Abstract
Clinical isolates of primate immunodeficiency viruses, including human immunodeficiency virus type 1 (HIV-1), enter target cells by sequential binding to CD4 and the chemokine receptor CCR5, a member of the seven-transmembrane receptor family. HIV-1 variants which use additional chemokine receptors are present in the central nervous system or emerge during the course of infection. Simian immunodeficiency viruses (SIV) have been shown to use CCR5 as a coreceptor, but no other receptors for these viruses have been identified. Here we show that two orphan seven-transmembrane segment receptors, gpr1 and gpr15, serve as coreceptors for SIV, and are expressed in human alveolar macrophages. The more efficient of these, gpr15, is also expressed in human CD4+ T lymphocytes and activated rhesus macaque peripheral blood mononuclear cells. The gpr15 and gpr1 proteins lack several hallmarks of chemokine receptors, but share with CCR5 an amino-terminal motif rich in tyrosine residues. These results underscore the potential diversity of seven-transmembrane segment receptors used as entry cofactors by primate immunodeficiency viruses, and may contribute to an understanding of viral variation and pathogenesis.
The human immunodeficiency viruses, HIV-1 and HIV-2, induce acquired immunodeficiency syndrome (AIDS) in humans, and simian immunodeficiency viruses (SIV)1 can induce AIDS-like illness in Old World monkeys (1–5). Isolates of HIV-1, the major cause of AIDS in humans, have been phylogenetically segregated into groups M and O (6). HIV-2 and SIV form a distinct group of phylogenetically and antigenically related viruses (2, 3, 6–8).
AIDS induced by HIV-1 or HIV-2 in humans or by SIV in monkeys is characterized by the depletion of CD4+ T lymphocytes, which represent a major target of viral infection in vivo (9). Infection of other CD4+ cell types, such as monocytes in the blood, tissue macrophages, and microglial cells in the brain, has been suggested to be important for the pathogenesis of primate immunodeficiency viruses in the central nervous system and in the lungs (10–14). Certain populations of dendritic cells in the blood and tissues may also be infected by these viruses (15, 16).
The tropism of primate immunodeficiency viruses for CD4+ cells is explained by the use of the CD4 glycoprotein as a primary receptor for virus entry into the cell (17– 19). The viral envelope glycoproteins, which mediate virus entry, consist of the gp120 exterior envelope glycoprotein and the gp41 transmembrane glycoprotein (20, 21). The gp120 glycoprotein binds the CD4 molecule, following which the gp120–CD4 complex binds one of the members of the chemokine receptor subgroup of seven-transmembrane segment (7-TMS) receptors (22–24). This binding is believed to promote conformational changes in the gp120 and gp41 glycoproteins which result in the fusion of viral and cellular membranes (25–27).
Viral variation, particularly that found in the gp120 glycoprotein sequences (28, 29), dictates the specific chemokine receptor which can be used as an entry cofactor. M-tropic HIV-1 variants which use the chemokine receptor CCR5 as a coreceptor predominate during the asymptomatic stages of infection (30–35). CCR5 is expressed on T lymphocytes, monocytes/macrophages, brain microglia, and dendritic cells (36–39). Individuals with defects in CCR5 expression are relatively resistant to HIV-1 infection (40–42), indicating the critical contribution of this chemokine receptor to virus transmission. Some M-tropic brain isolates of HIV-1 also use the chemokine receptor CCR3 as a coreceptor, consistent with the expression of CCR3 in brain microglia (39). Later in the course of infection, T-tropic HIV-1 variants emerge which can use chemokine receptors, especially CXCR4, but also CCR3 and CCR2b, in addition to CCR5 (34, 35, 43–45). The emergence of these viruses has been suggested to coincide with a less favorable clinical prognosis (45), perhaps through an expansion of the range of infectable CD4+ T cell subsets (46).
