Abstract
Inhibition of natural killer (NK) cells by the killer cell inhibitory receptor (KIR) involves recruitment of the tyrosine phosphatase SHP-1 by KIR and is prevented by expression of a dominant negative SHP-1 mutant. Another inhibitory receptor, the low affinity Fc receptor for immunoglobulin G (IgG) (FcγRIIb1), has been shown to bind SHP-1 when cocross-linked with the antigen receptor on B cells (BCR). However, coligation of FcγRIIb1 with BCR and with FcεRI on mast cells leads to recruitment of the inositol 5′ phosphatase SHIP and to inhibition of mast cells from SHP-1–deficient mice. In this study, we evaluated the ability of these two inhibitory receptors to block target cell lysis by NK cells, and the contribution of SHP-1 and SHIP to inhibition. Recombinant vaccinia viruses encoding chimeric receptors and dominant negative mutants of SHP-1 and SHIP were used for expression in mouse and human NK cells. When the KIR cytoplasmic tail was replaced by that of FcγRIIb1, recognition of HLA class I on target cells by the extracellular domain resulted in inhibition. A dominant negative mutant of SHP-1 reverted the inhibition mediated by the KIR cytoplasmic tail but not that mediated by FcγRIIb1. In contrast, a dominant negative mutant of SHIP reverted only the inhibition mediated by the FcγRIIb1 tail, providing functional evidence that SHIP plays a role in the FcγRIIb1-mediated negative signal. These data demonstrate that inhibition of NK cells by KIR involves primarily the tyrosine phosphatase SHP-1, whereas inhibition mediated by FcγRIIb1 requires the inositol phosphatase SHIP.
Activation of different types of cells in the immune system shares common signal transduction pathways, such as activation of tyrosine kinases, turnover of phosphoinositides, and calcium mobilization. The description of several inhibitory receptors that can interrupt the activation process in different cell types has generated interest in the mechanism of inhibition and raised questions about features that may be shared by the different receptors. It has been proposed that both the inhibitory Fc receptor FcγRIIb1 and the killer cell inhibitory receptor (KIR) mediate inhibition by recruitment of the SH2-containing protein tyrosine phosphatase SHP-1 (1–6). However, it has also been shown that FcγRIIb1 can inhibit mast cells that do not express SHP-1 (7). The aim of this study was to compare the inhibition mediated by FcγRIIb1 and KIR in the same cell type and to evaluate the contribution of SHP-1 and SH2-containing inositol polyphosphatase 5′ phosphatase (SHIP) to these negative signals.
FcγRIIb1 inhibits activation responses when cocross-linked with the B cell, T cell, and mast cell antigen receptors (8–10). The cytoplasmic tail of FcγRIIb1 contains an immunoreceptor tyrosine-based inhibitory motif (ITIM), which is necessary for the inhibitory function of the receptor (9, 10). In vitro, the phosphorylated FcγRIIb1 ITIM associates with SHP-1 and to a 145-kD protein designated as SHIP (7). SHIP is involved in inhibition of growth factor and cytokine responses (11, 12). Coligation of FcγRIIb1 with the IgE receptor on bone marrow–derived mast cells from me/me mice, which lack SHP-1, still resulted in inhibition of degranulation (7), demonstrating that SHP-1 was not necessary for inhibition and suggesting that SHIP might be involved in the negative signal mediated by FcγRIIb1. However, no functional evidence has been obtained for a role of SHIP in the inhibition mediated by FcγRIIb1.
