Abstract
We evaluated mature peripheral blood eosinophils for their expression of the surface tyrosine kinase, c-kit, the receptor for the stromal cell–derived cytokine, stem cell factor (SCF). Cytofluorographic analysis revealed that c-kit was expressed on the purified peripheral blood eosinophils from 8 of 8 donors (4 nonatopic and 4 atopic) (mean channel fluorescence intensity 2.0– 3.6-fold, average 2.8 ± 0.6-fold, greater than the negative control). The uniform and selective expression of c-kit by eosinophils was confirmed by immunohistochemical analysis of peripheral blood buffy coats. The functional integrity of c-kit was demonstrated by the capacity of 100 ng/ml (5 nM) of recombinant human (rh) SCF to increase eosinophil adhesion to 3, 10, and 30 μg/ml of immobilized FN40, a 40-kD chymotryptic fragment of plasma fibronectin, in 15 min by 7.7 ± 1.4-, 5.3 ± 3.3-, and 5.4 ± 0.2-fold, respectively, and their adhesion to 0.1, 0.5, and 1.0 μg/ml vascular cell adhesion molecule-1 (VCAM-1), by 12.7 ± 9.2-, 3.8 ± 2.5-, and 1.7 ± 0.6-fold, respectively. The SCF-stimulated adhesion occurred without concomitant changes in surface integrin expression, thereby indicating an avidity-based mechanism. rhSCF (100 ng/ml, 5 nM) was comparable to rh eotaxin (200 ng/ml, 24 nM) in stimulating adhesion. Cell adhesion to FN40 was completely inhibited with antibodies against the α4 and β1 integrin subunits, revealing that the SCF/c-kit adhesion effect was mediated by a single integrin heterodimer, very late antigen 4 (VLA-4). Thus, SCF represents a newly recognized stromal ligand for the activation of eosinophils for VLA-4–mediated adhesion, which could contribute to the exit of these cells from the blood, their tissue localization, and their prominence in inflammatory lesions.
Eosinophils are bone marrow–derived granulocytes with a dominant extravascular distribution primarily in mucosal tissues (1, 2). Eosinophils have been implicated beneficially in host defense against helminthic parasitic infection (3–6), in anti-tumor cytotoxicity (7–9), and in wound healing (10, 11). Conversely, the abundant eosinophils in the respiratory mucosal tissue from patients with asthma or rhinitis are believed to contribute to the inflammatory process by releasing preformed, highly cationic granule proteins with cytotoxic effects (12) and by generating lipid mediators, in particular the cysteinyl leukotriene, leukotriene C4, with attendant vascular and bronchial smooth muscle constrictor action (13). Eosinophils at the foci of tissue inflammation bear membrane markers of activation such as CD69 (14, 15) and exhibit extended survival, which is attributed to the attenuation of apoptosis by hematopoietic cytokines, particularly IL-5, and GM-CSF (16, 17).
Integrins, heterodimeric cell surface receptors, participate in the regulation of leukocyte endothelial cell adhesion, transendothelial cell/basement membrane migration, and localization in inflammatory tissues. Eosinophils express the very late antigen (VLA)1-4 (α4β1) and VLA-6 (α6β1) as well as α4β7 (18–20). VLA-4 mediates leukocyte attachment to VCAM-1 on activated endothelial cells (18, 21). Anti-α4 antibodies block eosinophil recruitment and prevent antigen-induced bronchial hyperreactivity in several animal models, suggesting a critical role for the α4 integrins in the tissue recruitment, activation, and/or accumulation of eosinophils in allergic disease (22–26). The VLA-4 integrin also binds to fibronectin through an alternatively spliced connecting segment-1 (CS-1) region of fibronectin (27). The interaction between VLA-4 and fibronectin results in prolonged eosinophil survival in culture by inducing the autocrine generation of GM-CSF and IL-3 (28). Inasmuch as a subpopulation of eosinophils in nasal polyps (29) and bronchoalveolar lavage fluid from individuals with asthma undergoing allergen challenge (30) expresses GM-CSF protein and/or mRNA, it is possible that in situ VLA-4–fibronectin interaction prolongs eosinophil retention and viability through an autocrine mechanism.
Stem cell factor (SCF, also known as steel factor) is a bone marrow stromal cytokine central to hematopoiesis (31–33). It is also a peripheral tissue product of fibroblasts and endothelial cells (34–37). SCF exists in two different forms, soluble and membrane bound, and is the ligand for the c-kit receptor that is found on primitive hematopoietic cells (38). Among hematopoietic cells, c-kit is believed to be retained only by mature tissue mast cells, and thus is a commonly used marker for the latter (39, 40). Interaction of the c-kit receptor with SCF stimulates the growth and early differentiation of hematopoietic cells (38) and sustains mast cell growth and differentiation in cultures of mouse bone marrow (41, 42) and human cord blood (43, 44). In response to cross-linking of the high affinity IgE receptor, FcεR1, SCF primes mature dispersed human lung mast cells for both augmented exocytosis of secretory granules (45) and cytokine production (46) and primes mouse bone marrow– derived mast cells (BMMC) for enhanced generation of membrane-derived eicosanoids (47). Additionally, SCF is a direct activator of BMMC, stimulating both exocytosis and eicosanoid generation with the same biochemical steps and kinetics as activation by FcεR1 (48). SCF promotes the adhesion of BMMC to fibronectin via β1 integrins, increasing the α5β1 (VLA-5) integrin avidity (49–51). Thus, SCF is a potentially critical regulatory factor in the localization, proliferation, priming, and direct activation of mast cells.
We now demonstrate by cytofluorographic and immunohistochemical analyses the surface expression of c-kit receptor in freshly isolated peripheral blood human eosinophils. That recombinant human (rh)SCF augments eosinophil adhesion to the VLA-4 ligands, fibronectin, and VCAM-1, establishes the functional integrity of the eosinophil-expressed c-kit. Thus, SCF represents an abundant stromal ligand with direct activating effects for human eosinophils as well as mast cells.
Materials and Methods
Reagents and Antibodies.
rhSCF (catalogue no. 1833-01, lot no. B6326; Genzyme, Cambridge, MA), rh eotaxin (Endogen, Inc., Boston, MA), 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM) (Molecular Probes, Eugene, OR), human serum albumin (HSA) (Sigma Chem. Co., St. Louis, MO), purified anti-human c-kit YB5.B8 (PharMingen, San Diego, CA), purified anti-human c-kit (95C3) (Coulter, Miami, FL), purified anti-human integrin β1 (CD29) (4B4) (Coulter), purified anti-human integrin α4 (CD49d) (A4-PUJ1) (Upstate Biotechnology, Lake Placid, NY), purified anti-human CD3 (HIT3a) (PharMingen), mouse laminin and the 40-kD chymotryptic fragment of human fibronectin (FN40) (GIBCO BRL, Gaithersburg, MD) were purchased. The preparation of purified VCAM-(mouse Cκ) fusion protein (VCAM-1-κ) was previously reported (52). Anti-human integrin α4 (CD49d) (B5G10, ascites) (53), anti-human integrin α6 (CD49f) (450-33D, ascites) (54), anti-human integrin β1 (CD29) (A-1A5, ascites) (55), purified anti-human integrin α4β7 (Act-1) (56), and the negative control antibody P3 (hybridoma culture supernatant) (57) were provided by Dr. M.E. Hemler (Dana-Farber Cancer Institute, Harvard Medical School). Purified anti-human c-kit mAb SR-1 (ascites) (58) was a gift from Dr. V. Broudy (University of Washington, Seattle, WA).
Isolation of Eosinophils from Peripheral Human Blood.
Blood was collected into sterile, heparinized syringes from the peripheral veins of nonatopic and atopic volunteer donors who gave informed consent. After the erythrocytes were sedimented with dextran for 45 min at 37°C, the granulocyte fraction was obtained by centrifugation through a cushion of Ficoll–Hypaque (1.77 g/ml; Pharmacia, Uppsala, Sweden) of the buffy coat at 350 g for 30 min. After the hypotonic lysis of residual erythrocytes, eosinophils were separated from neutrophils by negative immunomagnetic selection with a magnetic cell separation (MACS) column (Miltenyi Biotec, Sunnyvale, CA) (59). In brief, the erythrocyte-depleted granulocyte pellet was incubated for 45 min at 4°C with anti-CD16 mAb bound to immuno-magnetic beads. When the mixture was applied to a steel wire column in a strong magnetic field, the CD16+ neutrophils were retained, whereas the CD16− eosinophils were highly purified in the fraction that flowed through the column. Contaminating T lymphocytes and monocytes were further removed by incubating the CD16− fraction for another 15 min at 4°C with saturating concentrations of anti-CD3 and anti-CD14 magnetic beads, respectively (Miltenyi). Cytocentrifugation slides of the eosinophils stained with Wright's and Giemsa stains showed that the purity of the isolated eosinophils was greater than 95% in all experiments.