Primary isolates of HIV-2 and SIV have been shown to use rhesus macaque or human CCR5 as a coreceptor (47, 48) and are inhibited by the natural CCR5 ligands, MIP-1α, MIP-1β, and RANTES (49). None of the other known HIV-1 coreceptors has been shown to be used by SIV, whereas some isolates of HIV-2 can use CXCR4 for entry into CD4− cells (50). Several lines of evidence have suggested the existence of at least one other coreceptor for SIV. A human B cell/T cell hybrid, CEM×174, supports SIV entry, but lacks CCR5 and does not support efficient entry of HIV-1 viruses using CCR5 (48). A neuroglioma cell line, U87, stably transfected with CD4, similarly supports entry of SIVmac239 but does not allow for efficient entry of any known HIV-1 virus (51). Finally, PBMCs from humans lacking a functional CCR5 receptor can nonetheless be infected with SIV (48). Here we identify two additional SIV coreceptors, gpr1 and gpr15, which are expressed in U87 and CEM×174 cells, respectively. Both proteins are expressed in human alveolar macrophages, and the gpr15 protein is also expressed in CD4+ T lymphocytes.
Materials and Methods
Preparation of cDNA Libraries and cDNA.
Messenger RNA was isolated using the CsCl method and selection on magnetic beads with oligo-dT (Dynabeads; DYNAL, Inc., Lake Success, NY). RNA was obtained from purified human CD4+ peripheral blood T cells (gift of Dr. Linda Clayton, Dana-Farber Cancer Institutes, Boston, MA), human alveolar macrophages (gift of Dr. Hal Chapman, Brigham and Women's Hospital, Boston, MA), CEM×174 cells, and U87 neuroglioma cells. RNA was also isolated from phytohemagglutinin-treated, interleukin-2–stimulated PBMCs from a healthy rhesus macaque (New England Regional Primate Research Center, Foxboro, MA). The cDNA libraries from the U87 and CEM×174 cell lines were made by reverse transcription (Superscript; GIBCO BRL, Gaithersburg, MD) using a unidirectional primer supplied by the manufacturer. Size-selected cDNAs were cloned into a BstXI/NotI-digested pcDNA3.1 vector (Invitrogen, Carlsbad, CA). The human alveolar macrophage library was prepared by Invitrogen in pcDNA1. Double-stranded CD4+ T cell cDNA was synthesized using a kit from Boehringer Mannheim (Indianapolis, IN).
Cloning of cDNAs for 7-TMS Proteins.
The expression plasmids for rdc1, ebi2, gpr1, gpr15, and dez were prepared by PCR amplification of a cDNA library made from either CEM×174 or U87 cells, as described above. The amplified fragments were cloned into the pcDNA3 plasmid for expression. Expression plasmids for other chemokine receptors were generously supplied by Drs. Paul Ponath and Walter Newman (LeukoSite, Inc., Cambridge, MA) (v28, CXCR1, CXCR2), Dr. Elliot Kieff (Harvard Medical School, Boston, MA) (ebi1), and Dr. Monica Napolitano (Regina Elena Cancer Institut, Rome, Italy) (ter1).
Testing SIV Coreceptor Activity.
A previously described env-complementation method (27, 28, 47) was used to produce recombinant HIV-1 viruses which contained the SIV envelope glycoproteins and were capable of encoding chloramphenicol acetyltransferase (CAT) in target cells. Briefly, recombinant virus was incubated with Cf2Th cells transfected with plasmids expressing human CD4 and candidate coreceptors. Cells were harvested and assayed for CAT activity, which was determined by measuring the conversion of chloramphenicol to acetylated forms of chloramphenicol. The SIVmac239 and SIVmac316 envelope glycoproteins were expressed from the previously described pSIVΔgpv plasmid (47). The HIV-1 envelope glycoproteins were expressed as previously described (27, 28).
Analysis of Expression of 7-TMS Proteins in Cells and Tissues.
The expression of 7-TMS protein messenger RNA in CEM× 174 and U87 cells and primary human CD4+ T lymphocytes and alveolar macrophages was examined by synthesis of cDNA from polyadenylated RNA prepared from these cells, as described above. Primers corresponding to the nucleotide sequences encoding the first and third extracellular loops of the proteins were used for amplification by PCR. The identity of amplified fragments was confirmed by restriction enzyme digestion.