The KIR expressed on human natural killer (NK) cells deliver a negative signal upon recognition of MHC class I molecules on target cells (13, 14). Tyrosine phosphorylation of the ITIMs in the cytoplasmic tail of KIR is critical for recruitment of SHP-1 and for inhibition (2–6, 15). Expression of a dominant negative mutant of SHP-1 in NK cells prevented the inhibition mediated by KIR, suggesting an important role for SHP-1 in the negative signal (2). The possibility that SHIP may also be involved in the inhibition of NK cells mediated by KIR has not been investigated. To test rigorously whether KIR and FcγRIIb1 can deliver inhibitory signals in the same cell type through similar or distinct signaling pathways, and whether SHIP may be involved in the KIR-mediated inhibition of NK cells, we have constructed chimeric KIR molecules with the cytoplasmic tail of FcγRIIb1 and assessed their ability to inhibit NK cells. We report here that SHIP, but not SHP-1, is required for the negative signal transmitted by FcγRIIb1 and that the reverse is true for KIR.
Materials and Methods
Cells and Antibodies.
The human NK cell line NK92 (a gift from H.-G. Klingemann, The Terry Fox Laboratory, Vancouver, Canada) (16) was maintained in Myelocult H5100 medium (Stem Cell Technologies, Inc., Vancouver, Canada) and supplemented with 100 U/ml rIL-2. The B lymphoblastoid cell line 721.221 and its HLA class I transfectants .221-Cw3 and .221-Cw4 were provided by J. Gumperz and P. Parham (Stanford University, Stanford, CA). Mouse NK populations were generated from C57BL/6 mice as described (17). The anti-p58–KIR mAb GL183 (18) was provided by A. Moretta and C. Bottino (University of Genova, Italy) and anti-p58–KIR mAb EB6 was obtained from Immunotech, Inc. (Westbrook, ME). The FITC-conjugated F(ab′)2 goat anti– mouse IgG was from Jackson ImmunoResearch (West Grove, PA).
cDNAs and Construction of Chimeras.
The chimeric KIR-6/ RIIb1 cDNA was constructed by ligation of fragments encoding the extracellular and transmembrane regions of KIR-6 (a p58–KIR reactive with mAb GL183) with the cytoplasmic region of human FcγRIIb1. The cytoplasmic tail of FcγRIIb1 was amplified from a cDNA molecule containing the transmembrane and cytoplasmic regions of human FcγRIIb1 (a gift from J. Altrichter, National Institute of Allergy and Infectious Diseases, Bethesda, MD) using the forward primer 5′-TTGATCTACCTTAGGAAAAA GCGGATTTCAG-3′ containing a Bsu36I restriction site and reverse primer 5′-TGCACCGAATTCAGACTAAATACGG-3′ containing a stop codon followed by an EcoRI restriction site. The fragment encoding KIR-6 used for ligation was amplified from the plasmid pSPORT–p58–cl6 (19), using forward primer 5′-AGCTCCCGGAGCTCCTATGACATG-3′ containing a SacI site and the reverse primer 5′-GCAGCAGGATCCCCTAAGGAGAAAGAAGAGGAG-3′ containing a Bsu36I site and a BamHI site for cloning into pBluescript. The Bsu36I–EcoRI fragment encoding the cytoplasmic tail of FcγRIIB1 was ligated to the Bsu36I–EcoRI digested pBluescript–cl6, followed by excision of the SacI–EcoRV chimeric insert and subcloning into the SacI–StuI sites of pSPORT–cl6. The amino acids at the KIR-6/ FcγRIIb1 boundary are FFLL/RKKR. Chimeric KIR-42/RIIb1 was made by replacing the extracellular segment of KIR-6 between the restriction sites SalI–BstEII with that of KIR-42 (a p58-KIR reactive with mAb EB6). The BstEII site encodes the amino acids glycine and asparagine that are five residues upstream from the start of the transmembrane region in both KIR-6 and KIR-42. A truncated version of KIR-6 was generated by amplifying a fragment of pSPORT–cl6 (from the initiation codon to asparagine 258 of KIR-6) using the forward primer 5′-GTCAAACTCGAGTGACCCAC-3′ containing the XhoI site and the reverse primer 5′-CTGTTCTCCCGGGTCATCCTTGGTTGTCCATTACAACAGC-3′ containing StyI site followed by a stop codon and a SmaI site. The XhoI–SmaI fragment was then ligated back into the XhoI–StuI sites of pSPORT–cl6 such that the cytoplasmic tail is 17 amino acids long (the last three residues, asparagine, glutamine, and glycine were added before the stop codon during PCR amplification) and lacks the two ITIM sequences. The truncated KIR-6 (KIR-6tr) and the chimeric constructs KIR-6/RIIb1 and KIR-42/RIIb1 were subcloned into the plasmid pSC66 (gift of B. Moss, National Institute of Allergy and Infectious Diseases, Bethesda, MD) and used to generate recombinant vaccinia viruses as described (20).
