism of inhibition. In the present study, we show that engagement of the BCR is not required for the development of the NP-mediated inhibition of Ig synthesis inasmuch as NP downregulates the Ig response by CD40-activated B cells. One plausible explanation is that the sole cross-linking of FcR is sufficient to transduce a negative regulatory signal. This hypothesis is compatible with the fact that the recombinant NP has an oligomeric structure thus susceptible to efficiently cross-link the FcγRII on B cells. It is noteworthy that no inhibition of B cell proliferation was observed in the presence of NP (data not shown). These results extend previous studies showing that antigen–antibody complexes bound to murine B lymphocytes via FcγR inhibit their antibody response, but not their proliferation in response to F(ab′)2 anti-μ and lymphokines (43, 44). Our data thus support the notion that NP would rather interfere with the plasma cell differentiation pathway. Determination of the exact nature of inhibitory signal(s) generated by interaction between NP and FcγRII will need further investigations. It was previously demonstrated that in vitro, MV infection inhibits Ig secretion from PWM-stimulated PBL cultures (45) and impairs the proliferative response of B cells to mitogens (46). This inhibition affects the early phase of B lymphocyte differentiation and could be due to a blockade of the late G1 phase of the cell cycle (47). It is thus probable that, besides NP, additional mechanisms operate during the MV-induced immunosuppression. In line with such a hypothesis, recent data suggest that the MV H and F proteins could mediate inhibition of lymphocyte proliferation in uninfected cells (48). In vivo, the downregulation of antibody production during measles infection could involve two different mechanisms, one acting on B cell proliferation, and the other one mediated by NP acting on B cell differentiation in plasma cell.
During measles infection, defects in the responses of both lymphocytes and monocytes have been reported (49). Measles virus infection of primary human monocytes leads to a marked suppression of IL-12, some downregulation of IL-10, and an unaltered TNF-α and IL-6 production. The interaction between MV and its cellular receptor, the human CD46, could inhibit IL-12 production (50). However, the possibility that cross-linking of FcγR by NP is involved in modulating monokine production needs to be investigated.
It is very puzzling to note that viruses use molecules of the immune system for their entry, but also for the subversion of regulatory pathways. This subversion could not only implicate viral proteins of the envelope like H or F, but also internal viral protein such as MV NP that decreases in vitro Ig secretion. Other viral NP could perturb immune response as rabies and influenza NP. Rabies NP could act as a superantigen leading to T cell activation (21) and either clonal deletion or anergy. NP from influenza A virus inhibits polymorphonuclear neutrophils functions such as chemotaxis and superoxide production suggesting that NP could play a role in secondary bacterial infections observed in patients infected with influenza virus (51).
In conclusion, our present findings suggest that during acute phase of measles virus infection, NP specifically targets to the FcγRII-expressing cells and may play a role in the MV-induced immunosuppression.
Acknowledgments
We thank Marie-Claude Biémont for technical help in construction of NP recombinant virus and Rob Ruigrock for electron microscopy analysis. We also thank Christian Bonnerot and Sebastian Amigorena for providing IIA1.6 cells transfected with murine FcγRII, Jan G.J. Van de Winkel for providing IIA1.6 cells tranfected with human FcγRII, and David Y. Mason for the KB61 antibody. We are grateful to Patrick Bertolino, Patrice Dubois, and Jacqueline Marvel for helpful comments on the manuscript.
This work was supported by institutional grants from the Centre National de la Recherche Scientifique and Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche, and by additional supports from Association pour la Recherche sur le Cancer (CRC 6108) and Ligue Nationale contre le Cancer (CRC). K. Ravanel is a recipient of a grant from the Fondation pour la Recherche Médicale.
Footnotes
1 Abbreviations used in this paper: AcNPV, Autographa californica nuclear polyedrosis virus; BCR, B cell receptor; CD40L, CD40 ligand; F, fusion; FcγRII, Fc receptor for IgG; H, hemagglutinin; MV, measles virus; NP, nucleocapsid protein; Sf, Sporodoptera frugiperda.