Results
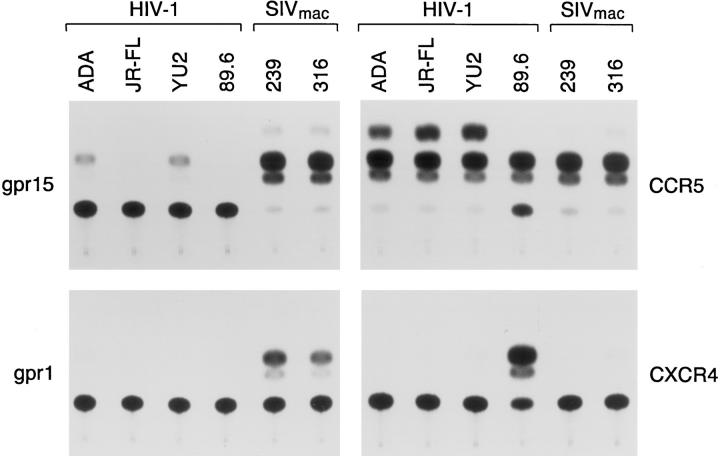
We had previously tested a number of human chemokine receptors (CCR1–CCR5, as well as CXCR4) and found that of these only CCR5 could support entry of an HIV-1 virus pseudotyped with the envelope glycoproteins of a pathogenic, molecularly cloned SIV, SIVmac239 (47). To identify additional coreceptors which might be used by SIV, we screened cDNA libraries from SIV-infectable cells, CEM×174 and U87, for the expression of mRNA encoding known 7-TMS proteins exhibiting some sequence similarity to chemokine receptors. The cDNAs which were shown to be expressed in either cell line were tested for the ability to support SIV and HIV-1 entry. Recombinant HIV-1 viruses which contained either HIV-1 or SIV envelope glycoproteins and expressed CAT were incubated with Cf2Th canine thymocytes transfected with plasmids expressing human CD4 and the 7-TMS proteins. Table 1 lists the 7-TMS proteins tested, summarizes their expression in CEM×174, U87, and human CD4+ T cells, and indicates coreceptor activity for viruses with the SIVmac239 envelope glycoproteins. Of the 7-TMS proteins tested, only gpr1, gpr15, and CCR5 supported the entry of viruses with the SIVmac239 envelope glycoproteins. These three 7-TMS proteins also supported the entry of viruses with the macrophage-tropic SIVmac316 envelope glycoproteins (Fig. 1). The SIV coreceptor activity exhibited by the gpr15 protein was greater than that of CCR5, whereas the coreceptor activity of gpr1 was ∼30% that of CCR5 (Table 2). Most of the viruses with HIV-1 envelope glycoproteins (HXBc2, JR-FL, 89.6) did not infect Cf2Th cells expressing CD4 and gpr15, although the viruses with the M-tropic HIV-1 ADA and YU2 envelope glycoproteins demonstrated a low but reproducible signal in these cells (Fig. 1 and Table 2). Following incubation with the ADA and YU2 viruses, the CAT conversion in the CD4+, gpr15+ Cf2Th cells was <1% of that seen in the CD4+, CCR5+ control cells (Table 2). Cf2Th cells expressing CD4 and gpr1 were not infected by viruses containing any of the HIV-1 envelope glycoproteins tested (Table 2).
Table 1.
Expression of 7-TMS Proteins and Activity as an SIVmac239 Coreceptor
| 7-TMS protein | Reference | Expression in: | SIVmac239 coreceptor activity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CEM×174 | U87 | Primary CD4+ T cells | ||||||||
| apj | 55 | − | − | − | ND | |||||
| blr1 | 56 | + | − | ND | − | |||||
| CCR5 | 38, 57 | − | − | + | + | |||||
| CXCR1 | 58 | ND | ND | ND | − | |||||
| CXCR2 | 59 | ND | ND | ND | − | |||||
| CXCR3 | 60 | ND | ND | ND | − | |||||
| CXCR4 | 61 | + | + | + | − | |||||
| dez | 62 | − | + | + | − | |||||
| ebi1 | 63 | + | + | + | − | |||||
| ebi2 | 63 | + | + | + | − | |||||
| gc96 | * | − | − | + | ND | |||||
| gcy4 | 64 | − | − | + | ND | |||||
| gpr1 | 53 | − | + | − | + | |||||
| gpr2 | 53 | − | − | − | ND | |||||
| gpr4 | 65 | − | − | + | ND | |||||
| gpr5 | 65 | − | − | − | ND | |||||
| gpr15 | 54 | + | − | + | + | |||||
| rdc1 | 66 | + | + | + | − | |||||
| ter1 | 67 | + | − | + | − | |||||
| v28 | 68 | ND | ND | ND | − | |||||
Positive expression values indicate the detection of a PCR product of expected size and restriction map amplified from the indicated cDNA source. Coreceptor activity for viruses with SIVmac239 envelope glycoproteins was determined as described in Materials and Methods.
