Abstract
Dendritic cells (DC) are a subset of leukocytes whose major function is antigen presentation. We investigated the phenotype and function of enriched (95–98.5%) rat DC. We show that both spleen and thymus DC express the natural killer cell receptor protein 1 (NKR-P1) as a disulfide linked homodimer of 60 kD. Freshly isolated DC express a low level of NKR-P1, which is strongly upregulated after overnight culture. Spleen, but not thymus DC, were able to kill the NK-sensitive YAC-1 cell line in vitro, and since this killing was Ca2+ dependent, a Fas ligand–Fas interaction was probably not involved. Besides their potent antigen-presenting function, DC can thus be cytotoxic for some tumor targets.
Dendritic cells (DC) are a specialized class of leukocytes found in many tissues and most abundantly in the T-dependent areas of lymphoid organs (1). They play a fundamental role in antigen presentation to T cells, being very potent since only small numbers of DC are sufficient to induce T cell responses (2, 3). DC can prime naive T-cells (CD4 and CD8) both in vitro and in vivo (1). A recent report suggests that DC may directly modulate B cell growth and differentiation (4). The antigen presenting function of DC is related to their high expression of both class I and class II MHC products, as well as different costimulatory and adhesion molecules (1). Moreover, they have special antigen handling mechanisms, including the lectin-type DEC-205 in mice and the mannose receptor in humans (5, 6) and abundant MHC class II rich vacuoles (7). In the absence of known unique DC markers, these cells are mainly recognized on the basis of their typical dendritic morphology and their high expression of class II molecules. However, several surface antigens have been found preferentially expressed by rodent DC, such as the integrin CD11c (8), the DEC-205 receptor (6), and a candidate integrin recognized by the OX-62 mAb (9).
The ontogeny of DC remains controversial (10); although most of these cells seem to have a myeloid origin, recent reports indicate that thymus DC are of lymphoid origin, sharing a common thymic precursor with T and NK cells (11). A common hematopoietic precursor for T, B, NK, and DC has also been described in humans (12) but, to the best of our knowledge, the presence of NK-related markers or functions on differentiated DC has not been previously described. Moreover, different DC subsets have been defined based on their phenotype and, more interestingly, on their function (13). Thus, murine spleen DC can be separated in two populations depending on whether they express the CD8α molecule (14). Although CD8− DC exhibit potent antigen-presenting function, CD8+ cells may inactivate mature peripheral CD4+ T cells using a Fas ligand– dependent mechanism (15), indicating that some DC may kill Fas-expressing cells.
Natural killer cell receptor protein 1 (NKR-P1) is a disulfide-linked homodimer expressed by all NK cells and a small subset of T cells in rodents, and it belongs to the group V of the C type lectin superfamily, which also includes the CD69, Ly49, and CD94 molecules (16). NKR-P1 molecules have also been described in humans (16). Previous studies have shown that NKR-P1 is an activation receptor on rat NK cells, leading to stimulation of granule exocytosis, and anti– NKR-P1 mAb (3.2.3) can redirect lysis of NK-resistant target cells by NK cells (17–19). Here, we describe that rat DC express NKR-P1. Furthermore, highly purified spleen, but not thymus DC exhibit a NK-like cytotoxicity in vitro. We suggest that this unusual function of DC could facilitate the phagocytosis of cell fragments and subsequent antigen presentation to T cells, creating a direct link between innate and adaptive immunity.
Materials and Methods
Animals.
4–5-wk-old male rats from the strains Wistar, Sprague-Dawley, Lewis.1A, or Lewis were purchased from the Centre d'Elevage Janvier (Le Genest-Saint-Isle, France).
Medium.
The culture medium used (complete medium) was RPMI 1640 (Sigma Chemical Co., St. Louis, MO) supplemented with heat-inactivated 10% FCS (Life Technologies, Eragny, France), 2 mM l-glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 1 mM sodium pyruvate, and 5 × 10−5 M 2-mercaptoethanol.
mAbs.