References
- 1.Naniche D, Varior-Krishnan G, Cervoni F, Wild TF, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 3.Graves M, Griffin DE, Johnson RT, Hirsch RL, de Soriano IL, Roedenbeck S, Vaisberg A. Development of antibody to measles virus polypeptides during complicated and uncomplicated measles virus infections. J Virol. 1984;49:409–412. doi: 10.1128/jvi.49.2.409-412.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norrby E, Gollmar Y. Appearance and persistence of antibodies against different virus components after regular measles infections. Infect Immun. 1972;6:240–247. doi: 10.1128/iai.6.3.240-247.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Binnendijk RS, Poelen MC, de Vries P, Voorma HO, Osterhaus AD, Uytdehaag FG. Measles virus-specific human T cell clones. Characterization of specificity and function of CD4+ helper/cytotoxic and CD8+cytotoxic T cell clones. J Immunol. 1989;142:2847–2854. [PubMed] [Google Scholar]
- 6.Ilonen J, Makela MJ, Ziola B, Salmi AA. Cloning of human T cells specific for measles virus haemagglutinin and nucleocapsid. Clin Exp Immunol. 1990;81:212–217. doi: 10.1111/j.1365-2249.1990.tb03320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson S, Sekaly RP, Jacobson CL, McFarland HF, Long EO. HLA class II–restricted presentation of cytoplasmic measles virus antigens to cytotoxic T cells. J Virol. 1989;63:1756–1762. doi: 10.1128/jvi.63.4.1756-1762.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bankamp B, Brinckmann UG, Reich A, Niewiesk S, ter Meulen V, Liebert UG. Measles virus nucleocapsid protein protects rats from encephalitis. J Virol. 1991;65:1695–1700. doi: 10.1128/jvi.65.4.1695-1700.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature (Lond) 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 10.Amigorena S, Bonnerot C, Drake JR, Choquet D, Hunziker W, Guillet JG, Webster P, Sautes C, Mellman I, Fridman WH. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science (Wash DC) 1992;256:1808–1812. doi: 10.1126/science.1535455. [DOI] [PubMed] [Google Scholar]
- 11.Esposito-Farese ME, Sautes C, de la Salle H, Latour S, Bieber T, de la Salle C, Ohlmann P, Fridman WH, Cazenave JP, Teillaud JL, et al. Membrane and soluble Fcγ RII/III modulate the antigen-presenting capacity of murine dendritic epidermal Langerhans cells for IgG-complexed antigens. J Immunol. 1995;155:1725–1736. [PubMed] [Google Scholar]
- 12.Von Pirquet C. Das verhalten der kutanen tuberkulin-reaktion wahrend der masern. Dtsch Med Wochenschr. 1908;34:1297–1300. [Google Scholar]
- 13.Hirsch RL, Griffin DE, Johnson RT, Cooper SJ, de Soriano IL, Roedenbeck S, Vaisberg A. Cellular immune responses during complicated and uncomplicated measles virus infections of man. Clin Immunol Immunopathol. 1984;31:1–12. doi: 10.1016/0090-1229(84)90184-3. [DOI] [PubMed] [Google Scholar]
- 14.Griffin DE, Ward BJ, Esolen LM. Pathogenesis of measles virus infection: an hypothesis for altered immune responses. J Infect Dis. 1994;170:S24–S31. doi: 10.1093/infdis/170.supplement_1.s24. [DOI] [PubMed] [Google Scholar]
- 15.Whittle HC, Bradley-Moore A, Fleming A, Greenwood BM. Effects of measles on the immune response of Nigerian children. Arch Dis Child. 1973;48:753–756. doi: 10.1136/adc.48.10.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coovadia HM, Parent MA, Loening WE, Wesley A, Burgess B, Hallett F, Brain P, Grace J, Naidoo J, Smythe PM, Vos GH. An evaluation of factors associated with the depression of immunity in malnutrition and in measles. Am J Clin Nutr. 1974;27:665–669. doi: 10.1093/ajcn/27.6.665. [DOI] [PubMed] [Google Scholar]
- 17.Tishon A, Manchester M, Scheiflinger F, Oldstone MB. A model of measles virus-induced immunosuppression: enhanced susceptibility of neonatal human PBLs. Nat Med. 1996;2:1250–1254. doi: 10.1038/nm1196-1250. [DOI] [PubMed] [Google Scholar]
- 18.Norrby E, Enders-Ruckle G, Meulen V. Differences in the appearance of antibodies to structural components of measles virus after immunization with inactivated and live virus. J Infect Dis. 1975;132:262–269. doi: 10.1093/infdis/132.3.262. [DOI] [PubMed] [Google Scholar]
