Abstract
Macrophage infiltration into inflammatory sites is generally preceded by neutrophils. This suggests neutrophils may be the source of chemotactic factors for monocytes. To identify these putative monocyte attractants, we have systematically prepared neutrophil granules, lysed them, and sequentially purified the released proteins by several reverse phase chromatography procedures. Assays for monocyte chemotactic activity of the chromatography fractions yielded a major peak of activity associated with a protein of 30 kD, according to SDS-PAGE analysis. NH2-terminal sequence of the protein revealed this to be identical to cathepsin G. The monocyte chemotactic activity of human cathepsin G was dose dependent with optimal concentration at 0.5–1 μg/ml. Cathepsin G is chemotactic rather than chemokinetic for monocytes, as demonstrated by checkerboard analysis. Cathepsin G–induced monocyte chemotaxis is partially pertussis toxin sensitive implying the involvement of a G protein–coupled receptor. Enzymatic activity of cathepsin G is associated with its monocyte chemotactic activity, since DFP- or PMSF-inactivated cathepsin G no longer induced monocyte migration. The chemotactic activity of cathepsin G can also be completely blocked by α1 antichymotrypsin, a specific inhibitor of chymotrypsin-like proteinases present in human plasma. In addition, cathepsin G is also a potent chemoattractant for neutrophils and a chemokinetic stimulant for T cells. In the course of pursuing these in vitro studies, we established that the T cell chemoattractant, azurocidin/CAP37 from human neutrophil granules, at doses of 0.05 to 5 μg/ml, was chemotactic for monocytes and neutrophils. As predicted from the in vitro chemotactic activity, subcutaneous injection of cathepsin G into BALB/c mice led to infiltration of both mononuclear cells and neutrophils. Thus, the transition of inflammatory exudate from neutrophil to mononuclear cells can be mediated, at least in part, by extracellular release of neutrophil granule proteins such as cathepsin G and azurocidin/CAP37.
Certain irritants and antigenic stimulants characteristically induce acute inflammatory responses dominated by neutrophils that evolve into chronic inflammatory reactions typified by mononuclear cell infiltrates. It has been proposed that monocyte infiltration may actually be neutrophil dependent (1, 2). Indeed, after subcutaneous injection of IL-8, we observed such a progression from rapid neutrophil infiltration to the subsequent appearance of a considerable infiltrate of T cells and monocytes (3). In vitro IL-8 is a considerably more potent chemoattractant of neutrophils than T cells, and is not chemotactic for monocytes at all (4). This led us to show that neutrophils release chemoattractants for T lymphocytes (5). Chemoattractants for T cells rapidly appeared in the supernatants of neutrophils incubated with IL-8 (3), suggesting that they were stored and released from neutrophil granules rather than newly synthesized. These T cell chemotactic activities were purified and shown to be attributable to defensins (human neutrophil peptide 1 and 2) and azurocidin/CAP37 (5), which are known to be stored in human neutrophil granules.
Although other laboratories had previously reported defensins and azurocidin/CAP37 to be chemotactic for monocytes (6, 7), we were previously unable to confirm these findings (5). However, since IL-8 injection sites also contained some monocytes, we decided to investigate this issue more systematically by assaying neutrophil products for monocyte chemotaxis. In this study, using improved procedures, the extracts of human neutrophil granules were therefore chromatographed and assayed for monocyte chemotactic activity. This approach identified cathepsin G as a monocyte chemoattractant. Furthermore, assays using a broader dosage range than previously available also showed azurocidin/CAP37 to be a monocyte as well as T cell chemoattractant. Finally, both azurocidin/CAP37 and, to an even greater extent, cathepsin G were shown to chemoattract neutrophils in vitro. These findings were directly correlated with the capacity of these two agents to attract neutrophils as well as mononuclear cells to injection sites in mice.
Materials and Methods
Reagents.
Human neutrophil cathepsin G was purchased from Calbiochem-Novabiochem Corp. (La Jolla, CA), human thrombin from Boehringer Mannheim (Indianapolis, IN) and human α1 antichymotrypsin from Sigma Chemical Co. (St. Louis, MO). Azurocidin/CAP37 was purified from human neutrophil granules as described (5).
Neutrophil Granule Preparation and Lysis. Human neutrophils were isolated from granulocyte packs obtained from the Department of Transfusion Medicine (Warren Grant Magnusson Clinical Center, National Institutes of Health, Bethesda, MD). Neutrophils were isolated by the method of Boyum (8). In brief, erythrocytes were removed by sedimentation with 1.5% dextran. Mononuclear cells were centrifuged on a Histopaque-1077 (Sigma Chemical Co.) cushion, and the residual erythrocytes were removed by hypotonic lysis. The neutrophils were resuspended in PBS, counted, and assessed for viability. The neutrophils were resuspended in disruption buffer containing 0.25 M sucrose, 10 mM Hepes (pH 7.4), and 4 mM EGTA to 108 cells/ml and lysed by nitrogen cavitation. Nuclei and debris were removed by centrifugation at 600 g for 20 min. The resulting lysate was layered over 48% Percoll and centrifuged at 29,000 rpm for 26 min (9). The granules were recovered from the Percoll fractions and assessed for granule enzymes (3). Dense granules containing azurophilic as well as specific granules were pooled and centrifuged at 35,000 rpm for 3 h. The granules were recovered as a white flocculent material just above the Percoll pellet.
Chemotaxis Assay.
Human peripheral blood monocytes were isolated from normal donors (National Institutes of Health Clinical Center Transfusion Department, Bethesda, MD) with an isoosmotic Percoll gradient as described in reference 10. The monocyte preparations were >90% pure as assessed by morphological criteria. Monocyte migration was evaluated using a 48-well microchamber technique (11). Lyophilized fractions were dissolved in 200 μl of chemotaxis medium (RPMI 1640 containing 10 mg/ml of bovine serum albumin, 25 mM Hepes) and placed in lower wells of the chemotaxis chamber (Neuro Probe Inc., Cabin John, MD). 50 μl of cell suspension in the same medium (1.5 × 106 cells/ml) were placed in the upper wells. The two compartments were separated by a Nucleopore polycarbonate filter (5 μm pore size; Neuro Probe Inc.). After incubation of the apparatus at 37°C for 90 min in humidified air with 5% CO2, the filter was removed, fixed, and stained with LeukoStat stain kit (Fisher Scientific, Pittsburgh, PA) and the number of migrating cells in three high-power fields (×400) was counted. The results are expressed as the mean number of migrating cells in one high-power field (HPF)1 in triplicate or as the chemotaxis index which represent the ratio of the number of cells in HPF in the test to control samples. Data were analyzed for statistical difference by Student's t test for unpaired or paired samples. To evaluate the involvement of G protein in cathepsin G–induced chemotaxis of monocytes, they were treated with pertussis toxin (Calbiochem-Novabiochem Corp.) at concentrations of 1–1,000 ng/ml for 30 min at 37°C before chemotaxis assay. T lymphocyte migration was assessed as described (5).