The following hybridomas (all mouse IgG) were obtained from the European Collection of Animal Cell Culture (Salisbury, UK): OX6 (class II), OX8 (CD8α), OX39 (CD25), R73 (TCR-α/β), OX12 (Ig k chain), ED3 (sialoadhesin), OX62 (integrin on DC), OX33 (CD45RA), OX22 (CD45-RC), and 341.1 (CD8β). V65 (TCR-γ/δ) was provided by T. Hunig (Wurzburg, Germany). The mAb 3.2.3 (NKR-P1) was purchased from Serotec (Oxford, UK). The fusion molecules CTLA4-Ig (provided by P.S. Linsley, Bristol-Myers Squibb, Seattle, WA) recognizing B7-1 and B7-2 and Fas-Ig (provided by F. Godeau, Institut National de la Sante et de la Recherche Medicale U373, Paris, France) were used at 2.5 μg/ml. H19-282 mAb (mouse IgG1 anti–human CD59) was used as negative control for staining and in vitro culture studies and was provided by A. Bernard (Hopital de l'Archet, Nice, France). Control mouse IgG2b was purchased from PharMingen (San Diego, CA).
Purification of DC.
Thymus and spleen fragments were digested with 2 mg/ml collagenase D (Boehringer-Mannheim, Indianapolis, IN) for 15 min at 37°C in the presence of 10 μM EDTA during the last 5 min (14). The cell suspension was washed and resuspended in 5 μM PBS EDTA containing 2% heat-inactivated FCS at 4°C at 1–2 × 108 cells/ml. 4 ml of this suspension was layered onto 4 ml of 14.5% (wt/vol) metrizamide (grade I, Sigma Chemical Co.) in PBS in 15-ml conical tubes and centrifuged 13 min at 1,800 g at 4°C. Low-density cells were recovered, washed twice, and resuspended at 107 cells/ml in complete medium for culture overnight at 37°C in 5% CO2. Nonadherent cells were then further enriched for DC by centrifugation over a 14.5% metrizamide column, and subjected to two rounds of plastic adherence for 1 h at 37°C. Using this method, we routinely obtained spleen or thymus cell suspensions containing 60–85 and 70–90% DC, respectively, as assessed by morphology and phenotype (high level of class II and B7 molecules expression, see Results section). A final enrichment for DC was routinely performed by removing T cells, B cells, and macrophages using magnetic beads. In brief, cells were incubated with a mixture of appropriate dilutions of R7.3, V65, OX12, OX33, OX22, OX8, 341.1, and ED3 mAbs for 30 min at 4°C, washed three times, and then incubated with anti–mouse IgG-coated Dynabeads (Dynal, Oslo, Norway) for 20 min at 4°C with agitation. After three rounds of magnetic depletion, the final cell populations contained between 95 and 98.5% DC. Enriched populations of fresh DC were also prepared (as indicated) by selection of low-density cells from collagenase-digested organs followed by magnetic bead depletion of TCR-α/β or -γ/δ, sIg, CD45R, CD4, CD8, and sialoadhesin positive cells. In all cases, the enriched DC lacked CD8+ cells, whereas CD8 is an independent marker for rat NK cells (20).
Immunofluorescence Analysis.
For flow cytometry analysis, 5 × 104 cells were incubated with mAb for 30 min at 4°C, washed twice with PBS/0.2% BSA/0.1% NaN3, and then incubated with appropriate FITC-conjugated secondary antibody (Jackson Labs., West Grove, PA). After three washes, the cells were analyzed on a Facscalibur® flow cytometer (Becton Dickinson, Mountain View, CA). Dead cells were excluded from analysis by briefly incubating the cells with propidium iodide. For two-color immunofluorescence, 105 cells were incubated with biotinylated OX6 or OX62 mAbs and FITC-conjugated 3.2.3, R7.3, or OX8 mAbs, followed by streptavidin-PE (Immunotech, Marseille, France).
Biotinylation of Cell Surface Proteins and Immunoprecipitation.