These sequence data are available from EMBL/GenBank/DDBJ under accession number U45982.
Figure 1.
CAT activity in Cf2Th cells expressing CD4 alone or together with gpr1, gpr15, CCR5, or CXCR4 after incubation with HIV-1 recombinant viruses carrying the SIVmac239, SIVmac316, or HIV-1 (YU2, HXBc2, 89.6, or ADA) envelope glycoproteins. A representative experiment is shown. The amount of target cell lysate used was equivalent for all the experiments shown. CAT activity was determined by calculating the percentage of chloramphenicol present in acetylated forms (three uppermost spots) to the total amount of chloramphenicol. The nonacetylated form of chloramphenicol is present in the spot closest to the origin, which is near the bottom of the figure.
Table 2.
CAT Activity in Cf2Th Cells Expressing CD4 and 7-TMS Proteins following Incubation with Viruses Containing Different Envelope Glycoproteins
| 7-TMS protein | Viral envelope glycoproteins | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SIV | HIV-1 | |||||||||||
| SIVmac239 | SIVmac316 | ADA | YU2 | JR-FL | 89.6 | |||||||
| CXCR4 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 9.3 | ||||||
| CCR5 | 19.0 | 12.1 | 113.9 | 290.8 | 203.7 | 9.4 | ||||||
| gpr1 | 7.0 | 2.7 | <0.1 | <0.1 | <0.1 | <0.1 | ||||||
| gpr15 | 30.3 | 30.5 | 0.7 | 0.9 | <0.1 | <0.1 | ||||||
The percent conversion of chloramphenicol to acetylated forms is shown following incubation of comparable amounts of lysates derived from Cf2Th cells exposed to recombinant viruses. The CAT activity was calculated as described in the legend to Fig. 1. In some cases, dilutions of the lysates were tested to bring the assay within the linear range and, thus, the reported values exceed 100%.
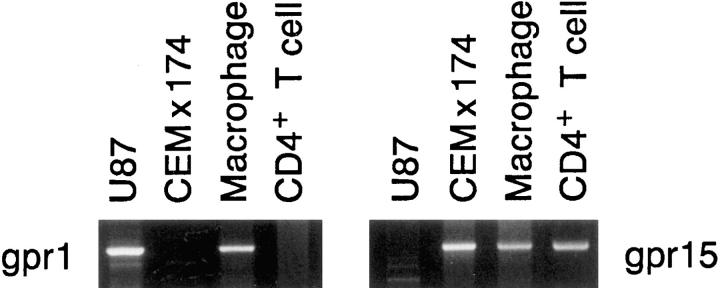
The expression of gpr15 and gpr1 in different cell types was examined. Since specific reagents to detect these proteins were not available, expression was examined by RNA analysis. A cDNA for gpr15 was readily detected in human CD4+ T lymphocytes, in human alveolar macrophages, in activated rhesus macaque PBMCs, and in CEM×174 cells, but not in U87 cells (Table 1, Fig. 2, and data not shown). By contrast, a cDNA for gpr1 could not be detected in primary human T lymphocytes, activated rhesus macaque PBMCs, or CEM×174 cells, but was detected in U87 cells and human alveolar macrophages.
Figure 2.
Expression of gpr1 and gpr15 RNA in cells. The cDNA libraries from U87 and CEM×174 cells and from human alveolar macrophages, as well as cDNA prepared from human CD4+ T lymphocytes, were PCR amplified using gpr1- or gpr15-specific primers.
Discussion
HIV-1, HIV-2, and SIV all use CCR5 as a coreceptor, indicating the importance of this protein in primate immunodeficiency virus pathogenesis (28, 30–33, 47, 48). The use of other chemokine receptors by HIV-1 has been suggested to be important for infection of anatomical compartments such as the brain or for more efficient T cell depletion (39, 45, 46). The identification of gpr1 and gpr15 as additional SIV coreceptors should assist efforts to understand the consequences of the use of coreceptors other than CCR5 in primate models of AIDS. While the in vivo contribution of gpr15 to SIV replication and pathogenesis requires further investigation, several lines of evidence indicate that gpr15 is an important SIV coreceptor. The gpr15 protein is expressed on CD4+ T lymphocytes, a major target cell for SIV infection in vivo (10), and on alveolar macrophages. The gpr15 protein is also expressed on CEM× 174 cells, which are routinely used to passage SIV obtained from monkey PBMCs (52). The rapid outgrowth of SIV viruses with minimal sequence changes on CEM×174 cells suggests that these cells express a receptor used by primary SIV viruses. The weak use of gpr15 by the ADA and YU2 HIV-1 viruses may be an inadvertent consequence of similarities in the amino-terminal regions of gpr15 and CCR5, or may indicate that adaptation to these receptors or to a related receptor occurs in some subsets of HIV-1.