Cytofluorographic and Immunohistochemical Analyses of Surface c-kit Expression on Eosinophils.
Cytofluorographic analyses of surface epitopes expressed by human peripheral blood eosinophils with or without rhSCF stimulation were performed by established procedures (52). Freshly isolated human peripheral blood eosinophils were resuspended in RPMI 1640 medium containing 10% FCS at a concentration of 106 cells/ml and were divided into two identical fractions. rhSCF was added to one fraction to a final concentration of 100 ng/ml (5 nM). Alternatively, rh eotaxin was used at a final concentration of 24 nM and both fractions were incubated for 15 min at 37°C. The cells were harvested and washed once with cold PBS containing 0.5% HSA and 0.02% sodium azide (FACS buffer). Samples of 5 × 105 cells were then incubated for 1 h on ice with primary antibodies (purified mAbs at a final concentration of 10 μg/ml or ascites at a final dilution of 1:500 or P3 culture supernatant at 1:4 dilution). The cells were washed once with FACS buffer and incubated in the dark for 1 h on ice with fluorescein isothiocyanate–conjugated goat anti–mouse IgG (GIBCO BRL) at a final dilution of 1:100. The cells were washed again with the FACS buffer, resuspended in 0.25 ml of PBS, and analyzed on a FACSort® machine (Becton Dickinson, Oxnard, CA). For c-kit expression (Fig. 1), the results are presented as overlaid histograms and the fold increase of mean fluorescence intensity (MFI). The fold increase of c-kit expression was calculated by dividing the MFI units of SR-1 staining by the MFI units of P3 control mAb staining in each donor.
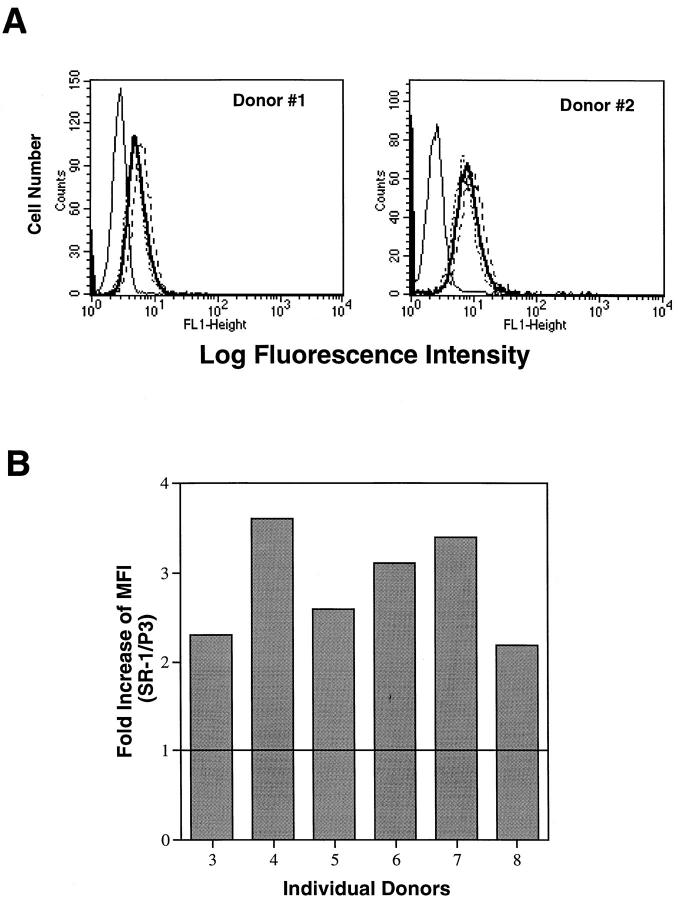
Figure 1.
Cytofluorographic analysis of c-kit receptor expression on freshly isolated, human peripheral blood eosinophils. (A) Eosinophils from donors 1 and 2 were analyzed with three different mouse anti–human c-kit mAbs, SR-1 (——), YB5.B8 (----), and 95C3 (.....), as well as a control mouse mAb, P3 (——), (IgG control). (B) Eosinophils from an additional 6 donors (3–8) were analyzed with SR-1 and P3 mAbs. The values expressed on the y axis are values of the MFI units of SR-1 staining divided by the MFI units of P3 control mAb staining in each donor.
For immunohistochemical analysis of the surface c-kit expression, peripheral blood buffy coats were prepared after dextran sedimentation of erythrocytes. Two fractions of the buffy coat, each containing 106 white blood cells, were incubated on ice for 1 h with SR-1 (1:500 diluted ascites) and control P3 antibody (1:4 diluted hybridoma culture supernatant) in a total volume of 100 μl of FACS buffer, respectively. Cells were washed once with 3 ml of FACS buffer, and cytocentrifugation slides were prepared, with 4 × 104 cells per slide. The slides were fixed in 4% paraformaldehyde (Polysciences, Inc., Warrington, PA) in PBS for 10 min at room temperature. After washing with PBS for 3–4 times, the slides were blocked with 2% chicken egg albumin (Sigma) for 30 min at room temperature, and were incubated with 1:10 diluted goat anti–mouse IgG(Fc)-conjugated gold particles (average size 10 nm) (Amersham International, Buckinghamshire, England) for 1 h at room temperature. The slides were washed first with PBS 2–3 times and then with distilled water 2–3 times. A silver enhancement procedure was carried out for 8 min at room temperature following the manufacturer's manual (AuroProbe™; Amersham). The slides were then counterstained with hematoxylin and eosin, mounted with Permount® (Fisher Scientific, Pittsburgh, PA), and analyzed with a Leica microscope (Model Dialux 20). Results were photographically recorded using Kodak Royal Gold film ASA25.
Cell Adhesion Assay.
Cells were attached in stasis to FN40, VCAM-1, and laminin as described (52). FN40 and laminin at concentrations of 3 μg/ml, 10 μg/ml, and 30 μg/ml were coated onto non–tissue culture grade 96-well microtiter plates (Nunc-Immuno Plate) in 0.1 M NaHCO3 (pH 8.3) (100 μl/well) for 16 h at 4°C. The plates were washed twice with PBS (150 μl/well), and the nonspecific binding sites were blocked by incubation with 5% HSA in PBS (100 μl/well) for 45 min at 37°C. After two washes with PBS (150 μl/well), the plates were ready to be used. For the VCAM-1 assay, plates were precoated with goat anti–mouse κ (GIBCO BRL) at a 1:1,000 final dilution in 0.1 M NaHCO3 (100 μl/well) for 16 h at 4°C. After two washes with PBS, the plates were coated with VCAM-1-κ at concentrations of 0.1, 0.5, and 1.0 μg/ml in 0.1 M NaHCO3 (100 μl/well) for 2 h at 4°C. The plates were washed with PBS and blocked with HSA as mentioned above. The freshly isolated eosinophils were incubated with BCECF-AM (5 μg/ml) in RPMI 1640 containing 10 mM Hepes and 0.1% HSA (RPMI–HSA) for 30 min at 37°C, washed twice with PBS, and resuspended in RPMI–HSA at 4 × 106 cells/ml. Samples (50 μl) of the cell suspension containing 2 × 105 cells were added to each well of the ligand-coated plate; each well had been preloaded with either 50 μl of RPMI–HSA or 50 μl of RPMI–HSA containing defined concentrations of rhSCF (generally 10 nM) or defined concentrations of rh eotaxin (generally 48 nM). The plates were incubated for 15 min at 37°C, and the fluorescence of total input cells was quantitated by a fluorescence analyzer (Idexx Laboratories, Westbrook, ME). Unbound cells were removed by washing the plates with RPMI/10 mM Hepes 4–5 times at 150 μl/well until the cells in the control wells (5% HSA alone) were less than 5% of their total input. Cells remaining attached to the plate were quantitated after every wash with the same fluorescence analyzer. The percentage of specific binding to immobilized integrin ligands was calculated as the fluorescence of the cells detected after the final wash divided by the fluorescence of the total starting cells × 100, and assay results are presented as means ± SD of three independent experiments, each performed in triplicate.
For antibody-blocking experiments, cells were resuspended in cold RPMI–HSA at a concentration of 4 × 106 cells/ml and divided into four identical lots. Purified mAbs were added to each lot to a final concentration of 10 μg/ml. The mAbs used in this study were anti-CD3 (negative control), anti-α4 (A4-PUJ1), anti-α4β7 (Act-1), and anti-β1 (4B4). Cells were incubated with the mAbs for 10 min on ice and added to each well of a FN40 (30 μg/ml) coated plate. During the 15-min incubation at 37°C, the mAb concentration in each well was reduced to 5 μg/ml by preloading the wells with RPMI–HSA with or without rhSCF or rh eotaxin. The remainder of the assay procedure was as described above.
Statistical Analysis.
The statistical significance of differences between sample means for each set of cells was based on comparison as determined by the Student's t test for matched pairs. Results are presented as mean ± SD.
