Abstract
T-dependent B cell responses in the spleen are initiated in the outer periarteriolar lymphoid sheath (PALS) and culminate in the generation of proliferative foci and germinal center reactions. By pulsing anti–hen egg lysozyme (HEL) immunoglobulin transgenic (IgTg) B cells with various concentrations of HEL in vitro before adoptive transfer into normal recipients, it was shown that a critical number of B cell receptors (BCRs) must be ligated for B cells to undergo arrest in the outer PALS. T cell help was manipulated independently of the BCR stimulus by incubating B cells expressing the appropriate major histocompatibility complex class II antigen with a peptide recognized by CD4+ TCR Tg T cells. B cells which either failed to arrest in the outer PALS due to a subthreshold BCR stimulus, or arrested only transiently due to the brevity of the BCR stimulus, underwent an abortive response within the follicles when provided with T cell help. In contrast, naive B cells stimulated by a sustained, suprathreshold concentration of either foreign or self-antigen and given T cell help, proliferated in the outer PALS and then differentiated. Outer PALS arrest was not influenced by the nature of the B cells occupying the follicle, but appeared to be determined solely by the magnitude of BCR stimulation. Thus antigen-pulsed B cells arrested in the outer PALS in an identical manner irrespective of whether the follicles comprised a population of normal B cells with multiple specificities, a monoclonal naive population, or a monoclonal population of tolerant B cells. In addition, tolerant B cells were found to relocate from the follicles to the outer PALS of HEL/anti-HEL double Tg mice in which the concentration of soluble self-antigen had been increased by zinc feeding. Similarly, when anti-HEL Tg mice were crossed with a second HEL Tg strain expressing a higher concentration of soluble HEL, the tolerant anti-HEL Tg B cells were located constitutively in the outer PALS. Thus, subtle variations in antigen concentration resulted in dramatic changes in positioning of B cells within the spleen. A series of mixed bone marrow chimeras in which the effective antigen concentration was inversely related to the number of self-reactive B cells due to absorption of antigen by transgene-encoded membrane and secreted Ig, was used to confirm that alteration in B cell position previously attributed to changes in follicular composition could be explained on the basis of available antigen concentration, rather than the diversity of the repertoire.
The immune system has evolved to enhance immunity to foreign antigens while limiting the risk of autoreactivity. The sophistication of mammalian immunoregulation is reflected not only in the complexity of molecular interactions between individual cells, but also in the anatomical organization of secondary lymphoid tissue in which immune responses take place. In this paper, the well-characterized hen egg lysozyme (HEL)1/anti-HEL transgenic (Tg) model (1) has been used to explore the interactions between splenic microarchitecture, pattern of cell migration, dynamics of antigen exposure, and effect of T cell help in regulating the B cell response.
B cells enter the splenic white pulp via the central arteriole and its penicillary branches which drain into the marginal sinuses surrounding the follicles (2, 3). They then migrate through the outer periarteriolar lymphoid sheath (PALS), the interface between the T cell–rich inner PALS and the follicles, and gain entry to the B cell–rich follicles (4, 5). Resting B cells migrate onwards to the red pulp and reenter the circulating pool within 24 h. Initiation of collaborative T-dependent B cell responses takes place in the outer PALS, and leads to the formation of proliferative foci at the junction between the red and white pulp, and of germinal centers within follicles (6–10).
Our data demonstrate that both arrest and proliferation of B cells in the outer PALS are required for the subsequent formation of proliferative foci and germinal centers. The stimulus for B cell arrest is the ligation of a critical number of B cell receptors (BCRs), whereas proliferation in the outer PALS is dependent on extended antigenic exposure and the provision of T cell help. Reduction in the strength or duration of the BCR signal below the threshold required for the B cells to arrest for a prolonged period in the outer PALS prevents differentiation into germinal centers and proliferative foci, but still allows a T-dependent B cell response to take place within the follicles.
It has previously been shown that outer PALS arrest also occurs during the induction of tolerance to self antigen (HEL) in the same Tg model (11, 12). This raises the question of whether the same mechanism is operating under these conditions or whether there is an alternative explanation as suggested by Cyster et al. in their follicular exclusion hypothesis (11–13). According to this hypothesis, arrest of tolerant self-reactive B cells in the outer PALS of normal mice occurs because of competition with the diverse repertoire of B cells located within the follicle. The follicular exclusion hypothesis was based on experiments in which transfer of tolerant B cells into recipients containing an identical tolerant B cell population resulted in survival of donor B cells within the follicles, whereas transfer of tolerant B cells into mice with a diverse follicular repertoire led to arrest in the outer PALS followed by death over the next few days. This censorship hypothesis implies that the B cell repertoire has developed some mechanism for monitoring its own diversity.
An alternative explanation for outer PALS arrest of both naive and tolerant B cells is that it is determined entirely by suprathreshold antigenic stimulation of the BCR, irrespective of the specificity of the B cells or the outcome of the interaction with antigen (14). The data presented here from the same HEL/anti-HEL Tg model used by Cyster et al. (11, 12) are consistent with the latter explanation. By manipulating the available antigen concentration and the follicular composition independently of each other, B cell location in the spleen was found to be a function of antigen receptor engagement, independent of follicular composition.
Materials and Methods
Mice.
Inbred and Tg mouse lines were housed under specific pathogen-free conditions at the Centenary Institute (Newtown, Australia). The ML5 Tg line expresses HEL as a soluble self-antigen under the control of the zinc-inducible mouse metallothionein 1 promoter (1), and AL3 mice express HEL under the control of the mouse albumin promoter (15). MD4 mice express rearranged heavy and light chains encoding a high affinity anti-HEL antibody of IgHa allotype which can be used as a marker of transgene-encoded Ig in mice bearing the endogenous IgHb allotype (1). The -D line of TCR Tg mice expresses a TCR specific for the COOH-terminal epitope of moth cytochrome c (MCC) in the context of I-Ek (16). Soluble HEL Tg (ML5 and AL3 lines) and anti-HEL IgTg (MD4) lines were maintained on a C57BL/6 (H-2b, IgHb) background, whereas cytochrome-specific TCR Tg mice were maintained on a B10.BR (H-2k, IgHb) background. H-2bk F1 mice were obtained by crossing the H-2b and H-2k Tg lines with non-Tg B10.BR and C57BL/6 mice, respectively.
Antibodies and Reagents.
The antibodies used for immunohistology were rat anti–mouse B220 (RA3.6B2; reference 17), polyclonal rabbit anti-HEL serum (a gift from Dr. H. Briscoe, University of Sydney, Sydney, Australia), anti–rat Texas red from CALTAG Labs. (S. San Francisco, CA) and anti–rabbit fluorescein from Silenus (Victoria, Australia). Biotinylated peanut agglutinin (PNA) was obtained from Vector Labs., Inc. (Burlingame, CA), avidin-FITC from Molecular Probes Inc. (Eugene, OR), and avidin-fluoroblue from Biomeda (Foster City, CA). HEL was obtained from Sigma Chemical Co. (St. Louis, MO). MCC COOH-terminal heptadecapeptide (KANERADLIAYLKQATK; MCC87-103) was purchased from the Queensland Institute of Medical Research. Additional reagents used for flow cytometry were biotinylated rat anti–mouse IgDa (AMS15.1; reference 18), anti-B220-PE (CALTAG Labs.), rat anti–IgMa-FITC (RS3.1) (19) and streptavidin–quantum red (Sigma Chemical Co.). Monoclonal antibodies used for T cell depletion were anti-CD4 (RL172.4; reference 20), anti-CD8 (3.155; reference 21), and anti-Thy 1.1 (HO13.4; reference 22). Young rabbit complement was obtained from C-six Diagnostics (Mequon, WI). 5-carboxyfluorescein diacetate succinimidyl ester (CFSE) was obtained from Molecular Probes.