The in vivo contribution of gpr1 to primate immunodeficiency virus infection is also unresolved. The gpr1 protein weakly supported SIV infection in our studies. Whether this inefficient coreceptor activity is an intrinsic property of gpr1 or merely reflects low cell surface expression of gpr1 requires further investigation. While gpr1 is not apparently expressed on primary CD4+ lymphocytes, it is expressed on tissue macrophages and in the brain (53) and thus may play a role in SIV infection of particular nonlymphoid target cells.
In primary structure, gpr1 and gpr15 resemble the angiotensin II receptor and the orphan receptors dez and apj more than they do any of the known chemokine receptors (53, 54). Gpr15, like dez and gpr1, lacks the cysteines in the NH2-terminal region and the third extracellular loop which, in the chemokine receptors, are thought to be disulfide linked. It is interesting that despite the general sequence divergence of gpr15/gpr1 and other identified primate immunodeficiency virus coreceptors the gpr15 and gpr1 amino termini contain three tyrosines which align with similarly positioned tyrosines in CCR5 (Fig. 3). Alteration of these tyrosines has been shown to decrease the efficiency with which CCR5 supports the entry of SIV and M-tropic HIV-1 isolates (Farzan, M., H. Choe, and J. Sodroski, unpublished observations). The identification of gpr15 and gpr1 as SIV coreceptors suggests a greater range and complexity of coreceptors for the primate immunodeficiency viruses than previously described. Comparative studies of these divergent coreceptors with the known coreceptors for these viruses should assist in the identification of common structural elements in 7-TMS proteins which serve as viral entry cofactors.
Figure 3.
An alignment of human gpr1, human gpr15, rhesus CCR5 (rccr5), and human CCR5 from the NH2-terminus through the first cysteine of CCR5 is shown. Tyrosines shown to be important for HIV-1 and SIVmac239 entry are shown in bold. Other residues similarly positioned in these proteins are underlined. Sequences for gpr1 and gpr15 are provided in references 53 and 54, respectively.
Acknowledgments
We thank Dr. Ronald Desrosiers for the gift of the SIVmac239 and SIVmac316 infectious proviral clones and Drs. Paul Ponath, Walter Newman, Elliot Kieff, and Monica Napolitano for plasmids expressing chemokine receptors. We thank Ms. Lorraine Rabb and Ms. Yvette McLaughlin for manuscript preparation.
This work was supported by a grant to Joseph Sodroski from the National Institutes of Health (AI-24755) and by a Center for AIDS Research grant to the Dana-Farber Cancer Institute (AI-28691). Dana-Farber Cancer Institute is also the recipient of a Cancer Center grant from the National Institutes of Health (CA-06516). Luisa Marcon was supported by National Cancer Institute National Research Science Award Training Grant (CA-09382) and by an award from Istituto Superiore di Sanitá and by the University of Padua. Craig Gerard was supported by National Institutes of Health grants HL-51366 and AI-36162 as well as by the Rubenstein/Cable Fund at the Perlmutter Laboratory. This work was made possible by gifts from the late William McCarty-Cooper, from the G. Harold and Leila Y. Mathers Charitable Foundation, and from the Friends 10.
Footnotes
Abbreviations used in this paper: 7-TMS, seven-transmembrane segment; CAT, chloramphenicol acetyltransferase; SIV, simian immunodeficiency virus.
