Abstract
T lymphocytes play a pivotal role in the immune response during viral infections. In a murine model of experimental respiratory syncytial virus (RSV) infection, mice sensitized to either of the two major glycoproteins of RSV develop distinct patterns of cytokine secretion and lung inflammation upon subsequent RSV infection. Mice sensitized to RSV-G (attachment) glycoprotein exhibit a strong interleukin (IL)-4 and IL-5 response and develop pulmonary eosinophilia, whereas mice sensitized to RSV-F (fusion) glycoprotein develop a predominantly T helper cell (Th)1 response and pulmonary inflammation characterized by mononuclear cell infiltration. In this study, we examined the potential role of virus-specific CD8+ T cytolytic T cells on the differentiation and activation of functionally distinct CD4+ T cells specific to these viral glycoproteins. Mice primed with recombinant vaccinia virus expressing RSV-F glycoprotein mounted a strong RSV-specific, MHC class I–restricted cytolytic response, whereas priming with recombinant vaccinia virus expressing RSV-G glycoprotein failed to elicit any detectable cytolytic response. Priming for a RSV-specific CD8+ T cell response, either with a recombinant vaccinia virus expressing RSV-G glycoprotein in which a strong CD8+ T cell epitope from RSV-M2 (matrix) protein has been inserted or with a combination of vaccinia virus expressing the matrix protein and the RSV-G glycoprotein, suppressed the eosinophil recruitment into the lungs of these mice upon subsequent challenge with RSV. This reduction in pulmonary eosinophilia correlated with the suppression of Th2 type cytokine production. The importance of CD8+ T cells in this process was further supported by the results in CD8+ T cell deficient, β2 microglobulin KO mice. In these mice, priming to RSV-F glycoprotein (which in normal mice primed for a strong cytolytic response and a pulmonary infiltrate consisting primarily of mononuclear cells on RSV challenge) resulted in the development of marked pulmonary eosinophilia that was not seen in mice with an intact CD8+ T cell compartment. These results indicate that CD8+ T cells may play an important role in the regulation of the differentiation and activation of Th2 CD4+ T cells as well as the recruitment of eosinophils into the lungs during RSV infection.
Multiple arms of the immune system are activated in response to infection by foreign organisms. The outcomes of the infection, such as the clearance of the organisms and the generation of tissue injury, depend on these immune effector mechanisms that are mobilized against the pathogen. The role of T cells in the immune response to viral infections has been clearly established (1, 2). Mature T cells that express α/β T cell receptors can be divided into cells expressing either CD4 or CD8 surface antigen. CD8+ T cells recognize processed antigen in the context of MHC class I molecules, whereas CD4+ T cells recognize peptides in the context of MHC class II molecules (3, 4). The conventional view of CD8+ T cells has been primarily the killing of virally infected cells by direct cytolysis or by cytokines secreted by these T cells such as IFN-γ and TNF (1, 2). Functional subsets of CD4+ T cells have been described based on the cytokines produced by these cells, Th1 CD4+ T cells that secrete IL-2 and IFN-γ and Th2 T cells that secrete IL-4 and IL-5 (5, 6). The physiological relevance of these subsets of T cells with diverse functions have been shown in several models of viral infection including respiratory syncytial virus (RSV)1.
In the murine model of RSV infection, the roles of T cells and their products in the eradication of the virus as well as the pathogenesis of lung inflammation have been well documented (7–12). Recent studies have shown that mice sensitized to either of the two major glycoproteins of this virus developed distinct lung pathology when subsequently infected with RSV (12–15). Mice sensitized to the attachment glycoprotein (G) of RSV developed pulmonary eosinophilia that correlated with a strong induction of Th2 type response, whereas mice primed to the fusion (F) glycoprotein mounted a weak Th2 response and developed lung inflammation characterized by mononuclear cell infiltration. The underlying mechanisms that lead to these apparent distinct patterns of cytokine production and lung injury still remain unclear.
The process by which CD4+ T cells mature and differentiate has been the subject of intense investigation (16). CD4+ T cells can be induced to differentiate into effector cells of either the Th1 or Th2 subsets, depending on the milieu in which these cells are activated. Cytokines such as IL-4 and IL-12 have been shown to play a crucial part in the regulation of CD4+ T cell differentiation (17–19). The presence of certain immune effector functions during the differentiation and expansion of CD4+ T cells may potentially be a key factor in determining the functional phenotypes acquired by these cells. In the murine model of experimental RSV infection, one important difference in the immune response to F and G glycoprotein of RSV is the lack of MHC class I–restricted cytolytic response to the G glycoprotein (13, 20). In the following studies, we began to investigate the impact of CD8+ T cells and MHC class I–restricted CTL responses on cytokine secretion and the subsequent development of pulmonary eosinophilia during experimental murine RSV infection. We have found that the induction of CD8+ T cell response to RSV proteins resulted in dramatically decreased levels of Th2 type cytokines and eosinophil recruitment into the lungs of RSV-infected mice previously sensitized to the RSV-G glycoprotein. The possible mechanisms underlying these observations and their potential significance are discussed.
Materials and Methods
Mice.
Female BALB/c (H-2d) mice age 8–12 wk old were purchased from Taconic Farms Inc. (Germantown, NY). Mice with disrupted β2 microglobulin (β2m) genes were bred from stock initially provided by T. Hansen (Washington University, St. Louis, MO). The generation and characterization of these mice have been described previously (21). These mice were backcrossed into the BALB/c background, and were screened for the absence of MHC class I expression. They were homozygous for the H-2d haplotype at the MHC class II locus as judged by the absence of the expression of I-Ab and the presence of I-Ad molecules detected by flow cytometric analysis of spleen cells. These mice were maintained in pathogen-free conditions.
Virus and Infection of Mice.
Recombinant vaccinia virus expressing RSV fusion glycoprotein (VF) and RSV attachment glycoprotein (VG) were obtained from J.L. Beeler (Federal Drug Administration, National Institutes of Health). The generation and characterization of these virus have been previously described (22, 23). Recombinant vaccinia viruses expressing only β-galactosidase (VSC11) insertion vector was used as a control. RSV (A2 strain) was a gift from P.L. Collins (National Institute of Allergy and Infectious Diseases, National Institutes of Health). RSV was grown on HEp-2 cells and plaque purified. The virus stock was grown in HEp-2 cells and titered for infectivity. Mice were infected with 3 × 106 PFU of recombinant vaccinia virus by scarification at the base of tail. In experiments where mice were primed with two recombinant vaccinia viruses, equal numbers (3 × 106 PFU) of each recombinant virus were used to prime the animals. In some experiments, the mice were given 106 PFU of RSV in 50 μl inoculum intranasally 3 wk after priming and killed 5 d later. Cells were isolated from the lungs for in vitro culture. Lung tissue was prepared for histopathology.
Construction of Recombinant Vaccinia Virus.