Purification of Monocyte Chemotactic Protein.
Human neutrophil granules (15 mg of wet pellet) were resuspended in 1 ml of 2 M NaCl containing 0.5% TFA and precipitated by low speed centrifugation. The supernatant did not contain protein material and was discarded. The pellet was resuspended in 3 ml of distilled water and clarified by low speed centrifugation. The supernatant (1.2 mg of total protein) was loaded onto a C4 Delta-Pak Radial- Pak cartridge column (8 × 100 mm; Waters Corp, Milford, MA) equilibrated in buffer A (0.1% TFA in water). Proteins were eluted with a linear gradient of buffer B (0.05% TFA in acetonitrile, gradient from 0 to 90% of B for 60 min) at a flow rate of 1 ml/min. 1-ml fractions were collected, and 200-μl aliquots of each fraction were lyophilized for testing of chemotactic activity and SDS-PAGE analysis. Lyophilized aliquots of fractions were subjected to SDS electrophoresis in 15% polyacrylamide minigel (Bio Rad Labs., Hercules, CA) or 10–27% gradient gel (Novex, San Diego, CA) in the Tris-glycine system and stained with Coomassie R-250. The resultant chemotactically active fraction 5 was diluted three times in buffer A and loaded onto a Nucleosil 300-5C18 column (4 × 250 mm, Macherey-Nagel, Düren, Germany) for further purification. Proteins were eluted with a gradient of buffer B (0.05% TFA in acetonitrile) at a flow rate of 0.5 ml/min. 1-ml fractions were collected, and 100-μl aliquots of each fraction were lyophilized for testing of chemotactic activity. 5 μg of protein of fraction 5 were subjected to sequence analysis on a protein sequencer (model 477A/120A; Applied Biosystems, Foster City, CA).
Determination of Enzymatic Activity.
Thiobenzylester substrate Succ-Phe-Leu-Phe-S-Bzl (Sigma Chemical Co.) was used to measure cathepsin G activity. To monitor enzyme activities, assays were performed at room temperature by using 0.5 mM 5, 5′-ditiobis-(2-nitrobenzoic acid) (DTNB; Sigma Chemical Co.) to detect the thiobenzyl leaving group (ε410 = 13,600 l/[cm × M]). The activity was determined using a microtiter assay (12). In brief, 50 μl of sample was added to 100 μl of 1 mM DTNB made up in 10 mM Hepes, 1 mM CaCl2, and 1 mM MgCl2, pH 7.2. The reaction was initiated by the addition of 50 μl of Succ-Phe-Leu-Phe-S-Bzl to give a final concentration of 62.5 μM. The duration of the assay depended on color development, the rate of which was measured on a microplate reader (MR 5,000; Dynatech, Chantilly, VA) at 410 nm. Medium control, DTNB alone, and DTNB and substrate were always run in parallel.
Diisopropylfluorophosphate and PMSF Modification of Cathepsin G and Inhibition by α1 Antichymotrypsin.
20 μl of 0.1 M solution of diisopropylfluorophosphate (DFP; Sigma Chemical Co.) or PMSF (Boehringer Mannheim, Indianapolis, IN) in isopropanol was added to 20 μg of cathepsin G (Calbiochem-Novabiochem) in 400 μl of DPBS. After 30 min incubation at 22°C, the protein preparation was desalted on a reverse phase guard column RP-4 (15 × 3.2 mm, NewGuard Brownlee column; Perkin-Elmer Corp., Norwalk, CT) cartridge column. Proteins were eluted with a linear gradient (from 0 to 90% of buffer B for 30 min) at a flow rate of 1 ml/min. After lyophilization, the preparations were dissolved in chemotaxis media and tested for chemotactic activity. When α1 antichymotrypsin was used, cathepsin G was preincubated for 10 min at room temperature with different concentrations of α1 antichymotrypsin before the chemotaxis assay.
Intracellular Ca2+ Measurements.
Flow cytometric measurements of intracellular calcium were performed as described (13), using a cytometer (Epics 753; Coulter Corp., Miami, FL). Changes in intracellular calcium concentration were determined by gated analysis of the ratio of fluorescence of Indo-1 bound to calcium (emission at 485 nm) to fluorescence of free Indo-1 (emission at 395 nm). The bound/free ratio was plotted against time. To assist in determining the number of fluxing cells, the baseline of the bound/free ratio was used to set the cut off. Cells with bound/ free ratio higher than baseline were considered to have increased [Ca2+] and their percentage calculated versus total number of gated cells.
In Vivo Studies.
Female BALB/c mice at 8–12 wk of age were obtained from the Animal Production Area (National Cancer Institute, Frederick Cancer Research and Development Center, Frederick, MD). Mice received a single injection of various doses of cathepsin G (Calbiochem Corp.) or boiled cathepsin G in 0.2 ml of PBS, or PBS alone subcutaneously. At 24 h after the injection, the injection site of skin was fixed in 10% phosphate-buffered formalin and stained with hematoxylin–eosin stain for histological examination. Chromogenic Limulus amebocyte lysate assays (Whittaker Bioproducts, Walkersville, MD) revealed the cathepsin G preparation to contain less than 0.125 endotoxin units/50 μg of protein.
Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 86-23, 1985).
Results
Identification of Cathepsin G as a Major Neutrophil-derived Chemotactic Factor for Monocytes.