M. Farzan and H. Choe contributed equally to this work.
References
- 1.Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Charmaret S, Gruest J, Dauget C, Axler-Bin C, Vezinet-Brun F, Rouzioux C, et al. Isolation of a T-lymphocyte retrovirus from a patient at risk for acquired immunodeficiency syndrome (AIDS) Science (Wash DC) 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 2.Clavel F. HIV-2, the West African AIDS virus. AIDS (Lond) 1987;1:135–140. [PubMed] [Google Scholar]
- 3.Desrosiers RC. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- 4.Gallo RC, Salahuddin SZ, Popovic M, Shearer GM, Kaplan M, Haynes BF, Palker TJ, Redfield R, Oleske J, Safai B, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science (Wash DC) 1984;272:872–877. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 5.Letvin NL, Daniel MD, Sehgal PK, Desrosiers RC, Hunt RD, Waldron LM, MacKey JJ, Schmidt DK, Chalifoux LV, King NW. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovrus STLV-III. Science (Wash DC) 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 6.Myers, G., S. Wain-Hobson, L. Henderson, B. Korber, K.-T. Jeang, and G. Pavlakis. 1994. Human Retroviruses and AIDS: A Compilation and Analysis of Nucleic Acid and Amino Acid Sequences. Los Alamos National Laboratory, Los Alamos, New Mexico. Section III:23–31.
- 7.Kanki P, McLane M, King N, Essex M. Serological identification and characterization of a macaque T-lymphocytic retrovirus closely related to HTLV-III. Science (Wash DC) 1985;228:1199–1201. doi: 10.1126/science.3873705. [DOI] [PubMed] [Google Scholar]
- 8.Weiss R, Clapham P, Weber J, Dalgleish A, Lasky L, Berman P. Variable and conserved neutralization antigens of human immunodeficiency virus. Nature (Lond) 1986;324:572–575. doi: 10.1038/324572a0. [DOI] [PubMed] [Google Scholar]
- 9.Fauci A, Macher A, Longo D, Lane HC, Rook A, Masur H, Gelmann E. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984;100:92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- 10.Desrosiers RC, Hansen-Moosa A, Mori K, Bouvier DP, King NW, Daniel MD, Ringler DJ. Macrophage-tropic variants of SIV are associated with specific AIDS-related lesions but are not essential for the development of AIDS. Am J Pathol. 1991;139:29–35. [PMC free article] [PubMed] [Google Scholar]
- 11.Gartner S, Markovits P, Markovitz D, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science (Wash DC) 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 12.Gendelman H, Orenstein J, Martin M, Ferrva C, Mitra R, Phipps T, Wahl L, Lane C, Fauci A, Burke D. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1–treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koenig S, Gendelman HE, Orenstein JM, Dal MC, Canto, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science (Wash DC) 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 14.Ringler DJ, Wyand MS, Walsh DG, MacKey JJ, Sehgal PK, Daniel MD, Desrosiers RC, King NW. The productive infection of alveolar macrophages by simian immunodeficiency virus. J Med Primatol. 1989;18:217–226. [PubMed] [Google Scholar]
- 15.Pope M, Betjes M, Romani N, Hirmand H, Cameron P, Hoffman L, Gezelter S, Schuler G, Steinman R. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 16.Weissman D, Li Y, Ananworanich J, Zhou L-J, Adelsberger J, Tedder T, Baseler M, Fauci A. Three populations of cells with dendritic morphology exist in peripheral blood only one of which is infectable with human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1995;92:826–830. doi: 10.1073/pnas.92.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalgleish AG, Beverley PCL, Clapham PR, Crawford DH, Greaves MF, Weiss RA. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature (Lond) 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 18.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman JC, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature (Lond) 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 19.Maddon PJ, Dalgleish AG, McDougal JS, Clapham PR, Weiss RA, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 20.Allan J, Lee TH, McLane MF, Sodroski J, Haseltine W, Essex M. Identification of the major envelope glycoprotein product of HTLV-III. Science (Wash DC) 1983;228:1091–1094. [Google Scholar]
- 21.Robey WG, Safai B, Oroszlan S, Arthur L, Gonda M, Gallo R, Fischinger PJ. Characterization of envelope and core structural gene products of HTLV-III with sera from AIDS patients. Science (Wash DC) 1985;228:593–595. doi: 10.1126/science.2984774. [DOI] [PubMed] [Google Scholar]