Tissue culture medium (TCM) comprised RPMI 1640 supplemented with 0.01 M sodium bicarbonate, 50 mg/liter penicillin, 100 mg/liter streptomycin, 5 × 10−5 M β-mercaptoethanol, and 10% FCS (Commonwealth Serum Labs., Victoria, Australia).
Adoptive Transfers.
Spleens and lymph nodes were harvested, and single cell suspensions were prepared by gently pushing the organs though an 80-gauge wire sieve. The cells were washed and red cells lysed. For purification of B cells, adherent cells were depleted by incubating the suspension on plastic petri dishes for 60 min at 37°C. T cells were depleted by treatment with a mixture of anti-Thy 1.1, anti-CD4, and anti-CD8 monoclonal antibodies for 30 min at 4°C, followed by addition of young rabbit complement for a further 30 min at 37°C. The remaining cells were washed in TCM. For pulsing with cytochrome peptide, B cells were resuspended at 5 × 107/ml in TCM and incubated with 10 μM MCC87-103 for 2 h at 37°C, and then washed three times with TCM before adoptive transfer. CFSE labeling was performed as described previously (14, 23). In brief, cells were washed twice in serum-free RPMI 1640, resuspended at 5 × 107/ml in RPMI 1640, and incubated for 10 min at 37°C with 5 μM CFSE. Labeling was quenched with three washes of cold RPMI/10% FCS.
For T cell help experiments, activated T cells were obtained by priming donor TCR Tg mice with an intravenous injection of 10 μg MCC87-103 20–24 h before harvesting lymph nodes. This resulted in activation of >80% of donor TCR Tg CD4+ T cells. Adoptive transfers were performed by intravenous injection into the lateral tail vein.
Bone Marrow Chimeras.
Recipient mice were irradiated (750 rads) and reconstituted 6 h later by intravenous injection of 106 bone marrow cells. Mixed chimeras were reconstituted with varying ratios of bone marrow cells obtained from IgTg (MD4) and nontransgenic donors as described in the results section. Bone marrow chimeras were used for experiments 8 wk after reconstitution.
Immunohistology.
Fluorescence immunohistology was performed on frozen sections of spleen. After killing experimental mice by cervical dislocation, organs were harvested and tissue samples were embedded immediately in Tissue Tek optimum cutting temperature compound (Miles, Elkhart, IN) and snap frozen in liquid nitrogen. 5-μm sections were cut from tissue blocks and thaw mounted on to glass slides. Sections were air dried for 1 h, and then fixed in acetone for 10 min at room temperature and stored at −20°C. Immediately before staining, sections were rehydrated with Tris-buffered saline, pH 7.6, and blocked with 30% horse serum (CSL). Slides were incubated with each antibody layer for 30 min at 37°C in a dark, humidified chamber, and then washed with three changes of Tris-buffered saline before addition of the next layer. After the final wash, slides were wet mounted and photomicrographs were obtained using a fluorescence microscope (DMR BE; Leitz, Wetzlar, Sweden).
Double staining for HEL-binding B cells was performed using a three-layer stain starting with 200 ng/ml HEL. After washing, sections were stained with polyclonal rabbit anti-HEL and rat anti-B220, washed again, and stained with a combination of anti– rabbit fluorescein and anti–rat Texas red. Triple staining for germinal center cells was performed as for double staining, but in addition, biotinylated PNA was included in the second layer and avidin fluoroblue in the third layer.
Flow Cytometry.
FACS® analysis of peripheral blood mononuclear cells was performed on a FACScan® (Becton Dickinson, Mountain View, CA). Blood was collected into Alsever's solution, and white cells were separated by density gradient centrifugation on Ficoll-Hypaque (specific gravity = 1.083). For staining, cells were suspended in PBS supplemented with 5% FCS and 0.05% sodium azide, and maintained at 4°C throughout. B cells were detected with anti-B220-PE, and anti-HEL IgTg cells were detected with anti-IgMa-FITC and biotinylated anti-IgDa followed by streptavidin–quantum red. Data were analyzed using winMDI software.
Results
B Cells Undergo Arrest in the Outer PALS in Response to Ligation of a Critical Number of BCRs.
The proposition that BCR ligation is the major determinant of outer PALS arrest was investigated using IgTg B cells expressing a high affinity anti-HEL receptor (1). Purified B cells obtained from the spleens of IgTg mice were pulsed with graded doses of soluble HEL in vitro and transferred into non-Tg syngeneic recipients (summarized in Table 1). In the absence of BCR ligation (Fig. 1 A), or after pulsing with 1 ng/ml of HEL (Fig. 1 B), the B cells migrated into the follicles unimpeded. A threshold for outer PALS arrest was observed at ∼20 ng/ml of HEL (Fig. 1 C), a concentration corresponding to occupation of ∼50% of surface Ig molecules on each B cell (24). When the B cells were pulsed with 100 ng/ml of HEL, which resulted in >95% receptor occupancy, there was near total arrest of B cells in the outer PALS for at least 24 h (Fig. 1 D). The crucial factor in determining the positioning of B cells at this site appeared to be the concentration of antigen rather than the duration of exposure to antigen in vitro, as similar results were obtained with B cells pulsed for 0.5, 4, and 12 h before transfer (not shown).
Table 1.
Summary of Adoptive Transfer Experiments
| Donor anti-HEL IgTg B cells | Recipient | Results | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location of transferred B cells in spleen (day 1) | Corresponding figure | |||||||||||
| MHC | BCR ligation* | Strain | Follicular repertoire | Tg T cell help‡ | ||||||||
| H-2b | 0 | Non-Tg | Diverse | − | Follicle | 1 A | ||||||
| 1 ng/ml | Non-Tg | Diverse | − | Follicle | 1 B | |||||||
| 20 ng/ml | Non-Tg | Diverse | − | Follicle/outer PALS | 1 C | |||||||
| 100 ng/ml | Non-Tg | Diverse | − | Outer PALS | 1 D | |||||||
| H-2bk | 100 ng/ml | Non-Tg | Diverse | + | Outer PALS | 2, A–C | ||||||
| 0 | HEL Tg | Diverse | + | Outer PALS | 2, D–F | |||||||
| 0 | Non-Tg | Diverse | + | Follicle | 2, G–I | |||||||
| H-2b | 0 | IgTg | Monoclonal | − | Follicle | 3 A | ||||||
| 100 ng/ml | IgTg | Monoclonal | − | Outer PALS | 3 B | |||||||
| 0 | Double Tg | Monoclonal | − | Follicle | 3 C | |||||||
| 100 ng/ml | Double Tg | Monoclonal | − | Outer PALS | 3 D | |||||||
In vitro pulse with HEL.
In vitro pulse with MCC87-103 to attract T cell help.
Figure 1.
Outer PALS arrest of naive B cells is induced by BCR ligation with antigen. Fluorescent micrographs of frozen sections of spleen. HEL-binding B cells are stained green (fluorescein) and B220+ cells are stained red (Texas red). Non-Tg recipients of unstimulated IgTg B cells (A) and IgTg B cells pulsed in vitro with 1 (B), 20 (C), and 100 ng/ml HEL (D). The follicle (F) and outer PALS (O) are indicated.
The Duration of BCR Ligation Determines the Site of T Cell–dependent Proliferation.