Dominant Negative Mutants of SHP-1 and SHIP.
A recombinant vaccinia virus encoding a mutant form of SHP-1 with a point mutation in the catalytic site (cysteine 453 to serine) has been described (2). A cDNA encoding SHIP was used to make a deletion mutant containing the SH2 domain alone (referred to as SHIP– SH2) by PCR amplification from the start site to leucine 166 followed by a stop codon and a SalI site. The PCR fragment was cloned into a modified version of the vaccinia vector pSC66, which contains at its NH2 terminus an ATG initiation site in a suitable context, a Flag epitope (21), and a NotI site in-frame with the coding sequence.
Vaccinia Virus Infections.
Purified viruses encoding KIR-6, KIR-6/RIIb1, KIR-6tr, KIR-42, KIR-42/RIIb1, SHP-1C453S, and SHIP–SH2 were used to infect mouse NK populations or the human cell line NK92 as described (14).
Cytotoxicity Assays.
The ability of mouse NK and human NK92 cells to lyse targets was measured in a 4-h 51Cr release assay as described (14).
Results and Discussion
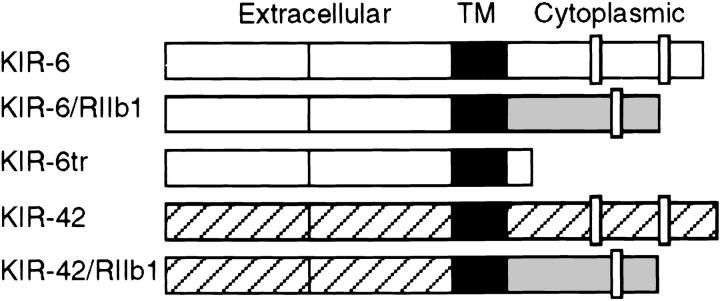
To test whether the cytoplasmic tail of FcγRIIb1 may inhibit NK cells, and to address the role of the tyrosine phosphatase SHP-1 and inositol phosphatase SHIP in the negative signal delivered by these receptors, chimeric molecules with the cytoplasmic tail of FcγRIIb1 and the extracellular domains of KIR-6 or KIR-42 were constructed (Fig. 1). As a control, a truncated version of KIR-6 was made (Fig. 1), which lacks both ITIM sequences that are essential for KIR-mediated inhibition (4, 15). KIR-6 prevents the lysis of HLA-Cw3 positive target cells, whereas KIR-42 prevents lysis of targets that express HLA-Cw4 (14). Recombinant vaccinia viruses were made that express the chimeric KIR-6/RIIb1, KIR-42/RIIb1, and KIR-6tr molecules.
Figure 1.
Schematic representation of the chimeric receptors. Hybrid molecules bearing the cytoplasmic tail of human FcγRIIb1 and the extracellular domains of KIR-cl6 or KIR-cl42 were generated as described. Open, hatched, and shaded boxes represent sequences from KIR-6, KIR-42, and FcγRIIb1, respectively. Closed boxes represent transmembrane regions and open bars in the cytoplasmic tails indicate the position of ITIMs.