Cells were washed twice in PBS and resuspended at 106/ml in 5 mM N-hydroxy succinimide ester-biotin (Sigma Chemical Co.) in biotinylation buffer (10 mM Na-borate, pH 8.8, 150 mM NaCl), incubated for 30 min at 4°C, washed twice in RPMI 1640, and twice in PBS at 4°C. Cells were then lysed at 2 × 106/ml for 30 min on ice in 1% NP-40 lysis buffer containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM MgCl2, and 1 mM EGTA in the presence of the protease inhibitors 10 μg/ml leupeptin and aprotinin. Postnuclear lysates were obtained after centrifugation (14,000 rpm for 15 min at 4°C) and were precleared for 2 h with 2 × 107 goat anti–mouse IgG-coated magnetic beads (Dynal)/ml of lysate prewashed in 1% NP-40 lysis buffer. Lysates were then subjected to immunoprecipitation for 2 h with mAbs, and then for 2 h with 4 × 107 beads/ml of lysate at 4°C. Immunoprecipitates were washed in 1% detergent lysis buffer and twice in 0.05% detergent lysis buffer, boiled in sample buffer, and separated on 5–13% SDS-PAGE under reducing or nonreducing conditions as indicated. Immunoblotting and protein detection were performed as described (21).
Cytotoxicity Assays.
Cytotoxic activity of DC populations was assessed in a standard 6-h 51Cr–release assay using the YAC-1, P815, L12-10, and K562 cell lines as targets. In brief, target cells were labeled with Na 51Cr for 45 min at 37°C in complete medium. Serial dilutions of effector cells in complete medium were mixed with 2,000 target cells in V-bottom 96-well plates, centrifuged 3 min at 1,500 g, and incubated for 6 h at 37°C, 5% CO2. The supernatants were harvested and specific 51Cr release was determined as described (22).
Results and Discussion
Rat DC Express the NKR-P1 Molecule.
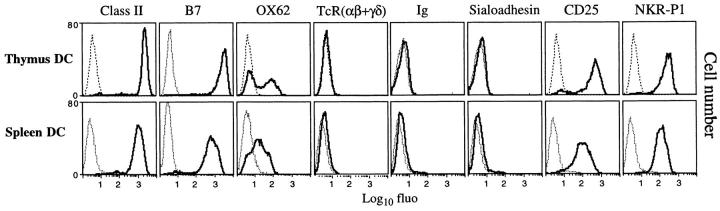
Using the protocol described in the Materials and Methods section, we routinely obtained spleen and thymus DC populations 95– 98% homogeneous. These cells exhibit a typical dendritic morphology (data not shown) and lack of esterase and myeloperoxidase (data not shown), and express very high levels of class II and B7 molecules (Fig. 1). They do not express T or B cell receptors, sialoadhesin (Fig. 1), nor CD8 (Fig. 2). Moreover, they express high levels of CD25, and the antigen recognized by the OX62 mAb was expressed at a low to moderate level by a subset of spleen and thymus DC (Fig. 1). The complete phenotype and morphological features of rat DC will be described elsewhere (our manuscript in preparation). DC preparations from both thymus and spleen had potent stimulatory activity (>300-fold superior to total spleen cells) in primary allogeneic mixed leukocyte culture (data not shown). Of particular interest was that >90% of spleen and thymus DC expressed the NKR-P1 molecule recognized by the 3.2.3 mAb (Fig. 1). DC expression of NKR-P1 was not strain-restricted since we have observed similar staining for this molecule on DC isolated from Lewis, Wistar, and Sprague-Dawley rats. In contrast, spleen DC from the C57Bl/6 mouse strain do not express the NKR-P1C molecule (23).
Figure 1.
The phenotype of thymus and spleen rat DC. DC were purified as described in the Materials and Methods section. Expression of the indicated antigens was analyzed in single color immunofluorescence. Propidium iodide was used to exclude dead cells from analysis. Dotted lines show the background with isotype-matched control antibody. Each staining was performed at least six times with similar results.