- 19.Hussey GD, Goddard EA, Hughes J, Ryon JJ, Kerran M, Carelse E, Strebel PM, Markowitz LE, Moodie J, Barron P, et al. The effect of Edmonston-Zagreb and Schwarz measles vaccines on immune response in infants. J Infect Dis. 1996;173:1320–1326. doi: 10.1093/infdis/173.6.1320. [DOI] [PubMed] [Google Scholar]
- 20.Auwaerter PG, Hussey GD, Goddard EA, Hughes J, Ryon JJ, Strebel PM, Beatty D, Griffin DE. Changes within T cell receptor Vβsubsets in infants following measles vaccination. Clin Immunol Immunopathol. 1996;79:163–170. doi: 10.1006/clin.1996.0063. [DOI] [PubMed] [Google Scholar]
- 21.Lafon M, Lafage M, Martinez-Arends A, Ramirez R, Vuillier F, Charron D, Lotteau V, Scott-Algara D. Evidence for a viral superantigen in humans. Nature (Lond) 1992;358:507–510. doi: 10.1038/358507a0. [DOI] [PubMed] [Google Scholar]
- 22.Ono SJ, Bazil V, Sugawara M, Strominger JL. An isotype-specific trans-acting factor is defective in a mutant B cell line that expresses HLA-DQ, but not -DR or -DP. J Exp Med. 1991;173:629–637. doi: 10.1084/jem.173.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calman AF, Peterlin BM. Mutant human B cell lines deficient in class II major histocompatibility complex transcription. J Immunol. 1987;139:2489–2495. [PubMed] [Google Scholar]
- 24.Accolla RS. Human B cell variants immunoselected against a single Ia antigen subset have lost expression of several Ia antigen subsets. J Exp Med. 1983;157:1053–1058. doi: 10.1084/jem.157.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Den Herik-Oudijk IE, Westerdaal NA, Henriquez NV, Capel PJ, Van De Winkel JG. Functional analysis of human FcγRII (CD32) isoforms expressed in B lymphocytes. J Immunol. 1994;152:574–585. [PubMed] [Google Scholar]
- 26.Giraudon P, Wild TF. Monoclonal antibodies against measles virus. J Gen Virol. 1981;54:325–332. doi: 10.1099/0022-1317-54-2-325. [DOI] [PubMed] [Google Scholar]
- 27.Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulford K, Ralfkiaer E, MacDonald SM, Erber WN, Falini B, Gatter KC, Mason DY. A new monoclonal antibody (KB61) recognizing a novel antigen which is selectively expressed on a subpopulation of human B lymphocytes. Immunology. 1986;57:71–76. [PMC free article] [PubMed] [Google Scholar]
- 29.Buckland R, Gerald C, Barker D, Wild F. Cloning and sequencing of the nucleoprotein gene of measles virus (Halléstrain) Nucleic Acids Res. 1988;16:11821. doi: 10.1093/nar/16.24.11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Udem SA, Cook KA. Isolation and characterization of measles virus intracellular nucleocapsid RNA. J Virol. 1984;49:57–65. doi: 10.1128/jvi.49.1.57-65.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Defrance T, Carayon P, Billian G, Guillemot JC, Minty A, Caput D, Ferrara P. Interleukin 13 is a B cell stimulating factor. J Exp Med. 1994;179:135–143. doi: 10.1084/jem.179.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birrer MJ, Bloom BR, Udem S. Characterization of measles polypeptides by monoclonal antibodies. Virology. 1981;108:381–390. doi: 10.1016/0042-6822(81)90446-3. [DOI] [PubMed] [Google Scholar]
- 33.Lund GA, Tyrrell DL, Bradley RD, Scraba DG. The molecular length of measles virus RNA and the structural organization of measles nucleocapsids. J Gen Virol. 1984;65:1535–1542. doi: 10.1099/0022-1317-65-9-1535. [DOI] [PubMed] [Google Scholar]
- 34.Chan PL, Sinclair NR. Regulation of the immune response. V. An analysis of the function of the Fc portion of antibody in suppression of an immune response with respect to interaction with components of the lymphoid system. Immunology. 1971;21:967–981. [PMC free article] [PubMed] [Google Scholar]
- 35.Silvy A, Lagresle C, Bella C, Defrance T. The differentiation of human memory B cells into specific antibody-secreting cells is CD40 independent. Eur J Immunol. 1996;26:517–524. doi: 10.1002/eji.1830260303. [DOI] [PubMed] [Google Scholar]