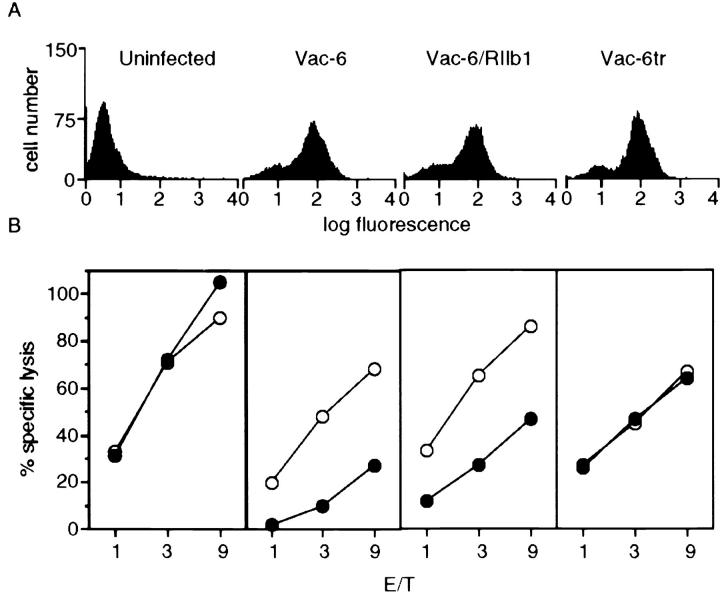
Expression of KIR-6 on mouse NK cells inhibits the antibody-dependent cell-mediated cytotoxicity of HLA-Cw3 positive .221 targets (15). NK1.1+, TCR− NK cells, prepared from the spleens of C57Bl/6 mice were infected with Vac-6, Vac-6/RIIb1, and Vac-6tr and tested for their ability to lyse .221 and .221-Cw3 target cells. The infected cells expressed comparable levels of the three receptors (Fig. 2 A). NK cells expressing KIR-6/RIIb1 lysed .221 cells but had reduced cytotoxic activity against .221-Cw3 cells. Uninfected cells and those infected with Vac-6tr lysed both .221 and .221-Cw3 to the same extent (Fig. 2 B). Thus, the cytoplasmic tail of FcγRIIb1 can deliver a signal that inhibits the cytotoxic function of NK cells.
Figure 2.
The cytoplasmic tail of human FcγRIIb1 can deliver an inhibitory signal in mouse NK cells. (A) Surface expression of KIR-6, KIR-6/RIIb1, and KIR-6tr on cells infected with 5, 5, and 10 PFU/cell of the indicated recombinant vaccinia viruses, respectively. (B) Specific lysis of the B cell line .221 (open circles), and its HLA-Cw3 transfectant (closed circles) by uninfected mouse NK cells or those infected with Vac-6, Vac-6/ RIIb1 or Vac-6tr. ADCC was induced by precoating the targets with 0.1 μg/ml of anti-HLA-DR mAb L243 for 30 min on ice. Effectors and targets were plated at the indicated ratios.
Expression of a catalytically inactive mutant of SHP-1 in human NK cells reverts the KIR-mediated inhibition of target cell lysis (2, 6). KIR-6, KIR-42, and their chimeric counterparts KIR-6/RIIb1 and KIR-42/RIIb1 were expressed in the human NK cell line NK92 either individually or together with the dominant negative mutant of SHP-1. The surface staining of Vac-6 and Vac-6/RIIb1 infected cells by GL183 and that of Vac-42 and Vac-42/RIIb1 infected cells by EB6 was comparable as seen by the mean fluorescence intensities (Fig. 3). Coinfection of NK92 cells with the receptors along with dominant negative SHP-1 did not alter their surface expression (Fig. 3). NK92 cells infected with Vac-6 or with Vac-6/RIIb1 killed .221 and .221-Cw4 targets but were inhibited from lysing .221-Cw3 targets. Reciprocally, cells infected with Vac-42 and Vac-42/RIIb1 lysed .221 and .221-Cw3 but not .221-Cw4 targets (Fig. 3). Therefore, as shown in mouse NK cells, the cytoplasmic tail of FcγRIIb1 was able to deliver an inhibitory signal in human NK cells. Expression of dominant negative SHP-1 in NK92 reverted the KIR-mediated inhibition of target cell lysis but not that mediated by the cytoplasmic tail of FcγRIIb1 (Fig. 3). Thus, SHP-1 appears to play an important role in KIR-mediated inhibition of NK cells, as reported earlier (2), but not in FcγRIIb1-mediated inhibition of NK cells.