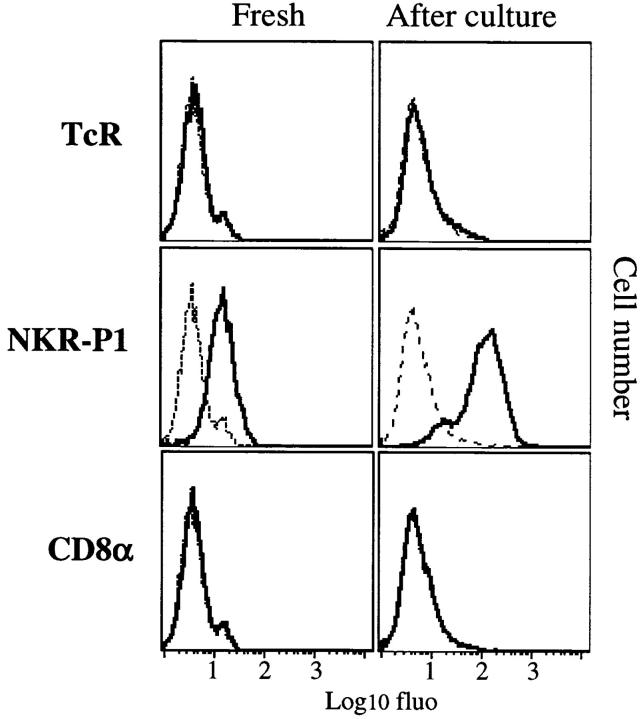
Figure 2.
Upregulation of NKR-P1 expression on DC upon overnight in vitro culture. Enriched populations of fresh DC were prepared by selecting low-density cells of collagenase-digested spleen and thymus followed by magnetic beads depletion of non-DC. An aliquot of the cells was then analyzed in two-color immunofluorescence with biotynylated OX6 or OX62 mAbs followed by FITC-conjugated R7.3, 3.2.3, OX8, or control mAb and streptavidin-PE. The remaining cells were cultured overnight in complete RPMI and analyzed as described above. The histograms show expression patterns of TCR, NKR-P1, and CD8 on OX62-positive gated cells. Dotted lines show the background with isotype-matched control antibodies. The results are representative of four different experiments.
Upregulation of NKR-P1 Expression on DC upon In Vitro Culture.
The expression of NKR-P1 was assessed on freshly extracted DC before culturing at 37°C, which is known to result in DC activation (1). Low-density cells were depleted of non-DC after treatment with mAbs and magnetic beads. Expression of NKR-P1 was assessed on both OX62-positive or OX6high cells in two-color immunofluorescence (Fig. 2). Low level expression was observed on spleen DC, and was strongly upregulated after overnight culture. Similar results were obtained on individual OX6high or OX62-positive (gated) populations and with thymus DC (data not shown). As expected, we did not observe de novo expression of TCR or CD8 (Fig. 2). The finding that after overnight culture, the expression of NKR-P1A was upregulated on DC excluded the possibility that the NKR-P1 molecule was adsorbed rather than synthesized. The overnight culture step currently used during the purification of rodent DC is thought to reproduce their in vivo activation (1). Thus, although it remains to be shown, it is likely that upregulation of NKR-P1 also occurs in vivo.
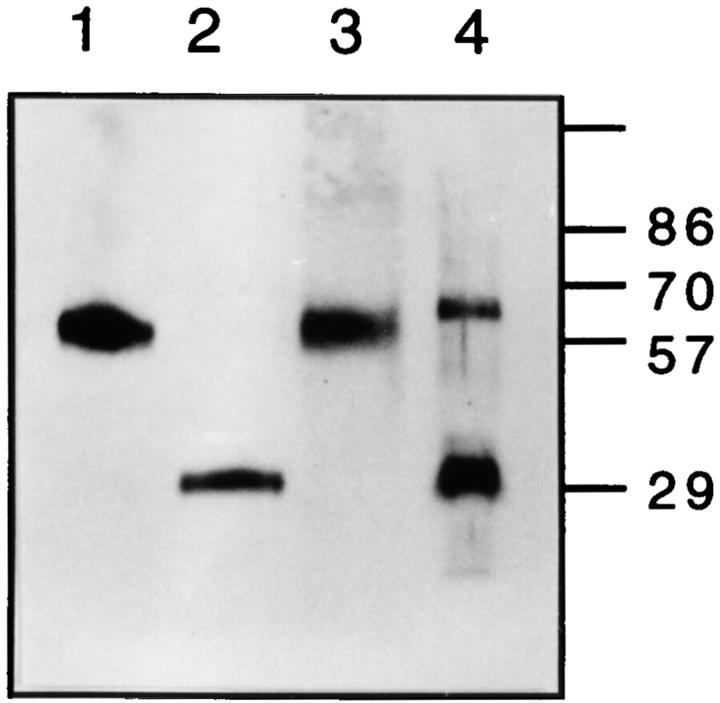
Immunoprecipitation of the Molecule Recognized by 3.2.3 mAb on Rat DC.
