Abstract
T cell receptor (TCR) recognition of peptide–major histocompatibility complex antigens can elicit a diverse array of effector activities. Here we simultaneously analyze TCR engagement and the production of multiple cytokines by individual cells in a clonal Th1 CD4+ cell population. Low concentrations of TCR ligand elicit only interferon-γ (IFN-γ) production. Increasing ligand recruits more cells into the IFN-γ+ pool, increases IFN-γ produced per cell, and also elicits IL-2, but only from cells already making IFN-γ. Most cells producing only IFN-γ show less TCR downmodulation than cells producing both cytokines, consistent with a requirement for more TCR signaling to elicit IL-2 than to evoke IFN-γ synthesis. These studies emphasize the hierarchical organization of TCR signaling thresholds for induction of distinct cytokine responses, and demonstrate that this threshold phenomenon applies to individual cells. The existence of such thresholds suggests that antigen dose may dictate not only the extent, but also the quality of an immune response, by altering the ratios of the cytokines produced by activated T cells. The quantitative relationships in this response hierarchy change in response to costimulation through CD28 or LFA-1, as well as the differentiation state of the lymphocyte, explaining how variations in these parameters in the face of a fixed antigen load can qualitatively influence immune outcomes. Finally, although the IFN-γ/IL-2 hierarchy is seen with most cells, among cells with the greatest TCR downmodulation, some produce only IFN-γ and not IL-2, and the amount of IFN-γ exceeds that in double producers. Thus, these single cell analyses also provide clear evidence of nonquantitative intraclonal heterogeneity in cytokine production by long-term Th1 cells, indicating additional complexity of T cell function during immune responses.
Antigen-receptor (TCR) recognition of peptide-MHC molecule ligands elicits a diverse array of effector activities from T cells, including secretion or surface expression of cytokines influencing cell function in an autocrine and paracrine fashion (1, 2). The ratios of individual cytokines to one another determine whether the immune response is predominantly of the humoral or inflammatory type (3), or whether T cells proliferate, become anergic, or undergo apoptosis (4). A key issue in understanding the relationship between antigen exposure and immune response is the relationship between antigen load and the relative production of each mediator. Upon TCR engagement, do T cells make all of their cytokines in a fixed proportion, with only the absolute quantity varying with occupancy, or does changing the level of TCR signaling affect the relative production of each cytokine differently? Evidence for the latter possibility could help explain why different classes of T cell responses occur at different antigen concentrations (5, 6). Some investigators have reported the selective production of IL-4 versus IFN-γ at low versus high antigen concentrations, respectively (7, 8). We have shown that for a mouse Th1 clone, the ligand concentration needed for half-maximal production of each of two cytokines can differ by 10–100-fold, due in part to the distinct effect of CD28-mediated costimulation on the two responses (9) and similar findings have been reported using human T cells (10, 11). These limited data suggest that the effect of antigen dose on immunity may reflect distinct TCR signaling thresholds for individual cytokine responses that can be modified by additional signals from the APC.
Most studies on the quantitative relationship between antigen-receptor engagement and T cell function have involved analysis of the pooled responses of cell populations exposed to variations in ligand density in the form of antireceptor antibodies or peptide-MHC molecule ligands. Even using cloned responding populations, this type of analysis does not directly reveal the relationship between the extent of TCR engagement and effector response, which might vary among the individual cells even in such putatively homogenous populations, or differences in the responses of single cells within a cloned population based on parameters other than TCR occupancy. Such diversity could arise due to epistatic differences among cells in the state or number of molecules involved in signaling or gene transcription, or because of variation in the exposure to APCs in the cultures, which themselves may be heterogeneous in terms of antigen display and accessory molecule function (12, 13). Most significantly, they cannot determine whether an increasing antigen dose leads to a greater response per cell, or to a greater number of responding cells, with production per cell remaining relatively constant. Evidence for the latter possibility has been reported using artificial promoter constructs expressed in transformed T cell lines (14, 15).
We were therefore especially interested in analyzing T cell responses to peptide-MHC molecule ligands using methods that would permit quantitation of TCR engagement on the responding T cells and at the same time allow measurement of multiple effector responses by each of the cells in the same antigen-exposed population. Several recent advances have made such an analysis feasible. A number of studies have shown that effective engagement of the TCR complex leads to downmodulation of TCR expression (16–18), apparently through endocytic uptake that is dependent on the activity of tyrosine kinases activated by ligand interaction with the receptor (19, 20). In addition, methods for intracellular staining of various cytokines permit quantitative analysis of the effector responses of individual T cells (21). Because both of these approaches depend on flow cytometric measurement, it is possible to simultaneously evaluate TCR engagement and multiple effector responses in each cell of a population under varying conditions.
Here we report the result of such experiments using several Th1 clones of mouse origin. Our studies reveal a hierarchical organization of TCR signal-dependent response thresholds for elicitation of different cytokines in individual cells. This result has significant implications for the mix of cytokines produced at a given antigen dose level, and hence, the quality of the ensuing immune response. The quantitative relationships between these hierarchical thresholds can be modulated by antigen-unspecific cosignals and by the state of differentiation of the T cell, suggesting a mechanism by which costimulatory signals affect the polarization of immune responses (22–25). Our data are also consistent with earlier proposals that the amount of IL-2 produced by single cells does not vary over a large range in the face of increasing antigen density (14, 15). Instead, the primary factor involved in the rising response of the cell population is growth in the proportion of cells making this cytokine at a relatively fixed level. The existing evidence that this latter phenomenon relates to cooperative promoter occupancy by transcription factors suggests a general molecular model for the regulatable hierarchical response thresholds we document here. Finally, we note the existence of intraclonal heterogeneity in the production of cytokines characteristic of fully differentiated Th1 cells that cannot be readily attributed to quantitative differences in TCR signaling. This latter observation agrees with previous reports concerning the low frequency of individual cells producing the complete set of cytokines considered prototypic of the Th1 or Th2 state (24–27). We suggest that this latter form of response heterogeneity may play a special role in the memory versus effector properties of individual T cells.