Figure 3.
SHP-1 is not required for inhibition of NK cells by FcγRIIb1. The KIR-negative human NK92 cell line was infected with recombinant vaccinia viruses expressing KIR-6, KIR-42, or their chimeric derivatives bearing the cytoplasmic tail of human FcγRIIb1 (KIR-6/RIIb1 and KIR-42/RIIb1), alone or with dominant negative SHP-1 (dnSHP-1), as indicated. The mean fluorescence intensity of staining with mAbs GL183 (for KIR-6 and KIR-6/RIIb1) and EB6 (for KIR-42 and KIR-42/RIIb1) is indicated next to each bar in the .221 panel. Lysis of .221, .221-Cw3, and .221-Cw4 targets was determined in a 4-h 51Cr release assay at an E/T of 4. Similar results were observed at an E/T of 1 and in two independent experiments.
A recent report showed that mast cells from SHP-1–deficient me/me mice are inhibited by FcγRIIb1 (7). Coclustering FcγRIIb1 with the BCR or FcεRI led to recruitment of SHIP, suggesting that SHIP is involved in inhibition mediated by FcγRIIb1 (7). We tested functionally whether SHIP has a role in FcγRIIb1-mediated inhibition and whether it may also contribute to KIR-mediated inhibition of NK cells. KIR or chimeric KIR were expressed in NK92 cells together with a dominant negative mutant of SHIP containing the SH2 domain alone. Expression of SHIP–SH2 in NK92 caused some reduction in the ability of NK92 to lyse target cells, as well as a small reduction in the expression of KIR during coinfections (Fig. 4). The partial inhibition mediated by KIR-6 when NK92 were tested with .221-Cw4 cells has not been consistently observed. Coexpression of SHIP–SH2 prevented the inhibition mediated by KIR-6/ RIIb1 and KIR-42/RIIb1 but not that mediated by KIR-6 or by KIR-42 (Fig. 4).
Figure 4.
SHIP is required for inhibition of NK cells by FcγRIIb1 but not by KIR. The experiment was as described in Fig. 3 except that a dominant negative SHIP (dnSHIP) was used. The data shown were obtained at an E/T of 6. Similar results were obtained at an E/T of 1 and in two independent experiments.
These data provide functional evidence that SHIP is required for the negative signal delivered by FcγRIIb1. In addition, they demonstrate that two distinct signaling pathways can turn off the cytotoxic activity of NK cells upon interaction with target cells. The inhibitory receptor normally used by human NK cells to detect self-MHC class I on target cells requires interaction with the tyrosine phosphatase SHP-1 to deliver the inhibitory signal (2, 6). In contrast, FcγRIIb1, whose function is to inhibit B cell and mast cell activation in the presence of soluble IgG, requires the inositol phosphatase SHIP to inhibit NK cells. KIR signaling interferes with early activation events, including tyrosine phosphorylation of FcR-associated ζ chain, ZAP-70, and PLC-γ2 (6), and calcium flux (22, 23). KIR recognition of HLA class I on target cells was also found to prevent association of the adaptor protein pp36 with PLC-γ1 (23). The lack of a role for SHIP in KIR-mediated inhibition is consistent with the identification of SHP-1 as the only major protein purified from lysates of T, B, and NK cells by phosphopeptides corresponding to the KIR ITIMs immobilized on beads (24). In contrast, a phosphopeptide corresponding to the ITIM of FcγRIIb1 precipitated both SHP-1 and SHIP from such cell lysates (24). However, the functional evidence provided here indicates that association of FcγRIIb1 with SHP-1 is not necessary for inhibition of NK cells.