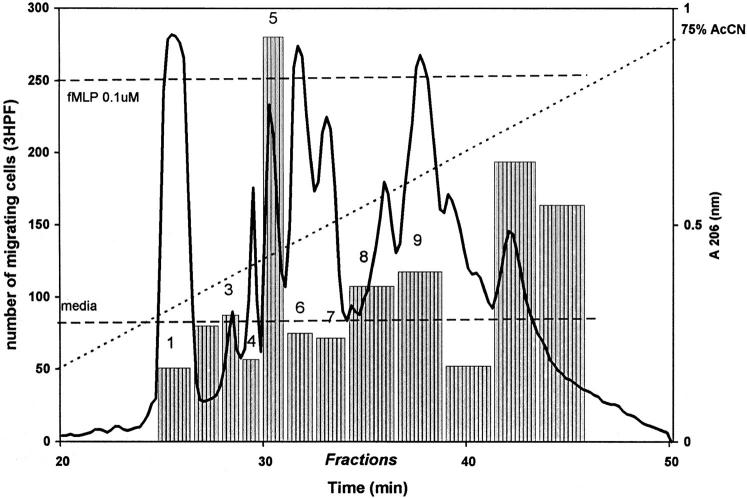
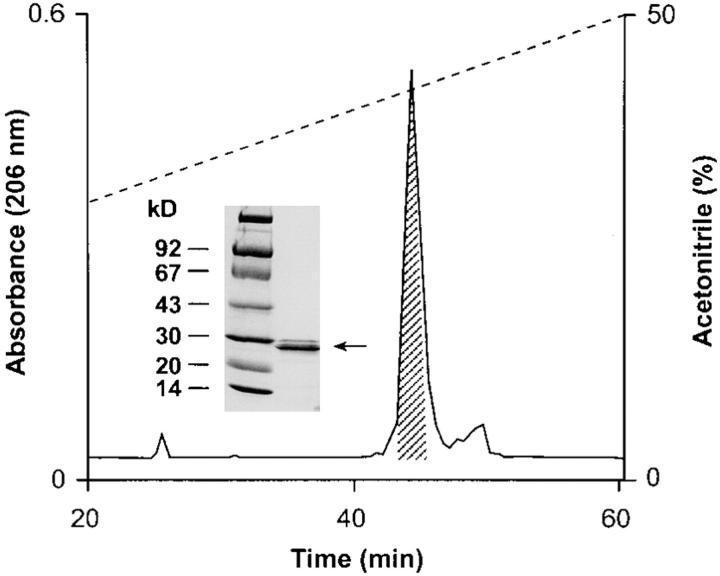
To determine if constituents of neutrophil granules are responsible for attracting monocytes, we extracted neutrophil granules and fractionated the extract on a C4 reverse phase column. Aliquots of the fractions were tested for monocyte chemotactic activity (Fig. 1). Fraction 5 manifested the highest monocyte activity. For further purification, this fraction was subjected to chromatography on a C18 column (Fig. 2). The major protein peak had monocyte chemotactic activity and was shown to be homogeneous (Fig. 2, inset). This protein was subjected to NH2-terminal amino acid sequence analysis and yielded a sequence of 15 amino acid residues I-I-G-G-R-E-S-R-P-H-S-R-P-Y-M. A search for the protein sequence in the data base (Protein Identification Resource) revealed that this protein is cathepsin G (EC 3.4.21.20), a serine proteinase with chymotrypsin-like specificity. For further experiments, cathepsin G purchased from Calbiochem was used.
Figure 1.
Fractionation of neutrophil granule extract by reverse phase HPLC. Neutrophil granule extract (1.2 mg of protein) was loaded onto a C4 Delta-Pak Radial-Pak cartridge column (8 × 100 mm) equilibrated in buffer A (0.1% TFA in water). Proteins were eluted with a linear gradient of buffer B (0.05% TFA in acetonitrile) at a flow rate of 1 ml/min. 1-ml fractions were collected, and 200-μl aliquots of each fraction were lyophilized for testing of monocyte chemotaxis activity using the microchamber assay. Monocyte chemotaxis to FMLP (0.1 μM) and media itself are indicated by horizontal broken lines.
Figure 2.
Chromatography of fraction 5 on Nucleosil 300-5C18 column. Chemotactically active fraction 5 after reverse phase HPLC on C4 Delta-Pak Radial-Pak column was diluted three times in buffer A before loading. Proteins were eluted with a gradient of buffer B at a flow rate of 0.5 ml/min. 1-ml fractions were collected, and 100-μl aliquots of each fraction were lyophilized for testing of chemotactic activity. (Inset) SDS-PAGE analysis of the chromatographic fraction in 15% polyacrylamide gel.
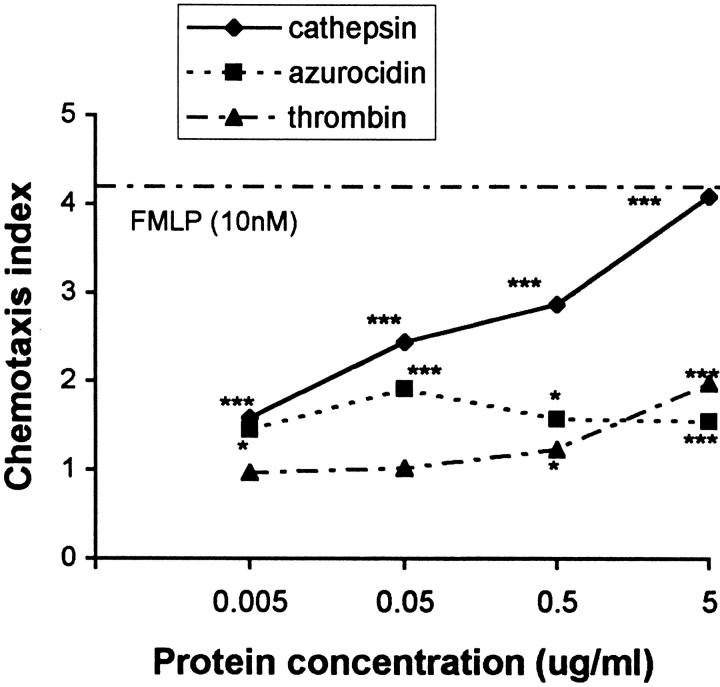
Since azurocidin/CAP37, a serine proteinase-like protein from neutrophils, as well as thrombin were previously reported to be chemotactic for monocytes (6, 14), we compared the chemotactic activity of cathepsin G for monocytes with that of azurocidin/CAP37 and thrombin. The monocyte chemotactic activity of cathepsin G appeared to be dose dependent with an optimal concentration of 0.5–1 μg/ml (Fig. 3). In other experiments using higher doses of cathepsin G (up to 10 μg/ml) a bell-shaped dose response was obtained (not shown). Cathepsin G appeared to be a much more potent chemoattractant for monocytes than either azurocidin or thrombin.
Figure 3.
Chemotactic activity of cathepsin G, azurocidin, and thrombin for human monocytes. Monocyte migration was evaluated using a 48-well microchamber technique (11) as described in Materials and Methods. The results are expressed as the chemotaxis index which represents the ratio of the number of cells in HPF in the test to control samples (media) *P <0.05; ***P <0.001. FMLP (10 nM) is included as a positive control.
To determine whether the activity of a cathepsin G is chemotactic or chemokinetic, we performed checkerboard analysis by adding cathepsin G into upper wells of a chemotaxis chamber together with monocytes. The results clearly indicated that the response of monocytes to cathepsin G is chemotactic rather than chemokinetic (Table 1).
Table 1.
Checkerboard Analysis of Monocyte Migration in Response to Cathepsin G
| Upper compartment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Lower compartment | 0 | 0.025 | 0.05 | 0.25 | ||||
| μg/ml | μg/ml | μg/ml | μg/ml | μg/ml | ||||
| 0 | 1.0 ± 0.05 | 0.80 ± 0.06 | 0.77 ± 0.08 | 0.68 ± 0.11 | ||||
| 0.025 | 1.95 ± 0.09** | 2.0 ± 0.5 | 0.79 ± 0.07 | 0.72 ± 0.09 | ||||
| 0.05 | 3.08 ± 0.08** | 1.99 ± 0.04** | 1.41 ± 0.18 | 0.83 ± 0.05 | ||||
| 0.25 | 6.48 ± 0.37** | 5.11 ± 0.23** | 3.53 ± 0.28** | 1.72 ± 0.20 | ||||
Cathepsin G was added into upper wells of chemotaxis chamber together with monocytes. Results are expressed as chemotaxis index ± standard error of migration. Migration in control buffer was 71.0 ± 3.5 cells. Data were analyzed for statistical difference by the Student's t test (
P <0.001).