The molecule recognized by the 3.2.3 mAb on rat spleen DC was immunoprecipitated from highly purified (>97%) DC preparations and NK-enriched populations (nylon wool nonadherent spleen cells) and analyzed on SDS-PAGE gel (Fig. 3). As expected (17), a molecule of ∼60 kD recognized by 3.2.3 mAb appeared composed of two homodimeric chains of ∼30 kD on NK-enriched spleen cell populations. The NKR-P1 molecule from DC resolved under reducing conditions into two bands, the major one corresponding to ∼30 kD, as expected, and a second one migrating slightly slower than the nonreduced molecule of ∼60 kD. The persistence of this band could be due to incomplete reduction, possibly reflecting a greater aggregate-forming tendency of the NKR-P1 monomers on DC compared to those expressed on NK cells.
Figure 3.
Immunoprecipitation of membrane NKR-P1. Immunoprecipitation was performed on spleen DC (97%) and NK-enriched populations (nylon wool nonadherent spleen cells) as described in Materials and Methods. Samples were loaded on 5– 13% SDS-PAGE and analyzed by immunoblotting and chemiluminescence. Lanes 1 and 2, NK-enriched spleen cells; lanes 3 and 4, spleen DC; lanes 1 and 3, nonreduced; lanes 2 and 4, reduced.
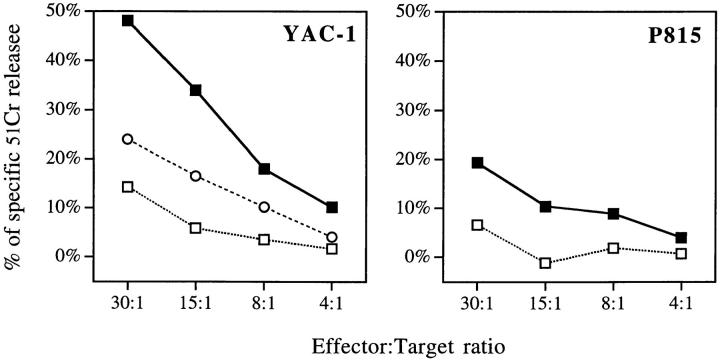
In Vitro Cytotoxicity of Rat DC.
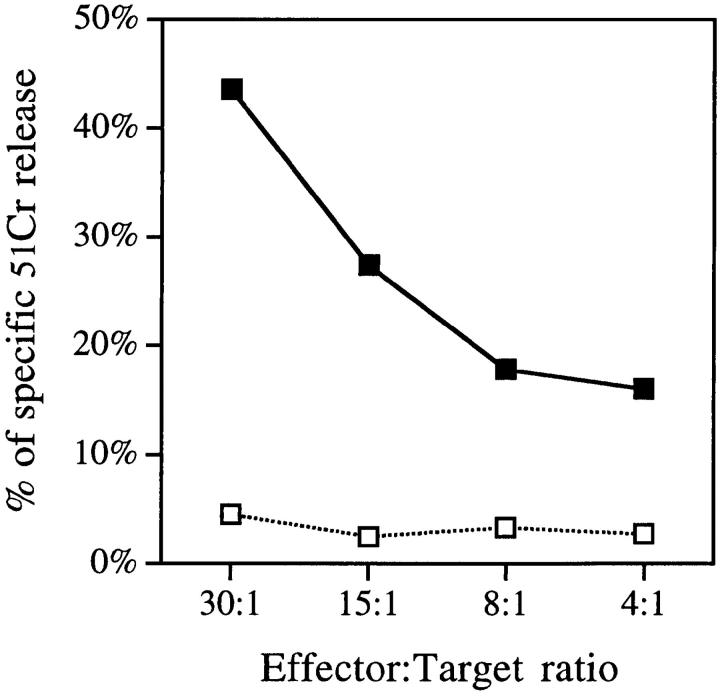
Based on our results that showed that rat DC expressed a NK-related molecule, we tested in 6-h Cr release assays the potential cytolytic activity of highly purified DC on target cells usually used for testing NK activity. As shown in Fig. 4, spleen DC efficiently killed the prototypical NK-sensitive YAC-1 cells. The level of killing observed was roughly similar to that reported with the rat NK cell line RNK-16 at the same E/T ratios (19). This unusual activity was also observed using spleen DC maintained in culture for >3 d (data not shown) and was reproduced in >10 independent experiments. The possibility that our DC preparations contained NK cells was excluded because the only non-DC contaminating our DC preparations (95–98.5% pure) corresponded to CD45− cells (fibroblasts, endothelial cells) and because our procedure included a depletion of CD8+ cells (20). Even if the contaminating cells were NK, it does not seem possible that they could be responsible for the lysis of YAC cells we observed as total spleen cells from the same animal, which contain 15–25% NK cells, exhibited an anti-YAC cytotoxic activity lower than that found for spleen DC at the same E/T ratios (Fig. 4). As compared to YAC-1 targets, the cytotoxic activity of spleen DC against P815 (Fig. 4), L12.10, or K562 (data not shown) was low.