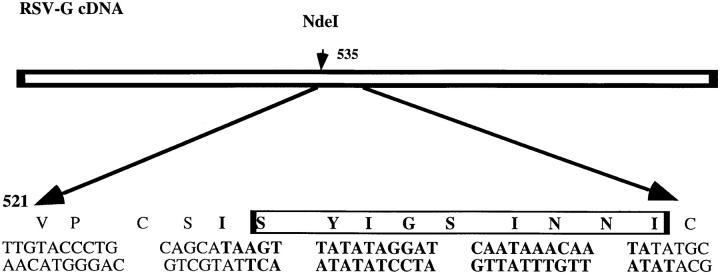
PGEM3 plasmid vector containing the cDNA for RSV-G glycoprotein was a gift from P.L. Collins (National Institute of Allergy and Infectious Diseases, National Institutes of Health). Synthetic double-stranded oligonucleotides encoding the immunodominant epitope from the RSV-M2 protein (amino acid residues 82–90; 24) were made (Biomolecular Research Facility, University of Virginia, Charlottesville, VA). The oligonucleotides were inserted into the NdeI site of cDNA encoding the RSV-G protein (Fig. 1). The correct sequence and the orientation of the inserted nucleotide were confirmed by sequencing. The portion of the cDNA encoding the G protein with inserted 22K epitope (G22K) was then cloned into a modified version of PSC11 plasmid vector. The recombinant vaccinia virus expressing this protein (VG22K) was then generated by a method previously described (22).
Figure 1.
Construction of cDNA encoding RSV-G glycoprotein containing a CTL epitope of RSV-M2(22K) protein. The indicated oligonucleotide heteroduplex (bold) was introduced into the NdeI site at base 535 of the RSV-G cDNA. The synthetic oligonucleotide encodes the nine residues SYIGSINNI from amino acids 82–90 of the RSV-M2(22K) matrix protein.
Histopathology.
Lungs of mice were harvested and fixed in 10% formalin in PBS. The specimens were processed, embedded, and sectioned by American Histolabs Inc. (Gaithersburg, MD). Sections of the lungs were prepared and stained for eosinophils by the Leinert-Giemsa technique. To compare the degree of tissue eosinophilia in the sections, we enumerated cells with characteristic eosinophil staining in and around blood vessel walls. The length of individual vessels was estimated using a micrometer attached to the eyepiece of microscope. The results were expressed as number of eosinophils present per millimeter of blood vessel.
Lymphocyte Culture.
Cells were isolated from lung tissue by collagenase digestion as previously described (25). In brief, lungs were minced in Iscove's media (GIBCO BRL, Gaithersburg, MD) containing 10% fetal calf serum and incubated with collagenase D (Boehringer Mannheim, Indianapolis, IN) at final concentration of 0.7 mg/ml. The digestion was done at 37°C for 90 min. The digested lung tissue was tapped through a wire screen. Particulate matter was removed by a quick centrifugation at 1,000 rpm. The cell suspension was layered over a Ficoll-Hypaque density gradient, (Lymphocyte-M; Cedarlane Labs. Ltd., Ontario, Canada) and centrifuged at 400 g for 15 min. The mononuclear cells at the interface were isolated and used in in vitro culture. Single cell suspensions were isolated from a spleen by grinding the spleen through a wire screen followed by a quick centrifugation. Cells were cultured at indicated cell numbers with irradiated naive spleen cells infected with RSV at a multiplicity of infection of 0.1. The ratio of responders to stimulators was, in general, 5:1.
Detection of Cytokines in Culture Supernatants.
Supernatants from in vitro culture of cells isolated from spleens or lungs were collected at 48 h after stimulation and kept at −70°C until analyzed. The concentrations of IL-2, IL-4, IL-5, and IFN-γ in these supernatants were measured using commercial ELISA reagents under conditions recommended by the manufacturer (PharMingen, San Diego, CA).
Assays for Cell-mediated Cytotoxicity.
The 51Cr–release cytotoxicity assay was performed as previously described (2). Mastocytoma cells (P815) expressing compatible MHC class I (H-2d) were used for these assays as targets. Target cells either uninfected or infected with indicated virus were incubated with 51Cr overnight at room temperature, and then washed twice and plated at 5 × 103 cells/well in a 96-well, flat-bottom, tissue culture plate. Effector cells were added at variable cell number to appropriate wells in quadruplicate. The plates were incubated at 37°C in 10% CO2 for 6 h. 100 μl of supernatant was harvested from each well and counted on a gamma counter (Isomedic; ICN Biomedicals, Inc., Costa Mesa, CA). The percent lysis was calculated as previously described (2).
Results
Cytolytic Activity of RSV-F– and RSV-G–specific Spleen Cells.
In previous reports, we and others have shown that in BALB/c mice, the cytokine response of CD4+ T lymphocyte primed to the two major glycoproteins of RSV are distinct (12–14). Mice primed with a recombinant vaccinia virus expressing the RSV-G glycoprotein mounted a strong Th2-like cytokine response and developed lung inflammation characterized by intense eosinophil infiltration upon challenge with infectious RSV. On the other hand, mice sensitized to the RSV-F glycoprotein mounted a Th1-like cytokine response and developed lung inflammation consisting of mononuclear cell infiltrates with few eosinophils after RSV challenge (12, 14).
To study the mechanisms underlying this difference in responsiveness of immune CD4+ T cells, we evaluated aspects of the immune response to these two proteins that could potentially influence the cytokine response of G- or F-specific CD4+ T cells and the type of pulmonary pathology observed after RSV infection. An important difference in the immune response to these two glycoproteins lies in the ability of these proteins to elicit an MHC class I–restricted cytolytic response. Previous studies in the mouse (13, 20) indicated that RSV-G does not induce a MHC class I–restricted cytolytic response either after RSV infection or after priming with VG, whereas a vigorous CTL response to RSV-F is observed after RSV infection or VF immunization. To verify this, we examined the induction of cytolytic activity in immune splenocyte cultures obtained from BALB/c mice primed with recombinant vaccinia virus expressing either RSV-F or RSV-G glycoprotein and restimulated in vitro with live RSV.
As reported previously (13), immune splenocytes from BALB/c mice primed with VF exhibited cytolytic activity on histocompatible RSV-F–expressing targets when tested 5 d after in vitro stimulation with RSV-infected stimulators. Immune splenocyte cultures from VG-primed mice, on the other hand, exhibited no specific cytolytic activity on RSV-G–expressing target cells over a range of effector to target ratios (Fig. 2 A). Target cells infected with a control vaccinia virus (VSCll) or uninfected target cells (not shown) were not lysed by either immune cell population (Fig. 2 A).
Figure 2.
RSV-specific (A) and vaccinia virus–specific (B) cytolytic responses in mice primed with recombinant vaccinia virus expressing RSV-F (VF) or RSV-G (VG) glycoprotein. Splenocytes from mice previously primed with VF or VG were stimulated in vitro with RSV-infected naive spleen cells (A) or spleen cells infected with recombinant vaccinia virus not expressing RSV antigen (B). RSV-specific and vaccinia-specific cytolytic activity of these bulk cultures against target cells uninfected or infected with indicated recombinant vaccinia virus were analyzed in a standard 51Cr assay.
One explanation for the lack of a memory CD8+ CTL response to RSV-G is that the expression of G glycoprotein in vaccinia virus–infected cells inhibits vaccinia replication and thereby leads to inefficient priming of either RSV-G or vaccinia-specific memory CD8+ T lymphocytes. To address this possibility, the immune splenocytes from the VG- or VF-primed mice examined above (Fig. 2 A) were also restimulated in parallel with a control vaccinia virus (VSCll) expressing no RSV gene products to assess the capacity of the two recombinant vaccinia viruses to prime for an in vitro secondary vaccinia-specific CTL response. As Fig. 2 B shows, the magnitude of the vaccinia-specific CTL response in immune splenocytes from VF- and VG-primed mice is comparable. This finding indicates that the expression of RSV-G in vaccinia-infected antigen presenting cells did not affect the processing of vaccinia epitopes and their presentation by MHC class I molecules or the priming of vaccinia-specific memory CD8+ T lymphocytes.