Enzymatic Activity of Cathepsin G Is Essential for Its Chemotactic Activity.
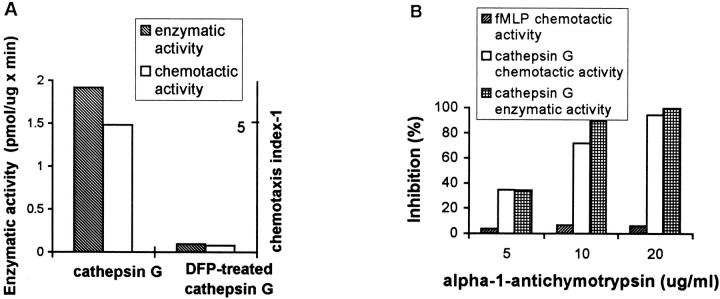
To determine the relationship of the chemotactic activity of cathepsin G to its enzymatic activity, cathepsin G was modified by DFP or PMSF. The inhibition of proteolytic activity of cathepsin G by DFP by 95% led to inactivation of its monocyte chemotactic activity also by 95% (Fig. 4 A). PMSF treatment of cathepsin G gave very similar results (data not shown).
Figure 4.
(A) Inhibition of monocyte chemotactic activity of cathepsin G by DFP treatment. Chemotactic activity of cathepsin G treated with DFP (2.5 μg/ml) was compared with enzymatically active cathepsin G (2.5 μg/ml). (B) Inhibition of monocyte chemotactic activity of cathepsin G by α1 antichymotrypsin. Cathepsin G (2.5 μg/ ml) was preincubated for 10 min at room temperature with different concentrations of human α1 antichymotrypsin before chemotaxis assay.
The activity of serine proteinases in the blood is regulated by specific acute phase proteins, serpins (serine proteinase inhibitors). Chymotryptic activity is specifically inhibited by α1 antichymotrypsin. We therefore measured the chemotactic activity of cathepsin G preincubated for 10 min with different concentrations of α1 antichymotrypsin. At a fourfold molar ratio, α1 antichymotrypsin almost completely inhibited monocyte chemotaxis to cathepsin G in parallel with inhibition of its enzymatic activity (Fig. 4 B), confirming the requirement of proteolytic activity for cathepsin G chemotactic activity.
Cathepsin G Is Also a Potent Chemokinetic Stimulant for T Cells and a Chemoattractant for Neutrophils.
We previously re- ported (5) that azurocidin/CAP37, which is homologous to cathepsin G, has T cell–specific chemotactic activity. It was therefore relevant to compare the chemotactic activities of cathepsin G and azurocidin for T cells. Checkerboard analysis, however, indicated the response of T lymphocytes to cathepsin G to be entirely chemokinetic (Table 2). Inactivation of enzymatic activity of cathepsin G by PMSF reduced the mobilizing activity of cathepsin G for T cells in chemotaxis chamber by 76% at a cathepsin G concentration of 1 μg/ml (data not shown).
Table 2.
Checkerboard Analysis of T Cells Migration in Response to Cathepsin G
| Upper compartment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower compartment | 0 | 0.004 | 0.02 | 0.1 | 0.05 | |||||
| μg/ml | μg/ml | μg/ml | μg/ml | μg/ml | μg/ml | |||||
| 0 | 1.0 ± 0.09 | 1.44 ± 0.38 | 10.68 ± 1.44 | 18.1 ± 2.21 | 11.3 ± 1.17 | |||||
| 0.004 | 2.04 ± 0.16 | 1.93 ± 0.15 | 5.68 ± 0.62 | 12.92 ± 1.38 | 11.22 ± 0.61 | |||||
| 0.02 | 5.14 ± 0.27 | 4.07 ± 0.42 | 15.39 ± 1.27 | 14.36 ± 1.15 | 10.62 ± 0.14 | |||||
| 0.1 | 6.25 ± 0.11 | 5.17 ± 0.43 | 16.09 ± 0.73 | 15.12 ± 1.88 | 12.78 ± 1.75 | |||||
| 0.5 | 8.64 ± 0.65 | 6.87 ± 0.80 | 5.97 ± 1.02 | 8.83 ± 0.34 | 6.85 ± 0.71 | |||||
Cathepsin G was added into upper and lower wells of chemotaxis chamber together with monocytes. Results are expressed as chemotaxis index ± standard error of migration. Migration in control buffer was 16.2 ± 1.5 cells HPF. Chemotaxis indexes equal or higher than two are statistically significant in comparison to migration of T cells to media alone.
Cathepsin G also manifested chemotactic activity for neutrophils (Fig. 5) and was more potent than azurocidin and thrombin. Checkerboard analysis showed that the effect of cathepsin G on neutrophils is chemotactic (data not shown).
Figure 5.
Chemotactic activity of cathepsin G, azurocidin, and thrombin for human neutrophils. FMLP (10 nM) is included as a positive control. The results are expressed as the chemotaxis index which represents the ratio of the number of cells in HPF in the test to control samples (media). *P <0.05; ***P <0.001.
Cathepsin G–induced Chemotaxis of Monocytes is Partially Pertussis Toxin Sensitive.
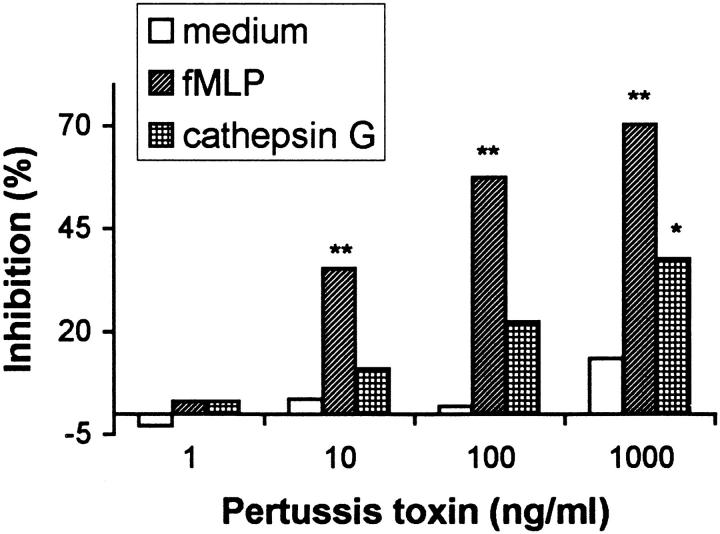
Directed movement of cells along a chemotaxis gradient is often based on signal transduction by a receptor coupled Gi protein that is sensitive to pertussis toxin (15). Pretreatment of monocytes with pertussis toxin partially reduced monocyte chemotaxis to cathepsin G, although the response to FMLP was more significantly decreased (Fig. 6), suggesting that the chemotactic response of monocytes to cathepsin G is only partially mediated by pertussis toxin–sensitive G protein(s).