To test whether persistence of antigen after the initial stimulus could influence the nature and site of the subsequent T-dependent B cell response, IgTg B cells were pulsed in vitro with both soluble HEL and MCC87-103, and transferred with activated TCR Tg T cells as a source of T cell help (Table 1). In non-Tg hosts, the B cell stimulus was limited by the half-life of the BCR–ligand association, making it unlikely that such a stimulus would be available for a prolonged period of time. As expected, the donor B cells were arrested in the outer PALS on day 1 after transfer (Fig. 2 A). By day 3, the number of donor B cells within the follicles had increased significantly (Fig. 2 B), but the response failed to generate proliferative foci (Fig. 2 C) or germinal centers (not shown) at later times. On the other hand, transfer of MCC87-103– pulsed IgTg B cells together with TCR Tg T cells into soluble HEL Tg recipients resulted in outer PALS arrest on day 1 (Fig. 2 D) and extensive proliferation of the B cells (as indicated by FACS® analysis of CFSE-labeled donor cells, not shown) throughout the T cell zone on day 3 (Fig. 2 E). Furthermore, proliferative foci and germinal centers, the latter indicated by the presence of PNA+ HEL-binding B cells in the follicles, were present on day 5 (Fig. 2 F; see also reference 14). In other words, when exposure to HEL was prolonged rather than transient, the collaborative response between B and T cells could proceed to completion.
Figure 2.
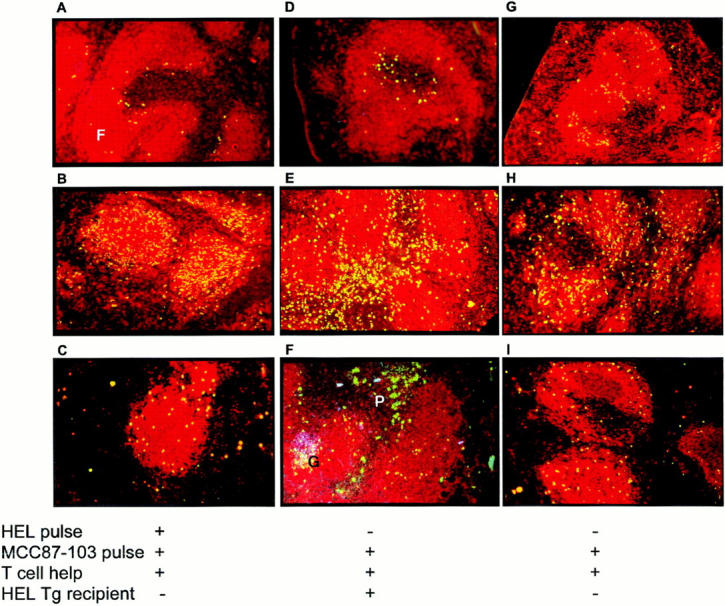
Location of T-dependent B cell proliferation depends on duration of BCR ligation with antigen. Fluorescent micrographs of frozen sections of spleen. HEL-binding B cells are stained green (fluorescein) and B220+ cells are stained red (Texas red). In (F) the section was also stained blue with PNA fluoroblue to reveal germinal center cells. (A–C) H-2bk IgTg B cells were pulsed in vitro with MCC87-103 and HEL (100 ng/ml) and transferred with activated cytochrome-specific TCR Tg T cells into H-2bk non-Tg recipients. The day 1 section (A) shows the HEL-binding B cells located in the outer PALS. The day 3 section (B) shows that there has been a marked increase in the number of intrafollicular HEL-binding B cells. The day 7 section (C) shows reduction in the number of intrafollicular B cells and no evidence of differentiation into proliferative foci or germinal centers. (D–F) H-2bk IgTg B cells were pulsed with MCC87-103, but not HEL, and transferred with activated cytochrome-specific TCR Tg T cells into H-2bk HEL Tg (ML5) recipients. The day 1 section (D) shows arrest of HEL-binding B cells in the outer PALS. The day 3 section (E) shows a dramatic increase in the number of HEL-binding B cells in the outer PALS. The day 5 section (F) shows that HEL-binding B cells have differentiated into proliferative foci adjacent to the follicles (P) and germinal centers (G), which costain with PNA (blue) and B220 (red) within the follicles. (G–I) H-2bk IgTg B cells were pulsed in vitro with MCC87-103 but not HEL, and transferred with activated cytochrome-specific TCR Tg T cell help into H-2bk non-Tg recipients. The day 1 section (G) shows HEL-binding B cells located in the follicles. The day 3 section (H) shows an increase in the number of HEL-binding B cells in the follicles. The day 7 section (C) shows a reduction in the number of intrafollicular B cells and no evidence of differentiation.
T Cell Help Without BCR Ligation Results in Abortive Intrafollicular Proliferation.
When self-antigen is present at concentrations below a certain threshold, potentially self-reactive B cells remain in a state of clonal indifference and may present foreign epitopes to antiforeign CD4+ T cells, giving rise to autoantibody production (25, 26). Although the majority of T cells occupy the inner PALS of the spleen, small numbers are present in the marginal zone, the periphery of the follicles, and germinal centers where they play an integral role in the selection of B cells undergoing somatic mutation (10, 27). This raises the issue of whether B cells located in the follicles rather than the PALS can initiate and maintain a T-dependent response.
The response of B cells to T cell help in the absence of BCR stimulation (i.e., when B cells were located in the follicle rather than the outer PALS) was investigated by pulsing IgTg B cells in vitro with MCC87-103 but not HEL, and then transferring them with activated TCR Tg CD4+ T cells into syngeneic non-Tg recipients (Table 1). 1 d after transfer, the B cells had migrated into the follicles (Fig. 2 G), although their distribution differed from that observed after the transfer of resting naive B cells (Fig. 1 A). The peptide-pulsed B cells were located in discrete clusters around the periphery of the follicles distal to the PALS in a distribution resembling that of the resident follicular T cell population (28). There were no donor B cells in the PALS or the red pulp, suggesting that a distinct migration pathway had been followed. By day 3, the number of B cells within the follicles had increased significantly (Fig. 2 H). As with IgTg B cells transiently exposed to both HEL and MCC87-103 (vide supra), intrafollicular accumulation was not accompanied by B cell differentiation into plasma cells (Fig. 2 I) or germinal centers (not shown).
BCR Ligation Results in Outer PALS Arrest Even in the Presence of Monoclonal Follicles Comprised of Naive Antiforeign B Cells or Tolerant Self-reactive B Cells.
The above results pointed to the importance of an adequate BCR signal in determining the arrest of antigen-stimulated B cells in the outer PALS. They did not, however, exclude a role for other factors, in particular, the repertoire of B cells occupying the follicle, as suggested by Cyster et al. (11). According to the follicular exclusion hypothesis, B cell migration in response to antigenic stimulation is influenced by the composition of the follicles. This hypothesis was based on a series of observations in the same HEL/anti-HEL Tg model as used here (11, 12). Included among them was the finding that tolerant B cells from ML5 × MD4 double Tg mice undergo arrest in the outer PALS when the follicle contains normal B cells with a polyclonal repertoire, but migrate unimpeded into follicles composed of IgTg-tolerant B cells of the same anti-HEL specificity. To investigate the relevance of follicular composition to outer PALS arrest in a more stringent way, two approaches were used. The first was designed to test whether follicles consisting of a monoclonal population of naive IgTg B cells could exclude IgTg B cells of identical anti-HEL specificity. Purified IgTg B cells were labeled with CFSE to distinguish them from those of the recipients and transferred into syngeneic IgTg recipients with or without prior in vitro pulsing with a suprathreshold dose of soluble HEL (100 ng/mL; Table 1). Those B cells stimulated with cognate antigen underwent arrest in the outer PALS surrounding the monoclonal follicles in a manner identical to that observed when BCR-stimulated B cells were transferred into recipients containing a diverse follicular repertoire (Fig. 3, A and B).
Figure 3.