Figure 4.
In vitro cytolytic activity of spleen and thymus DC against YAC-1 and P815. 2 × 103 51Cr-labeled YAC-1 (left) or P815 (right) target cells were incubated with total spleen cells (open circle), purified (95– 98.5) spleen (closed square), or thymus (open square) DC in triplicate for 6 h at 37°C. 51Cr release was then assessed in the supernatant. Results of one experiment representative of at least eight for each target are shown.
Despite a very similar phenotype compared to splenic DC, those isolated from thymus exhibited a very low in vitro killing activity (Fig. 4). It is possible that the spleen DC population is heterogenous and that the cytolytic function that we observed was dependent on a specialized subpopulation not related to thymus DC. Indeed, at least some markers such as OX62 (Fig. 1), CD5, or OX41 mAb (our manuscript in preparation) seem to define subsets of rat DC. Alternatively, this discrepancy could be related to different maturation stages between thymus and spleen DC. The ability of the different types of DC to kill other target cells in longer assays, or the same targets after cytokine activation, is under investigation.
Two arguments indicated that rat spleen DC probably do not kill YAC-1 target cells through the FasL/Fas pathway. First, we could not detect membrane FasL expression on spleen or thymus DC, as determined by FACS® using a Fas-Ig chimeric molecule (data not shown). Second, the in vitro cytolytic activity of splenic DC was abrogated upon addition of EGTA (2 mM) to the culture, together with 2 mM MgCl2 to ensure excess Mg2+ concentrations (Fig. 5). The viability of spleen DC during the assay was not modified by the presence of EGTA (data not shown). These results indicated that the mechanism of spleen DC killing was Ca2+ dependent, and thus unlikely to be related to FasL/Fas interaction (24). This contrasts with the recent report of Süss and Shortman showing that a subset of spleen DC in mice express a functional Fas ligand molecule (15). It is unlikely that the complete inhibition of splenic DC killing by the chelating agent EGTA was related to the inhibition of the ligand binding capacity of NKR-P1 molecule because EGTA concentration used in our assays (2 mM) is not sufficient to inhibit its carbohydrate-binding capacity (25). The dependence on Ca2+ of rat splenic DC killing rather suggests a mechanism of granule exocytosis as described for CTL and NK cells. Whether rat spleen DC contain perforin-containing granules is under investigation.
Figure 5.
Spleen DC kill the YAC-1 target cells using a Ca2+-dependent mechanism. 51Cr-labeled YAC-1 target cells (2 × 103) were incubated with purified spleen DC (95–98.5%) in triplicate for 6 h at 37°C in the presence (open square) or absence (closed square) of 2 mM EGTA and 2 mM MgCl2. 51Cr release was then assessed in the supernatant. Results of one experiment representative of eight are shown.