Stimulation of B cells by receptor cross-linking involves rapid release of Ca2+ from intracellular stores and a sustained influx of extracellular calcium (25). A significant feature of FcγRIIb1-mediated inhibition is a selective block in calcium influx through the plasma membrane while not affecting release from intracellular stores (7, 26, 27). The enzymatic activities of SHIP include removal of the 5′ phosphate moiety from substrates like phosphatidylinositol 3,4,5-trisphosphate (PIP3) and inositol 1,3,4,5-tetrakisphosphate (IP4) (11, 12). Therefore, downstream events dependent on these phosphoinositol metabolites are likely to be inhibited by SHIP (28). Maximal tyrosine phosphorylation of SHIP and association with Shc was observed in B cells upon cocross-linking of the BCR with FcγRIIb1 (29). Thus, SHIP association with the FcγRIIb1 ITIM could sequester Shc and uncouple the early tyrosine phosphorylation events from late events like p21ras activation (30).
The evolution of distinct mechanisms to block lymphocyte activation may relate to the consequences of partial activation of various types of lymphocytes. Cocross-linking of surface Ig with FcγRIIb1 results in accelerated entry into apoptosis by B cells as compared with that caused by cross-linking surface Ig alone (31). The function of FcγRIIb1 is to inhibit Ig-producing and proliferating B cells after soluble Ig has been produced. Therefore, apoptosis may be useful to eliminate these cells. The function of KIR is to protect normal cells from attack by NK cells. Therefore, it is essential for KIR to provide a complete and reliable shutdown of the NK cell. The most suited target in this inhibitory pathway would be tyrosine-phosphorylated substrates that are proximal in the activation pathway. Recruitment of SHP-1 by KIR followed by rapid dephosphorylation of proximal substrates may fulfill this requirement. Thus, the dichotomy in the use of SHP-1 by KIR and SHIP by FcγRIIb1, as demonstrated by our data, appears well suited to the function of the cells in which these receptors operate.
Acknowledgments
The authors wish to thank M. Weston for technical assistance, J. Altrichter for helpful discussions and a plasmid, H.-G. Klingemann for the cell line NK92, B. Moss for the plasmid pSC66, A. Moretta and C. Bottino for the mAb GL183, and J. Gumperz and P. Parham for the 721.221 HLA transfectants.
References
- 1.D'Ambrosio D, Hippen KL, Minskoff SA, Mellman I, Pani G, Siminovitch KA, Cambier JC. Recruitment and activation of PTP1C in negative regulation of antigen receptor signaling by FcγRIIB1. Science (Wash DC) 1995;268:293–297. doi: 10.1126/science.7716523. [DOI] [PubMed] [Google Scholar]
- 2.Burshtyn DN, Scharenberg AM, Wagtmann N, Rajagopalan S, Berrada K, Yi T, Kinet JP, Long EO. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitor receptor. Immunity. 1996;4:77–85. doi: 10.1016/s1074-7613(00)80300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olcese L, Lang P, Vély F, Cambiaggi A, Marguet D, Bléry M, Hippen KL, Biassoni R, Moretta A, Moretto L, Cambier JC, Vivier E. Human and mouse killer– cell inhibitory receptors recruit PTP1C and PTPlD protein tyrosine phosphatases. J Immunol. 1996;156:4531–4534. [PubMed] [Google Scholar]
- 4.Fry AM, Lanier LL, Weiss A. Phosphotyrosines in the killer cell inhibitory receptor motif of NKB1 are required for negative signaling and for association with protein tyrosine phosphatase 1C. J Exp Med. 1996;184:295–300. doi: 10.1084/jem.184.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell KS, Dessing M, Lopez-Botet M, Cella M, Colonna M. Tyrosine phosphorylation of a human killer inhibitory receptor recruits protein tyrosine phosphatase 1C. J Exp Med. 1996;184:93–100. doi: 10.1084/jem.184.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binstadt BA, Brumbaugh KM, Dick CJ, Scharenberg AM, Williams BL, Colonna M, Lanier LL, Kinet JP, Abraham RT, Leibson PJ. Sequential involvement of lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity. 1996;5:629–638. doi: 10.1016/s1074-7613(00)80276-9. [DOI] [PubMed] [Google Scholar]