Results
Cytofluorographic and Immunohistochemical Analyses of Surface c-kit Expression on Eosinophils.
To determine whether freshly isolated, peripheral human blood eosinophils expressed the c-kit receptor, the purified cells from eight separate donors (four nonatopic and four atopic) were analyzed by cytofluorography for the expression of c-kit. Three mouse mAbs against three independent epitopes of the human c-kit (58, 60) gave virtually identical expression for two of the donors (Fig. 1 A), with mean log fluorescence intensities of 2.0- and 3.3-fold over control, respectively, with mAb SR-1. The surface c-kit expression was subsequently confirmed in six additional donors with one of the three mAbs, SR-1 (Fig. 1 B). In every case, the c-kit expression was readily detectable, with a mean log fluorescence intensity of 2.0 to 3.6-fold greater than the negative control (IgG control mAb P3) (mean 2.8 ± 0.6 fold, n = 8).
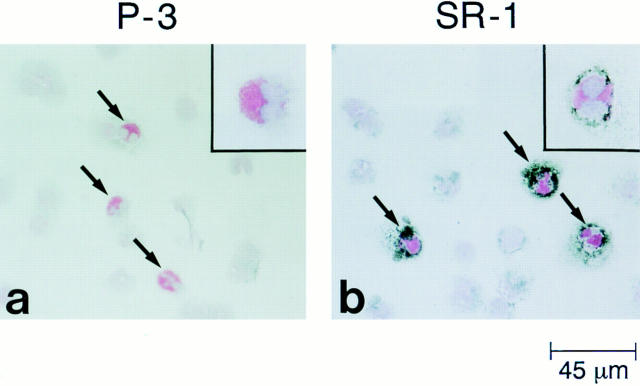
To confirm c-kit expression by peripheral blood eosinophils and determine its potential expression by other circulating leukocytes, peripheral blood buffy coats from two separate donors (1 atopic and 1 nonatopic) were incubated with either SR-1 or control P3 antibody, and were subjected to immunohistochemical analysis using secondary antibody-conjugated gold particles and a silver enhancement procedure, followed by counterstaining with hematoxylin and eosin. As indicated by the counterstaining and shown for the nonatopic donor (Fig. 2), 100% of the eosinophils in the buffy coats were positive for c-kit, and eosinophils were the only cells displaying a signal for c-kit receptor. Identical results were obtained for the atopic donor (data not shown). A similar positive signal was also detected on freshly isolated human peripheral blood eosinophils after MACS column purification (data not shown).
Figure 2.
Immunohistochemical analysis of c-kit receptor expression on freshly prepared human peripheral blood buffy coats. Peripheral blood buffy coats were incubated with either negative control mAb P3 (IgG matched) (a), or anti-human c-kit mAb SR-1 (b), and cytocentrifugation slides were prepared. After application of secondary antibody-conjugated gold particles and a silver enhancement procedure, the slides were counterstained with hematoxylin and eosin, and analyzed with a Leica microscope. Arrows indicate the eosinophils. Other leukocytes, as shown in the same field, were negative for surface c-kit expression. Higher magnification views of individual eosinophils are shown in the upper right corners.
Effect of rhSCF on Eosinophil Adhesion to FN40 and rVCAM-1.
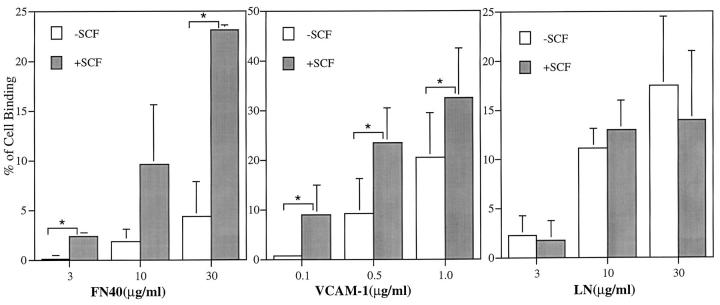
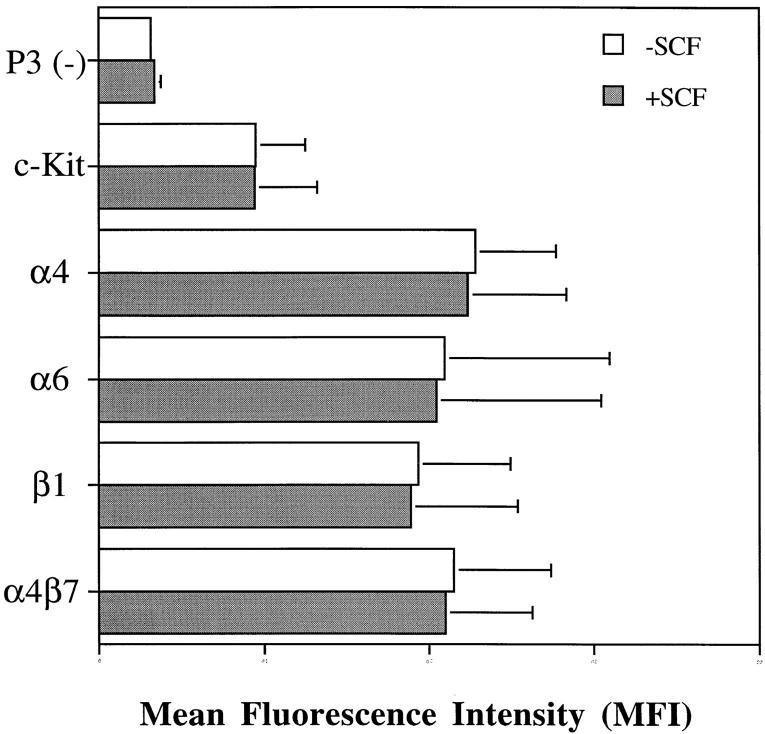
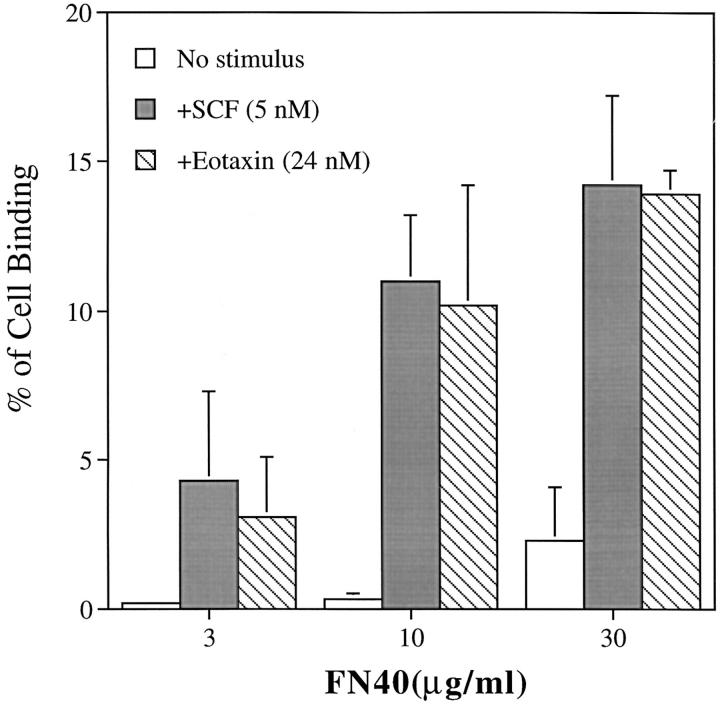
To determine the functional integrity of the expressed c-kit receptor on eosinophils, the ability of rhSCF to augment their adhesion to ligands selective for integrin α4 and α6 was evaluated in a static adhesion assay. The 15-min time course for the adhesion assay was selected because a kinetic study showed that augmented adhesion peaked at 15 min, persisted for 30 min, and decreased to baseline by 45 min (data not shown). Adhesion to FN40 increased at all three inputs of 3, 10, and 30 μg/ml of immobilized FN40. In the absence of rhSCF, the adhesion of FN40 was limited (specific binding of 4.3 ± 3.5% at 30 μg/ml FN40). Concomitant stimulation with rhSCF (5 nM) augmented adhesion to 3, 10, and 30 μg/ml FN40 by 7.7 ± 1.4-fold, 5.3 ± 3.3-fold, and 5.4 ± 0.2-fold, respectively, compared with baseline (Fig. 3). Adhesion to rVCAM-1 also increased in relation to the input of ligand. In the absence of rhSCF, rVCAM-1 supported greater adhesion than FN40 (specific binding of 9.3 ± 9% and 22.5 ± 10% at 0.5 μg/ml and 1.0 μg/ml of rVCAM-1, respectively). Stimulation with rhSCF augmented adhesion to 0.1, 0.5, and 1.0 μg/ml rVCAM-1 by 12.7 ± 9.2-fold, 3.8 ± 2.5-fold, and 1.7 ± 0.6-fold, respectively, compared with baseline (Fig. 3). Higher baseline adhesion mediated by VCAM-1 compared with fibronectin has been previously reported (22). Cell adhesion to laminin also increased in a dose-dependent fashion with respect to ligand input but did not increase further with stimulation by rhSCF (Fig. 3). Treatment with rhSCF did not change the surface expression of integrin α4, α6, β1, α4β7, or c-kit receptor as evaluated by cytofluorographic analysis (Fig. 4), indicating that the increases in adhesion to the α4 ligands were not due to increased receptor expression.