Materials and Methods
Peptides.
Peptides were synthesized, purified, and the products analyzed by the National Institute of Allergy and Infectious Diseases Peptide Facility. The sequences of PCC88-104 and MCC93-103(97I) are KAERADLIAYLKQATAK and DLIAILKQATK, respectively.
T Cell Clones and APCs.
A.E7 and 3C6 are Th1-cell clones specific for pigeon cytochrome c (PCC) 88-104 and I-Ek (28). These clones were maintained by intermittent stimulation with B10.A spleen cells and PCC88-104 and expansion in IL-2–containing medium, followed by rest in the absence of APC, antigen, or growth factor. All cells studied here were used no earlier than 2 wk after restimulation and cytokine expansion. Live cells were isolated from such resting cultures using Lympholyte M (Cedarlane Labs Ltd., Hornby, Ontario, Canada). P13.9 is a supertransfected derivative of the DAP.3 fibroblast-derived transfectant DCEK Hi7 that expresses high constitutive levels of I-Ek, CD80, and ICAM-1. The DCEK Hi7 parent expresses similar levels of I-Ek, but has low CD80 and little or no ICAM-1 (29).
ELISA Measurement of Functional Responses.
P13.9 were cultured with peptide overnight at 37°C, then treated with mitomycin c (20 μg/ml) at 37°C for 1 hr, and washed four times with PBS. T cell clones (2.5 × 104/well) were cultured with APC (5 × 104/well) in 96-well flat-bottomed plates. Supernatants were collected 24 h later. ELISA assays were done using mAb JES6-1A12 and biotinylated JES6-5H4 for IL-2, MP2-8F8 and biotinylated MP2-43D11 for IL-3, and R4-6A2 and biotinylated XMG1.2 for IFN-γ (PharMingen, San Diego, CA). To detect the binding of biotinylated antibodies, alkaline phosphatase–conjugated avidin (Sigma Chemical Co., St. Louis, MO) was followed by p-nitrophenyl phosphate (Sigma Chemical Co.). The reaction product was measured at 405 nm and the results converted into cytokine units using standard curves.
Single Cell Analysis of Cytokine Production and TCR Expression.
In most experiments, P13.9 were cultured with peptide overnight at 37°C and unbound peptide was washed away. T cell clones (5 × 105) and APC (106) were cultured in 5 ml polypropylene tubes (FALCON 2058; Becton Dickinson, Franklin Lakes, NJ) in the presence of monensin (2 μM; Sigma Chemical Co.). In other experiments, T cells (2.5 × 105) with or without precoating with anti-CD28 (2 μg/ml) were stimulated using anti-CD3ε immobilized in 48-well plates. After culture, cells were washed with PBS containing 0.5 mM EDTA. Surface molecules were stained with biotinylated anti-Vβ3, anti-TCR Cβ, or anti-CD40L antibody followed by streptavidin-CyChrome (PharMingen). After washing with PBS, cells were fixed with PBS containing 4% paraformaldehyde for 5 min at 37°C, then washed with PBS (1% BSA) once. After two additional washes with PBS (0.1% saponin, 0.1% BSA, and 0.01 M Hepes), FITC– anti IFN-γ and PE–anti IL-2 antibodies (PharMingen) were added (21). Before flow cytometry acquisition, stained cells were washed twice with PBS (0.1% saponin, 0.1% BSA, and 0.01 M Hepes). T cells were gated using FSC/SSC parameters and 2 × 104 cells were analyzed.
Quantitative Reverse Transcriptase–PCR.
T cell clones (5 × 106) were cultured with P13.9 (107) for 5 h as above. Total RNA was extracted with RNA STAT-60 (Tel-Test “B”, Inc., Friendswood, TX). cDNA was synthesized using random hexamers and reverse transcriptase. The first PCR was performed using 1 μl of cDNA reaction sample, β-actin primers (0.4 μM), Taq DNA polymerase (2.5 U, Perkin Elmer, Norwalk, CT), dNTP (200 μM), [32P]dCTP (1 μCi; Amersham Corp., Arlington Heights, IL), and MgCl2 (2.5 mM) in a total volume 50 μl. The reaction was 25 cycles of denaturation 94°C for 1 min, annealing 55°C for 1 min, and extension 72°C for 2 min. After electrophoresis in 8% polyacrylamide gels and autoradiography, densitometric analysis was performed. Based on these data, the volumes of cDNA solutions containing the same amount of β-actin templates were calculated. After a second PCR was done in order to confirm that the adjusted cDNA amounts give the same level of amplification of β-actin among samples, specific primers for β-actin, IL-2, or IFN-γ were used in a third PCR. Incorporated radioactivity in bands of appropriate size was quantified by densitometer analysis of the autoradiographs. Results of these experiments are presented as the percentage of the maximum response of the cells to antigen. The primer sequences used were: β-actin sense 5′-GTGGGCCGCTCTAGGCACCAA-3′ and antisense 5′-CTCTTTGATGTCACGCACGATTTC-3′; IL-2 sense 5′-ATGTACAGCATGCAGCTCGCATC-3′ and antisense 5′-GGCTTGTTGAGATGATGCTTTGACA-3′; and IFN-γ sense 5′-TGAACGCTACACACTGCATCTTGG-3′ and antisense 5′-CGACTCCTTTTCCGCTTCCTGAG-3′.
Induction and Response Analysis of Anergic Cells.