- 7.Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor FcγRIIB. Nature (Lond) 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 8.Amigorena S, Bonnerot C, Drake JR, Choquet D, Hunziker W, Guillet JG, Webster P, Sautes C, Mellman I, Fridman WH. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science (Wash DC) 1992;256:1808–1812. doi: 10.1126/science.1535455. [DOI] [PubMed] [Google Scholar]
- 9.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JV. A 13-amino-acid motif in the cytoplasmic domain of FcγRIIB modulates B-cell receptor signalling. Nature (Lond) 1994;368:70–73. doi: 10.1038/368070a0. [DOI] [PubMed] [Google Scholar]
- 10.Daeron M, Latour S, Malbec O, Espinosa E, Pina P, Pasmans S, Fridman WH. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of FcγRIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 1995;3:635–646. doi: 10.1016/1074-7613(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 11.Damen JE, Liu L, Rosten P, Humphries RK, Jefferson AB, Majerus PW, Krystal G. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci USA. 1996;93:1689–1693. doi: 10.1073/pnas.93.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lioubin MN, Algate PA, Tsai S, Carlberg K, Aebersold R, Rohrschneider LR. p150Ship, a signal transduction molecule with inositol polyphosphate-5-phosphatase activity. Genes Dev. 1996;10:1084–1095. doi: 10.1101/gad.10.9.1084. [DOI] [PubMed] [Google Scholar]
- 13.Moretta A, Vitale M, Bottino C, Orengo AM, Morelli L, Augugliaro R, Barbaresi M, Ciccone E, Moretta L. P58 molecules as putative receptors for major histocompatibility complex (MHC) class I molecules in human natural killer (NK) cells. Anti-p58 antibodies reconstitute lysis of MHC class I–protected cells in NK clones displaying different specificities. J Exp Med. 1993;178:597–604. doi: 10.1084/jem.178.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagtmann N, Rajagopalan S, Winter CC, Peruzzi M, Long EO. Killer cell inhibitory receptors specific for HLA-C and HLA-B identified by direct binding and by functional transfer. Immunity. 1995;3:801–809. doi: 10.1016/1074-7613(95)90069-1. [DOI] [PubMed] [Google Scholar]
- 15.Rojo S, Burshtyn DN, Long EO, Wagtmann N. Type I transmembrane receptor with inhibitory function in mouse mast cells and NK cells. J Immunol. 1997;158:9–12. [PubMed] [Google Scholar]
- 16.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–658. [PubMed] [Google Scholar]
- 17.Karlhofer FM, Yokoyama WM. Stimulation of murine natural killer (NK) cells by a monoclonal antibody specific for the NK1.1 antigen. IL-2–activated NK cells possess additional specific stimulation pathways. J Immunol. 1991;146:3662–3673. [PubMed] [Google Scholar]
- 18.Moretta A, Tambussi G, Bottino C, Tripodi G, Merli A, Ciccone E, Pantaleo G, Moretta L. A novel surface antigen expressed by a subset of human CD3− CD16+ natural killer cells. Role in cell activation and regulation of cytolytic function. J Exp Med. 1990;171:695–714. doi: 10.1084/jem.171.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagtmann N, Biassoni R, Cantoni C, Verdiani S, Malnati M, Vitale M, Bottino C, Moretta L, Moretta A, Long EO. Molecular clones of the p58 natural killer cell receptor reveal Ig-related molecules with diversity in both the extra- and intracellular domains. Immunity. 1995;2:439–449. doi: 10.1016/1074-7613(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 20.Earl, P.L., and B. Moss. 1988. Generation of recombinant vaccinia viruses. In Current Protocols in Molecular Biology. F.M. Ausubel, R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl, editors. John Wiley & Sons, New York. 16.17.1–16.17.16.