CD8+ Cytolytic T Lymphocyte Recognition of a Chimeric RSV-G Glycoprotein/22K Matrix Protein Product.
If the induction of a Th2-like CD4+ T cell response and pulmonary eosinophilia after RSV challenge were linked to the failure of G glycoprotein to prime a CD8+ T lymphocyte response, then immunization of mice with an altered G protein capable of priming both RSV-specific CD4+ and CD8+ T lymphocyte responses should result in reduced lung eosinophilia after RSV challenge. To examine this hypothesis, we constructed a mutant G gene containing the amino acids residues 82 to 90 of the RSV 22K matrix (M2) protein (see Materials and Methods, Fig. 1). The RSV matrix protein has been shown to be an important target for MHC class I–restricted CD8+ T lymphocytes in both mice and humans (26, 27). In BALB/c mice, residues 82-90 have been reported to be a dominant matrix-specific H-2d-restricted CTL epitope (24, 28).
When expressed in a recombinant vaccinia virus, this mutant G/matrix chimeric protein (VG22K) showed a similar pattern of expression and glycosylation as wild-type G protein (not shown). When the G/22K protein was tested for recognition by matrix-specific CTL generated by priming of BALB/c mice with recombinant vaccinia virus expressing RSV matrix (M2 or 22K) protein (V22K) and in vitro restimulation with RSV-infected splenocyte stimulators, target cells infected with VG22K were recognized by 22K-specific bulk spleen cells to the same extent as targets infected with V22K (Fig. 3). This recognition is specific to the 22K epitope since targets infected with VG were not lysed by the T cells. This result indicates that the 22K epitope, when expressed in the context of the RSV-G glycoprotein, was properly processed and presented to 22K-specific cytolytic T cells by target cells in vitro.
Figure 3.
Target cells expressing G/22K protein are recognized by 22K-specific CTL. Target cells infected with indicated recombinant vaccinia virus were tested in a standard 51Cr assay for recognition by 22K-specific CTL generated from splenocytes of mice previously primed with V22K. Note that target cells infected with recombinant vaccinia virus expressing the native RSV-G glycoprotein were not recognized by 22K-specific CTL.
Pulmonary Eosinophilia, Cytokine Production and Cytotoxicity in Mice Primed with the Chimeric G/22K Protein.
Since the 22K matrix epitope contained within the chimeric G/22K protein could be processed in target cells and presented to 22K-specific CTL, it was of interest to determine if the G/22K protein could prime for an in vitro secondary 22K-specific CTL response. As Fig. 4 shows, this was the case. Immune splenocytes from BALB/c mice primed with VG22K mounted a vigorous in vitro secondary CTL response detectable at day 5 after the in vitro restimulation with infectious RSV. Priming was specific for the 22K matrix protein since target cells expressing the 22K matrix protein, but not target cells infected with control vaccinia virus (VSC11), were recognized by CTL. In contrast, splenocytes from mice primed in parallel with vaccinia virus expressing wild-type G did not mount a detectable CTL response on 22K expressing target cells (Fig. 4). Also, splenocytes from mice primed with the control vaccinia virus (VSC11), which expresses no RSV proteins, also failed to mount a detectable cytolytic response on 22K-expressing target cells after in vitro stimulation with RSV. This latter finding suggests that the cytolytic activity on 22K-expressing targets exhibited by G/22K immune splenocytes was not due to a primary CTL response to the 22K matrix protein induced in vitro by infectious RSV.
Figure 4.
Splenocytes from mice primed with VG22K, but not VG or VSC11 exhibit 22K-specific cytolytic activity. Splenocytes from mice primed with VSC11, VG, or VG22K were stimulated in vitro with RSV-infected spleen cells. Cytolytic activity against target cells infected with V22K (gray bar) or control vaccinia virus VSC11 (black bar) was analyzed in a standard 51Cr assay.
If priming of an RSV-specific CD8+ T cell response accounts for the difference in the pattern of lung inflammation between RSV-F and -G immune mice after intranasal RSV challenge, then G/22K-immune mice should, like F-immune mice, exhibit minimal eosinophil accumulation after intranasal RSV infection. To examine this hypothesis, groups of BALB/c mice were primed with recombinant vaccinia virus expressing either RSV-F protein (VF), wild-type G protein (VG), or the chimeric G/22K protein (VG22K), and challenged 3 wk later with infectious RSV. BALB/c mice primed with the VSC11 recombinant vaccinia and challenged with RSV served as controls. 5 d later lungs of individual mice were harvested for histologic evaluation of eosinophil accumulation or disrupted for isolation of infiltrating lung mononuclear cells.
In agreement with earlier reports (12, 14), priming with VG resulted in a marked perivascular eosinophil infiltration upon challenge with live RSV, as determined by quantitative morphometry (Fig. 5). In contrast, pulmonary eosinophilia among VG22K-primed mice was significantly diminished when compared to mice primed with VG, and was comparable with mice primed with VF. Scattered foci of eosinophil infiltration were occasionally observed in some of the mice primed with VG22K, which probably reflected variability in the response of individual mice to the 22K epitope.
Figure 5.
Perivascular eosinophils in the lungs of mice sensitized to RSV-F, RSV-G, or RSV-G/22K glycoproteins. Mice were primed with the indicated recombinant vaccinia virus 3 wk before intranasal challenge with RSV. Mice were killed 5 d later and lung sections were stained for eosinophils. Eosinophils around the blood vessels were counted and the lengths of the vessels were measured by micrometer. Each data point represents cell counts from individual animals.
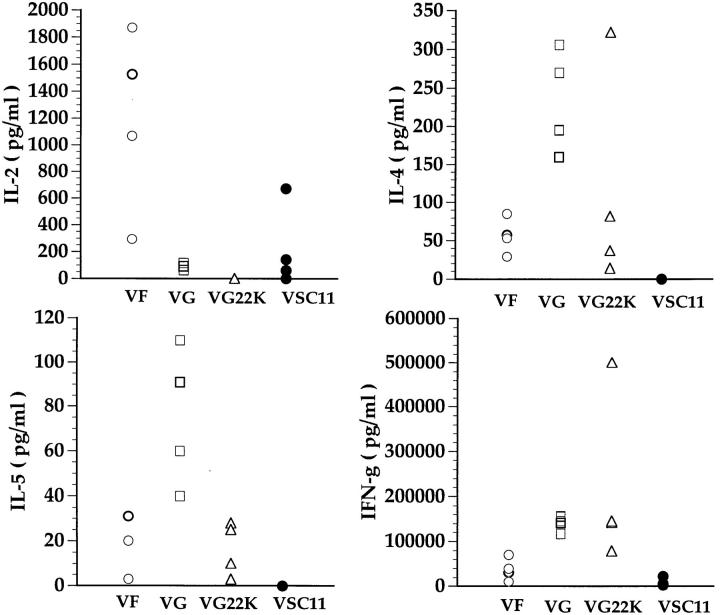
We previously reported that antigen-dependent cytokine production by mononuclear cells isolated at day 5 after intranasal RSV challenge from the lungs of RSV-F– or RSV-G–primed mice directly correlates with the type of pulmonary inflammatory response demonstrable histologically in these primed animals at day 5 after RSV challenge (14). Thus, lung mononuclear cells isolated from infected G-primed mice secreted high levels of IL-4 and particularly IL-5 commensurate with the eosinophil accumulation in the lungs of these animals. A similar analysis was carried out on lung mononuclear cells from RSV-challenged mice primed with VF, VG, or VG22K. Fig. 6 showed the cytokine production by mononuclear cells isolated from the lungs of these animals in response to RSV stimulation in vitro. As reported previously (14), cells isolated from the VF-primed animals produced high levels of IL-2 and IFN-γ. Consistent with the histologic findings reported above (Fig. 5), cells isolated from mice primed with VG, on the other hand, secreted high levels of IL-4 and IL-5. Low but detectable levels of IL-4 and IL-5 were found in the culture supernatants of lung cell cultures from VF-primed animals. Also consistent with the histologic finding, the levels of the Th2 type cytokines (IL-4 and IL-5) produced by infiltrating lung mononuclear cells from mice primed with VG22K were markedly lower than the levels produced by cells from mice primed with recombinant vaccinia virus expressing the native RSV-G glycoprotein and were comparable to the levels detected in the VF-primed animals. IFN-γ production by lung mononuclear cells were comparable among mice primed with G, G22K, or F. As expected, cells isolated from the lungs of RSV-infected mice primed with the control recombinant vaccinia (VCS11) produced low or nondetectable levels of all cytokines.