P13.9 were cultured with or without peptide and Dynabeads (Dynal Inc., Oslo, Norway) overnight. A.E7 (2 × 106) was cultured with APC (2 × 106) for 24 h in 5-ml tubes. After washing with 0.5 mM EDTA-PBS, APC were depleted with a magnet. After 7 d of rest, A.E7 was restimulated with P13.9 and 10 μM PCC88-104 for 5 h and stained as described above.
Results
Hierarchical Arrangement of Antigen Dose Thresholds for Distinct Cytokine Responses of CD4+ Th1 Cells.
We have shown previously that the ligand concentration needed to elicit 50% of the maximum response by the 3C6 Th1 clone for each of two cytokines (IL-2 and IL-3) can differ by up to 100–fold, and that this relationship depends in large measure on the availability of CD28 costimulation (9). These data were interpreted as evidence for hierarchical TCR occupancy thresholds for triggering individual effector responses. Similar findings have been made in studies using human T cells, which show that induction of IFN-γ production and proliferation require substantially more antigen-induced TCR downmodulation than cytotoxicity (10). To expand on these previous observations, ELISA was used to measure cytokine secretion in response to agonist peptide–MHC class II ligand presented by transfected cells whose CD80/CD86-mediated costimulatory function does not change in response to T cell signals. Fig. 1 shows IFN-γ, IL-2, and IL-3 production by two different Th1 clonal cell populations (3C6 and A.E7), displayed as percentage of maximum response to allow direct comparison of the fractional response for each mediator at each antigen concentration. The ligand amounts eliciting the minimally measurable and maximal responses are different for each cytokine, with the overall hierarchy being IFN-γ > IL-2 > IL-3 for 3C6 and IFN-γ > IL-3 = IL-2 for A.E7. These findings confirm earlier data suggesting that the numbers of TCR ligands needed to elicit a fixed fractional response for different cytokines are distinct. They also demonstrate that among individual clones, some effector responses (IL-3 in this case) may vary in their relative position in this dose- response hierarchy.
Figure 1.
Hierarchical cytokine production thresholds detected by ELISA. 3C6 and A.E7 were cultured with P13.9 and PCC88-104. Supernatants were collected 24 h later and IL-2, IL-3, and IFN-γ secretion was determined by ELISA. The results were converted into cytokine units using standard curves and expressed as the percentage of the maximum response obtained for each cytokine at plateau. Absolute cytokine production representing the maximal response on this scale ranged in various experiments from 50 to 200 U/ml for IL-2, 6,000 to 19,000 U/ml for IFN-γ for both cells, 5 to 25 U/ml for 3C6, and 80 to 700 /ml for A.E7 for IL-3.
Evidence for Hierarchical Response Thresholds at the Single Cell Level.
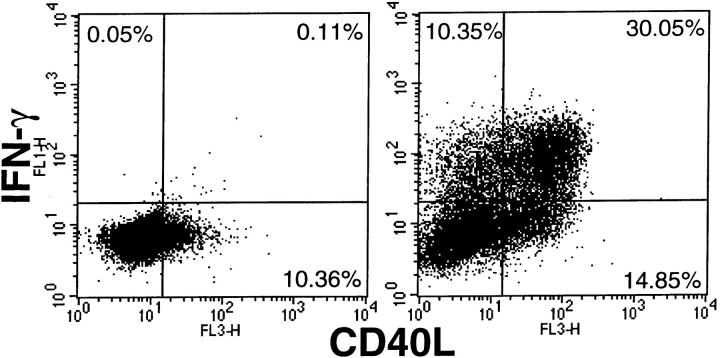
Multiparameter flow cytometric assays for intracellular cytokine levels were conducted to examine whether these ELISA findings reflect the properties of individual cells in a population. In accord with the lower antigen dose requirement for IFN-γ production seen by ELISA, staining of activated A.E7 for IFN-γ and IL-2 after low level (0.1 nM) antigen exposure reveals only two populations, IFN-γ single producers and cells making neither IFN-γ nor IL-2 at detectable levels (Fig. 2). As the antigen concentration is increased, cells producing IL-2 become apparent, but these IL-2 producers also make IFN-γ. Both the number of cells making IFN-γ and the amount of this cytokine per cell increase with antigen concentration. In contrast, IL-2 production remains more constant per cell as ligand increases, with the primary change being in the number of cells making this cytokine at a fixed level. 3C6 shows a similar pattern as antigen dose is increased, although somewhat more cells making IL-2 with low accompanying IFN-γ production are observed at a high antigen concentration (Fig. 2). These single cell data thus confirm the results of the ELISA, showing that IFN-γ is made by the cells at lower offered antigen doses than those required for IL-2. They further show that this hierarchical relationship reflects the behavior of individual cells in the population, each of which makes either IFN-γ or both IFN-γ and IL-2, but not IL-2 alone. Each cell thus shows a higher response threshold for IL-2 production, rather than there being two different sets of cells in the population each making either IL-2 or IFN-γ, and each with distinct sensitivities to TCR signals. In contrast to this relationship for IL-2 and IFN-γ, a similar analysis examining single cell IFN-γ and CD40L responses shows a different result (Fig. 3). Virtually no cells make only one or the other cytokine, and only a small fraction of cells makes responses strongly biased to one or the other mediator. Instead, the majority of cells make similar levels of both cytokines, indicating that for these two responses, the triggering thresholds are similar.
Figure 2.
Cytokine production analyzed at the single cell level. Intracellular IFN-γ and IL-2 were determined by flow cytometry after 5 h of culture in monensin with the indicated concentration of PCC88-104 and P13.9 APC. The numbers in each figure refer to the percentage of the total cell number and the FL1 (IFN-γ) and FL2 (IL-2) mean intensities of cells in the indicated quadrant of the two-parameter histograms.