Figure 3.
Effect of rhSCF on the adhesion of human eosinophils to FN40, VCAM-1, and laminin. Freshly isolated, human peripheral blood eosinophils were incubated for 15 min at 37°C in 96-well plates coated with FN40, VCAM-1, or laminin (LN) in the presence (▪) or absence of (□) rhSCF (100 ng/ ml, 5 nM) and assayed for adhesion. Data are presented as mean ± SD of three independent experiments for FN40 and LN and four independent experiments for VCAM-1, each performed in triplicate. Asterisks indicate P <0.05.
Figure 4.
Cytofluorographic analysis of the effect of rhSCF (100 ng/ml) on c-kit and integrin expression by eosinophils after incubation for 15 min at 37°C. Values on the x axis are the MFI units of each mAb staining. Data are presented as mean ± SD of MFI units from three independent donors. Medium alone (□); medium plus rhSCF (▪).
Effect of Antibody Neutralization on rhSCF-stimulated Adhesion of Eosinophils to FN40.
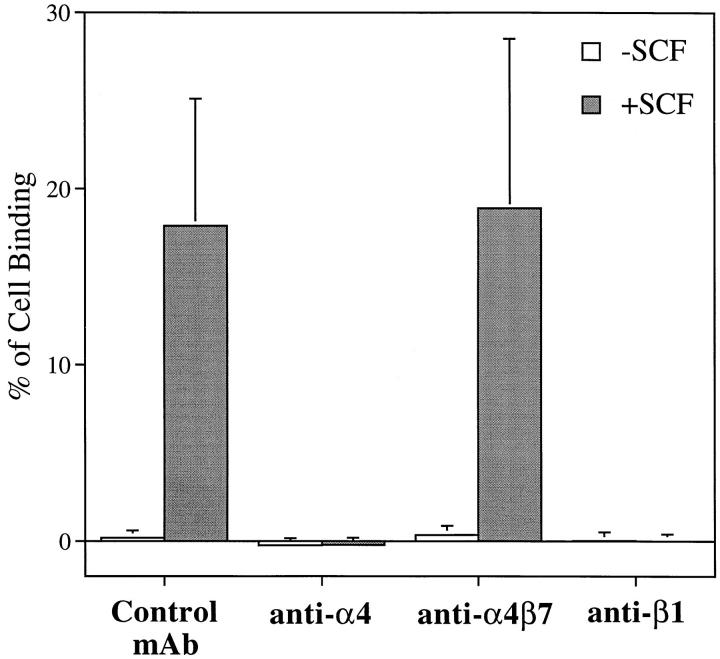
Both of the α4 integrins expressed on eosinophils, VLA-4 (α4β1) and α4β7, serve as receptors for FN40 and VCAM-1 (19). Antibody-blocking experiments were performed in the assay for adhesion of eosinophils to 30 μg/ml FN40 with or without rhSCF stimulation (5 nM). Although the fold increase in response to rhSCF was similar at each concentration of FN40 (see Fig. 3), the greatest absolute increment in adhesion occurred at 30 μg/ml FN40. mAbs against α4 (A4-PUJ1) and β1 (4B4) completely abolished eosinophil adherence to FN40, whereas the anti-α4β7 mAb (Act-1) had no effect and was comparable to the control mAb of irrelevant specificity (anti-CD3) (Fig. 5). Similarly, cell adhesion to rVCAM-1 in the absence or presence of rhSCF (5 nM) was also completely blocked by anti-α4 and anti-β1 mAbs (data not shown). Therefore, rhSCF–c-kit ligation specifically augmented the adhesion of VLA-4 to its ligands, fibronectin, and VCAM-1.
Figure 5.
Effect of rhSCF on the adhesion of human eosinophils to FN40 (30 μg/ml) in the presence of anti-integrin mAbs and control IgG mAb. Isolated human eosinophils were incubated with mouse anti–human α4 (A4-PUJ1), anti-human α4β7 (Act-1), anti-human β1 (4B4), or IgG control mAb anti-human CD3 (HIT3a) for 10 min at 4°C before being assayed for adhesion with or without rhSCF (100 ng/ml, 5 nM) in 96-well plates coated with FN40 (30 μg/ml) for 15 min at 37°C. Data are presented as mean ± SD of three independent experiments each performed in triplicate.
Dose Effect of rhSCF on Eosinophil Adhesion to FN40.
The effect of rhSCF at concentrations ranging from 12.5 ng/ml (0.625 nM) to 200 ng/ml (10 nM) on eosinophil adhesion to 30 μg/ml FN40 was studied. Enhancement of VLA-4 binding to FN40 by rhSCF was significant at 2.5, 5, and 10 nM as compared with the unstimulated replicate eosinophils (P <0.05) (Fig. 6). Each fourfold increment in rhSCF concentration produced a statistically significant gain in binding (P <0.05) except for the final increment between 2.5 and 10 nM, suggesting that a plateau was reached.
Figure 6.
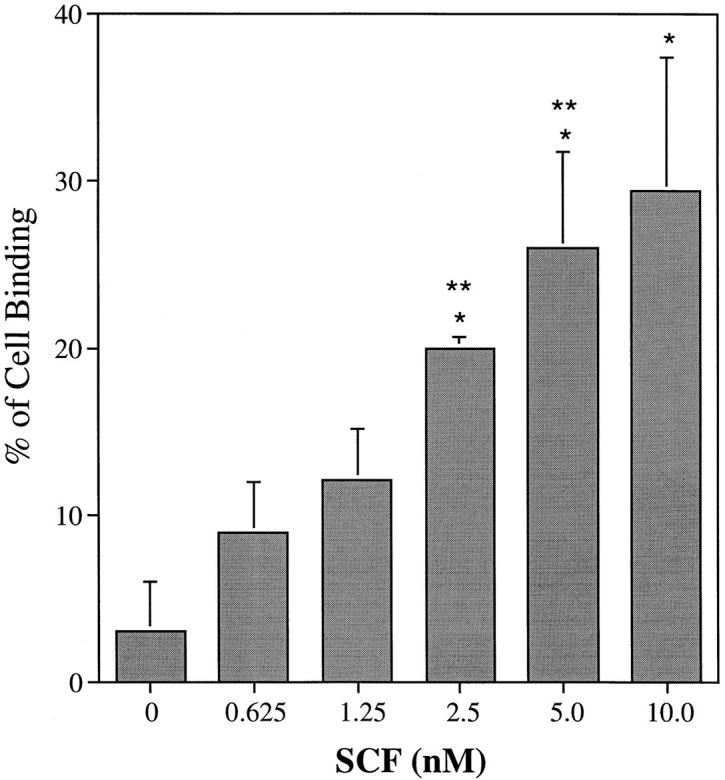
Dose effect of rhSCF on eosinophil adhesion to FN40. Human eosinophils were assayed for adhesion to FN40 (30 μg/ml)–coated 96-well plates in the presence of increasing concentrations of rhSCF. Data are presented as mean ± SD of three independent experiments each performed in triplicate. Single asterisk indicates significant stimulation relative to the unstimulated eosinophils and double asterisk indicates significant stimulation relative to a fourfold lesser concentration.
Comparison of the Effects of rhSCF and rh Eotaxin on Eosinophil Binding to FN40.