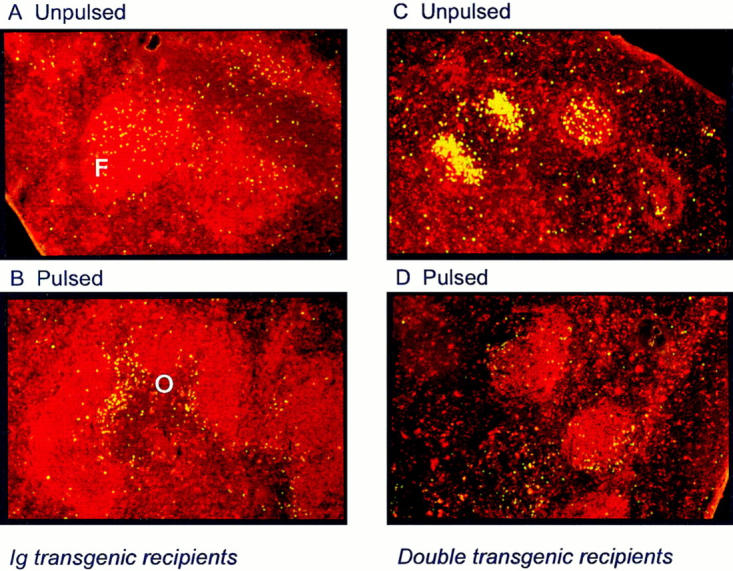
Outer PALS arrest of naive B cells is induced by BCR ligation with antigen and is independent of follicular repertoire diversity. Fluorescent micrographs of frozen sections of spleen. HEL-binding B cells are stained green (CFSE) and B220+ cells are stained red (Texas red). (A) Unstimulated IgTg B cells are located in the follicles (F) of IgTg recipients. (B) IgTg B cells pulsed with 100 ng/ml HEL are located in the outer PALS (O) of IgTg recipients. (C) Unstimulated IgTg B cells are located in the follicles of double Tg (MD4 × ML5) recipients. (D) IgTg B cells pulsed with 100 ng/ml HEL are located in the outer PALS of double Tg recipients.
In the second experiment, IgTg B cells were transferred into ML5 × MD4 double Tg recipients (Table 1). Under these conditions, the follicles also contained a monoclonal repertoire of B cells, but they now were comprised of a population of tolerant cells with self rather than foreign specificity. Again the strength of the BCR signal determined the positioning of the B cells (Fig. 3, C and D). Thus, unpulsed B cell entered the follicles as reported previously by Cyster et al. (11, 12), whereas B cells exposed to HEL before transfer underwent arrest in the outer PALS. When taken together with the earlier observation that the serum HEL concentration in double Tg mice is below the threshold required for outer PALS arrest (14), the results from these two experiments led to two conclusions. First they confirmed the importance of the degree of receptor occupancy as opposed to follicular composition in determining the outcome of B cell exposure to antigen. Second, they indicated that B cell migration was not influenced by the nature of the antigen (foreign versus self ).
Tolerant B Cells in Double Tg Mice Recirculate Through Primary Follicles Until the Antigen Concentration Is Increased beyond the Threshold for Outer PALS Arrest.
The phenotype of tolerant B cells from the soluble HEL double Tg mice resembles that of a subset of primary follicular and follicular mantle zone B cells from normal mice (IgMloIgDhi) (29). Histological examination of the spleens of double Tg mice has revealed that they are deficient in marginal zones in which memory cells are thought to reside (30), whereas the organization of PALS and follicles appears to be relatively normal (31). An alternative method of investigating the importance of follicular composition on B cell location was to determine the effect of the concentration of serum HEL on the in situ distribution of tolerant B cells in intact double Tg mice. This was achieved by administering zinc supplements to MD4 × ML5 double Tg mice in which the HEL transgene is under the transcriptional regulation of the zinc-inducible mouse metallothionein 1 promoter (15; summarized in Table 2). Before induction, the HEL-binding B cells were distributed throughout the primary splenic follicles (Fig. 4 A). However, on day 4 after zinc induction, i.e., 24 h after the serum HEL concentration had peaked, the HEL-binding B cells had redistributed to the outer PALS (Fig. 4 B), despite the fact that the percentage of these cells had remained unchanged during this period.
Table 2.
Summary of Experiments Investigating B cell Location in Double Transgenic Mice
| Anti-HEL/HEL double Tg line | Determinant of effective serum HEL concentration | B cell repertoire | Location of IgTg B cells in spleen | Corresponding figure | ||||
|---|---|---|---|---|---|---|---|---|
| IgTg × ML5 | Zinc induction of HEL | Predominantly tolerant IgTg (monoclonal) | ||||||
| Day 1 and 2: baseline | Follicle | 4 A | ||||||
| Day 3 and 4: induced | Outer PALS | 4 B | ||||||
| IgTg × AL3 | Sex | Predominantly tolerant IgTg (monoclonal) | ||||||
| M ∼80 ng/ml | Follicle | 4 C | ||||||
| F ∼160 ng/ml | Outer PALS | 4 D | ||||||
| BM chimaeras IgTg/non-Tg BM into ML5 | Proportion of HEL-binding B cells in repertoire | % HEL-binding B cells | ||||||
| 100:0 | 94 | Follicle | ||||||
| 99:1 | 63 | Follicle | ||||||
| 95:5 | 13 | Follicle/outer PALS | 4 E | |||||
| 80:20 | 5 | Outer PALS | 4 F |
Figure 4.
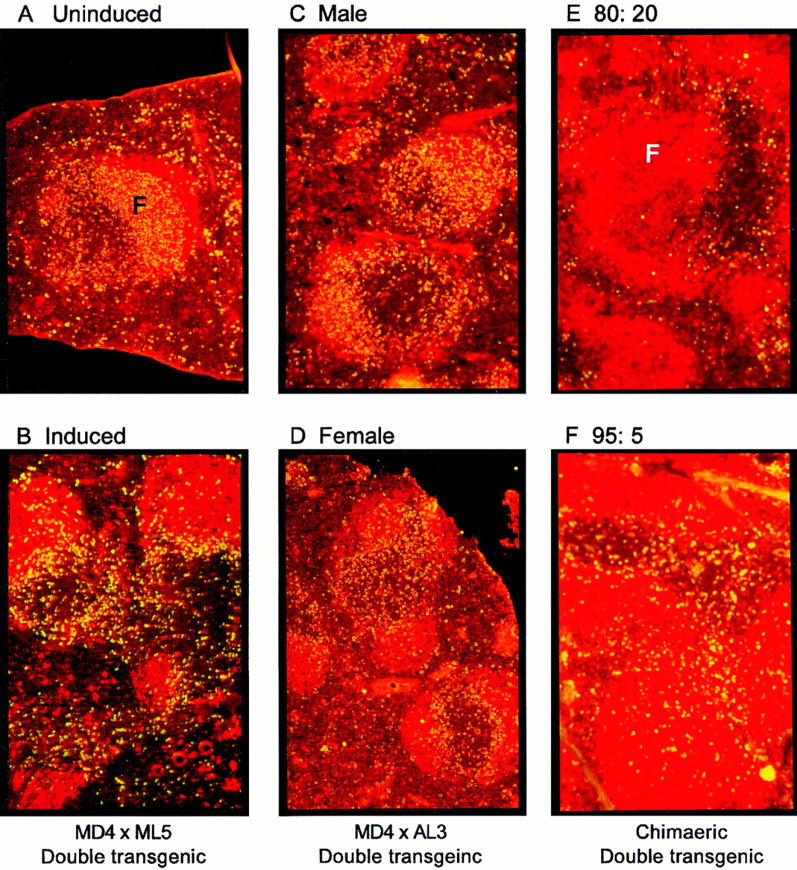
Location of self-reactive B cells is determined by the prevailing concentration of self-antigen. Fluorescent micrographs of frozen sections of spleen. HEL-binding B cells are stained green (fluorescein) and B220+ cells are stained red (Texas red). (A and B) Sections of spleen from MD4 × ML5 double Tg mice treated with oral zinc sulfate (25 mM). The section obtained 1 d after starting zinc but before a change in serum HEL concentration (A) shows HEL-binding B cells predominantly within the follicles (F), whereas the section obtained 4 d after starting zinc, 1 d after the increase in serum HEL concentration, shows relocation of HEL-binding B cells to the outer PALS (B). (C and D) Sections of spleen from unmanipulated MD4 × AL3 double Tg mice. HEL-binding B cells are located in the follicles in male mice (C), but in the outer PALS in females (D). (E and F) Sections of spleen from mixed bone marrow chimeras. The section from a HEL Tg (ML5) mouse reconstituted with IgTg/non-Tg bone marrow in a ratio of 95:5 shows HEL-binding B cells distributed throughout the follicles and PALS (E). A section from a HEL Tg (ML5) mouse reconstituted with IgTg/non-Tg marrow in a ratio of 80:20 shows HEL-binding B cells predominantly in the outer PALS (F).