Figure 3.
Similar stimulation thresholds for IFN-γ and CD40L production. 3C6 cells were stimulated for 8 h with P13.9 APC alone (left) or with P13.9 pulsed with 10 μM PCC88-104 (right) in the absence of monensin, then stained surface CD40L expression and intracellular IFN-γ content.
One possible explanation for the asymmetries seen in the ELISA and flow cytometric data for IL-2 and IFN-γ versus the lack of such asymmetry for IFN-γ and CD40L is in the characteristics of the antibodies used in the assays. It was possible that the anti–IFN-γ antibodies are more sensitive detectors of their ligand than the anti–IL-2 antibodies, but comparable to anti-CD40L in this regard. To address this issue, reverse transcriptase–PCR assays were run using mRNA from cells treated similarly to those used for the experiments shown in Figs. 1 and 2. This provides an antibody-independent approach to analysis of cytokine responses, although not on a single cell basis. The data confirm the results of the two assays using antibody detection of product, in that the IFN-γ response is first detected at a 10–30-fold lower antigen concentration than is the IL-2 response (Fig. 4).
Figure 4.
Reverse transcriptase–PCR analysis of T cell cytokine responses. IFN-γ and IL-2 mRNA levels were analyzed for 3C6 and A.E7 after stimulation with P13.9 APC pulsed with varying PCC88-104 concentrations.
Evidence for Hierarchical Triggering Thresholds at the TCR Engagement Level.
TCR downmodulation serves as a convenient single cell measure of TCR occupancy (10, 18). The same cell populations analyzed for cytokine production by intracellular staining were therefore also analyzed for TCR expression before and after antigen exposure for 5 h, a time point we find to be near the plateau of TCR downmodulation under these conditions (Itoh, Y., and R.N. Germain, manuscript in preparation). For 3C6, both the majority of cells producing only IFN-γ and many of the IFN-γ–negative cells have a similar TCR level, lower than that on cells not exposed to specific antigen (Fig. 5 B). These data indicate that a subpopulation of these cloned T cells engage their TCR, but fail to make either of the studied cytokines. In contrast, the TCR expression of IL-2– producing cells is lower than that of IL-2–negative cells (Fig. 5 B), suggesting that the former have undergone more TCR downmodulation, and have therefore received more antigen-dependent signals.
Figure 5.
TCR downmodulation and cytokine production. T cell clone 3C6 was cultured with P13.9 + PCC88-104 (10 μM) in the presence of monensin. After 5 h of incubation, surface TCR Vβ3, as well as intracellular IFN-γ and IL-2, were analyzed. (A) IFN-γ and IL-2 production by 3C6. R1 = cells producing both IFN-γ and IL-2; R2 = cells producing IFN-γ alone; R3 = cells producing neither IFN-γ nor IL-2. (B) TCR expression of each cell subpopulation after incubation with 10 μM PCC88-104 + P13.9. Gates R1, R2, and R3 are as in A. The histogram for cells in R2 largely overlaps that for cells in R3. (C) Ungated TCR expression of 3C6 incubated with either with 10 μM PCC88-104 (solid line) or without peptide (dotted line) + P13.9. The gates set to analyze cytokine expression by the cells with the 15% lowest (TCRlow) and 15% highest (TCRhi) receptor levels after incubation are indicated. (D) IFN-γ and IL-2 production profiles of TCRlow and TCRhi populations as detailed in C. The percentage of total cells and the mean intensity of cytokine staining are shown for cells in the respective quadrants of the two-parameter histograms.
These same data have also been analyzed by identifying the cells with the 15% highest and 15% lowest TCR levels after antigen exposure (Fig. 5 C) and examining their cytokine profiles (Fig. 5 D). The TCRlow population is greatly enriched in cells producing both IL-2 and IFN-γ as compared with the TCRhi population. This population also contains the very small number of cells making substantial IL-2 and a low level of IFN-γ as well as those producing the greatest amount of IFN-γ alone. In contrast, many of the TCRhi cells produce high amounts of IFN-γ without IL-2. These results demonstrate at the individual cell level that IL-2 production requires more TCR triggering (has a higher signaling threshold) than IFN-γ production, indicating that the response hierarchies seen when varying offered antigen concentration reflect the requirement at the single cell level for different amounts of TCR-derived signals for the production of each cytokine. These observations also indicate that for some cells, the failure to make IL-2 is not the result of TCR engagement inadequate to reach the quantitative signaling threshold for production of this cytokine. These cells produce a greater amount of IFN-γ than cells making both IFN-γ and IL-2 and they have undergone similar, extensive TCR downmodulation as the double producers, suggesting an intraclonal heterogeneity in response potential distinct from the quantitative hierarchy seen in other cells for IFN-γ versus IL-2 production.
Alteration in Relative Response Thresholds for IFN-γ and IL-2 Production by Variation of Costimulatory Signals.