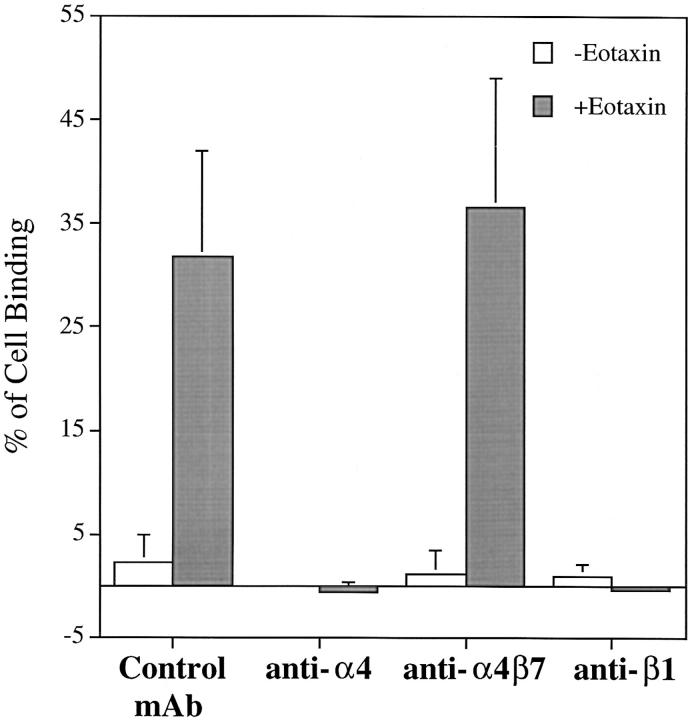
Eotaxin, a selective eosinophil chemoattractant (61, 62), belongs to the CC chemokine family. Other members of this family are known to be stimuli for eosinophil adhesion via integrin α4 (63). Preliminary dose-dependence experiments indicated that the effect of 24 nM rh eotaxin was similar to the effect of 5 nM rhSCF for augmenting eosinophil adhesion to 30 μg/ml FN40. Thus, the effects of these concentrations on eosinophil adhesion were compared over three concentrations of FN40 (Fig. 7). Eotaxin at 24 nM increased eosinophil binding to FN40 to the same extent as 100 ng/ml (5 nM) rhSCF at each static input of FN40 ligand. Eotaxin did not change the surface expression of c-kit, α4, α6, β1, and α4β7 by cytofluorographic analysis (data not shown), and the augmented adhesion of these cells to FN40 produced by eotaxin was completely blocked by mAbs against α4 and β1 (Fig. 8).
Figure 7.
Comparison of the effects of rhSCF and eotaxin on eosinophil adhesion to FN40. Human eosinophils were assayed for adhesion to FN40-coated 96-well plates for 15 min at 37°C in medium alone (□) or in the presence of rhSCF (100 ng/ml, 5 nM) (▪) or rh eotaxin (24 nM) (▨ ). Data are presented as mean ± SD of three independent experiments each performed in triplicate.
Figure 8.
Effect of rh eotaxin on the adhesion of human eosinophils to FN40 (30 μg/ml) in the presence of anti-integrin mAbs and control IgG mAb. Isolated human eosinophils were incubated with mouse anti–human α4 (A4-PUJ1), anti–human α4β7 (Act-1), anti–human β1 (4B4) or IgG control mAb anti–human CD3 (HIT3a) for 10 min at 4°C before being assayed for adhesion with or without rh eotaxin (24 nM) in 96-well plates coated with FN40 (30 μg/ml) for 15 min at 37°C. Data are presented as mean ± SD of three independent experiments, each performed in triplicate.
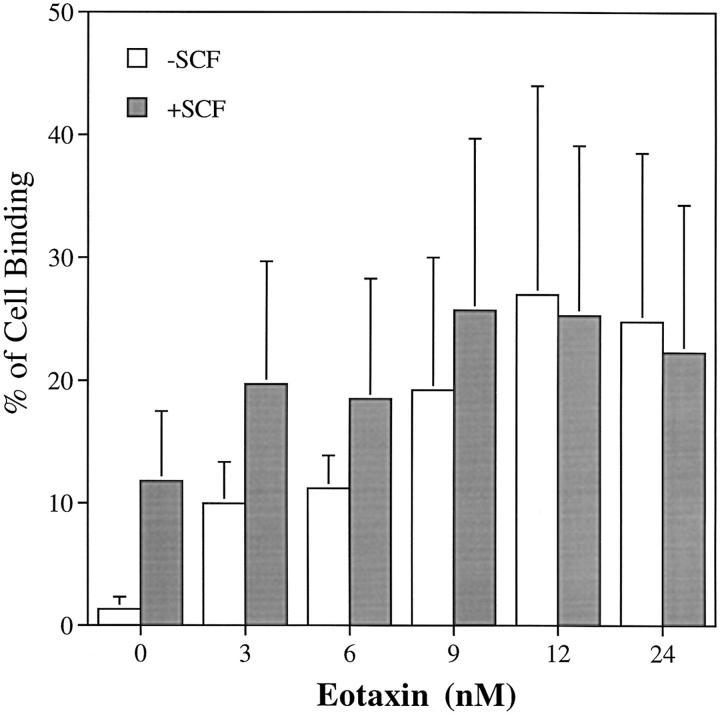
The dose-related effect of rh eotaxin on human eosinophil adhesion to FN40 (30 μg/ml) was analyzed in the absence and presence of 1.25 nM rhSCF. The approximate EC50 for rh eotaxin-augmented adhesion occurred at the lowest concentration studied, 3 nM, and the plateau was reached at 12 nM (20.8 ± 13-fold, n = 3). The effect of the concomitant presence of rhSCF at slightly less than its EC50 (see Fig. 6) was generally somewhat additive and did not extend the maximum reached with the plateau doses of rh eotaxin alone (Fig. 9).
Figure 9.
Effects of the combination of rhSCF and rh eotaxin on the adhesion of human eosinophils to FN40. Human eosinophils were assayed for adhesion to FN40 (30 μg/ml)–coated 96-well plates in the presence of increasing concentrations of rh eotaxin, either alone (□) or with a constant dose of rhSCF (25 ng/ml, 1.25 nM) (▪). Data are presented as mean ± SD of three independent experiments, each performed in triplicate.
Discussion
The finding that mature human peripheral blood eosinophils express a functional c-kit receptor for SCF reveals that a stromal ligand can activate effector cells conventionally linked to allergic inflammation. Although originally appreciated for its central role in the hematopoiesis of all lineages, SCF is elaborated in the peripheral microenvironment by diverse cell types, including endothelial cells and fibroblasts (34–37). In addition to the functional implications for eosinophil- and mast cell–directed inflammation, the study also uncovers a potential limitation to the use of c-kit detection as a marker of mature mast cells.
In the initial experiments, the surface expression of the c-kit receptor was established by cytofluorographic analysis of freshly isolated human peripheral blood eosinophils. The expression was similar among the cells of all eight donors tested (Fig. 1), irrespective of the presence of donor atopy by history. To exclude an unsuspected specificity, three mAbs directed against separate epitopes of the human c-kit were shown to yield nearly identical fluorescence signals. The positive surface c-kit expression was also demonstrated by immunohistochemical analysis of peripheral blood buffy coats (Fig. 2). Eosinophils were uniformly positive for c-kit expression and were the only cell type expressing c-kit in these preparations. The presence of c-kit was then confirmed by the functional, dose-related response of the eosinophils to SCF signal in static adhesion assays (Fig. 6). An earlier study did not detect a c-kit signal on peripheral blood eosinophils, and functional assays with SCF were not performed (64). However, this same study did demonstrate low level c-kit expression by human peripheral blood basophils and a priming effect of SCF on their IgE-dependent release of histamine (64). Because eosinophils and basophils are closely related and arise from a common progenitor (65), the expression of c-kit and their activation via SCF can now be added to the list of their shared characteristics. Moreover, a triad of hematopoietic allergic effector cells, mast cells, basophils, and eosinophils, would appear to share the expression of c-kit.
rhSCF–c-kit stimulated eosinophil adhesion to FN40 and VCAM-1, but not to laminin (Fig. 3), implying a response via an α4 integrin. Antibody-blocking studies revealed that the adhesion of eosinophils to FN40 (Fig. 5) and VCAM-1 (data not shown) was mediated by VLA-4 without the involvement of α4β7. Because the surface integrin receptor numbers were unchanged after treatment of the cells with rhSCF, including α4, α6, β1, and α4β7 (Fig. 4), the enhanced adhesiveness of VLA-4 induced by rhSCF was likely due to increased avidity of VLA-4 to its ligands. This observation is supported by an earlier study with the transformed human cell line MO7E (66). The α4β7 integrin did not contribute to the fibronectin and VCAM-1 binding, suggesting that the avidity change of VLA-4 by SCF may be conveyed through either the unique β subunit or the combined effect on the α and β heterodimer. The fact that rhSCF did not increase VLA-6 (α6β1)–mediated adhesion to laminin favors the latter hypothesis. Thus, the SCF–c-kit engagement on human peripheral blood eosinophils provides an inside–out signal to increase transiently the avidity of an integrin, VLA-4, for its cell surface and matrix ligands.
Recently, Weber and colleagues (63) demonstrated that the CC chemokines RANTES and MCP-3, as well as the anaphylatoxin C5a, rapidly increased α4 integrin avidity on eosinophils and augmented their adhesion to VCAM-1 and fibronectin with a peak at 15 min after stimulation. This result prompted us to examine the effect of another CC chemokine, eotaxin, on eosinophil adhesive function. The EC50 values of 1.25–2.5 nM for rhSCF (Fig. 6) and of 3 nM for rh eotaxin (Fig. 9) were similar with 30 μg/ml of FN40. Similarly, the magnitudes of eosinophil binding to the inputs of 3, 10, and 30 μg/ml of FN40 were similar at the plateau concentrations of rhSCF and of rh eotaxin (Fig. 7). The cell adhesion response to each stimulus (rhSCF and rh eotaxin) for the ligand FN40 depended entirely on VLA-4, as shown by mAb inhibition (Figs. 5 and 8). Stimulation of the peripheral blood eosinophils with less than the EC50 for the stromal cytokine, rhSCF, and incremental amounts of the chemokine, rh eotaxin, appeared additive, and the combination neither increased the maximal cellular response nor revealed a negative counterregulatory action for the common target, VLA-4.