- 21.Hopp TP, Prickett KS, Price VL, Libby RT, March CJ, Cerretti DP, Urdal DL, Conlon PJ. A short polypeptide marker sequence for recombinant protein identification and purification. Biotechnology. 1988;6:1204–1210. [Google Scholar]
- 22.Kaufman DS, Schoon RA, Robertson MJ, Leibson PJ. Inhibition of selective signaling events in natural killer cells recognizing major histocompatibility complex class I. Proc Natl Acad Sci USA. 1995;92:6484–6488. doi: 10.1073/pnas.92.14.6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valiante NM, Phillips JH, Lanier LL, Parham P. Killer cell inhibitory receptor recognition of human leukocyte antigen (HLA) class I blocks formation of a pp36/ PLC-γ signaling complex in human natural killer (NK) cells. J Exp Med. 1996;184:2243–2250. doi: 10.1084/jem.184.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burshtyn DN, Yang W, Yi T, Long EO. A novel phosphotyrosine motif with a critical amino acid at position -2 for the SH2 domain–mediated activation of the tyrosine phosphatase SHP-1. J Biol Chem. 1997;272:13066–13072. doi: 10.1074/jbc.272.20.13066. [DOI] [PubMed] [Google Scholar]
- 25.Cambier JC, Pleiman CM, Clark MR. Signal transduction by the B cell antigen receptor and its coreceptors. Annu Rev Immunol. 1994;12:457–486. doi: 10.1146/annurev.iy.12.040194.002325. [DOI] [PubMed] [Google Scholar]
- 26.Choquet D, Partiseti M, Amigorena S, Bonnerot C, Fridman WH, Korn H. Cross-linking of IgG receptors inhibits membrane immunoglobulin-stimulated calcium influx in B lymphocytes. J Cell Biol. 1993;121:355–363. doi: 10.1083/jcb.121.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diegel ML, Rankin BM, Bolen JB, Dubois PM, Kiener PA. Cross-linking of Fcγ receptor to surface immunoglobulin on B cells provides an inhibitory signal that closes the plasma membrane calcium channel. J Biol Chem. 1994;269:11409–11416. [PubMed] [Google Scholar]
- 28.Scharenberg AM, Kinet JP. The emerging field of receptor-mediated inhibitory signaling: SHP or SHIP? . Cell. 1996;87:961–964. doi: 10.1016/s0092-8674(00)81790-0. [DOI] [PubMed] [Google Scholar]
- 29.Chacko GW, Tridandapani S, Damen JE, Liu L, Krystal G, Coggeshall KM. Negative signaling in B lymphocytes induces tyrosine phosphorylation of the 145-kDa inositol polyphosphate 5-phosphatase, SHIP. J Immunol. 1996;157:2234–2238. [PubMed] [Google Scholar]
- 30.Sarmay G, Koncz G, Gergely J. Human type II Fcγ receptors inhibit B cell activation by interacting with the p21ras-dependent pathway. J Biol Chem. 1996;271:30499–30504. doi: 10.1074/jbc.271.48.30499. [DOI] [PubMed] [Google Scholar]
- 31.Ashman RF, Peckham D, Stunz LL. Fc receptor off-signal in the B cell involves apoptosis. J Immunol. 1996;157:5–11. [PubMed] [Google Scholar]