The above experiments were conducted using APCs with a fixed CD80 and ICAM-1 display. Costimulatory signals produced by CD28 interaction with CD80 or CD86 markedly augment the total amount of IL-2 secreted at a given level of offered TCR ligand, with a lesser effect on other cytokines such as IL-3 and IFN-γ (4, 12, 13). The quantitative aspects of the hierarchical relationships just described might thus be modified by changing the costimulatory environment in which the T cell is signaled, an idea for which some support already exists (9, 11, 24, 25, 30). DCEK Hi7 is a transfected APC that has the same I-Ek levels as P13.9, but expresses low CD80 and little or no ICAM-1 (29, 31). At 10 μM peptide, DCEK induces a similar fraction of the cells to produce IFN-γ as does P13.9 (Fig. 6 B), although the amount of IFN-γ per cell is somewhat lower than with the CD80+ cells. However, the difference between P13.9 and DCEK is most remarkable looking at IL-2 production. P13.9 and 10 μM of PCC88-104 induce numerous IL-2– positive cells, whereas DCEK + PCC88-104 stimulate only very few IL-2–producing cells at the same antigen dose, despite promoting the same level of TCR downmodulation (Fig. 6, B and C). These data directly demonstrate that CD28- and ICAM-1–mediated costimulation can change the relative TCR occupancy thresholds required for elicitation of different cytokines. This effect on the functional response to TCR signaling occurs without substantially altering the extent of TCR engagement, indicating an intracellular site of action rather than a change in the efficiency of ligand recognition. Fig. 6 also shows that the change in threshold upon removal of CD28 costimulation reveals itself not in a grossly different amount of IL-2 produced per cell, but in a smaller number of cells producing IL-2 at all. A similar result is obtained if the peptide ligand is replaced by anti-CD3 and CD80 signaling replaced by anti-CD28 antibody, to reduce the possibility that variations in cell–cell contact contribute to these findings (Fig. 7). Thus, the threshold change appears to affect the cells individually in an all-or-none fashion, as also observed when examining the antigen-dose response in the presence of fixed costimulation (Fig. 2).
Figure 6.
Effect of costimulation on signaling thresholds for effector responses. A.E7 was cultured with P13.9 (costimulationhi) or DCEK Hi 7 (costimulationlo) APC plus 10 μM PCC88-104 for 5 h in the presence of monensin, then stained as described in Fig. 2. The left and middle figures show IFN-γ and IL-2 production stimulated by P13.9 and DCEK Hi 7, respectively. The right histogram shows TCR expression after stimulation.
Figure 7.
Effect of CD28 costimulation on response threshold, the number of responding cells, and the response per cell for IL-2 and IFN-γ induced by anti-CD3 antibody. A.E7 cells were precultured without (top) or with (bottom) anti-CD28 antibody and then added for 5 h to the wells of plates precoated with the indicated concentration of anti-CD3 antibody. The cells were then stained for intracellular IL-2 and IFN-γ.
Altered Response Thresholds in Anergic T cells.
If Th1 cells are first exposed to TCR ligand and deprived of IL-2, subsequent stimulation in the presence of CD80/86+ APC reveals a relatively selective loss of IL-2 production (proliferative anergy) (4, 12, 13). The predominant effect on IL-2 has been attributed to a specific signaling lesion affecting IL-2 gene induction, possibly mediated through a block in the ras-raf-ERK pathway (32, 33). Like elimination of CD28 costimulation, this may also represent a shift in the relative TCR occupancy thresholds for individual cytokine responses. To explore this possibility, A.E7 cells were rendered anergic using the partial agonist peptide 97I (31), then rested. The recovered cells were restimulated using CD80+/ICAM-1+ APC and agonist PCC88-104 peptide. Cells precultured with antigen-free APC show the expected IFN-γ only and IFN-γ + IL-2 double-producing populations upon restimulation (Fig. 8 A). In contrast, T cells precultured with APC bearing 97I show very few cells producing IL-2, and the population of high IFN-γ only producers requiring extensive TCR engagement under normal conditions is also diminished (Fig. 8, B and C). Cells from both treatment groups have the same TCR levels at the beginning and end of the assay culture (Fig. 8 D), indicating that there is no defect in occupancy-induced downmodulation in the 97I-exposed (anergic) cells. Thus, the cytokine response of Th1 cells rendered anergic resembles that of cells exposed to low levels of antigen or deprived of CD28 costimulation (compare Figs. 2, 6, 7, and 8 B).
Figure 8.
Cytokine production by anergized T cells. A.E7 was cultured with P13.9 alone (A) or P13.9 with 50 μM MCC97I (B) for 24 h. After separation from APC and resting for a week, cells were restimulated with P13.9 and 10 μM PCC88-104 in the presence of monensin, then stained with anti–IFN-γ, IL-2, and TCR antibodies. IFN-γ production and TCR expression are shown after the second incubation in C and D, respectively. Cells precultured with P13.9 and MCC97I (—) or P13.9 without peptide (----) were restimulated with P13.9 and PCC88-104. Cells without antigen restimulation were shown (·····) for both cell populations.
Discussion
Many studies of T cell activation involve either populations rather than single cells, analysis of only one response parameter, or assay at only one or a few antigen concentrations. The simultaneous study of multiple T cell effector responses over a wide dose range and at the single cell level can provide substantial additional insight into how the extent of TCR signaling relates to a T cell's functional response. Some single cell analyses involving only one or two of these several parameters have been reported previously. Kelso et al. (26) measured multiple cytokine responses from individually cloned cells and reported on the diversity of responses seen under putatively homogenous stimulation conditions. Based on these data, they have argued for a stochastic pattern of effector responses, with available cytokines and cosignals in the environment determining the relative frequency of a particular type of response but not unequivocally inducing it. Bucy and co-workers (25, 27) have conducted elegant double-label in situ hybridization studies of cytokine mRNA levels in bulk and cloned T cell populations polarized to the Th1 or Th2 types. These experiments have shown a low frequency of cells producing more than one of the prototypic cytokines of the Th1 (IL-2 or IFN-γ) or Th2 (IL-4, IL-5, or IL-10) phenotype. These data suggest intraclonal heterogeneity of response, but because the extent of signaling in the cells could not be coanalyzed, quantitative versus qualitative explanations for these findings could not be distinguished. Lanzavecchia and co-workers (11, 18) have introduced flow cytometric measurement of TCR levels as a single cell measure of TCR signal intensity. Using this method together with functional analysis on a population basis, they have concluded that hierarchical thresholds of the type reported for mouse Th1 cytokine responses (9) are seen for human T cells and, like the mouse hierarchy, can be influenced by costimulatory signals. However, neither these latter studies nor our own previous work determined whether cell-to-cell heterogeneity and statistical bias in response of the type described by Kelso et al. or a common and consistent hierarchy seen in each cell in the population accounted for the relationship between antigen dose and induction of distinct responses.