- 36.Fooks AR, Stephenson JR, Warnes A, Dowsett AB, Rima BK, Wilkinson GW. Measles virus nucleocapsid protein expressed in insect cells assembles into nucleocapsid-like structures. J Gen Virol. 1993;74:1439–1444. doi: 10.1099/0022-1317-74-7-1439. [DOI] [PubMed] [Google Scholar]
- 37.Spehner D, Kirn A, Drillien R. Assembly of nucleocapsidlike structures in animal cells infected with a vaccinia virus recombinant encoding the measles virus nucleoprotein. J Virol. 1991;65:6296–6300. doi: 10.1128/jvi.65.11.6296-6300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cambier JC. Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM) J Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- 39.Bankamp B, Horikami SM, Thompson PD, Huber M, Billeter M, Moyer SA. Domains of the measles virus N protein required for binding to P protein and self-assembly. Virology. 1996;216:272–277. doi: 10.1006/viro.1996.0060. [DOI] [PubMed] [Google Scholar]
- 40.Buckland R, Giraudon P, Wild F. Expression of measles virus nucleoprotein in Escherichia coli: use of deletion mutants to locate the antigenic sites. J Gen Virol. 1989;70:435–441. doi: 10.1099/0022-1317-70-2-435. [DOI] [PubMed] [Google Scholar]
- 41.Rima BK, Earle JA, Yeo RP, Herlihy L, Baczko K, ter Meulen V, Carabana J, Caballero M, Celma ML, Fernandez-Munoz R. Temporal and geographical distribution of measles virus genotypes. J Gen Virol. 1995;76:1173–1180. doi: 10.1099/0022-1317-76-5-1173. [DOI] [PubMed] [Google Scholar]
- 42.Scharenberg AM, Kinet JP. The emerging field of receptor-mediated inhibitory signaling: SHP or SHIP? . Cell. 1996;87:961–964. doi: 10.1016/s0092-8674(00)81790-0. [DOI] [PubMed] [Google Scholar]
- 43.Uher F, Lamers MC, Dickler HB. Antigen-antibody complexes bound to B-lymphocyte Fcγ receptors regulate B-lymphocyte differentiation. Cell Immunol. 1985;95:368–379. doi: 10.1016/0008-8749(85)90324-7. [DOI] [PubMed] [Google Scholar]
- 44.Uher F, Dickler HB. Cooperativity between B lymphocyte membrane molecules: independent ligand occupancy and cross-linking of antigen receptors and Fcγ receptors down-regulates B lymphocyte function. J Immunol. 1986;137:3124–3129. [PubMed] [Google Scholar]
- 45.Casali P, Rice GP, Oldstone MB. Viruses disrupt functions of human lymphocytes. Effects of measles virus and influenza virus on lymphocyte-mediated killing and antibody production. J Exp Med. 1984;159:1322–1337. doi: 10.1084/jem.159.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McChesney MB, Fujinami RS, Lampert PW, Oldstone MB. Viruses disrupt functions of human lymphocytes. II. Measles virus suppresses antibody production by acting on B lymphocytes. J Exp Med. 1986;163:1331–1336. [PMC free article] [PubMed] [Google Scholar]
- 47.McChesney MB, Kehrl JH, Valsamakis A, Fauci AS, Oldstone MB. Measles virus infection of B lymphocytes permits cellular activation but blocks progression through the cell cycle. J Virol. 1987;61:3441–3447. doi: 10.1128/jvi.61.11.3441-3447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlender J, Schnorr JJ, Spielhoffer P, Cathomen T, Cattaneo R, Billeter MA, ter Meulen V, Schneider-Schaulies S. Interaction of measles virus glycoproteins with the surface of uninfected peripheral blood lymphocytes induces immunosuppression in vitro. Proc Natl Acad Sci USA. 1996;93:13194–13199. doi: 10.1073/pnas.93.23.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Griffin DE. Immune responses during measles virus infection. Curr Top Microbiol Immunol. 1995;191:117–134. doi: 10.1007/978-3-642-78621-1_8. [DOI] [PubMed] [Google Scholar]
- 50.Karp CL, Wysocka M, Wahl LM, Ahearn JM, Cuomo PJ, Sherry B, Trinchieri G, Griffin DE. Mechanism of suppression of cell-mediated immunity by measles virus. Science (Wash DC) 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 51.Cooper JA, Jr, Carcelen R, Culbreth R. Effects of influenza A nucleoprotein on polymorphonuclear neutrophil function. J Infect Dis. 1996;173:279–284. doi: 10.1093/infdis/173.2.279. [DOI] [PubMed] [Google Scholar]