Figure 6.
Inhibition of monocyte migration in response to cathepsin G (5 μg/ml, chemotaxis index [c.i.] 3.9) by pertussis toxin. Monocytes were treated with pertussis toxin for 30 min at 37°C before chemotaxis assay. The results are expressed as the c.i., *P <0.05; **P <0.01. FMLP (10 nM) is included as a positive control (c.i. 7.4).
Cathepsin G Induces Ca2+ Mobilization in Human Monocytes.
Induction of Ca2+ mobilization in responsive cells is another indicator of receptor function. We therefore examined whether at concentrations effective for chemotaxis, cathepsin G could induce Ca2+ mobilization in human monocytes. At concentrations of 2.5 μg/ml, cathepsin G effectively increased cytoplasmic Ca2+ concentrations in monocytes, DFP-treated cathepsin G was inactive (Fig. 7). α1 antichymotrypsin also inhibited cathepsin G–induced Ca2+ mobilization (data not shown).
Figure 7.
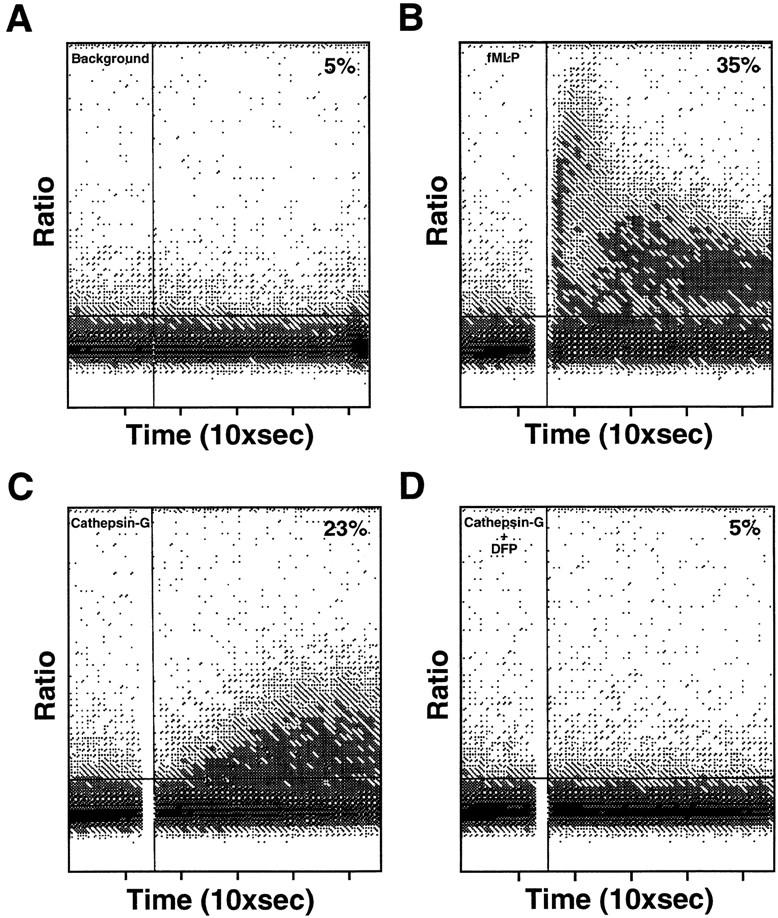
Ca2+ mobilization in human monocytes loaded with Indo-1; the bound/free ratio was continuously recorded. (A) No stimulus, (B) FMLP (10 nM), (C) cathepsin G (2.5 μg/ml), (D) DFP-treated cathepsin G (2.5 μg/ml).
In Vivo Cathepsin G Induces Infiltration of Mononuclear Cells and Neutrophils.
The in vivo effect of cathepsin G was investigated by subcutaneous injection of various doses of cathepsin G into BALB/c mice. After 24 h, the injection site was excised, and the extent and types of cells infiltrating the site were examined histologically (Table 3). Cathepsin G induced the infiltration of mononuclear cells and neutrophils at the injection site. The infiltrates consisted of slightly more mononuclear cells than neutrophils. A 0.5 μg dose of cathepsin G induced the most marked inflammatory cell infiltrate. In contrast, sites injected with the same amount of boiled cathepsin G exhibited only a low degree of infiltration by mononuclear cells and neutrophils.
Table 3.
Histological Evaluation of Cellular Infiltration after Subcutaneous Injection of Cathepsin G in BALB/c Mice
| Dermis | Subcutaneous | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal No. | Injection | μg | PMN | MNC | PMN | MNC | ||||||
| 1 | PBS | – | – | – | – | |||||||
| 2 | PBS | – | – | – | – | |||||||
| 3 | PBS | – | – | – | – | |||||||
| 4 | CathG | 0.005 | – | – | 1F | 1F | ||||||
| 5 | CathG | 0.005 | 1F | – | 1F | – | ||||||
| 6 | CathG | 0.005 | – | – | – | 1F | ||||||
| 7 | CathG | 0.05 | 1F | – | 2F | 1F | ||||||
| 8 | CathG | 0.05 | 1F | 1F | 1F | 1F | ||||||
| 9 | CathG | 0.05 | – | – | – | – | ||||||
| 10 | CathG | 0.5 | 1F | 2MF | 2F | 2MF | ||||||
| 11 | CathG | 0.5 | – | 1F | – | 1MF | ||||||
| 12 | CathG | 0.5 | 2F | 2MF | 3MF | 2F | ||||||
| 13 | CathG | 5.0 | – | – | – | 1F | ||||||
| 14 | CathG | 5.0 | – | 1MF | 1MF | 1F | ||||||
| 15 | CathG | 5.0 | 1F | 1F | 2F | 2F | ||||||
| 16 | Boiled CathG | 0.5 | – | – | – | – | ||||||
| 17 | Boiled CathG | 0.5 | – | – | – | – | ||||||
| 18 | Boiled CathG | 0.5 | – | 1F | – | 1F | ||||||
Mice received a single injection of various doses of either cathepsin G (CathG; Calbiochem Corp.) boiled cathepsin G in 0.2 ml of PBS, or PBS alone subcutaneously. At 24 h after the injection, the injection site of skin was excised and fixed in 10 % phosphate-buffered formalin and stained with hematoxylin–eosin stain for histological examination. Grading as follows: 1, minimal; 2, mild; 3, moderate. –, No significant lesion; F, focal; MF, multifocal.
Discussion
Macrophage infiltration into inflammatory sites is normally preceded by an initial influx of neutrophils. In contrast, in cases of clinical cyclic or experimental neutropenia, influx of mononuclear cells into inflammatory sites was significantly decreased and delayed (1–3). Restoration of circulating neutrophils reestablished normal sequence of events in the development of inflammatory response (1–3). The molecular basis for this phenomenon has not been adequately explained. Although Ward first detected a chemotactic factor for mononuclear cells in the lysate of neutrophils in 1968 (16), the identity of the responsible molecule remains uncertain. The goal of the present work was to identify a neutrophil factor(s) signaling monocyte inflammatory influx.