Figure 6.
Cytokines produced by cells isolated from lungs of mice primed with recombinant vaccinia virus and challenged with RSV. Mice were primed with indicated recombinant vaccinia virus and challenged with live RSV intranasally 3 wk after priming. Mononuclear cells were isolated from lungs of these mice 5 d after challenge and stimulated with RSV. Supernatants were collected 48 h later and analyzed for cytokines by ELISA.
Although immunization with the G/22K chimeric protein did not evoke pulmonary eosinophilia after intranasal RSV challenge, the G/22K protein did appear to efficiently prime for a memory T lymphocyte response. This was evident both from the extent of lung inflammation in these mice in response to RSV challenge (which was comparable to that of wild-type G- or F-primed mice) and from the magnitude of the cytokine response of lung mononuclear cells collected after RSV challenge that was considerably higher than that of control nonimmune mice (Fig. 6).
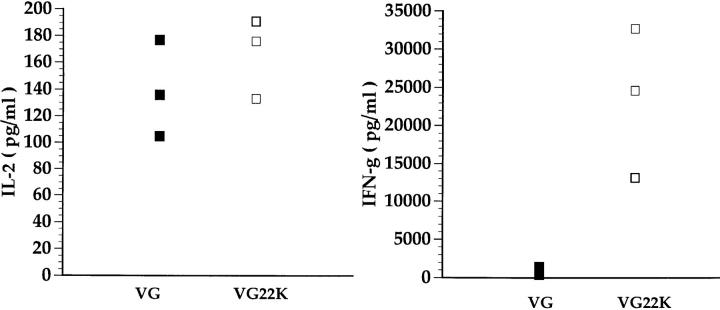
To further establish that the G/22K can efficiently prime an RSV-specific memory T lymphocyte response, we examined the cytokine response of immune splenocytes from VG- and VG22K-primed mice to in vitro stimulation with RSV. As Fig. 7 shows, G-immune and G/22K-immune splenocytes produced comparable levels of IL-2 in response to RSV suggesting that the wild-type G and the chimeric G/22K proteins induced a comparable expansion of IL-2– producing memory T lymphocytes. It is also noteworthy that neither G/22K- nor G-immune splenocyte cultures produced detectable IL-4 or IL-5 after a single cycle of in vitro stimulation with RSV (data not shown). This result is consistent with our earlier findings that RSV-specific memory CD4+ T lymphocytes in the spleen, unlike the polarized effector populations found in the lung after RSV infection, produce predominantly IL-2 and IFN-γ after the first in vitro stimulation with antigen (14). The high level of IFN-γ produced by immune splenocytes from mice primed with VG22K most likely reflects the activation of 22K-specific CD8+ T cells in these bulk cultures.
Figure 7.
VG22K effectively primes for RSV-specific memory T lymphocyte response. Splenocytes from mice previously primed with VG or VG22K were stimulated in vitro with RSV-infected naive spleen cells. Supernatants were collected 48 h later and analyzed for IL-2, IL-4, IL-5, and IFN-γ. IL-4 and IL-5 were not detected in culture supernatants from either group of animals (data not shown).
Priming with V22K Results in Decreased Pulmonary Eosinophilia in Mice Sensitized to RSV-G Glycoprotein.
Our results indicate that priming for a CD8+ T cell response to the 22K epitope in the context of RSV-G glycoprotein reduced Th2 response and lung eosinophilia upon challenge with RSV. However, insertion of this epitope into the RSV-G glycoprotein could alter immune recognition of the RSV-G glycoprotein and result in the reduction of eosinophil infiltration independent of the priming of CD8+ T cell memory response. To examine this issue, we immunized mice with a combination of VG and V22K to simultaneously prime a memory CD4+ T lymphocyte response to G glycoprotein and a memory CD8+ T lymphocyte response to the 22K matrix epitope without altering the RSV-G structure.
Fig. 8 shows the result of a quantitative morphometric analysis of lung eosinophil numbers after RSV challenge in mice primed with VG alone or with a mixture of VG and V22K, or VG22K and V22K. Priming with the combination of V22K and VG resulted in a significant reduction in the number of eosinophils found around the blood vessels when compared to the mice that were primed with VG alone. An even greater reduction was observed in the group of mice primed with the combination of V22K and VG22K. This result suggests that altered immune recognition of G in the chimeric G/22K protein does not account for the diminished eosinophilia evoked by this protein. This finding also suggests a direct role of CD8+ T cells in the regulation of eosinophil recruitment into the lungs during RSV infection. The more effective suppression of tissue eosinophilia in mice co-primed with the V22K and VG22K is most likely due to a more effective priming of a strong memory CD8+ T cell response by this combination of viruses.
Figure 8.
Priming with V22K results in decreased pulmonary eosinophilia in mice sensitized to RSV-G glycoprotein. Mice were primed with a single or a mixture of two recombinant vaccinia viruses as indicated and subsequently challenged with RSV intranasally. Mice were killed 5 d later, and lung sections were stained for eosinophils. Eosinophils around the blood vessels were counted and the lengths of the vessels were measured by micrometer. Each data point represents cell counts from individual animals.
Pulmonary Eosinophilia in CD8+ T Lymphocyte–deficient, β2m Knockout Mice.
The findings so far suggest that the induction of a memory CD8+ T lymphocyte response to RSV suppresses lung eosinophilia and downregulates IL-4 and IL-5 production in RSV-G immune mice after intranasal RSV challenge. If this is the case, then priming to either RSV-F or RSV-G in CD8+ T cell–deficient mice should result in enhanced pulmonary eosinophilia with RSV challenge. Figs. 9 and 10 show the results of this analysis carried out in β2m-deficient (β2mKO) mice.
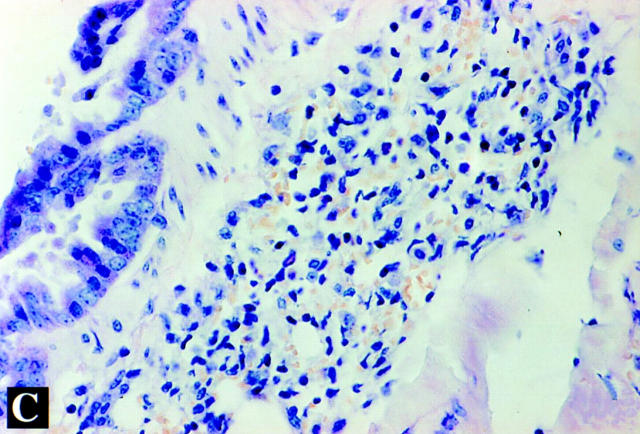
Figure 9.