Cell migration is a complex process requiring constant changes of the cell-substratum bonds with formation of new bonds (adhesion) at the leading edge of a cell and breakdown of old bonds (deadhesion) at the rear of the cell (67). This transient response is essential for adhesion to be followed by random or directed cell migration. Progressive movement within a tissue may require integrated activation of one integrin and resultant negative regulation of another integrin on the same cell as the ligand binding requirements change. For example, in Chinese hamster ovary cells, engagement of the transfected human αIIbβ3 with its specific ligand caused a transdominant inhibition of other integrins and resulted in suppressed adhesion of endogenous hamster α5β1 to fibronectin, as well as transfected human α2β1 to collagen (68). In another study, the activation of the transfected integrin αIIbβ3 in Chinese hamster ovary cells, detected by mAb PAC1 binding, was suppressed by the introduction of H-Ras and Raf-1 to these cells (69). This suppressive activity was correlated with activation of the ERK MAP kinase pathway and did not involve other dual specificity kinase sequences such as SEK–c-Jun or MKK–p38 MAP kinase (69). Thus, the activation of a particular integrin by cytokines such as SCF, which leads to an enhanced binding of that integrin to its ligand, may contribute to an integrated signal for coordinated cellular events such as cell migration.
Although found in small numbers in the circulation of healthy people, eosinophils mainly reside in the mucosal tissues in vivo, with an estimated tissue:circulation ratio of 200:1 (1). The mechanisms involving the tissue-specific localization of eosinophils are not commonly addressed, as most studies have focused on the incremental recruitment with immunologically elicited inflammation. SCF is a normal constitutive product of endothelial cells and fibroblasts and can be detected in the serum (36, 70). Therefore, it is a candidate to mediate a basal level interaction between circulating eosinophils and endothelial cells by facilitating interaction between VLA-4 and VCAM-1 at very low levels of VCAM-1 expression (71). Alternatively, exposure of eosinophils to SCF in the extracellular matrix may mediate their transient attachment to fibronectin, altering their migration speed and influencing their tissue localization. Indeed, Palecek and colleagues (72) have demonstrated that at low concentrations of ligand, cell migration speed increased as integrin binding affinity increased. Thus, SCF is a candidate for participating in the tissue distribution of eosinophils under normal physiologic conditions.
The interaction of VLA-4 and VCAM-1 plays a key role in lymphocyte and eosinophil adhesion and extravasation in both in vitro and in vivo models of allergic inflammation (18, 22–26). VCAM-1 expression is upregulated in the eosinophil-rich, inflamed airway tissue of individuals with asthma (73, 74). VLA-4 is expressed on eosinophils and lymphocytes, but not neutrophils; and the c-kit receptor expression is limited to eosinophils among these three cell types. Thus, under conditions of allergic inflammation, local SCF could contribute both by priming mast cells to elaborate IL-4 (46, 75) and TNF-α (75) with consequent upregulation of endothelial cell VCAM-1 expression (76), and by mediating a transiently increased avidity for that ligand by eosinophil VLA-4. This possibility is supported by a recent finding in mice of the association of allergen challenge-induced eosinophilic airways inflammation with increased levels of histamine and SCF in bronchoalveolar lavage fluid and of SCF in serum (70). In that study, the administration of antibody to SCF before allergen challenge markedly decreased histamine release and pulmonary eosinophil infiltration. The inflammatory response induced by allergen challenge also increases the production of the eosinophil-selective chemoattractant, eotaxin (61), which is a product of a number of cell types including epithelial cells, endothelial cells, fibroblasts, and even eosinophils (62). Therefore, increased concentrations of eotaxin and SCF could provide a coordinated signal for the selective recruitment of eosinophils, beginning with transiently enhanced eosinophil adhesion to endothelial cells within the vasculature through VLA-4–VCAM-1 interaction, followed by directed tissue movement through adhesion/deadhesion in a chemotactic gradient, and final eosinophil tissue retention through regulated integrin–matrix interaction. Such a matrix interaction may also prolong eosinophil survival through autocrine production of IL-3 and GM-CSF initiated by VLA-4–fibronectin interaction (28).
We have provided evidence that SCF is an agonist for eosinophil adhesion. These findings have potential relevance for physiologic eosinophil trafficking and for allergic inflammation, in which mast cell activation and eosinophil accumulation are both prominent features.
Acknowledgments
This work was supported by National Institutes of Health grants AI-22531, AI-31599, AI-01304, and HL-36110. Q. Yuan is the recipient of a research fellowship from the Charles A. King Trust and The Medical Foundation.
Footnotes
1 Abbreviations used in this paper: BCECF-AM, 2′,7′-bis-(2-carboxyethyl)- 5-(and 6)-carboxyfluorescein acetoxymethyl ester; BMMC, bone marrow–derived mast cells; CS-1, connecting segment-1; HSA, human serum albumin; MACS, magnetic cell separation; MFI, mean fluorescence intensity; rh, recombinant human; SCF, stem cell factor; VCAM, vascular cell adhesion molecule; VLA, very late antigen.
References
- 1.Resnick MB, Weller PF. Mechanisms of eosinophil recruitment. Am J Respir Cell Mol Biol. 1993;8:349–355. doi: 10.1165/ajrcmb/8.4.349. [DOI] [PubMed] [Google Scholar]
- 2.Spry CJ. Mechanisms of eosinophilia. V. Kinetics of normal and accelerated eosinopoiesis. Cell Tissue Kin. 1971;4:351–364. [PubMed] [Google Scholar]
- 3.Butterworth AE, Sturrock RF, Houba V, Rees PH. Antibody-dependent cell-mediated damage to schistosomula in vitro. Nature (Lond) 1974;252:503–505. doi: 10.1038/252503a0. [DOI] [PubMed] [Google Scholar]
- 4.Kipnis TL, James SL, Sher A, David JR. Cell-mediated cytotoxicity by Trypanosoma cruzi. II. Antibody-dependent killing of bloodstream forms by mouse eosinophils and neutrophils. Am J Trop Med Hyg. 1981;30:47–53. [PubMed] [Google Scholar]
- 5.Folkard SG, Hogarth PJ, Taylor MJ, Bianco AE. Eosinophils are the major effector cells of immunity to microfilariae in a mouse model of onchocerciasis. Parasitology. 1996;112:323–329. doi: 10.1017/s0031182000065847. [DOI] [PubMed] [Google Scholar]
- 6.Rotman HL, Yutanawiboonchai W, Brigandi RA, Leon O, Gleich GJ, Nolan TJ, Schad GA, Abraham D. Strongyloides stercoralis: eosinophil-dependent immune-mediated killing of third stage larvae in BALB/cByJ mice. Exp Parasitol. 1996;82:267–278. doi: 10.1006/expr.1996.0034. [DOI] [PubMed] [Google Scholar]
- 7.Enblad G, Sundstrom C, Glimelius B. Infiltration of eosinophils in Hodgkin's disease involved lymph nodes predicts prognosis. Hematol Oncol. 1993;11:187–193. doi: 10.1002/hon.2900110404. [DOI] [PubMed] [Google Scholar]
- 8.Ownby HE, Roi LD, Isenberg RR, Brennan MJ. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer. 1983;52:126–130. doi: 10.1002/1097-0142(19830701)52:1<126::aid-cncr2820520123>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Rivoltini L, Viggiano V, Spinazze S, Santoro A, Colombo MP, Takatsu K, Parmiani G. In vitro anti-tumor activity of eosinophils from cancer patients treated with subcutaneous administration of interleukin 2. Int J Cancer. 1993;54:8–15. doi: 10.1002/ijc.2910540103. [DOI] [PubMed] [Google Scholar]
- 10.Todd R, Donoff BR, Chiang T, Chou MY, Elovic A, Gallagher GT, Wong DT. The eosinophil as a cellular source of transforming growth factor alpha in healing cutaneous wounds. Am J Pathol. 1991;138:1307–1313. [PMC free article] [PubMed] [Google Scholar]
- 11.Wong DT, Donoff RB, Yang J, Song SZ, Matossian K, Nagura N, Elovic A, McBride J, Gallagher G, Todd R, et al. Sequential expression of transforming growth factors α and β 1 by eosinophils during cutaneous wound healing in the hamster. Am J Pathol. 1993;143:130–142. [PMC free article] [PubMed] [Google Scholar]