Monocyte-specific chemotactic factor(s), along with markers of azurophilic (β glucuronidase, myeloperoxidase) and specific granules (lactoferrin), are rapidly released by neutrophils upon stimulation by IL-8 (3). Biochemical purification of monocyte chemotactic factor from neutrophil granules led to the identification of cathepsin G. Cathepsin G is a serine proteinase found in the granules of neutrophils, monocytes, and mast cells (17–19). It is referred to as a chymotrypsin-like enzyme because it hydrolyzes peptide bonds after leucine, methionine, and phenylalanine residues. Cathepsin G is considered to be a rather inefficient proteinase, degrading collagen and proteoglycan more slowly than neutrophil elastase (20). Genes encoding cathepsin G, cathepsin G-like lymphocyte granzymes (B and H), and α/δ chains of the T cell receptor are closely linked on chromosomal band 14q11.2 (21).
Various physiological effects are ascribed to this enzyme: antimicrobial activity, degradation of extracellular matrix, vasoregulation (20), activation of neutrophil elastase (22), and cytokine processing (23). Furthermore, cathepsin G binds to and cleaves the V3 loop of HIV envelope protein gp120, pointing to possible involvement of cathepsin G in HIV target cell interaction (24).
We compared cathepsin G with several other related proteins reported to exhibit chemotactic activity for monocytes. Azurocidin/CAP37, a neutrophil granule protein with homology to serine proteinases but without proteolytic activity, is chemotactic for monocytes (6). Furthermore, a trypsin-like serine proteinase thrombin was also reported to have chemotactic activity for monocytes. The proteolytic activity of thrombin is not required for chemotactic activity (14). We have established that cathepsin G is a much more potent chemoattractant for monocytes than thrombin and azurocidin, and in contrast with azurocidin and thrombin, the enzymatic activity of cathepsin G is required for its chemotactic activity. Inactivation of cathepsin G enzymatic activity by DFP or PMSF abolished its monocyte chemotactic activity. This implies that the proteolytic activity of cathepsin G is associated with its chemotactic activity. However, we cannot exclude the possibility that any change in the vicinity of cathepsin G proteolytic active site could reduce its chemotactic activity due to structural changes rather than effects on proteolysis. The final solution to this uncertainty can only be resolved by studies of the cathepsin G receptor and the mechanism of its activation. Even if the enzymatic activity of cathepsin G is essential for chemotactic activity, it is probably not sufficient since bovine chymotrypsin, which has similar substrate specificity as cathepsin G, does not manifest significant chemotactic activity for human monocytes (data not shown). Therefore, the interaction of cathepsin G with its receptor(s) is determined not only by cathepsin G proteolytic substrate specificity, but presumably also involves more complex binding interactions.
Binding sites for cathepsin G have been reported on alveolar macrophages (25). Recently, it was reported that cathepsin G exhibits specific, saturable and reversible binding to T cells and NK cells (26). According to Selak and Smith there are 2 × 107 binding sites for cathepsin G on a platelet with maximum binding occurring at 35 μg/ml (27). Selak presented the evidence that platelets, although being activated by thrombin and cathepsin G, possess different receptors for these enzymes (28). Antibody directed against the thrombin receptor blocked thrombin-induced, but not cathepsin G–induced, platelet responses and, second, platelets pretreated with neutrophil elastase failed to respond to thrombin but responded to cathepsin G.
The activity of many chemotactic polypeptides are mediated by seven transmembrane receptors coupled to a pertussis toxin–sensitive G protein. The chemotactic activity of cathepsin G appeared to be at least partially pertussis toxin–sensitive, suggesting that it depends in part on a Gi protein–mediated signal. Further suggestive evidence that the activity of cathepsin G is mediated by a G protein–coupled receptor is the capacity of cathepsin G to mobilize intracellular Ca2+ ions in monocytes, which is also inhibited by DFP and α1 antichymotrypsin. The dependence of cathepsin G chemotactic activity on its enzymatic activity suggests that the chemotactic activity of cathepsin G is mediated by a proteolytic activation of receptor(s). Our preliminary attempts to detect competitive binding sites for cathepsin G on monocytes did not succeed (data not shown), and identification of the cathepsin G receptor(s) of monocytes and T lymphocytes is an important and challenging problem that remains to be solved.
We previously reported that azurocidin/CAP37 is chemotactic for T lymphocytes (5). Comparison of chemotactic activities of azurocidin and cathepsin G showed that cathepsin G has chemokinetic effects on T cells. We regard the increased motility of T cells as indication of their activation. Relevant to this, it was reported and confirmed by us that cathepsin G can mitogenically stimulate T lymphocytes (29). The mitogenic effect is also dependent on cathepsin G enzymatic activity (Tani, K., W.J. Murphy, O. Chertov, D. Taub, J.J. Oppenheim, and J.M. Wang, manuscript submitted for publication).
The overall results of this research suggest that cathepsin G may be the most potent neutrophil granule–derived chemoattractant for monocytes and chemokinetic stimulant for T lymphocytes, at least in vitro. It was reported that stimulation of 106 neutrophils with PMA (1 μM) can release 1 μg of cathepsin G (22), a quantity that is sufficient, according to our in vitro results, for induction of an inflammatory cell influx. There are other reports showing that neutrophils release cathepsin G extracellularly in response to different stimuli, e.g., the outer membrane protein of Treponema dendicola (30). There is also indirect evidence for cathepsin G extracellular release since IL-8 can stimulate the release of other azurophilic granules protein myeloperoxidase (3, 31) and elastase (32). Cathepsin G injected subcutaneously into BALB/c mice elicited a local inflammatory response, as predicted by its in vitro chemotactic effects, and it is not neutralized by serine proteinase inhibitors (serpins).
The in vitro chemotactic activity of cathepsin G is inhibited by α1 antichymotrypsin suggesting that α1 antichymotrypsin does function to downregulate inflammatory response by neutralizing chemotactic and mitogenic activity of cathepsin G in addition to its function of protecting host tissues from proteolytic damage. Perhaps because of this important immunoregulatory role of α1 antichymotrypsin, no homozygous-deficient α1 antichymotrypsin conditions have been reported and such a phenotype would not be compatible with normal development (33). α1 antichymotrypsin is synthesized and secreted mainly by hepatocytes, although it can also be produced by alveolar macrophages, cells of epithelial origin, and some tumor cells (33, 34). The plasma concentration of this acute phase protein rapidly increases several fold from normal concentration of ∼25 μg/ml (33) during tissue injury, autoimmune diseases, malignancies, and infections in response to systemic proinflammatory cytokines such as IL-1, IL-6, TNF-α, and TNF-β. In mice, the role of α1 antichymotrypsin is possibly fulfilled by the homologous protein contrapsin (35), which can interact with human cathepsin G (36). Induction of inflammation by subcutaneous injection of cathepsin G in mice, despite the fact that human serum did efficiently inhibit in vitro chemotactic effect of cathepsin G (data not shown), presumably indicates that the inhibitor(s) are not present in sufficient concentration in the tissue during initial stages of inflammation. There are two sources of proteinase inhibitors in an inflamed tissue: transudation and local synthesis in the tissue as a result of stimulation by proinflammatory cytokines (36). Apparently, it takes time for the inhibitor to achieve concentration sufficient to counterbalance the secreted proteinase. The fact that cathepsin G can stimulate an influx of inflammatory cells into the site of injection in spite of high concentration of proteinase inhibitors in plasma may indicate that the outcome of proteinase–inhibitor interaction depends on the available concentrations, the rate of diffusion of an inhibitor from circulation, and kinetics of the reaction. Therefore, it may be possible to interfere with local inflammatory conditions by therapy with cathepsin G inhibitors. Recent determination of cathepsin G three-dimensional structure provided a structural basis for the development of new highly efficient inhibitors (37).