To determine whether the NKR-P1 molecule on spleen DC is involved in the killing mechanism of YAC-1 cells, we performed studies in which 3.2.3 mAb was preincubated with DC or added to the assay medium. No significant changes were observed in the lysis of target YAC-1 cells by purified DC. However, the 3.2.3 mAb was able to significantly redirect lysis of P815 target cells by splenic DC, since the killing was increased approximately twofold as compared to that in the presence of control isotype-matched mAb (data not shown). Ligation of NKR-P1 on NK cells initiates a transmembrane signal that mimics the response of these cells to target cells (16), stimulates granule exocytosis and mediates redirected lysis of NK-resistant target cells (17). Our results also suggest that NKR-P1 could also activate cytotoxic mechanisms of DC. Moreover, preliminary results indicate that ligation of NKR-P1 on DC during allogeneic mixed leukocyte culture could substantially increase the proliferative response of T cells. The hypothesis that this stimulatory function of NKR-P1 is due to the production of a soluble factor by DC is consistent with the fact that NKR-P1 cross-linking on mouse NK cells induces not only cytotoxicity but also IFN-γ production (26). As recently demonstrated for other important C-type lectin receptors present on DC such as DEC-205 (6) and the mannose receptor (5), NKR-P1 could also play a role in receptor-mediated endocytosis and thus enhance antigen-presenting function of DC to syngeneic T cells.
It has been reported that DC can infiltrate primary tumors in experimental animals and humans and cause tumor regression (27). In light of the results presented here, it may be possible that this effect depends on a Ca2+-dependent NK-like cytotoxic function of DC similar to that we observed in vitro. Destruction of tumoral target cells by DC could then facilitate the phagocytosis of cell fragments and the presentation of peptides from tumor-associated antigens to T cells, leading to efficient T cell responses against the tumor.
Of interest, Fossum and Rolstad have shown that after intravenous injection of allogeneic lymphocytes, large fragments of these cells were found inside interstitial DC of secondary lymphoid organs (28). These authors failed to demonstrate NK activity, as determined by lysis of K562 target cells, with enriched population of DC collected from the thoracic duct, and concluded that NK cells are required for killing, and thus for cell fragments to be phagocytosed by DC. However, although the NK-like cytotoxic activity of our DC preparations was easily observed with YAC-1 target cells, the killing of K562 (data not shown) target cells was extremely low, and only spleen but not thymus exhibited this activity. Whether rat splenic DC kill allogeneic cells in vitro and in vivo and whether they express killer inhibitory receptors for specific class I molecule (29) is under investigation.
In conclusion, we showed in this study that rat DC exhibited both a NK-like phenotype and function. Together with the potent antigen processing and presenting function of DC, their ability to kill some target cells could create a direct link between innate and adaptive immunity. It remains to be determined whether this function occurs in vivo and whether it is restricted to the rat or is also present in mice and humans.
Acknowledgments
We thank Bice Perussia, Ralph Steinman, and Bryce van Denderen for critically reading the manuscript.
References
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Romani N, Kuide S, Crowley M, Witmer-Pack M, Livingstone AM, Fathman CG, Inaba K, Steinman RM. Presentation of exogenous protein antigens by dendritic cells to T cell clones: intact protein is presented best by immature, epidermal Langerhans cells. J Exp Med. 1989;169:1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois B, Vanbervliet B, Fayette J, Massacrier C, van Kooten C, Brière F, Banchereau J, Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J Exp Med. 1997;185:941–951. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature (Lond) 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 7.Nijman H, Kleijmeer M, Ossevort M, Oorschot V, Vierboom M, van de Keur M, Kenemans P, Kast W, Geuze H, Melief C. Antigen capture and MHC class II compartments of freshly isolated and cultured human blood dendritic cells. J Exp Med. 1995;182:163–174. doi: 10.1084/jem.182.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1980;171:1753–1772. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenan M, Puklavec M. The MRC OX-62 antigen: a useful marker in the purification of rat veiled cells with the biochemical properties of an integrin. J Exp Med. 1992;175:1457–1465. doi: 10.1084/jem.175.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Neill HC. The lineage relationship of dendritic cells with other hematopoietic cells. Scand J Immunol. 1994;39:513–516. doi: 10.1111/j.1365-3083.1994.tb03407.x. [DOI] [PubMed] [Google Scholar]