- 22.Lapham C, Ouyang J, Chandraseklar B, Nguyen N, Dimitrov D, Golding H. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science (Wash DC) 1996;274:602–605. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 23.Wu L, Gerard N, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A, Desjardin E, Newman W, et al. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR5. Nature (Lond) 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 24.Trkola A, Dragic T, Arthos J, Binley JM, Olson WC, Allaway GP, Cheng-Mayer C, Robinson J, Maddon PJ, Moore JP. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature (Lond) 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 25.Stein B, Gouda S, Lifson J, Penhallow R, Bensch K, Engelman E. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987;49:659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- 26.Kowalski M, Potz J, Basiripour L, Dorfman T, Goh WG, Terwilliger E, Dayton A, Rosen C, Haseltine W, Sodroski J. Functional regions of the human immunodeficiency virus envelope glycoproteins. Science (Wash DC) 1987;237:1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- 27.Helseth E, Kowalski M, Gabuzda D, Olshevsky U, Haseltine W, Sodroski J. Rapid complementation assays measuring replicative potential of HIV-1 envelope glycoprotein mutants. J Virol. 1990;64:2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, et al. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 29.Cocchi F, DeVico A, Garzino-Demo A, Cara A, Gallo RC, Lusso P. The V3 domain of the HIV-1 gp120 glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 30.Alkhatib G, Combadiere C, Broder CC, Feng Y, Murphy PM, Berger E. CC-CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science (Wash DC) 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 31.Deng HK, Choe S, Ellmeier W, Liu R, Unutmaz D, Burkhart M, di Marzio P, Marmon S, Sutton RE, Hill CM, et al. Identificaation of a major co-receptor for primary isolates of HIV-1. Nature (Lond) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 32.Doranz B, Rucker J, Yi Y, Smyth R, Samson M, Peiper S, Parmentier M, Collman R, Doms R. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 33.Dragic T, Litwin V, Allaway GP, Martin S, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+cells is mediated by the chemokine receptor CC-CKR-5. Nature (Lond) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Hsung Y, He T, Cao Y, Ho DD. HIV-1 subtype and second-receptor use. Nature (Lond) 1996;383:768. doi: 10.1038/383768a0. [DOI] [PubMed] [Google Scholar]
- 35.Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. Change in coreceptor use correlates with disease progression in HIV-1 infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu L, Paxton WA, Kassam N, Ruffing N, Rottman JB, Sullivan N, Choe H, Sodroski J, Newman W, Koup RA, Mackay CR. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1 in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O'Doherty U, Paxton W, Koup R, Mojsov S, et al. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raport CJ, Gosling J, Schweickart VL, Gray PW, Charo IF. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1β, and MIP-1α. J Biol Chem. 1996;271:17161–17166. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 39.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature (Lond) 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 40.Liu R, Paxton W, Choe S, Ceradini D, Martin S, Horuk R, MacDonald M, Stuhlmann H, Koup R, Landau N. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–378. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 41.Dean M, Carrington M, Winkler C, Huttley GA, Smith MW, Allikmets R, Goedert JJ, Buchbinder SP, Vittinghoff E, Gomperts E, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science (Wash DC) 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 42.Samson M, Libert F, Doranz B, Rucker B, Liesnard C, Farber C, Saragosti S, Lapouméroulie C, Cognaux J, Forceille C, et al. Resistance to HIV-1 infection of Caucasian individuals bearing a mutant allele of the CCR5 chemokine receptor gene. Nature (Lond) 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 43.Simmons G, Wilkinson D, Reeves JD, Dittmar MT, Beddows S, Weber J, Carnegie G, Desselberger U, Gray PW, Weiss RA, Clapham PR. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either LESTR or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein–coupled receptor. Science (Wash DC) 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 45.Schuitemaker H, Koot M, Koostra NA, Dercksen MW, deGoede REY, van Steenwijk RP, Lange JMA, Eeftink-Schattenkert JKM, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell– tropic virus populations. J Virol. 1992;65:356–363. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1996;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marcon L, Choe H, Martin KA, Farzan M, Ponath PD, Wu L, Newman W, Gerard N, Gerard C, Sodroski J. Utilization of C-C chemokine receptor 5 by the envelope glycoproteins of a pathogenic simian immunodeficiency virus (SIVmac239) J Virol. 