B Cells Are Located Constitutively in the Outer PALS in Double Tg Mice Expressing High Concentrations of Soluble HEL.
The findings reported in the previous section were consistent with the notion that the amount of available antigen in resting MD4 × ML5 double Tg mice is below the threshold required for outer PALS arrest, and supported the crucial role of signal strength rather than follicular composition in determining B cell positioning after exposure to antigen. The importance of the BCR signal and the status of the B cells were also examined in another line of soluble HEL double Tg mice in which HEL transcription is under the control of the mouse albumin promoter (line AL3; reference 15). In AL3 mice, serum HEL concentrations lie between 80 and 160 ng/ml, with females expressing higher levels than males. Double Tg offspring were created by crossing AL3 mice with MD4 IgTg mice (Table 2). Despite the high level of serum self-antigen, HEL-binding B cells were not deleted in these double Tg mice, but acquired a phenotype similar to that observed in the ML5 × MD4 combination used previously (32). In male AL3 × MD4 double Tg mice, the HEL-binding B cells were located in the primary follicles in a distribution indistinguishable from that in MD4 × ML5 double transgenics (Fig. 4 C, Table 2). However, in female AL3 × MD4 mice that expressed approximately twice as much serum HEL as male littermates, the HEL-binding B cells were located in the outer PALS (Fig. 4 D). Thus, histological location and outer PALS arrest are clearly dependent on the intensity of the BCR signal irrespective of whether the B cells are anergic or not.
The Location and Phenotype of IgTg B Cells in Chimeric Mice Is Determined by the Effective Serum HEL Concentration and Degree of Receptor Occupancy.
Under normal circumstances, autoreactive B cells represent only a minute fraction of the total B cell repertoire. Cyster et al. in their experiments on follicular exclusion mimicked this scenario by creating mixed chimeric double Tg mice in which the tolerant B cell population represented a small minority of the B cell repertoire (11, 12). In this situation, self-reactive B cells underwent arrest in the outer PALS and were deleted, which they claimed reflected the influence of the diverse follicular repertoire. Based on our findings (vide supra; 14), however, there could be an alternative explanation, namely, that subtle changes in the serum HEL concentration may occur in these mixed chimeras depending on the number of HEL-binding cells present. The experiments described above confirmed the previous demonstration that the constitutive serum HEL concentration in MD4 × ML5 mice was below the threshold required for outer PALS arrest, whereas ML5 recipients of adoptively transferred MD4 IgTg B cells expressed HEL at a level close to that of ML5 single Tg mice, which was sufficient to induce outer PALS arrest (14).
To investigate whether there was a correlation between the amount of available antigen and splenic location of B cells, mixed bone marrow chimeras designed to create mice with varying proportions of anti-HEL B cells were made using mixtures of non-Tg and IgTg bone marrow (Table 2). Irradiated H-2b ML5 recipient mice were reconstituted with either 80:20, 95:5, 99:1, or 100:0 ratios of Ig/non-Tg syngeneic bone marrow, yielding chimeras in which the IgTg B cells represented, on average, 5, 13, 63, and 94% of the peripheral B cell repertoire, respectively (Fig. 5 A). Since accurate quantitation of the serum HEL concentration in double Tg mice by immunoassays is problematic due to the presence of anti-HEL Ig in the serum, the degree of surface IgM downregulation was used as a measure of the effective antigen concentration, and more importantly, the intensity of the in vivo stimulus delivered through the BCR. There was a clear relationship between the number of HEL-binding B cells and the mean channel fluorescence for surface IgMa (Fig. 5 B). Moreover, the splenic histology mirrored these findings, with near total outer PALS arrest of HEL-binding B cells in the 80:20 chimeras (Fig. 4 E), and partial arrest in the 95:5 chimeras (Fig. 4 F), whereas in the 99:1 and 100:0 chimeras, all HEL-binding B cells were located in the primary follicles (not shown). Thus, the serum HEL concentration was sufficient to induce outer PALS arrest only when <15% of the B cells expressed anti-HEL Ig. These results are consistent with the known location of Tg B cells in the follicles of old double Tg mice in which ∼70% of the B cells express anti-HEL Ig (33).
Figure 5.

Anti-HEL-specific surface IgM downregulation is determined by the number of HEL-binding B cells. (A) Scatter plot of the percentage of circulating B cells bearing the Tg BCR in mixed bone marrow chimeras. IgTg cells were identified by IgDa expression. (B) Scatter plot of IgMa mean channel fluorescence on IgTg B cells obtained from mixed bone marrow chimeras. Cells were gated on IgDa. Viable lymphocytes were purified from peripheral blood by density gradient centrifugation on Ficoll-Hypaque.
Discussion
The initial event after stimulation of B cells through the BCR is migration to the outer PALS of the T cell zone. Arrest at this site is essential for subsequent differentiation of antiforeign B cells (34) and expedites deletion of tolerant B cells with antiself specificity (35). According to the findings reported here, the crucial factor responsible for outer PALS arrest is exposure to antigen above a critical threshold that operates irrespective of the repertoire of B cells occupying the follicles, the nature of the antigenic stimulus (foreign versus self ), or the functional state of the B cells (naive versus tolerant). Subsequently, the fate of the B cells is determined by the presence or absence of T cell help (Fig. 2, D–F; 14).
Several different experimental approaches pointed to the singular importance of BCR ligation in outer PALS arrest of B cells. In each case, a role for follicular composition could be effectively excluded (summarized in Table 2). When ML5 × MD4 double transgenic mice were fed zinc to increase expression of soluble HEL, B cells moved from the primary follicles to the outer PALS within 24 h of the increase in HEL concentration (Fig. 4, A and B). The B cell repertoire was identical in both phases of the experiment, comprising predominantly tolerant B cells. The only variable was the quantity of available HEL. The B cells undergoing arrest in the outer PALS after HEL induction are likely to have comprised both mature follicular cells that received an augmented BCR signal during recirculation through blood and lymphoid tissue, and newly formed B cells that were exposed to the increased HEL concentration during ontogeny.