- 11.Ardavin C, Wu L, Li C-L, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature (Lond) 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 12.Galy A, Travis M, Cen D, Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3:459–473. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 13.Caux C, Liu Y-J, Banchereau J. Recent advances in the study of dendritic cells and follicular dendritic cells. Immunol Today. 1996;16:2–4. doi: 10.1016/0167-5699(95)80061-1. [DOI] [PubMed] [Google Scholar]
- 14.Vremec D, Zorbas M, Scollay R, Saunders D, Ardavin C, Wu L, Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;17:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Süss G, Shortman K. A subclass of dendritic cells kill CD4 T cells via Fas/Fas-ligand–induced apoptosis. J Exp Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama WM, Seaman WE. The Ly-49 and NKR-P1 gene families encoding lectin-like receptors on natural killer cells: the NK gene complex. Annu Rev Immunol. 1993;11:613–635. doi: 10.1146/annurev.iy.11.040193.003145. [DOI] [PubMed] [Google Scholar]
- 17.Chambers WH, Vujanovic NL, DeLeo AB, Olszowy MW, Herberman RB, Hiserodt JC. Monoclonal antibody to a triggering structure expressed on rat killer cells and adherent lymphokine-activated killer cells. J Exp Med. 1989;169:1373–1389. doi: 10.1084/jem.169.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan JC, Niemi EC, Nakamura MC, Seaman WE. NKR-P1A is a target-specific receptor that activates natural killer cell cytotoxicity. J Exp Med. 1995;181:1911–1915. doi: 10.1084/jem.181.5.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan JC, Niemi EC, Goldfien RD, Hiserodt JC, Seaman WE. NKR-P1, an activating molecule on rat natural killer cells, stimulates phosphoinositide turnover and a rise in intracellular calcium. J Immunol. 1991;147:3244–3250. [PubMed] [Google Scholar]
- 20.Woda BA, Biron CA. Natural killer cell number and function in the spontaneously diabetic BB/W rat. J Immunol. 1986;137:1860–1866. [PubMed] [Google Scholar]
- 21.Thome M, Duplay P, Guttinger M, Acuto O. Syk and ZAP-70 mediate recruitment of p56lck/CD4 to the activated T cell receptor/CD3/δ complex. J Exp Med. 1995;181:1997–2006. doi: 10.1084/jem.181.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuturi M, Josien R, Cantarovich D, Douillard P, Smit H, Menoret S, Pouletty P, Clayberger C, Soulillou J. Prolongation of allogeneic heart graft survival in rats by administration of a peptide (a.a. 75–84) from the α1 helix of the first domain of HLA-B7 01. Transplantation (Baltimore) 1995;59:661–669. doi: 10.1097/00007890-199503150-00003. [DOI] [PubMed] [Google Scholar]
- 23.Maraskosky E, Brasel K, Teepe M, Roux E, Lyman S, Shortman K, McKenna H. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouvier E, Luciani M, Goldstein P. Fas involvement in Ca2+-independent T cell–mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bezouska K, Vlahas G, Horvath O, Jinochova G, Fiserova A, Giorda R, Chambers WH, Feizi T, Pospisil M. Rat natural killer cell antigen, NKR-P1, related to C-type animal lectins is a carbohydrate-binding protein. J Biol Chem. 1994;269:16945–16952. [PubMed] [Google Scholar]
- 26.Arase H, Arase N, Saito T. Interferon γ production by natural killer (NK) cells and the NK1.1+T cells upon NKR-P1 cross-linking. J Exp Med. 1996;183:2391–2396. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker Y. Dendritic cell activity against primary tumors: an overview. In Vivo. 1993;7:187–191. [PubMed] [Google Scholar]
- 28.Fossum S, Rolstad B. The roles of interdigitating cells (IDC) and natural killer (NK) cells in the rapid rejection of allogeneic lymphocytes. Eur J Immunol. 1986;16:440–451. doi: 10.1002/eji.1830160422. [DOI] [PubMed] [Google Scholar]
- 29.Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari MC, Moretta L. Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol. 1996;14:619–648. doi: 10.1146/annurev.immunol.14.1.619. [DOI] [PubMed] [Google Scholar]