- 12.Frigas E, Loergering DA, Gleich GJ. Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal epithelium. Lab Invest. 1980;42:35–43. [PubMed] [Google Scholar]
- 13.Owen WF, Jr, Soberman RJ, Yoshimoto T, Sheffer AL, Lewis RA, Austen KF. Synthesis and release of leukotriene C4by human eosinophils. J Immunol. 1987;138:532–538. [PubMed] [Google Scholar]
- 14.Bentley AM, Menz G, Storz C, Robinson DS, Bradley B, Jeffery PK, Durham SR, Kay AB. Identification of T lymphocytes, macrophages and activated eosinophils in the bronchial mucosa in intrinsic asthma. Relationship to symptoms and bronchial hyperresponsiveness. Am Rev Respir Dis. 1992;146:500–506. doi: 10.1164/ajrccm/146.2.500. [DOI] [PubMed] [Google Scholar]
- 15.Walsh GM, Williamson ML, Symon FA, Willars GB, Wardlaw AJ. Ligation of CD69 induces apoptosis and cell death in human eosinophils cultured with granulocyte–macrophage colony-stimulating factor. Blood. 1996;87:2815–2821. [PubMed] [Google Scholar]
- 16.Rothenberg ME, Petersen J, Stevens RL, Silberstein DS, McKenzie DT, Austen KF, Owen WF. IL-5–dependent conversion of normodense eosinophils to the hypodense phenotype uses 3T3 fibroblasts for enhanced viability, accelerated hypodensity, and sustained antibody-dependent cytotoxicity. J Immunol. 1989;143:2311–2316. [PubMed] [Google Scholar]
- 17.Owen WF, Rothenberg ME, Silberstein DS, Gasson JC, Stevens RL, Austen KF, Soberman RJ. Regulation of human eosinophil viability, density, and function by granulocyte/macrophage colony-stimulating factor in the presence of 3T3 fibroblasts. J Exp Med. 1987;166:129–141. doi: 10.1084/jem.166.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bochner BS, Luscinaskas FW, Gimbrone MA, Jr, Newman W, Sterbinsky SA, Derse-Anthony CP, Klunk D, Schleimer RP. Adhesion of human basophils and eosinophils to IL-1 activated human vascular endothelial cells: contribution of endothelial cell adhesion molecules. J Exp Med. 1991;173:1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin α4β7, on human leukocytes. J Immunol. 1994;153:517–528. [PubMed] [Google Scholar]
- 20.Georas SN, McIntyre BW, Ebisawa M, Bednarczyk JL, Sterbinsky SA, Schleimer RP, Bochner BS. Expression of a functional laminin receptor α6β1 (very late antigen 6) on human eosinophils. Blood. 1993;82:2872–2879. [PubMed] [Google Scholar]
- 21.Elices MJ, Osborn L, Takada Y, Crouse C, Lubowsky S, Hemler ME, Lobb RR. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 22.Lobb RR, Hemler ME. The pathophysiologic role of α4 integrins in vivo. J Clin Invest. 1994;94:1722–1728. doi: 10.1172/JCI117519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weg VB, Williams TJ, Lobb RR, Nourshargh S. A monoclonal antibody recognizing very late activation antigen-4 inhibits eosinophil accumulation in vivo. J Exp Med. 1993;177:561–566. doi: 10.1084/jem.177.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. Role of vascular cell adhesion molecule 1/ very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function–associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into tissue. J Exp Med. 1994;179:1145–1154. doi: 10.1084/jem.179.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pretolani MC, Ruffie C, Lapa e Silva J-R, Joseph D, Lobb RR, Vargaftig B. Antibody against very late antigen-4 prevents antigen-induced bronchial hyperreactivity and cellular infiltration in guinea-pig airways. J Exp Med. 1994;180:795–805. doi: 10.1084/jem.180.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abraham WM, Sielczak MW, Ahmed A, Cortes A, Lauredo IT, Kim J, Pepinsky B, Benjamin CD, Leone DR, Lobb RR, Weller PF. α4-integrins mediate antigen-induced late bronchial responses and prolonged airway hyperresponsiveness in sheep. J Clin Invest. 1994;93:776–787. doi: 10.1172/JCI117032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan JL, Hynes RO. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor α4β1. Cell. 1990;60:53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- 28.Anwar ARF, Moqbel R, Walsh GM, Kay AB, Wardlaw AJ. Adhesion to fibronectin prolongs eosinophil survival. J Exp Med. 1993;177:839–843. doi: 10.1084/jem.177.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohno I, Lea R, Finotto S, Marshall J, Denburg J, Dolovich J, Gauldie J, Jordana M. Granulocyte/macrophage colony-stimulating factor (GM-CSF) gene expression by eosinophils in nasal polyposis. Am J Respir Cell Mol Biol. 1991;5:505–510. doi: 10.1165/ajrcmb/5.6.505. [DOI] [PubMed] [Google Scholar]
- 30.Broide DH, Paine M, Firestein GS. Eosinophils express interleukin 5 and granulocyte–macrophage colony-stimulating factor mRNA at sites of allergic inflammation. J Clin Invest. 1992;90:1414–1424. doi: 10.1172/JCI116008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell ES. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- 32.Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol. 1989;7:59–76. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- 33.Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P, Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, WV, W41 and W. . EMBO (Eur Mol Biol Organ) J. 1990;9:1805–1813. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu R-Y, Birkett NC, Okino KH, Murdock DC, et al. Stem cell factor is encoded at the sl locus of the mouse and is the ligand for the c-kittyrosine receptor. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- 35.Anderson DM, Lyman SD, Baird A, Wignall JM, Eisenman J, Rauch C, March CJ, Boswell HS, Gimpel SD, Cosman D, Williams SE. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane and soluble forms. Cell. 1990;63:235–243. doi: 10.1016/0092-8674(90)90304-w. [DOI] [PubMed] [Google Scholar]
- 36.Langley KE, Bennet LG, Wypych J, Yancik SA, Liu X-D, Westcott KR, Chang DG, Smith KA, Zsebo KM. Soluble stem cell factor in human serum. Blood. 1993;81:656–660. [PubMed] [Google Scholar]
- 37.Heinrich MC, Dooley DC, Freed AC, Band L, Hoatlin ME, Keeble WW, Peters ST, Silvey KS, Ey FS, Kabat D, Maziarz RT, Bagby GC., Jr Constitutive expression of steel factor gene by human stromal cells. Blood. 1993;82:771–783. [PubMed] [Google Scholar]
- 38.Ogawa M, Matsuzaki Y, Nishikawa S, Hayashi S-I, Kunisada T, Sudo T, Kina T, Nakauchi H, Nishikawa S-I. Expression and function of c-kitin hemopoietic progenitor cells. J Exp Med. 1991;174:63–71. doi: 10.1084/jem.174.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Copeland NG, Gilbert DJ, Cho BC, Donovan PJ, Jenkins NA, Cosman D, Anderson D, Lyman SD, Williams DE. Mast cell growth factor maps near the steel locus on mouse chromosome 10 and is deleted in a number of steel alleles. Cell. 1990;63:175–183. doi: 10.1016/0092-8674(90)90298-s. [DOI] [PubMed] [Google Scholar]
- 40.Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, Geissler EN, Galli SJ. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat-kit ligand, stem cell factor. Proc Natl Acad Sci USA. 1991;88:6382–6386. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murakami M, Austen KF, Bingham CO, III, Friend DS, Penrose JF, Arm JP. Interleukin-3 regulates development of the 5-lypoxygenase/leukotriene C4synthase pathway in mouse mast cells. J Biol Chem. 1995;270:22653–22656. doi: 10.1074/jbc.270.39.22653. [DOI] [PubMed] [Google Scholar]
- 42.Tsuji K, Koike K, Komiyama A, Miyajima A, Arai K, Nakahata T. Synergistic action of interleukin-10 (IL-10) with IL-3, IL-4 and stem cell factor on colony formation from murine mast cells in culture. Int J Hematol. 1995;61:51–60. doi: 10.1016/0925-5710(95)00351-r. [DOI] [PubMed] [Google Scholar]
- 43.Saito H, Ebisawa M, Tachimoto H, Shichijo M, Fukagawa K, Matsumoto K, Likura Y, Awaji T, Tsujimoto G, Yanagida M, et al. Selective growth of human mast cells induced by steel factor, IL-6, and prostaglandin E2from cord blood mononuclear cells. J Immunol. 1996;157:343–350. [PubMed] [Google Scholar]