The second experiment demonstrating the primary role played by the strength of the antigenic signal involved the use of another set of double Tg mice created by crossing MD4 IgTg mice with AL3 HEL Tg mice which express much higher basal levels of HEL than ML5 (mean levels 80 ng/ml for males and 160 ng/ml for females; Table 2). On this occasion, outer PALS arrest was observed constitutively in female AL3 × MD4 mice, despite the fact that they contained the same monoclonal population of anti-HEL B cells as did ML5 and male AL3 double transgenics (Fig. 4, C and D). B cells from all three lines of mice showed a tolerant phenotype, although those from AL3 double Tg mice expressed lower levels of membrane IgMa than ML5 double Tg mice (not shown). Thus, once again antigen concentration rather than follicular composition dictated whether B cells migrated to the outer PALS.
Thirdly, IgTg B cells stimulated with antigen in vitro underwent arrest in the outer PALS surrounding monoclonal follicles comprising either naive or tolerant B cells of the same anti-HEL specificity (Fig. 3, summarized in Table 1). This was a dose-dependent phenomenon in which the transition from follicular entry to outer PALS arrest occurred across a range of antigen concentrations corresponding to the steep part of the ligand–receptor occupancy curve for the IgTg B cells used in the transfer system (24). The threshold of receptor occupancy required for outer PALS arrest was estimated to be 30–50% (indicated in Fig. 6 by the dotted line). A comparable conclusion had been reached previously by Fulcher and Basten (36) from in vitro experiments designed to quantitate the amounts of available antigen in serum from different lines of HEL Tg mice. According to their findings, serum from ML5 HEL Tg mice in which transferred B cells undergo outer PALS arrest contains sufficient HEL to saturate ∼50% of receptors on IgTg B cells as estimated flow cytometrically. On the other hand, the receptor occupancy was only half that level when the source of antigen was serum obtained from ML5 × MD4 double Tg mice in which tolerant B cells occupied the follicles. The functional significance of these small changes in receptor occupancy was indicated by the ability of serum from soluble HEL Tg mice to induce downregulation of IgMa on naive Tg B cells in vitro, in contrast to serum from HEL/anti-HEL double Tg mice (14).
Figure 6.
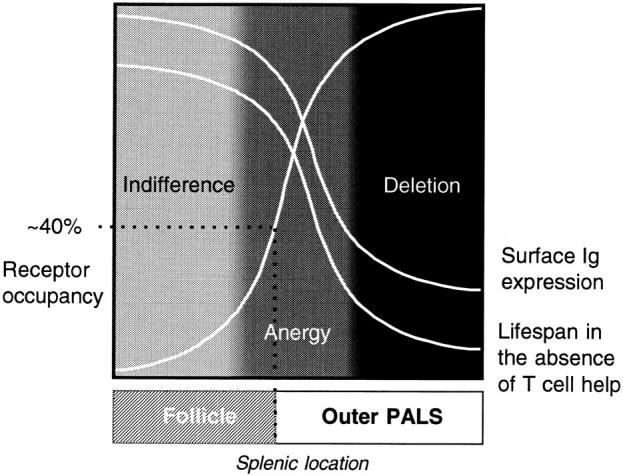
Model summarizing the relationship between antigen concentration, B cell responsiveness, surface Ig expression, splenic location, and life span.
Although precise measurement of serum HEL concentration is somewhat problematic in double Tg mice, the relative differences in amount of available antigen between single and double Tg mice led us to hypothesize that the large number of HEL-binding IgTg B cells present in the ML5 × MD4 double Tg mice acted as an antigen sink, thereby sequestering HEL that would otherwise be available to signal transferred B cells. The physiological significance of subtle changes in effective serum HEL concentration was confirmed in the mixed bone marrow chimera experiment (Table 2). Thus, the intensity of the stimulus delivered through the BCR, as measured by surface IgMa downregulation, was shown to be inversely proportional to the number of IgTg B cells present in the various chimeras (Fig. 5). In other words, the higher the number of HEL-binding B cells, the lower the signal received by each B cell as it reached the spleen. The changes in IgMa level indicated that even subtle variations in serum antigen concentration were registered by the circulating B cells, and affected their migration (Figs. 4, E and F, and 5). When taken in conjunction with other data illustrating the importance of BCR ligation, the results of the chimera experiments provided an alternative explanation to follicular exclusion of B cells in the presence of a competing polyclonal population as proposed by Cyster et al. (11) on the basis of their experiments in similar bone marrow chimeras. Moreover, their finding of follicular entry of tolerant double Tg B cells after transfer into non-Tg recipients lacking HEL but containing a polyclonal B cell repertoire also supports our contention that BCR ligation, rather than follicular composition, accounts for outer PALS arrest.
The fate of B cells on exposure to antigen in the absence of second signals can be related to a number of other parameters that are also a function of effective antigen concentration. Our data defined a few crucial points along the antigen dose response curve which led to the conclusion that splenic position, Ig downregulation, and life span can all be related to the degree of BCR occupancy (Fig. 6). At low doses of soluble antigen, mature B cells are located in the follicle in an indifferent or naive state with a normal life span (50% turnover time of 4–5 wk; 33). As the strength of the antigen signal delivered via the BCR increases, the first change to occur at a receptor occupancy of 25–30% is downregulation of membrane IgM, which is associated with the onset of anergy and a reduction in B cell life span (50% turnover time of ∼3 d; 33). Nevertheless, the B cells remain within the follicles until receptor occupancy attains the critical threshold of between 30 and 50% required for arrest in the outer PALS. Once the tolerant B cells have reached that site, their short life span ensures that all of them die within the next 3–4 d, unless rescued by T cell help (14). The lower antigen threshold required for induction of anergy as opposed to outer PALS arrest is graphically illustrated by the demonstration that anergic HEL-binding B cells are located in the follicles of MD4 × ML5 and male MD4 × AL3 double Tg mice, but in the outer PALS of female MD4 × AL3 double transgenics expressing a higher concentration of antigen (Fig. 4, A, C, and D, respectively).
The duration of antigen exposure in the outer PALS also appears to play a crucial role in B cell differentiation. When mature IgTg B cells were exposed to a single pulse of HEL in vitro and then transferred together with T cell help into normal recipients, they arrested as expected in the outer PALS (Fig. 2 A, Table 1). However, after moving rapidly into the follicle, the response was confined to that site (Fig. 2, B and C) and did not lead to formation of extrafollicular foci or germinal centers. In contrast, transfer of IgTg B cells into Tg recipients expressing soluble HEL in the presence of T cell help did result in formation of extrafollicular proliferative foci, germinal centers, and antibody (Fig. 2, D–F; 14). In other words, antigen must persist if sufficient outer PALS proliferation is to occur to permit development of a downstream B cell response. The phases and sites of B cell proliferation have been carefully characterized by Liu et al. who showed that, for haptenated T-dependent and type I T-independent antigens, BCR ligation leads rapidly to a phase of proliferation in the outer PALS, preceding intrafollicular proliferation and differentiation (37). These authors also showed that the proliferative phase of the response could be prolonged by supplying extra antigen and speculated that proliferation in the outer PALS continues until the supply of soluble antigen is exhausted, as would be expected to occur during the primary immune response to a finite quantity of antigen after immunization or acute infection. However, our findings demonstrate that the outer PALS phase of proliferation is completed within 5 d, even when the B cells are provided with an inexhaustible supply of antigen as occurred after transfer of mature B cells into soluble antigen-containing recipients (Fig. 2, D–F). Failure to curtail this initial proliferative response may lead to autoantibody formation, as exemplified by Fas-deficient lpr mice in which antibody secreting cells accumulate in the PALS rather than the follicle (38), possibly as a result of a defect in regulation of the T cell response.