The data reported here use a multiparameter single cell approach to investigate these and related issues. Our findings provide strong support for a model of T cell activation in which different effector functions have a hierarchical arrangement of elicitation thresholds, such that the ratio of elicited effector molecules changes with antigen concentration. This threshold phenomenon affects individual cells in a clonal population and primarily determines at what level of TCR signaling a cytokine response occurs, rather than, or in addition to, how much of the cytokine is synthesized by the cell. This is especially true for IL-2. These results concerning the hierarchical organization of cytokine response thresholds provide a possible explanation for how antigen dose controls the quality of immune responses (5, 6), based on the differing ratios of (counterregulatory) cytokines produced at distinct antigen concentrations. Another intriguing implication of the present findings is that the lower threshold for IFN-γ production by Th1 cells may allow them to amplify their responses by induction of antigen processing and presentation-related genes (DM, MHC class II) (34, 35) which in turn help provide the additional ligand needed to elicit IL-2 production and clonal expansion.
An important and as yet unresolved issue is how the hierarchies distribute among cells in an evolving polyclonal T cell response. Although the order for some specific responses is similar for related cells (IFN-γ versus IL-2 in our Th1 clones), there are clear variations among different clones in the relative positioning of other responses (IL-3 in this case). This heterogeneity in relative threshold positioning could account for the data of Kelso et al. (26), in which at a single antigen dose, different T cells in a nonclonal population produce a varying mix of cytokines that do not follow a clear Th1 or Th2 pattern. The extent to which such variation will influence the effect of response threshold hierarchy on the quality of in vivo immune responses is not yet known. The range of TCR affinities in heterogeneous responding T cell populations, combined with hierarchical cytokine thresholds in each cell, might account for data suggesting that entirely different cytokines are specifically elicited at low and high antigen concentrations (7, 8). Finally, CD80 and CD86 have been reported to have a different potential to promote T cell differentiation towards a Th1 or Th2 phenotype (22, 23), while ICAM-1 has been found to increase both the number of responding T cells in a population and their production of IFN-γ (24). Here we have shown that the relative production of IL-2 versus IFN-γ changes in the presence versus the absence of ICAM-1 and CD80-dependent signaling under the same conditions of TCR engagement. It seems plausible that similar shifts in the relative elicitation of IFN-γ versus IL-4 in Th0 cells (rather than the IL-2 studied here using long-term Th1 clones) could account for the predilection of one or the other cosignal to favor a particular response polarization.
One surprising result was that the variation in response among a clonal T cell population extended to cells that showed clear evidence of TCR downmodulation, and yet failed to make any detectable cytokine, or that showed extensive TCR downmodulation and made even higher levels of IFN-γ than cells producing both cytokines without detectable IL-2. We have isolated the cells that make neither IL-2 nor IFN-γ but show extensive TCR engagement by sorting for CD40L-negative cells generated in the absence of monensin. Upon reculture, some of these cells regain the ability to respond to antigen by producing cytokine, suggesting that after prolonged rest a fraction of the T cells in these clones is in a reversible state of reduced responsiveness to TCR engagement. Preliminary data indicate that this may correlate with the level and/or function of src kinases in these T cells (Itoh, Y., I. Stefanova, and R.N. Germain, unpublished observations). The cells producing IFN-γ without IL-2 are perhaps an even more intriguing intraclonal population. If this IL-2 nonproducer state is not a readily reversible one, they would be excellent candidates for “terminally differentiated effectors” lacking the capacity for extensive self-renewal, whereas the double producers would be able to respond to additional antigen exposure with clonal expansion. This possible segregation of clonal progeny into proliferating memory cells versus high-rate secreting effectors is reminiscent of the distinction between renewing memory B cells and fully differentiated plasma cells. This raises the intriguing possibility that selective loss of proliferative responses to TCR signaling in the face of costimulation, a state akin to classical clonal anergy (4), might be a regular part of the immune response, permitting delivery of potent effector function without overexpansion of a potentially dangerous T cell pool.
In addition to the existence of a critical signaling threshold for elicitation of a particular cytokine response, as antigen concentration rises beyond this point, there is an increase in the overall amount of cytokine produced by a cell population. There is some evidence in model systems that this rise largely depends on the recruitment of more cells into the responding pool, with each cell making a relatively constant cytokine amount (digital response) (14, 15, 25). In this study, evidence that such digital effects play a major role in the quantitative responses of cell populations was seen for IL-2 responses under the control of an intact endogenous regulatory region, confirming these earlier results. The IFN-γ response was more complex, with what appears to be up to a fourfold increase in intracellular cytokine levels as antigen concentration rises above the threshold level, as well as the recruitment of more cells into the responding pool over the same dose range. This contrasts with the report of similar amounts of IFN-γ mRNA detected by in situ hybridization in individual cells activated at both high and low dose of antigen (25).