- 44.Mitsui H, Furitsu T, Dvorak AM, Irani AMA, Schwartz LB, Inagaki N, Takei M, Ishizaka K, Zsebo KM, Gills S, Ishizaka T. Development of human mast cells from umbilical cord blood cells by recombinant human and murine c-kitligand. Proc Natl Acad Sci USA. 1993;90:735–739. doi: 10.1073/pnas.90.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bischoff SC, Dahinden CA. c-kit ligand: a unique potentiator of mediator release by human lung mast cells. J Exp Med. 1992;175:237–244. doi: 10.1084/jem.175.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okayama Y, Petit-Frere C, Kassel O, Semper A, Quint D, Tunon-de-Lara MJ, Bradding P, Holgate ST, Church MK. IgE-dependent expression of mRNA for IL-4 and IL-5 in human lung mast cells. J Immunol. 1995;155:1796–1808. [PubMed] [Google Scholar]
- 47.Murakami M, Matsumoto R, Urade Y, Austen KF, Arm JP. c-kitligand mediates increased expression of cytosolic phospholipase A2, prostaglandin endoperoxidase synthase-1, and hematopoietic prostaglandin D2 synthase and increased IgE-dependent prostaglandin D2 generation in immature mouse mast cells. J Biol Chem. 1995;270:3239–3246. doi: 10.1074/jbc.270.7.3239. [DOI] [PubMed] [Google Scholar]
- 48.Murakami M, Austen KF, Arm JP. The immediate phase of c-kit ligand stimulation of mouse bone marrow–derived mast cells elicits rapid leukotriene C4 generation through posttranslational activation of cytosolic phospholipase A2 and 5-lipoxygenase. J Exp Med. 1995;182:197–206. doi: 10.1084/jem.182.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dastych J, Metcalfe DD. Stem cell factor induces mast cell adhesion to fibronectin. J Immunol. 1994;152:213–219. [PubMed] [Google Scholar]
- 50.Kinashi T, Springer TA. Steel factor and c-kit regulate cell-matrix adhesion. Blood. 1994;83:1033–1038. [PubMed] [Google Scholar]
- 51.Kinashi T, Escobedo JA, Williams LT, Takatsu K, Springer TA. Receptor tyrosine kinase stimulates cell-matrix adhesion by phosphatidylinositol 3 kinase and phospholipase C-γ1 pathways. Blood. 1995;86:2086–2090. [PubMed] [Google Scholar]
- 52.Yuan Q, Strauch KL, Lobb RR, Hemler ME. Intracellular single-chain antibody inhibits integrin VLA-4 maturation and function. Biochem J. 1996;318:591–596. doi: 10.1042/bj3180591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hemler ME, Huang C, Takada Y, Schwartz L, Strominger JL, Clabby ML. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem. 1987;262:11478–11485. [PubMed] [Google Scholar]
- 54.Kennel SJ, Epler RG, Lankford TK, Foote LT, Dickas V, Canamucio M, Cavalierie R, Cosimelli M, Venturo I, Falcioni R, Sacchi A. Second generation monoclonal antibodies to the human integrin α6β4. Hybridoma. 1990;9:243–255. doi: 10.1089/hyb.1990.9.243. [DOI] [PubMed] [Google Scholar]
- 55.Hemler ME, Ware CF, Strominger JL. Characterization of a novel differentiation antigen complex recognized by a monoclonal antibody (A-1A5): unique activation-specific molecular forms on stimulated T cells. J Immunol. 1983;131:334–340. [PubMed] [Google Scholar]
- 56.Lazarovits AI, Moscicki RA, Kurnick JT, Camerini D, Bhan AK, Baird LG, Erikson M, Colvin RB. Lymphocyte activation antigens. I. A monoclonal antibody, anti-Act I, defines a new late lymphocyte activation antigen. J Immunol. 1984;133:1857–1862. [PubMed] [Google Scholar]
- 57.Lemke H, Hammerling GJ, Hohmann C, Rajewsky K. Hybrid cell lines secreting monoclonal antibody specific for major histocompatibility antigens of the mouse . Nature (Lond) 1978;271:249–251. doi: 10.1038/271249a0. [DOI] [PubMed] [Google Scholar]
- 58.Broudy VC, Lin N, Zsebo KM, Birkett NC, Smith KA, Bernstein ID, Papayannopoulou T. Isolation and characterization of a monoclonal antibody that recognizes the human c-kit receptor. Blood. 1992;79:338–346. [PubMed] [Google Scholar]
- 59.Hansel TT, De Vries IJ, Iff T, Rihs S, Wandzilak M, Betz S, Blaser K, Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Methods. 1991;145:105–110. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- 60.Buhring, H.-J., L.K. Ashman, V. Gattei, B. Kniep, A. Larregina, A. Pinto, P. Valent, and J.J. van den Oord. 1995. Stem-cell factor receptor (p1459 (c-kit)) summary report (CD117). In Leucocyte Typing V: White Cell Differentiation Antigens. S.F. Schlossman, L. Boumsell, W. Gilks, J.M. Harlan, T. Kishimoto, C. Morimoto, J. Ritz, S. Shaw, R. Silverstein, T. Springer, T.F. Tedder, and R.F. Todd, editors. Oxford University Press, Oxford. 1882–1888.
- 61.Jose PJ, Griffiths-Johnson DA, Collins PD, Walsh DT, Moqbel R, Totty NF, Truong O, Hsuan JJ, Williams TJ. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of airways inflammation. J Exp Med. 1994;179:881–887. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam N, Smith H, Shi X, Gonzalo JA, Newman W, et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–612. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weber C, Kitayama J, Springer TA. Differential regulation of β1 and β2 integrin avidity by chemoattractants in eosinophils. Proc Natl Acad Sci USA. 1996;93:10939–10944. doi: 10.1073/pnas.93.20.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Columbo M, Horowitz EM, Botana LM, MacGlashan DW, Jr, Bochner BS, Gillis S, Zsebo KM, Galli SJ, Lichtenstein LM. The human recombinant c-kitreceptor ligand, rhSCF, induces mediator release from human cutaneous mast cells and enhances IgE-dependent mediator release from both skin mast cells and peripheral blood basophils. J Immunol. 1992;149:599–608. [PubMed] [Google Scholar]
- 65.Boyce JA, Friend D, Matsumoto R, Austen KF, Owen WF. Differentiation in vitro of hybrid eosinophil/basophil granulocytes: autocrine function of an eosinophil developmental intermediate. J Exp Med. 1995;182:49–57. doi: 10.1084/jem.182.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kovach NL, Lin N, Yednock T, Harlan JM, Broudy VC. Stem cell factor modulates avidity of α4β1 and α5β1 integrins expressed on hematopoietic cell lines. Blood. 1995;85:159–167. [PubMed] [Google Scholar]
- 67.Lauffenburger DA. Models for receptor-mediated cell phenomena: adhesion and migration. Annu Rev Biophys Biophys Chem. 1991;20:387–414. doi: 10.1146/annurev.bb.20.060191.002131. [DOI] [PubMed] [Google Scholar]
- 68.Diaz-Gonzalez F, Forsyth J, Steiner B, Ginsberg MH. Transdominant inhibition of integrin function. Mol Biol Cell. 1996;7:1939–1951. doi: 10.1091/mbc.7.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hughes PE, Renshaw MW, Pfaff M, Forsyth J, Keivens VM, Schwartz MA, Ginsberg MH. Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell. 1997;88:521–530. doi: 10.1016/s0092-8674(00)81892-9. [DOI] [PubMed] [Google Scholar]
- 70.Lukacs NW, Strieter RM, Lincoln PM, Brownell E, Pullen DM, Schock HJ, Chensue SW, Taub DD, Kunkel SL. Stem cell factor (c-kit ligand) influences eosinophil recruitment and histamine levels in allergic airway inflammation. J Immunol. 1996;156:3945–3951. [PubMed] [Google Scholar]
- 71.Bevilacqua MP. Endothelial–leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 72.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz Af. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature (Lond) 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 73.Bentley AM, Durham SR, Robinson DS, Menz G, Storz C, Cromwell O, Kay AB, Wardlaw AJ. Expression of endothelial cell and leukocyte adhesion molecules, intercellular adhesion molecule-1, E-selectin and vascular cell adhesion molecule-1 in the bronchial mucosa in steady state and allergen-induced asthma. J Allergy Clin Immunol. 1993;92:857–868. doi: 10.1016/0091-6749(93)90064-m. [DOI] [PubMed] [Google Scholar]
- 74.Ohkawara Y, Yamauchi K, Maruyama N, Hoshi H, Ohno I, Honma M, Tanno Y, Tamura G, Shirato K, Ohtani H. In situ expression of the cell adhesion molecules in bronchial tissues from asthmatics with air flow limitation: in vivo evidence of VCAM-1/VLA-4 interaction in selective eosinophil infiltration. Am J Respir Cell Mol Biol. 1995;12:4–12. doi: 10.1165/ajrcmb.12.1.7529029. [DOI] [PubMed] [Google Scholar]
- 75.Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH, Holgate ST. Interleukin-4, interleukin-5, and interleukin-6 and tumor necrosis factor-α in normal and asthmatic airways—evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994;10:471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- 76.Iademarco MF, Barks JL, Dean DC. Regulation of vascular cell adhesion molecule-1 expression by IL-4 and TNF-α in cultured endothelial cells. J Clin Invest. 1995;95:264–271. doi: 10.1172/JCI117650. [DOI] [PMC free article] [PubMed] [Google Scholar]