In vitro studies have produced data that question the importance of BCR signaling in B cell activation (39–43). This is an important issue because at self-antigen concentrations below the threshold for outer PALS arrest and the induction of anergy, self-reactive B cells exist in a state of clonal indifference. If antigen recognition via the BCR was not required for antibody production, provision of T cell help to these potentially autoreactive cells could lead to autoimmunity. We have demonstrated transient intrafollicular accumulation of B cells when the outer PALS phase of the response was bypassed by providing T help in the absence of BCR ligation (Fig. 2, G–I). However, this response failed to yield germinal centers or proliferative foci. In other words, although potent T cell help may stimulate B cell proliferation, the BCR signal is necessary to generate effector and memory B cells in vivo, thereby limiting the danger from bystander activation of B cells given T cell help in the absence of effective BCR stimulation. Intrafollicular proliferation can also occur when B cells receive a T-independent antigenic stimulus, and represents another circumstance in which self-reactive B cells may be activated (44). Once again, however, intrafollicular expansion of B cells is transient and fails to induce germinal center reactions. Presumably this reflects not only the importance of BCR ligation in modulating the molecular phenotype required for B cell differentiation, but also the part played by compartmentalization of the cellular players involved (45). Indeed, the transient phase of intrafollicular proliferation after T cell help in the absence of BCR ligation is consistent with the findings of Rothstein et al. (46), who demonstrated that CD40 ligation enhanced Fas-mediated apoptosis of B cells by CD4+ Th1 cells, whereas the addition of a BCR stimulus afforded protection. In addition, evidence exists for the transient expression of CD40 ligand by memory T cells in response to antigen presentation by activated B cells, thereby focusing T cell help to antigen-specific B cells (47).
Outer PALS tropism appears to be a critical immunoregulatory event. Migration of B cells to the outer PALS after BCR ligation has also been observed in studies of B cells undergoing proliferation within an established germinal center reaction. When B cells were ligated by soluble cross-reacting antigen in this situation, they migrated from the intrafollicular germinal center to the outer PALS where they died in the absence of T cell help (48). Furthermore, the importance of antigen-specific lymphocyte localization for immunoregulation has been explored in histological comparisons of immunogenic and tolerogenic T cell responses (49). These experiments suggest that key events during the phase of PALS proliferation determine the outcome of the response. In the presence of inflammation, generated for example by the administration of antigen in complete Freund's adjuvant, PALS proliferation was followed by intrafollicular T cell proliferation, whereas soluble antigen resulted in curtailment of proliferation in the PALS. Thus, the PALS would appear to be a crucial site of immunoregulation for T as well as B cells. For T cells, the nature and state of activation of the antigen presenting cell in the PALS is likely to determine the outcome. For the B cell, the extent of antigen receptor ligation inducing PALS tropism and subsequent differentiation is determined by the presence or absence of T cell help (50, 51).
Finally, the results reported here and from other studies using this model lead to the conclusion that there are stringent spatiotemporal constraints on T–B interactions in vivo for which in vitro studies can provide only an approximation. In vitro, the necessity for two signals for B cell activation remains contentious, whereas in vivo, the anatomical constraints mean that signal one is necessary to localize B cells to the appropriate environment where T cell help (signal two) is available. In addition to stimulating proliferation and differentiation of naive B cells, T cells are also responsible for in vivo deletion of tolerant B cells via ligation of both Fas and CD40 (34, 52). Under these circumstances, retention of the outer PALS tropic response to BCR ligation by tolerant B cells facilitates their elimination by T cells, leading to the conclusion that the trafficking response to suprathreshold BCR ligation can enhance the immune responsiveness of naive B cells while reinforcing B cell tolerance.
Acknowledgments
The authors thank Karen Knight and her staff for expert animal husbandry. We also thank Kate Scott for screening the transgenic mice.
Footnotes
M.C. Cook was supported by a postgraduate scholarship from the University of Sydney Faculty of Medicine (Sydney, Australia). B. Fazekas de St. Groth is a Wellcome Trust Senior Research Fellow. This work was supported by the National Health and Medical Research Council of Australia.
Abbreviations used in this paper: BCR, B cell receptor; CFSE, 5-carboxyfluorescein diacetate succinimidyl ester; HEL, hen egg lysozyme; MCC, moth cytochrome c; PALS, periarteriolar lymphoid sheath; PNA, peanut agglutinin; TCM, tissue culture medium; Tg, transgenic.
References
- 1.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill S, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature (Lond) 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 2.Nieuwenhuis PF, Ford WL. Comparative migration of T and B lymphocytes in the rat spleen and lymph nodes. Cell Immunol. 1976;23:254–267. doi: 10.1016/0008-8749(76)90191-x. [DOI] [PubMed] [Google Scholar]
- 3.van Ewijk W, Nieuwenhuis P. Compartments, domains and migration pathways of lymphoid cells in the splenic pulp. Experientia (Basel) 1985;41:199–208. doi: 10.1007/BF02002614. [DOI] [PubMed] [Google Scholar]
- 4.Brelinska R, Pilgrim C, Reisert I. Pathways of lymphocyte migration within the periarteriolar lymphoid sheath of rat spleen. Cell Tissue Res. 1984;236:661–667. doi: 10.1007/BF00217236. [DOI] [PubMed] [Google Scholar]
- 5.Pabst R. The spleen in lymphocyte migration. Immunol Today. 1988;9:43–45. doi: 10.1016/0167-5699(88)91258-3. [DOI] [PubMed] [Google Scholar]
- 6.Thorbecke GJ, Keuning FJ. Antibody and gamma globulin formation in vitro in hemopoietic organs. J Infect Dis. 1956;98:157–169. doi: 10.1093/infdis/98.2.157. [DOI] [PubMed] [Google Scholar]
- 7.van Rooijen N, Claasen E, Eikelenboom P. Is there a single differentiation pathway for all antibody forming cells in the spleen? . Immunol Today. 1986;7:193–195. doi: 10.1016/0167-5699(86)90100-3. [DOI] [PubMed] [Google Scholar]
- 8.Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith KGC, Hewitson TD, Nossal GJV, Tarlington DM. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur J Immunol. 1996;26:444–448. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- 10.Kelsoe G. In situ studies of the germinal center reaction. Adv Immunol. 1996;60:267–288. doi: 10.1016/s0065-2776(08)60587-8. [DOI] [PubMed] [Google Scholar]
- 11.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B cell repertoire. Nature (Lond) 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 12.Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995;3:691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 13.Goodnow CC, Cyster JG, Hartley SB, Bell SE, Cooke MP, Healy JI, Akkaraju S, Rathmell JC, Pogue SL, Shokat KP. Self-tolerance checkpoints in B lymphocyte development. Adv Immunol. 1996;59:279–368. doi: 10.1016/s0065-2776(08)60633-1. [DOI] [PubMed] [Google Scholar]
- 14.Fulcher DA, Lyons AB, Korn SL, Cook MC, Koleda C, Parish C, Fazekas de St B, Groth, Basten A. The fate of self-reactive B cells depends primarily on the degree of antigen receptor engagement and the availability of T cell help. J Exp Med. 1996;183:2313–2328. doi: 10.1084/jem.183.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Mason DY, Jorgensen H, Brink RA, Pritchard-Briscoe H, Loughnan M, et al. Clonal silencing of self-reactive B lymphocytes in a transgenic model. Cold Spring Harbor Symp Quant Biol. 1989;54:907–920. doi: 10.1101/sqb.1989.054.01.106. [DOI] [PubMed] [Google Scholar]
- 16.Fazekas de St. Groth, B., P.A. Patten, W.Y. Ho, E.P. Rock, and M.M. Davis. 1992. An analysis of T cell receptor ligand interaction using a transgenic antigen model for T cell tolerance and T cell receptor mutagenesis. In Molecular Mechanisms of Immunological Self-recognition. F.W. Alt and A.H. Vogel, editors. Academic Press, San Diego. 123–127.