Genetic defects affecting the cathepsin G content in neutrophil granules have been described. Neutrophils of patients with Chediak-Higashi syndrome do not secrete cathepsin G and elastase efficiently. These patients suffer from frequent severe bacterial infections and often develop an atypical lymphoproliferative syndrome (38). Gallin et al. have described a human neutrophil granule disorder in a patient with recurrent infections (39). The monocytes of this patient responded very well to FMLP in vitro, but appeared at significantly lower numbers in Rebuck skin window sites. Moreover, the patient's PMN extract did not manifest chemotactic activity for normal monocytes in vitro, whereas the same extract from normal neutrophils was chemotactic for monocytes. In view of our findings that cathepsin G is a potent chemoattractant for monocytes, the immune defects in these patients may be at least partially attributed to insufficient release of cathepsin G which is necessary for proper development of inflammatory response.
Our findings that neutrophil-derived cathepsin G, azurocidin/CAP37, and defensins (5) can serve as signals that amplify inflammatory reactions by attracting mononuclear cells in addition to neutrophils lend further support to the hypothesis that neutrophils may be necessary for the development of mononuclear cell infiltration into inflammatory sites. Although these three neutrophil granule constituents have been identified as contributors that can convert acute into chronic inflammatory responses, it is possible that our purification procedures have not identified all the participant signals. Cathepsin G and defensins appear to have activating as well as chemotactic effects on the attracted cells. This contention is based on our studies showing that administration of cathepsin G or defensins, along with an antigen, to mice results in enhancing the antibody response and also leads to greater production of interferon γ and IL-4 by the injected mice (Tani, K., W.J. Murphy, O. Chertov, D. Taub, J.J. Oppenheim, and J.M. Wang, manuscript submitted). This indicates that these chemoattractants may also augment in vivo immune responses by activating lymphocytes. Consequently, neutrophils do appear to facilitate not only subsequent nonspecific mononuclear cell inflammatory responses, but also specific immune reactions.
Acknowledgments
We thank Dr. L.E. Henderson, Ray Sowder, and D.G. Johnson (Antiviral Vaccine Program, Science Applications International Corporation Frederick, Frederick, MD) for amino acid and protein sequence analyses. We are also grateful to Dr. Douglas Kuhns and Dr. Adit Ben-Baruch for careful reading of the manuscript and valuable suggestions.
Footnotes
Abbreviations used in this paper: DFP, diisopropylfluorophosphate; DTNB, 5,5′-ditiobis-(2-nitrobenzoic acid); HPF, high-power field.
References
- 1.Page AR, Good RA. A clinical and experimental study of the function of neutrophils in the inflammatory response. Am J Pathol. 1958;34:645–669. [PMC free article] [PubMed] [Google Scholar]
- 2.Doherty DE, Downey GP, Worthen GS, Haslett C, Henson PM. Monocyte retention and migration in pulmonary inflammation. Requirements for neutrophils. Lab Invest. 1988;59:200–213. [PubMed] [Google Scholar]
- 3.Taub DD, Anver M, Oppenheim JJ, Longo DL, Murphy WJ. T lymphocyte recruitment by interleukin-8 (IL-8). IL-8–induced degranulation of neutrophils releases potent chemoattractants for human T lymphocytes both in vitro and in vivo. J Clin Invest. 1996;97:1931–1941. doi: 10.1172/JCI118625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen CG, Anderson AO, Appella E, Oppenheim JJ, Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science (Wash DC) 1989;243:1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- 5.Chertov O, Michiel DF, Xu L, Wang JM, Tani K, Murphy WJ, Longo DL, Taub DD, Oppenheim JJ. Identification of defensin-1, defensin-2, and CAP37/ azurocidin as T-cell chemoattractant proteins released from interleukin-8–stimulated neutrophils. J Biol Chem. 1996;271:2935–2940. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 6.Pereira HA, Shafer WM, Pohl J, Martin LE, Spitznagel JK. CAP37, a human neutrophil-derived chemotactic factor with monocyte specific activity. J Clin Invest. 1990;85:1468–1476. doi: 10.1172/JCI114593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Territo MC, Ganz T, Selsted ME, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyum, A. 1968. Separation of leukocytes from blood and bone marrow. Scand. J. Clin. Lab. Invest. 21(Suppl.):77–89. [PubMed]
- 9.Millard PJ, Henkart MP, Reynolds CW, Henkart PA. Purification and properties of cytoplasmic granules from cytotoxic rat LGL tumors. J Immunol. 1984;132:3197–3204. [PubMed] [Google Scholar]
- 10.Colotta F, Bersani L, Lazzarin A, Poli G, Mantovani A. Rapid killing of actinomycin D–treated tumor cells by human monocytes. II.Cytotoxicity is independent of secretion of reactive oxygen intermediates and is suppressed by protease inhibitors. J Immunol. 1985;134:3524–3531. [PubMed] [Google Scholar]
- 11.Falk W, Goodwin RH, Jr, Leonard EJ. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33:239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- 12.Green GDJ, Shaw E. Thiobenzyl benzyloxycarbonyl-l-lysine, substrate for a sensitive colorimetric assay for trypsin-like enzymes. Anal Biochem. 1979;93:223–226. doi: 10.1016/s0003-2697(79)80141-4. [DOI] [PubMed] [Google Scholar]
- 13.Badalato R, Johnston JA, Wang JM, McVicar D, Xu LL, Oppenheim JJ. Serum amyloid A induced calcium mobilization and chemotaxis of human monocytes by activating a pertussis toxin signaling pathway. J Immunol. 1995;155:4004–4010. [PubMed] [Google Scholar]
- 14.Bar-Shavit R, Kahn A, Wilner GD, Fenton JW., II Monocyte chemotaxis: stimulation by specific exosite region in thrombin. Science (Wash DC) 1983;220:728–731. doi: 10.1126/science.6836310. [DOI] [PubMed] [Google Scholar]