Lung histopathology of β2mKO mice sensitized to RSV glycoproteins. Mice were primed with VG (A), VF (B), or VSC11 (C) and challenged with live RSV intranasally 3 wk after priming. Mice were killed 5 d after challenge, and lung sections were stained for eosinophils with Leinert-Giemsa stain. Original magnification: 200.
Figure 10.
Perivascular eosinophils in the lungs of β2mKO mice sensitized to RSV glycoproteins. Mice were primed with the indicated recombinant vaccinia virus 3 wk before intranasal challenge with RSV. Mice were killed 5 d later and lung sections were stained for eosinophils. Eosinophils around the blood vessels were counted and the lengths of the vessels were measured by micrometer. Note that the magnitude of lung eosinophilia is four to five times higher in β2mKO mice than BALB/c mice.
As observed in RSV-G–primed conventional mice, β2mKO mice primed with VG showed extensive lung eosinophil accumulation after RSV challenge (Fig. 9 A). However, unlike RSV-F immune conventional mice, β2mKO mice primed with VF show extensive pulmonary eosinophilia in response to RSV challenge (Fig. 9 B). Control β2mKO mice primed with the control VSC11 vaccinia virus showed no eosinophil accumulation after RSV challenge (Fig. 9 C).
Fig. 10 shows a quantitative morphometric analysis of lung eosinophil numbers in groups of β2mKO primed with VG, VF, or VSC11. This analysis reveals that the extent of pulmonary eosinophilia in RSV-F– and -G–immune animals is comparable. It is also noteworthy that the magnitude of lung eosinophil accumulation is four- to fivefold higher on β2mKO mice than in conventional mice. Also, as the morphometric data on β2mKO mice primed with the control VSC11 virus suggests this enhanced eosinophil response in these animals is dependent upon earlier exposure to RSV antigens and not due to an abnormal or exaggerated response of β2mKO mice to exposure to infectious vaccinia virus during priming or to RSV virus during challenge.
Discussion
In the studies described above, we have demonstrated that priming for a CD8+ T cell response resulted in a reduction in the production of IL-4 and IL-5 in BALB/c mice sensitized to RSV-G glycoprotein. This reduction in a Th2 type cytokine response correlated with the suppression of eosinophil recruitment into the lungs of these mice. This effect was observed by priming either with a recombinant vaccinia virus expressing RSV-G glycoprotein in which a strong CD8+ T cell epitope from RSV-M2 protein has been inserted (VG22K) or with a combination of vaccinia virus expressing the 22K (M2) protein and the RSV-G glycoprotein, respectively. The importance of CD8+ T cells in this process was further supported by the results in CD8+ T cell–deficient, β2mKO mice. In these mice, priming to RSV-F glycoprotein, which in normal mice primes for a strong cytolytic response and a pulmonary infiltrate consisting primarily of mononuclear cells on RSV challenge, resulted in the development of marked pulmonary eosinophilia that was not seen in mice with an intact CD8+ T cell compartment.
CD8+ T cells can potentially regulate the differentiation and activation of CD4+ T cells at various stages of differentiation (29, 30). Most studies on the factors that regulate the differentiation of CD4+ T cells have focused on the differentiation of naive CD4+ T cells to Th1 or Th2 effector T cells (16, 18, 31, 32). Recent studies have indicated that the differentiation of memory CD4+ T cells is also regulated by the same mechanisms that regulate the differentiation of naive CD4+ T cells toward a Th1 or Th2 effector phenotype (33, 34). In this connection, it is noteworthy that immunization with RSV-G in the form of a recombinant vaccinia virus primes for a vaccinia-specific memory CD8+ CTL response. Yet G-specific memory CD4+ T lymphocytes primed by this mechanism still give rise to effector cells producing high levels of IL-4 and IL-5 upon challenge with RSV. This result suggests that the environment in which the naive G specific CD4+ T cells differentiated, i.e., in the presence of activated vaccinia-specific CD8+ T cells did not dictate the eventual phenotype of the effector CD4+ T cells generated from the G-specific memory CD4+ T cells in response to RSV challenge. Rather, in this model at least, CD4+ T cell differentiation into activated effectors can be regulated by antigen-specific CD8+ T lymphocytes at the level of the antigen-specific memory CD4+ T cells.
It is not clear if CD8+ T cells regulate the cytokine response of CD4+ T cells by acting at the level of memory CD4+ T cells or differentiated effector CD4+ T cells derived from activated memory CD4+ T cells. In one report, adoptive transfer of activated RSV-22K–specific CD8+ CTL along with activated RSV-G–specific (IL-4– and IL-5–producing) CD4+ T cells markedly diminished the degree of pulmonary eosinophilia normally seen when only G-specific CD4+ T cells were transferred before RSV challenge (8). Since long-term lines of activated CD4+ and CD8+ T cells were used in this study, the downregulation of eosinophilia was due to an effect of activated 22K-specific CD8+ T cells on the function of an activated population of G-specific CD4+ T cells with a Th2-like effector phenotype. The findings reported here are in agreement with the above results and suggest that CD8+ T cells may regulate the cytokine response of CD4+ T cells at various stages of CD4+ T cell activation and differentiation. Further analysis will be required to define the stages of CD4+ T cell activation/differentiation that are susceptible to CD8+ T cell regulation.
The mechanisms that regulate the differentiation of CD4+ T cells toward the Th1 or Th2 effector pathways have been recently reviewed (16, 35). Perhaps the best documented mechanism is the polarizing effect of certain cytokines on the differentiation of CD4+ T cells (36). In particular, IL-4 and IL-12 have been shown to skew the differentiation of CD4+ T cells toward a Th2 or Th1 phenotype, respectively (17, 19, 32). Upon activation, CD8+ T cells secrete a number of soluble mediators that can potentially affect the differentiation of CD4+ T cells. In the RSV model described here, IFN-γ would appear to be the most likely candidate cytokine. It has been well documented that IFN-γ can suppress the proliferation of Th2 CD4+ T cells and that the presence of IFN-γ during CD4+ T cell activation/differentiation favors the development of Th1 type effector cells (37). However, several observations argue against IFN-γ as the CD8+ T cell effector molecule regulating CD4+ T cell differentiation in the RSV model. First, as noted above, the priming of RSV-G–specific CD4+ T cells using a recombinant vaccinia virus would be expected to result in a high level of IFN-γ production by activated vaccinia-specific CD8+ (and CD4+) T lymphocytes during priming with the recombinant vaccinia virus. Nonetheless, G-specific memory CD4+ T cells were not skewed toward a Th1 effector pathway. More importantly, as reported here and elsewhere, G-specific CD4+ T cell effectors produce high levels of IFN-γ at the site of RSV challenge in the lung, but still secrete high levels of IL-4 and IL-5 (14). Finally, we previously reported that mice rendered genetically deficient in IFN-γ production by targeted gene disruption had the same pattern of pulmonary inflammation as conventional mice after priming with RSV-F or RSV-G and challenge with RSV (14). Taken together, these findings suggest that products of activated CD8+ T cells other than IFN-γ may play a dominant role in regulating CD4+ T cell differentiation. Additional studies will be necessary to examine this possibility.