The interpretation given to the sharp threshold for response in previous experiments was the cooperative nature of transcription factor function (14). An increasing number of reports have provided evidence that little or no gene transcription is seen until all the proteins necessary for making a complete assembly on the promoter are available in the cell at an adequate concentration, following which transcription ensues at a relatively fixed level (36, 37). In vivo footprinting studies of the IL-2 gene in particular (38) have provided direct evidence in favor of this model of cooperative transcription factor binding for effective promoter loading and mRNA synthesis (39). This evidence for gene activation thresholds set by the limiting nature of certain transcription factors can explain not only the discrete cell recruitment seen as antigen dose is increased, but may also explain the differing response thresholds for individual effector responses and their modification by costimulation or anergy induction. If we consider that several distinct factors are needed for optimal transcription of a given cytokine gene, it is reasonable to postulate that the response hierarchy reflects to a large extent the composite efficiency with which TCR-activated and cosignal-activated transcription factors load onto different cytokine gene promoters, based on the arrangement, number, and nucleotide sequence of their factor binding sites (40–42). Those promoters most dependent for function on either factors uniquely induced by costimulation or a specific subset of TCR signals would be most affected by modifications in these parameters. This model can explain why variation in antigen dose with constant costimulation, changing costimulation with fixed antigen dose, or intracellular interference with the TCR signaling pathway (anergy) in the face of fixed TCR occupancy and costimulation can all result in the same phenotypic response from a T cell, seen here as the relatively selective loss of IL-2 production with maintenance of IFN-γ generation.
The altered downstream intracellular signaling induced by partial agonists (43, 44) might also change the relative extent of activation of distinct transcription factors within the cell. This in turn will affect the relative activity of certain genes whose promoter function differs with respect to the need for these factors. Such differences could account for the shift of >10-fold seen for the ED50 for activation-induced cell death versus effector cytokine secretion by A.E7 cells exposed to wild-type versus variant peptide ligands (Combadiére, B., C. Reis e Sousa, R.N. Germain, and M.J. Lenardo, manuscript submitted for publication), the selective loss of IL-2 in contrast to the IL-3 production seen with antagonists of the 3C6 TCR (9), or the induction of IL-4 secretion without proliferation in a Th2 clone by a variant ligand (45). Because of the reinforcing and counterregulatory effects of cytokines (3) and because in the thymus even a small change in ligand-induced signaling can convert a deletional response to a positive selection event (46–48), such changes in the relative positioning of two hierarchical responses may have major effects on the biological outcome. Thus, the quality as well as the magnitude of an evoked immune response will be influenced by both antigen dose and the pharmacologic properties of the TCR ligand(s) to which the antigen gives rise.
Acknowledgments
The authors wish to thank all the members of the Lymphocyte Biology Section for helpful discussion and B. Hemmer, R. Martin, I. Stefanova, L. Nicholson, M. Collins, and V. Kuchroo for sharing data before publication.
References
- 1.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA, Jr, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–285. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 3.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? . J Exp Med. 1996;184:1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parish CR. Immune response to chemically modified flagellin. II. Evidence for a fundamental relationship between humoral and cell-mediated immunity. J Exp Med. 1971;134:21–47. doi: 10.1084/jem.134.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bretscher PA, Wei GJ, Menon JN, Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. . Science (Wash DC) 1992;257:539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 7.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+T helper cell phenotype development in a T cell receptor-αβ transgenic model. J Exp Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+T cells. J Exp Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Racioppi L, Ronchese F, Matis LA, Germain RN. Peptide–major histocompatibility complex class II complexes with mixed agonist/antagonist properties provide evidence for ligand-related differences in T cell receptor- dependent intracellular signaling. J Exp Med. 1993;177:1047–1060. doi: 10.1084/jem.177.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valitutti S, Müller S, Dessing M, Lanzavecchia A. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J Exp Med. 1996;183:1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable threshold. Science (Wash DC) 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 13.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 14.Fiering S, Northrop JP, Nolan GP, Mattila PS, Crabtree GR, Herzenberg LA. Single cell assay of a transcription factor reveals a threshold in transcription activated by signals emanating from the T cell antigen receptor. Genes Dev. 1990;4:1823–1834. doi: 10.1101/gad.4.10.1823. [DOI] [PubMed] [Google Scholar]
- 15.Karttunen J, Shastri N. Measurement of ligand-induced activation in single viable T cells using the lacZ reporter gene. Proc Natl Acad Sci USA. 1991;88:3972–3976. doi: 10.1073/pnas.88.9.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinherz EL, Meuer S, Fitzgerald KA, Hussey RE, Levine H, Schlossman SF. Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell. 1982;30:735–743. doi: 10.1016/0092-8674(82)90278-1. [DOI] [PubMed] [Google Scholar]
- 17.Saito T, Weiss A, Miller J, Norcross MA, Germain RN. Specific antigen Ia activation of transfected human T cells expressing murine Ti αβ-human T3 receptor complexes. Nature (Lond) 1987;325:125–130. doi: 10.1038/325125a0. [DOI] [PubMed] [Google Scholar]