- 17.Coffman R. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre–B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 18.Stall A, Loken M. Allotypic specificities of murine IgD and IgM recognized by monoclonal antibodies. J Immunol. 1984;132:787–795. [PubMed] [Google Scholar]
- 19.Schuppel R, Wilke RJ, Weiler E. Monoclonal anti-allotype antibody towards BALB/c IgM. Analysis of specificity and site of a V–C cross-over in recombinant strain BALB/c-IgH-Va/IgH-Cb. Eur J Immunol. 1987;17:739–741. doi: 10.1002/eji.1830170527. [DOI] [PubMed] [Google Scholar]
- 20.Ceredig R, Lowenthal M, Nabholz M, MacDonald HK. Expression of interleukin 2 receptors as a differentiation marker in intrathymic stem cells. Nature (Lond) 1985;314:98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- 21.Sarmiento M, Glasebrook AL, Fitch FW. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T-cell–mediated cytolysis in the absence of complement. J Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- 22.Marshak-Rothstein A, Fink P, Gridley T, Raulet DH, Bevan MJ, Gefter ML. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979;122:2491–2497. [PubMed] [Google Scholar]
- 23.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 24.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature (Lond) 1989;342:385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 25.Zinkernagel RM, Cooper S, Chambers J, Lazzarini RA, Hengartner H, Arnheiter H. Virus-induced autoantibody response to a transgenic viral antigen. Nature (Lond) 1990;345:68–70. doi: 10.1038/345068a0. [DOI] [PubMed] [Google Scholar]
- 26.Steinhoff U, Burkhart C, Arnheiter H, Hengartner H, Zinkernagel RM. Virus or a hapten complex can activate autoreactive B cells by providing linked T help. Eur J Immunol. 1994;24:773–776. doi: 10.1002/eji.1830240343. [DOI] [PubMed] [Google Scholar]
- 27.Fuller KA, Kanagawa O, Nahm MH. T cells within germinal centers are specific for the immunising antigen. J Immunol. 1993;151:4505–4512. [PubMed] [Google Scholar]
- 28.Bowen MB, Butch AW, Parvin CA, Levine A, Nahm MH. Germinal center T cells are distinct helper-inducer T cells. Hum Immunol. 1991;31:67–75. doi: 10.1016/0198-8859(91)90050-j. [DOI] [PubMed] [Google Scholar]
- 29.Hardy RR, Hayakawa K, Parks DR, Herzenberg LA. Demonstration of B-cell maturation in X-linked immunodeficient mice by simultaneous three-colour immunofluorescence. Nature (Lond) 1983;306:270–272. doi: 10.1038/306270a0. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y-J, Oldfield S, MacLennan ICM. Memory B cells in T cell–dependent antibody responses colonize the splenic marginal zones. Eur J Immunol. 1988;18:355–362. doi: 10.1002/eji.1830180306. [DOI] [PubMed] [Google Scholar]
- 31.Mason DY, Jones M, Goodnow CC. Development and follicular localization of tolerant B lymphocytes in lysozyme/anti-lysozyme IgM/IgD transgenic mice. Int Immunol. 1992;4:163–175. doi: 10.1093/intimm/4.2.163. [DOI] [PubMed] [Google Scholar]
- 32.Adelstein S, Pritchard-Briscoe H, Anderson TA, Crosbie J, Gammon G, Loblay RH, Basten A, Goodnow CC. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science (Wash DC) 1991;251:1223–1225. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- 33.Fulcher DA, Basten A. Reduced life span of tolerant self-reactive B cells in a double transgenic model. J Exp Med. 1994;179:125–134. doi: 10.1084/jem.179.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacob J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J Exp Med. 1992;176:679–687. doi: 10.1084/jem.176.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathmell JC, Townsend SE, Xu JC, Flavell RA, Goodnow CC. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell. 1996;87:319–329. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- 36.Fulcher DA, Basten A. B cell activation versus tolerance–the central role of immunoglobulin receptor engagement and T cell help. Int Rev Immunol. 1997;15:33–53. doi: 10.3109/08830189709068170. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y-J, Zhang J, Lane P, Chan EY-T, MacLennan ICM. Sites of specific B cell activation in primary and secondary responses to T-cell–dependent and T-cell– independent antigens. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson BA, Panka DJ, Nguyen K-A, Erikson J, Abbas AK, Marshak-Rothstein A. Anatomy of autoantibody production: dominant localization of antibody producing cells to T cell zones in Fas-deficient mice. Immunity. 1995;3:509–519. doi: 10.1016/1074-7613(95)90179-5. [DOI] [PubMed] [Google Scholar]
- 39.DeFranco A, Ashwell JD, Schwartz RH, Paul WE. Polyclonal stimulation of resting B lymphocytes by antigen-specific T lymphocytes. J Exp Med. 1984;159:861–880. doi: 10.1084/jem.159.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tony H-P, Phillips NE, Parker DC. Role of membrane Ig cross-linking in membrane Ig-mediated, major histocompatibility–restricted T cell B cell co-operation. J Exp Med. 1985;162:1695–1708. doi: 10.1084/jem.162.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirohata S, Jelinek DF, Lipsky PE. T cell dependent activation of B cell proliferation and differentiation by immobilized monoclonal antibodies to CD3. J Immunol. 1988;140:3726–3744. [PubMed] [Google Scholar]
- 42.Owens T. A non-cognate interaction with anti-receptor antibody-activated helper T cells induces small resting murine B cells to proliferate and secrete antibody. Eur J Immunol. 1988;18:395–401. doi: 10.1002/eji.1830180312. [DOI] [PubMed] [Google Scholar]
- 43.Croft M, Swain SL. Analysis of CD4+T cells that provide contact-dependent bystander help to B cells. J Immunol. 1992;149:3157–3165. [PubMed] [Google Scholar]
- 44.Primi D, Hammerstrom L, Smith CIE, Moller G. Characterisation of self-reactive B cells by polyclonal B cell activators. J Exp Med. 1977;145:21–32. doi: 10.1084/jem.145.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kroemer G, Cuende E, Martinez-A C. Compartmentalization of the peripheral immune system. Adv Immunol. 1993;53:157–216. doi: 10.1016/s0065-2776(08)60500-3. [DOI] [PubMed] [Google Scholar]
- 46.Rothstein TL, Wang JKM, Panka DJ, Foote LC, Wang Z, Stanger B, Cui H, Ju S-T, Marshak-Rothstein A. Protection against Fas-dependent Th1 mediated apoptosis by antigen receptor engagement in B cells. Nature (Lond) 1995;374:163–166. doi: 10.1038/374163a0. [DOI] [PubMed] [Google Scholar]
- 47.Casamayer-Palleja M, Khan M, MacLennan ICM. A subset of CD4+memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. J Exp Med. 1995;181:1293–1301. doi: 10.1084/jem.181.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shokat KM, Goodnow CC. Antigen-induced B cell death and elimination during germinal centre immune responses. Nature (Lond) 1995;375:334–338. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- 49.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–329. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 50.Fazekas de St. Groth B, Cook MC, Smith A. The role of T cells in regulating B cell tolerance. Int Rev Immunol. 1997;15:73–100. doi: 10.3109/08830189709068172. [DOI] [PubMed] [Google Scholar]
- 51.Fazekas de St. Groth B. Regulation of the immune response-lessons from transgenic models. Aust NZ J Med. 1995;25:761–767. doi: 10.1111/j.1445-5994.1995.tb02879.x. [DOI] [PubMed] [Google Scholar]
- 52.Rathmell JC, Goodnow CC. Effects of the lpr mutation on elimination and inactivation of self-reactive B cells. J Immunol. 1994;153:2831–2842. [PubMed] [Google Scholar]