Both antigen dose and antigen form have been implicated as important regulators of effector CD4+ T cell differentiation along a Th1-like or Th2-like pathway (11, 38, 39, 40). In particular, the role of antigen dose in regulating Th2 differentiation of CD4+ T cells is controversial (39, 40). For example, sensitization to aeroallergens and allergic responses occur at extremely low antigen doses sustained over a prolonged period, whereas in many models of microbial infection, Th2-like responses are frequently associated with chronicity and a high microbial antigen burden (41, 42). Since RSV-specific CD8+ CTL have been shown to promote virus clearance from the lungs in experimental RSV infection (8), the regulatory effect of CD8+ T cells on memory CD4+ T cell differentiation into Th2-like effectors could reflect an effect of CD8+ CTL effectors on RSV antigen load. Thus, mice primed with the RSV-F, 22K, or the G/22K chimeric protein would produce CD8+ CTL in response to RSV challenge. This would, in turn, lead to more accelerated virus clearance from lungs than occurs in RSV-G–primed mice that lack memory CD8+ T cells. According to this view, a higher virus load and prolonged antigen availability would favor the differentiation of G-specific memory CD4+ T cells into effector cells with a Th2-like phenotype. Our observation that G-specific memory CD4+ T cells require multiple rounds of in vitro stimulation with RSV before displaying a Th2-like effector phenotype (14) is consistent with this interpretation.
Although the concept of viral load as a primary regulator of RSV-specific CD4+ T cell differentiation in the lung is attractive, there is little direct experimental support for it. In particular, a number of reports using either RSV virion subunits, inactivated vaccines, or vaccinia recombinants (including VF and VG) to prime mice have demonstrated that with any of these priming schemes, infectious virus can not be detected in the lungs of mice by 4–5 d after RSV challenge (22, 43–46). Therefore, if RSV antigen persistence in the lung is influencing T lymphocyte activation/differentiation, persistent antigen may not be reflected in the infectious virion content of the lungs. Also, we cannot exclude the possibility that there is a subtle difference in the kinetics of virus clearance or the magnitude of virus replication in the lungs of VG- and VF-primed mice that might account for the dramatic differences in T cell effector phenotypes after RSV infection. Studies to examine these issues are currently in progress.
Our findings in the β2mKO mice provide further support for a role of CD8+ T cells in the control of CD4+ T cell differentiation. β2mKO mice lack mature CD8+ T cells and, when primed with either VF or VG, develop a marked eosinophilia upon challenge with live RSV. Several aspects of this result are noteworthy. First, the magnitude of eosinophilia in these animals are four to five times higher than conventional BALB/c mice. This may be due to more extensive replication of recombinant vaccinia virus in these mice because they lack a vaccinia-specific CD8+ CTL response. This, in turn, could result in a higher dose of RSV-F and RSV-G and more effective priming of RSV-specific CD4+ T memory cells. Second, the absence of β2m in these animals may result in a deficiency of MHC class I–dependent, CD8+, γ/δ TCR-expressing T cells. γ/δ T cells have been reported to suppress allergic responses in the lung (47, 48). The absence of this regulatory T cell subset could lead to the exaggerated Th2 response and tissue eosinophilia observed by us in these animals. Third, the ability of these animals to develop a strong Th2 type response argues against a requirement for CD1-restricted NK1.1+, CD4+ T cells (49) in the differentiation of CD4+ T cells toward a Th2 phenotype in this model. Our results are in agreement with recent reports which also showed the induction of Th2 responses to unrelated antigens in β2mKO mice (50, 51).
A number of factors appear to determine whether epitopes present in a foreign protein are processed, presented, and recognized by CD8+ CTL (52). In the case of RSV-G, the reason for the inability of this viral glycoprotein to stimulate CD8+ CTL is not clear. Inspection of the amino acid sequence of G indicates that it has multiple potential peptide fragments with the appropriate MHC-binding motif for interaction with H-2d haplotype MHC class I molecules (53, 54). Based on our analysis of the recognition of the G/22K chimeric protein by CTL, the expression of the RSV-G protein in infected cells does not interfere with antigen-processing and -presentation events along the MHC class I–presentation pathway, nor does its structure preclude efficient processing and presentation of epitopes contained within the protein.
A relationship between viral respiratory tract infection and the exacerbation of allergic airway diseases has long been appreciated. Emerging experimental evidence has begun to reveal the mechanisms by which respiratory viruses potentiate the release of proinflammatory mediators by infected airway epithelial cells and sensitized mast cells from allergic individuals (55, 56). The mechanisms by which viral respiratory tract infections influence the development of allergic airway diseases, however, remain unclear. Several studies have shown that lower respiratory tract infection with RSV is an important risk factor for the subsequent development of asthma (57, 58). It is therefore tempting to speculate that in individuals with a genetic predisposition to atopic responses, immune responses to respiratory viruses such as RSV may alter the immune response and, in particular, the pathway of CD4+ T cell differentiation in response to aeroallergens present concomitantly or subsequently in the airways. Therefore, in some individuals, the development of a CD4+ T cell response to a virus in the absence of a CD8+ T cell response may not only lead to a strong Th2 type response to viral antigens, but also provide a cytokine milieu conducive to CD4+ Th2 responses to “bystander” aeroantigens. This could in turn lead to the subsequent development of an allergic airway disease.
At present there is no effective vaccine against primary RSV infection in infants and children. In an early clinical vaccine trial using a formalin-inactivated whole virion RSV preparation to vaccinate seronegative infants and children, administration of this inactivated vaccine resulted in a paradoxical response in vaccine recipients upon subsequent natural RSV infection (59, 60). Vaccine recipients exhibited an exaggerated pulmonary inflammatory response after natural infection, which resulted in enhanced morbidity and mortality compared to unvaccinated controls and a prominent eosinophil response in peripheral blood and lung tissue indicative of a Th2 type response (59). Immunization of mice with formalin-inactivated RSV also induces a Th2 pattern of lung inflammation and cytokine production after challenge with RSV (11). The findings reported here provide an explanation for the effect of formalin-inactivated RSV in children and experimental animals. Efficient processing and presentation of viral proteins to CD8+ T lymphocytes requires access to the MHC class I presentation pathway, usually by de novo expression of viral gene products in the infected cell (61). Viral proteins in formalin-inactivated RSV virion preparations are unlikely to be efficiently processed and presented to CD8+ T cells, but should efficiently enter the MHC class II processing pathway for presentation to CD4+ T cells. Therefore, in the aforementioned clinical trial, formalin-inactivated RSV may have primed a memory RSV-specific CD4+ T cell response in vaccine recipients without effective CD8+ T cell priming in a fashion analogous to the VG priming reported here. The activation of memory CD4+ T cells after natural RSV infection of children or experimental animals without a concomitant response of RSV-specific memory CD8+ T cells would then result in the exaggerated Th2-like inflammatory pattern observed in lungs of the inactivated vaccine recipients. The pronounced Th2-like response to inactivated RSV vaccine seen in both human subjects and experimental animals further underscores the potential importance of CD8+ T cells in downregulating CD4+ Th2-like effector T cell responses (62).