- 15.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 16.Ward PA. Chemotaxis of mononuclear cells. J Exp Med. 1968;128:1201–1221. doi: 10.1084/jem.128.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvesen G, Farley G, Shuman J, Przybyla A, Reily C, Travis J. Molecular cloning of human cathepsin G: structural similarity to mast cell and cytotoxic T lymphocyte proteinases. Biochemistry. 1987;26:2289–2293. doi: 10.1021/bi00382a032. [DOI] [PubMed] [Google Scholar]
- 18.Campbell EJ, Silverman EK, Campbell MA. Elastase and cathepsin G of human monocytes. Quantitation of cellular content, release in response to stimuli, and heterogeneity in elastase-mediated proteolytic activity. J Immunol. 1989;143:2961–2968. [PubMed] [Google Scholar]
- 19.Schechter NM, Mei-Wang Z, Blacher RW, Lessin SR, Lazarus GS, Rubin H. Determination of the primary structures of human skin chymase and cathepsin G from cutaneous mast cells of urticaria pigmentosa lesions. J Immunol. 1994;152:4062–4069. [PubMed] [Google Scholar]
- 20.Travis, J. 1988. Structure, function, and control of neutrophil proteinases. Am. J. Med. 84 (Suppl.):37–42. [DOI] [PubMed]
- 21.Caughey GH, Schaumberg TH, Zerweck EH, Butterfield JH, Hanson RD, Silverman GA, Ley TJ. The human mast cell chymase gene (CMA1): mapping to the cathepsin G/granzyme gene cluster and lineage-restricted expression. Genomics. 1993;15:614–620. doi: 10.1006/geno.1993.1115. [DOI] [PubMed] [Google Scholar]
- 22.Kubes P, Smith P, Grisham MD, Granger DN. Neutrophil-mediated proteolysis. Differential roles for cathepsin G and elastase. J Inflamm. 1993;17:321–332. doi: 10.1007/BF00918993. [DOI] [PubMed] [Google Scholar]
- 23.Padrines M, Wolf M, Walz A, Baggolini M. Interleukin-8 processing by neutrophil elastase, cathepsin G and proteinase-3. FEBS Lett. 1994;352:231–235. doi: 10.1016/0014-5793(94)00952-x. [DOI] [PubMed] [Google Scholar]
- 24.Avril LE, di Martino-Ferrer M, Brillard-Boudet M, Gauthier F. Inhibition of U-937 membrane-associated cathepsin G by gp120(IIIB) and V3 loop-derived peptides from several strains of HIV-1. FEBS Lett. 1995;367:251–256. doi: 10.1016/0014-5793(95)00571-p. [DOI] [PubMed] [Google Scholar]
- 25.Campbell EJ. Human leukocyte elastase, cathepsin G, and lactoferrin: family of neutrophil granule glycoproteins that bind to an alveolar macrophage receptor. Proc Natl Acad Sci USA. 1982;79:6941–6945. doi: 10.1073/pnas.79.22.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamazaki T, Aoki Y. Cathepsin G binds to human lymphocytes. J Leukocyte Biol. 1997;61:73–79. doi: 10.1002/jlb.61.1.73. [DOI] [PubMed] [Google Scholar]
- 27.Selak MA, Smith JB. Cathepsin G binding to human platelets. Evidence for a specific receptor. Biochem J. 1990;266:55–62. doi: 10.1042/bj2660055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selak MA. Cathepsin G and thrombin: evidence for two different platelet receptors. Biochem J. 1994;297:269–275. doi: 10.1042/bj2970269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hase-Yamazaki T, Aoki Y. Stimulation of human lymphocytes by cathepsin G. Cell Immunol. 1995;160:24–32. doi: 10.1016/0008-8749(95)80005-4. [DOI] [PubMed] [Google Scholar]
- 30.Ding Y, Uitto VJ, Haapasalo M, Lounatmaa K, Konttinen YT, Salo T, Sorsa T. Membrane components of Treponema dendicolatrigger proteinase release from human polymorphonuclear leukocytes. J Dent Res. 1996;75:1986–1993. doi: 10.1177/00220345960750121101. [DOI] [PubMed] [Google Scholar]
- 31.Knall C, Young S, Nick JA, Buhl AM, Worthen GS, Johnson GL. Interleukin-8 regulation of the Ras/ Raf/mitogen-activated protein kinase pathway in human neutrophils. J Biol Chem. 1996;271:2832–2838. doi: 10.1074/jbc.271.5.2832. [DOI] [PubMed] [Google Scholar]
- 32.Jones SA, Wolf M, Qin S, Mackay CR, Baggolini M. Different functions for interleukin 8 receptors (IL-8R) of human neutrophil leukocytes: NADPH oxidase and phospholipase D are activated through IL-8R1 but not IL-8R2. Proc Natl Acad Sci USA. 1996;93:6682–6686. doi: 10.1073/pnas.93.13.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubin H. The biology and biochemistry of antichymotrypsin and its potential role as a therapeutic agent. Biol Chem Hoppe-Seyler. 1992;373:497–502. doi: 10.1515/bchm3.1992.373.2.497. [DOI] [PubMed] [Google Scholar]
- 34.Cichy J, Potempa J, Chawla RK, Travis J. Regulation of α1antichymotrypsin synthesis in cells of epithelial origin. FEBS Lett. 1995;359:262–266. doi: 10.1016/0014-5793(95)00064-g. [DOI] [PubMed] [Google Scholar]
- 35.Hill RE, Shaw PH, Boyd PA, Baumann H, Hastie ND. Plasma protease inhibitors in mouse and man: divergence with the reactive centre regions. Nature (Lond) 1984;311:175–177. doi: 10.1038/311175a0. [DOI] [PubMed] [Google Scholar]
- 36.Kanemaru K, Meckelein B, Marshall DC, Abraham CR. Synthesis and secretion of active alpha 1-antichymotrypsin by murine primary astrocytes. Neurobiol Aging. 1996;17:767–771. doi: 10.1016/0197-4580(96)00111-x. [DOI] [PubMed] [Google Scholar]
- 37.Hof P, Mayr I, Huber R, Korzus E, Potempa J, Travis J, Powers JC, Bode W. The 1.8 Å crystal structure of human cathepsin G in complex with Suc-Val-Pro-PheP- (OPh)2: a Janus-faced proteinase with two opposite specificities. EMBO (Eur Mol Biol Organ) J. 1996;15:5481–5491. [PMC free article] [PubMed] [Google Scholar]
- 38.Ganz T, Metcalf JA, Gallin JI, Boxer LA, Lehler RI. Microbicidal/cytotoxic proteins of neutrophils are deficient in two disorders: Chediak-Higashi syndrome and “specific” granule deficiency. J Clin Invest. 1988;82:552–556. doi: 10.1172/JCI113631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallin JI, Fletcher MP, Seligmann BE, Hoffstein S, Cehrs K, Mounessa N. Human neutrophil-specific granule deficiency: a model to assess the role of neutrophil-specific granules in the evolution of the inflammatory response. Blood. 1982;59:1317–1329. [PubMed] [Google Scholar]