- 18.Valitutti S, Müller S, Cella E, Padovan E, Lanzavecchia A. Serial triggering of many T cell receptors by a few peptide-MHC complexes. Nature (Lond) 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 19.Boyer C, Auphan N, Gabert J, Blanc D, Malissen B, Schmitt-Verhulst AM. Comparison of phosphorylation and internalization of the antigen receptor/CD3 complex, CD8, and class I MHC-encoded proteins on T cells. Role of intracytoplasmic domains analyzed with hybrid CD8/class I molecules. J Immunol. 1989;143:1905–1914. [PubMed] [Google Scholar]
- 20.Luton F, Buferne M, Davoust J, Schmitt-Verhulst AM, Boyer C. Evidence for protein tyrosine kinase involvement in ligand-induced TCR/CD3 internalization and surface redistribution. J Immunol. 1994;153:63–72. [PubMed] [Google Scholar]
- 21.Lee CLY, Lee SHS, Jay FT, Rozee KR. Immunobiological study of interferon-gamma–producing cells after staphylococcal enterotoxin B stimulation. Immunology. 1990;70:94–99. [PMC free article] [PubMed] [Google Scholar]
- 22.Kuchroo VK, Prabhu M, Das, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 23.Freeman GJ, Boussiotis VA, Anumanthan A, Bernstein GM, Ke XY, Rennert PD, Gray GS, Gribben JG, Nadler LM. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–532. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 24.Maraskovsky E, Troutt AB, Kelso A. Co-engagement of CD3 with LFA-1 or ICAM-1 adhesion molecules the frequency of activation of single murine CD4+ and CD8+T cells and induces synthesis of IL-3 and IFN-γ but not IL-4 or IL-6. Int Immunol. 1992;4:475–485. doi: 10.1093/intimm/4.4.475. [DOI] [PubMed] [Google Scholar]
- 25.Bucy RP, Panoskaltsis-Mortari A, Huang GQ, Li J, Karr L, Ross M, Russell JH, Murphy KM, Weaver CT. Heterogeneity of single cell cytokine gene expression in clonal T cell populations. J Exp Med. 1994;180:1251–1262. doi: 10.1084/jem.180.4.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelso A, Groves P, Troutt AB, Francis K. Evidence for the stochastic acquisition of cytokine profile by CD4+ T cells activated in a T helper type 2–like response in vivo. . Eur J Immunol. 1995;25:1168–1175. doi: 10.1002/eji.1830250506. [DOI] [PubMed] [Google Scholar]
- 27.Bucy RP, Karr L, Huang GQ, Li J, Carter D, Honjo K, Lemons JA, Murphy KM, Weaver CT. Single cell analysis of cytokine gene coexpression during CD4+T cell phenotype development. Proc Natl Acad Sci USA. 1995;92:7565–7569. doi: 10.1073/pnas.92.16.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hecht TT, Longo DL, Matis LA. The relationship between immune interferon production and proliferation in antigen-specific, MHC-restricted T cell lines and clones. J Immunol. 1983;131:1049–1055. [PubMed] [Google Scholar]
- 29.Ding L, Linsley PS, Huang L-Y, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 30.Muraille E, De Becker G, Bakkus M, Thielemans K, Urbain J, Moser M, Leo O. Co-stimulation lowers the threshold for activation of naive T cells by bacterial superantigens. Int Immunol. 1995;7:295–304. doi: 10.1093/intimm/7.2.295. [DOI] [PubMed] [Google Scholar]
- 31.Madrenas J, Schwartz RH, Germain RN. Interleukin 2 production, not the pattern of early T cell antigen receptor-dependent tyrosine phosphorylation, controls anergy induction by both agonists and partial agonists. Proc Natl Acad Sci USA. 1996;93:9736–9741. doi: 10.1073/pnas.93.18.9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W, Whaley CD, Mondino A, Mueller DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+T cells. Science (Wash DC) 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 33.Fields PE, Gajewski TF, Fitch FW. Blocked Ras activation in anergic CD4+T cells. Science (Wash DC) 1996;271:1276–1278. doi: 10.1126/science.271.5253.1276. [DOI] [PubMed] [Google Scholar]
- 34.Glimcher LH, Kara CJ. Sequences and factors: a guide to MHC class-II transcription. Annu Rev Immunol. 1992;10:13–49. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]
- 35.Chang C-H, Flavell RA. Class II transactivator regulates the expression of multiple genes involved in antigen presentation. J Exp Med. 1995;181:765–767. doi: 10.1084/jem.181.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang J, Levine M. Binding affinities and cooperative interactions with bHLH activators delimit threshold responses to the dorsal gradient morphogen. Cell. 1993;72:741–752. doi: 10.1016/0092-8674(93)90402-c. [DOI] [PubMed] [Google Scholar]
- 37.Chung JD, Stephanopoulos G, Ireton K, Grossman AD. Gene expression in single cells of Bacillus subtilis:evidence that a threshold mechanism controls the initiation of sporulation. J Bacteriol. 1994;176:1977–1984. doi: 10.1128/jb.176.7.1977-1984.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garrity PA, Chen D, Rothenberg EV, Wold BJ. Interleukin-2 transcription is regulated in vivoat the level of coordinated binding of both constitutive and regulated factors. Mol Cell Biol. 1994;14:2159–2169. doi: 10.1128/mcb.14.3.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen D, Rothenberg EV. Interleukin 2 transcription factors as molecular target of cAMP inhibition: delayed inhibition kinetics and combinational transcription roles. J Exp Med. 1994;179:931–942. doi: 10.1084/jem.179.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka N, Taniguchi T. Cytokine gene regulation: regulatory cis-elements and DNA binding factors involved in the interferon system. Adv Immunol. 1992;52:263–281. doi: 10.1016/s0065-2776(08)60877-9. [DOI] [PubMed] [Google Scholar]
- 41.Howard, M.C., A. Miyajima, and R. Coffman. 1993. T cell– derived cytokines and their receptors. In Fundamental Immunology. W.E. Paul, editor. Raven Press, New York. 763– 800.
- 42.Rao A. NF-ATp: a transcription factor required for the co-ordinate induction of several cytokine genes. Immunol Today. 1994;15:274–281. doi: 10.1016/0167-5699(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 43.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-ζ and lack of Zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 44.Madrenas J, Wange RL, Wang JL, Isakov N, Samelson LE, Germain RN. ζ phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science (Wash DC) 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 45.Evavold BD, Allen PM. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science (Wash DC) 1991;252:1308–1310. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]
- 46.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 47.Jameson SC, Hogquist KA, Bevan MJ. Specificity and flexibility in thymic selection. Nature (Lond) 1994;369:750–752. doi: 10.1038/369750a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hogquist KA, Jameson SC, Bevan MJ. Strong agonist ligands for the T cell receptor do not mediate positive selection of functional CD8+T cells. Immunity. 1995;3:79–86. doi: 10.1016/1074-7613(95)90160-4. [DOI] [PubMed] [Google Scholar]