Since CD8+ T cells have also been implicated as effectors in immune-mediated injury during experimental RSV infection (8, 63), an effective vaccine strategy against this virus will require the priming of a protective MHC class I–restricted cytolytic CD8+ T cell response. Enhancement of a protective cytolic T cell response might be achieved by manipulating the cytokine environment during the initial priming, as has been observed in studies of the effect of anti–IL-4 antibody on the response of mice to RSV infection after vaccination with inactivated RSV (64). A better understanding of the complex interplay between RSV and the immune effectors that respond to it will be crucial to the development of an effective immunoprophylactic agent against this virus.
Acknowledgments
The authors wish to thank J. Beeler of Federal Drug Administration for providing recombinant vaccinia viruses expressing RSV proteins under the auspices of the World Health Organization program for vaccine development, P. McInnes and C. Heilman of the National Institute of Allergy and Infectious Diseases, National Institutes of Health for advice and support, and Yu Quing Shan for technical assistance. We thank M. Kurilla and V. Braciale for helpful discussions.
This work was supported by grants from the National Institute of Allergy and Infectious Diseases.
Footnotes
Abbreviations used in this paper: β2m, β2 microglobulin; G22K, portion of cDNA encoding the G protein with inserted 22K epitope; RSV, respiratory syncytial virus; V22K, recombinant vaccinia virus expressing RSV matric (M2 or 22K) protein; VF, recombinant vaccinia virus expressing RSV fusion glycoprotein; VG, recombinant vaccinia virus expressing RSV attachment glycoprotein; VG22K, recombinant vaccinia virus expressing chimeric G/22K glycoprotein.
References
- 1.Doherty PC, Allan W, Eichelberger M, Carding SR. Roles of α/β and γ/δ T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- 2.Lukacher AE, Braciale VL, Braciale TJ. In vivo effector function of influenza virus–specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984;160:814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braciale TJ, Braciale VL. Viral antigen presentation and MHC assembly. Semin Immunol. 1992;4:81–84. [PubMed] [Google Scholar]
- 4.Braciale TJ, Braciale VL. Antigen presentation: structural themes and functional variations. Immunol Today. 1991;12:124–129. doi: 10.1016/0167-5699(91)90096-C. [DOI] [PubMed] [Google Scholar]
- 5.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 6.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 7.Alwan WH, Record FM, Openshaw PJ. CD4+ T cells clear virus but augment disease in mice infected with respiratory syncytial virus. Comparison with the effects of CD8+T cells. Clin Exp Immunol. 1992;88:527–536. doi: 10.1111/j.1365-2249.1992.tb06482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alwan WH, Kozlowska WJ, Openshaw PJ. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bangham CR, Cannon MJ, Karzon DT, Askonas BA. Cytotoxic T-cell response to respiratory syncytial virus in mice. J Virol. 1985;56:55–59. doi: 10.1128/jvi.56.1.55-59.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC, III, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham BS, Henderson GS, Tang YW, Lu X, Neuzil KM, Colley DG. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 12.Openshaw PJ, Clarke SL, Record FM. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol. 1992;4:493–500. doi: 10.1093/intimm/4.4.493. [DOI] [PubMed] [Google Scholar]
- 13.Alwan WH, Record FM, Openshaw PJ. Phenotypic and functional characterization of T cell lines specific for individual respiratory syncytial virus proteins. J Immunol. 1993;150:5211–5218. [PubMed] [Google Scholar]
- 14.Srikiatkhachorn A, Braciale TJ. Virus-specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection. J Virol. 1997;71:678–685. doi: 10.1128/jvi.71.1.678-685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hancock GE, Speelman DJ, Heers K, Bortell E, Smith J, Cosco C. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J Virol. 1996;70:7783–7791. doi: 10.1128/jvi.70.11.7783-7791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seder RA. Acquisition of lymphokine-producing phenotype by CD4+T cells. J Allergy Clin Immunol. 1994;94:1195–1202. doi: 10.1016/0091-6749(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 17.Seder RA, Paul WE, Davis MM, Fazekas de St B, Groth The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh CS, Heimberger AB, Gold JS, O'Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an αβ T-cell– receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh CS, Macatonia SE, O'Garra A, Murphy KM. Pathogen-induced Th1 phenotype development in CD4+αβ-TCR transgenic T cells is macrophage dependent. Int Immunol. 1993;5:371–382. doi: 10.1093/intimm/5.4.371. [DOI] [PubMed] [Google Scholar]
- 20.Nicholas JA, Rubino KL, Levely ME, Adams EG, Collins PL. Cytolytic T-lymphocyte responses to respiratory syncytial virus: effector cell phenotype and target proteins. J Virol. 1990;64:4232–4241. doi: 10.1128/jvi.64.9.4232-4241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in β2m, MHC class I proteins and CD8+T cells. Science (Wash DC) 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 22.Wertz GW, Stott EJ, Young KK, Anderson K, Ball LA. Expression of the fusion protein of human respiratory syncytial virus from recombinant vaccinia virus vectors and protection of vaccinated mice. J Virol. 1987;61:293–301. doi: 10.1128/jvi.61.2.293-301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ball LA, Young KK, Anderson K, Collins PL, Wertz GW. Expression of the major glycoprotein G of human respiratory syncytial virus from recombinant vaccinia virus vectors. Proc Natl Acad Sci USA. 1986;83:246–250. doi: 10.1073/pnas.83.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkarni AB, Morse HC, III, Bennink JR, Yewdell JW, Murphy BR. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+cytotoxic T cells. J Virol. 1993;67:4086–4092. doi: 10.1128/jvi.67.7.4086-4092.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollard AM, Lipscomb MF. Characterization of murine lung dendritic cells: similarities to Langerhans cells and thymic dendritic cells. J Exp Med. 1990;172:159–167. doi: 10.1084/jem.172.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherrie AH, Anderson K, Wertz GW, Openshaw PJ. Human cytotoxic T cells stimulated by antigen on dendritic cells recognize the N, SH, F, M, 22K, and 1b proteins of respiratory syncytial virus. J Virol. 1992;66:2102–2110. doi: 10.1128/jvi.66.4.2102-2110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Openshaw PJ, Anderson K, Wertz GW, Askonas BA. The 22,000-kilodalton protein of respiratory syncytial virus is a major target for Kd-restricted cytotoxic T lymphocytes from mice primed by infection. J Virol. 1990;64:1683–1689. doi: 10.1128/jvi.64.4.1683-1689.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulkarni AB, Collins PL, Bacik I, Yewdell JW, Bennink JR, Crowe JJ, Murphy BR. Cytotoxic T cells specific for a single peptide on the M2 protein of respiratory syncytial virus are the sole mediators of resistance induced by immunization with M2 encoded by a recombinant vaccinia virus. J Virol. 1995;69:1261–1264. doi: 10.1128/jvi.69.2.1261-1264.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diaz-Sanchez D, Noble A, Staynov DZ, Lee TH, Kemeny DM. Elimination of IgE regulatory rat CD8+T cells in vivo differentially modulates IL-4 and interferon-γ but not interleukin-2 production by splenic T cells. Immunology. 1993;78:513–519. [PMC free article] [PubMed] [Google Scholar]
- 30.Holmes BJ, Diaz-Sanchez D, Lawrence RA, Bell EB, Maizels RM, Kemeny DM. The contrasting effects of CD8+ T cells on primary, established and Nippostrongylus brasiliensis–induced IgE responses. Immunology. 1996;88:252–260. doi: 10.1111/j.1365-2567.1996.tb00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science (Wash DC) 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 32.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+T cells to enhance priming for interferon-γ production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley LM, Yoshimoto K, Swain SL. The cytokines IL-4, IFN-γ, and IL-12 regulate the development of subsets of memory effector helper T cells in vitro. J Immunol. 1995;155:1713–1724. [PubMed] [Google Scholar]
- 34.Marshall JD, Secrist H, DeKruyff RH, Wolf SF, Umetsu DT. IL-12 inhibits the production of IL-4 and IL-10 in allergen-specific human CD4+T lymphocytes. J Immunol. 1995;155:111–117. [PubMed] [Google Scholar]
- 35.Swain SL. CD4+T cell development and cytokine polarization: an overview. J Leukocyte Biol. 1995;57:795–798. doi: 10.1002/jlb.57.5.795. [DOI] [PubMed] [Google Scholar]
- 36.Coffman RL, Varkila K, Scott P, Chatelain R. Role of cytokines in the differentiation of CD4+T-cell subsets in vivo. Immunol Rev. 1991;123:189–207. doi: 10.1111/j.1600-065x.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 37.Gajewski TF, Joyce J, Fitch FW. Antiproliferative effect of IFN-γ in immunoregulation. III. Differential selection of Th1 and Th2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989;143:15–22. [PubMed] [Google Scholar]
- 38.Del Pretes GF, de Carli M, Mastromauro C, Biagiotti R, Macchia D, Falagiani P, Ricci M, Romagnani S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canisexpand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991;88:346–350. doi: 10.1172/JCI115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+T helper cell phenotype development in a T cell receptor–αβ– transgenic model. J Exp Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Secrist H, DeKruyff RH, Umetsu DT. Interleukin 4 production by CD4+T cells from allergic individuals is modulated by antigen concentration and antigen-presenting cell type. J Exp Med. 1995;181:1081–1089. doi: 10.1084/jem.181.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plaut, M., and E.M. Zimmerman. 1993. Allergy and mechanisms of hypersensitivity. In Fundamental Immunology. W.E. Paul, editor. Raven Press, New York. 1399–1425.