1997;71:2522–2527. doi: 10.1128/jvi.71.3.2522-2527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Z, Zhou P, Ho DD, Landau N, Marx P. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J Virol. 1997;71:2705–2714. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cocchi F, DeVico A, Garzino-Demo A, Arya S, Gallo R, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science (Wash DC) 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 50.Endres M, Clapham P, Marsh M, Ahuja M, Turner J, McKnight A, Thomas J, Stoebenau-Haggarty B, Choe S, Vance P, et al. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 51.Clapham P, Blanc D, Weiss R. Specific cell surface requirements for the infection of CD4-positive cells by human immunodeficiency virus types 1 and by simian immunodeficiency virus. Virology. 1991;181:703–715. doi: 10.1016/0042-6822(91)90904-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kestler H, Ringler D, Mori K, Panicali D, Sehgal P, Daniel M, Desrosiers R. Importance of the nefgene for maintenance of high virus loads and for the development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 53.Marchese A, Docherty J, Nguyen T, Heiber M, Cheng R, Heng H, Tsui L, Shi X, George S, O'Dowd B. Cloning of human genes encoding novel G protein–coupled receptors. Genomics. 1994;23:609–618. doi: 10.1006/geno.1994.1549. [DOI] [PubMed] [Google Scholar]
- 54.Heiber M, Marchese A, Nguyen T, Heng H, George S, O'Dowd B. A novel human gene encoding a G-protein–coupled receptor (gpr15) is located on chromosome 3. Genomics. 1996;32:462–465. doi: 10.1006/geno.1996.0143. [DOI] [PubMed] [Google Scholar]
- 55.O'Dowd B, Heiber M, Chan A, Heng H, Tsui L, Kennedy J, Shi X, Petronis A, George S, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene (Amst) 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- 56.Dobner T, Wolf I, Emrich T, Lipp M. Differentiation-specific expression of a novel G protein–coupled receptor from Burkitt's lymphoma. Eur J Immunol. 1992;22:2795–2799. doi: 10.1002/eji.1830221107. [DOI] [PubMed] [Google Scholar]
- 57.Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. Molecular cloning and functional expression of a new human CC-chemokine receptors gene. Biochemistry. 1996;35:3362–3367. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- 58.Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science (Wash DC) 1991;253:1280–1283. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- 59.Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science (Wash DC) 1991;253:1278–1280. [Google Scholar]
- 60.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Federsppiel B, Melhado IG, Duncan AM, Delaney A, Schappert K, Clarke-Lewis I, Jirik FR. Molecular cloning of the cDNA and chromosomal localization of the gene for a putative seven-transmembrane segment (7-TMS) receptor isolated from human spleen. Genomics. 1993;16:706–712. doi: 10.1006/geno.1993.1251. [DOI] [PubMed] [Google Scholar]
- 62.Methner A, Hermey G, Schinke B, Hermans-Borgmeyer I. A novel G protein–coupled receptor with homology to neuropeptide and chemoattractant receptors expressed during bone development. Biochem Biophys Res Commun. 1997;238:836–842. doi: 10.1006/bbrc.1997.6455. [DOI] [PubMed] [Google Scholar]
- 63.Birkenbach M, Josefsen K, Yalamanchili R, Lenoir G, Kieff E. Epstein-Barr virus–induced genes: first lymphocyte-specific G protein-coupled peptide receptors. J Virol. 1993;67:2209–2220. doi: 10.1128/jvi.67.4.2209-2220.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liao F, Lee HH, Farber JM. Cloning of STRL22, a new human gene encoding a G-protein–coupled receptor related to chemokine receptors and located on chromosome 6q27. Genomics. 1997;40:175–180. doi: 10.1006/geno.1996.4544. [DOI] [PubMed] [Google Scholar]
- 65.Heiber M, Docherty JM, Shah G, Nguyen T, Cheng R, Heng H, Marchese A, Tsui L, Shi X, George SR, O'Dowd BF. Isolation of three novel human genes encoding G protein-coupled receptors. DNA Cell Biol. 1995;14:25–35. doi: 10.1089/dna.1995.14.25. [DOI] [PubMed] [Google Scholar]
- 66.Libert F, Parmentier M, Lefort A, Dumont JE, Vassart G. Complete nucleotide sequence of a putative G protein coupled receptor: RDC1. Nucleic Acids Res. 1990;18:1917. doi: 10.1093/nar/18.7.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Napolitano M, Zingoni A, Bernardini G, Spinett G, Nista A, Storlazzi CT, Rocchi M, Santoni A. Molecular cloning of TER1, a chemokine receptor-like gene expressed by lymphoid tissues. J Immunol. 1996;157:2759–2763. [PubMed] [Google Scholar]
- 68.Combadiere C, Ahuja SK, Murphy PM. Cloning, chromosomal localization, and RNA expression of a human beta chemokine receptor–like gene. DNA Cell Biol. 1995;14:673–680. doi: 10.1089/dna.1995.14.673. [DOI] [PubMed] [Google Scholar]