- 42.Bretscher BA, Wu G, Menon N, Ohmann H. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. . Science (Wash DC) 1992;257:539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 43.Piedra PA, Wyde PR, Castleman WL, Ambrose MW, Jewell AM, Speelman DJ, Hildreth SW. Enhanced pulmonary pathology associated with the use of formalin-inactivated respiratory syncytial virus vaccine in cotton rats is not a unique viral phenomenon. Vaccine. 1993;11:1415–1423. doi: 10.1016/0264-410x(93)90170-3. [DOI] [PubMed] [Google Scholar]
- 44.Oien NL, Brideau RJ, Thomsen DR, Homa FL, Wathen MW. Vaccination with a heterologous respiratory syncytial virus chimeric FG glycoprotein demonstrates significant subgroup cross-reactivity. Vaccine. 1993;11:1040–1048. doi: 10.1016/0264-410x(93)90131-g. [DOI] [PubMed] [Google Scholar]
- 45.Hancock GE, Speelman DJ, Frenchick PJ, Mineo KM, Baggs RB, Hahn DJ. Formulation of the purified fusion protein of respiratory syncytial virus with the saponin QS-21 induces protective immune responses in Balb/c mice that are similar to those generated by experimental infection. Vaccine. 1995;13:391–400. doi: 10.1016/0264-410x(95)98263-a. [DOI] [PubMed] [Google Scholar]
- 46.Homa FL, Brideau RJ, Lehman DJ, Thomsen DR, Olmsted RA, Wathen MW. Development of a novel subunit vaccine that protects cotton rats against both human respiratory syncytial virus and human parainfluenza virus type 3. J Gen Virol. 1993;7:1995–1999. doi: 10.1099/0022-1317-74-9-1995. [DOI] [PubMed] [Google Scholar]
- 47.McMenamin C, Holt PG. The natural immune response to inhaled soluble protein antigens involves major histocompatibility complex (MHC) class I–restricted CD8+ T cell–mediated but MHC class II–restricted CD4+T cell– dependent immune deviation resulting in selective suppression of immunoglobulin E production. J Exp Med. 1993;178:889–899. doi: 10.1084/jem.178.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γδ T cells. Science (Wash DC) 1994;265:1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 49.Yoshimoto T, Bendelac A, Watson C, Hu LJ, Paul WE. Role of NK1.1+T cells in a TH2 response and in immunoglobulin E production. Science (Wash DC) 1995;270:1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Rogers KH, Lewis DB. β2-microglobulin–dependent T cells are dispensable for allergen-induced T helper 2 responses. J Exp Med. 1996;184:1507–1512. doi: 10.1084/jem.184.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown DR, Fowell DJ, Corry DB, Wynn TA, Moskowitz NH, Cheever AW, Locksley RM, Reiner SL. β2-microglobulin–dependent NK1.1+T cells are not essential for T helper cell 2 immune responses. J Exp Med. 1996;184:1295–1304. doi: 10.1084/jem.184.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heemels M, Ploegh H. Generation, translocation, and presentation of MHC class I–restricted peptides. Annu Rev Biochem. 1995;64:463–491. doi: 10.1146/annurev.bi.64.070195.002335. [DOI] [PubMed] [Google Scholar]
- 53.Wertz GW, Collins PL, Huang Y, Gruber C, Levine S, Ball LA. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci USA. 1985;82:4075–4079. doi: 10.1073/pnas.82.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engelhard VH. Structure of peptides associated with MHC class I molecules. Curr Opin Immunol. 1994;6:13–23. doi: 10.1016/0952-7915(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 55.Einarsson O, Geba GP, Zhu Z, Landry M, Elias JA. Interleukin-11 stimulation in vivo and in vitro by respiratory syncytial virus and induction of airways hyperresponsiveness. J Clin Invest. 1997;97:915–924. doi: 10.1172/JCI118514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graziano FM, Tilton R, Hirth T, Segaloff D, Mullins T, Dick E, Buckner CK, Busse WW. The effect of parainfluenza 3 infection on guinea pig basophil and lung mast cell histamine release. Am Rev Respir Dis. 1989;139:715–720. doi: 10.1164/ajrccm/139.3.715. [DOI] [PubMed] [Google Scholar]
- 57.Welliver RC, Duffy L. The relationship of RSV-specific immunoglobulin E antibody responses in infancy, recurrent wheezing, and pulmonary function at age 7–8 years. Pediatr Pulmonol. 1993;15:19–27. doi: 10.1002/ppul.1950150104. [DOI] [PubMed] [Google Scholar]
- 58.Sigurs N, Bijarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–505. [PubMed] [Google Scholar]
- 59.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 60.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 61.Braciale TJ, Morrison LA, Sweetser MT, Sambrook J, Gething MJ, Braciale VL. Antigen presentation pathway to class I and class II MHC-restricted T lymphocytes. Immunol Rev. 1987;98:95–114. doi: 10.1111/j.1600-065x.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- 62.Graham BS. Immunological determinants of disease caused by respiratory syncytial virus. Trends Microbiol. 1996;4:290–293. doi: 10.1016/0966-842x(96)10032-9. [DOI] [PubMed] [Google Scholar]
- 63.Cannon MJ, Openshaw PJ, Askonas BA. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang YW, Graham BS. Anti–IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus. J Clin Invest. 1994;94:1953–1958. doi: 10.1172/JCI117546. [DOI] [PMC free article] [PubMed] [Google Scholar]