The immune system appears to be rigid, restricting one lymphocyte to make one antibody (1) and the peripheral B cell pool to a constant number (108 in mice) (2– 4). To make a rapid immune response to an unlimited number of antigens at any anatomical site, it has developed at least three major strategies: (a) continuous production of 2 × 107 lymphocytes/d from bone marrow (mice; 5), displaying a part of the 1011 potential immunoglobulin repertoire (6); (b) the establishment of a long-lived B cell pool (108), under the influence of environmental antigens (7) or an idiotypic network (8, 9); and (c) the ability of long-lived B cells to migrate between different lymphoid tissues (10, 11), thus monitoring sites of antigen invasion. Since the 108 peripheral B cells have an average life span of months, <10% of the 2 × 107 B cells produced every day are expected to be recruited into the long-lived peripheral B cell pool (2–4). The question is: where and how does this selection take place?
Splenic Outer Periarteriolar Lymphoid Sheath, the Site of Heavy Chain of the Valuable Region of Ig Gene (IgVH) Region–targeted Selection of Newly Produced B Cells into the Long-lived Peripheral B Cell Pool.
Most newly produced B cells from bone marrow first migrate into the spleen through the terminal branches of central arterioles, arriving in the marginal zone blood sinusoids (Fig. 1 A). Both newly produced B cells and long-lived recirculating B cells can penetrate the marginal zone sinus, reaching the outer zone of the periarteriolar lymphocytic sheath (outer periarteriolar lymphoid sheath [PALS]). Although the long-lived recirculating B cells migrate further into the follicle, the majority of newly produced B cells appear to die within the outer PALS (2, 12). Thus, outer PALS may represent the site where a small proportion of the newly produced B cells are recruited into the long-lived pool. The selection signal has the following features: (a) it selects B cells according to heavy chain of the valuable region of Ig gene (IgVH) expression (7); (b) it may respond to environmental antigen at a low dose (7) or an idiotypic element such as serum Ig (8); (c) it appears to positively select cells (7); and (d) it does not induce somatic hypermutation and isotype switch (7, 13). Three aspects of this ligand selection process are unclear: (a) Does the ligand induce B cell activation and proliferation? (b) How does this ligand selection differ from the negative selection of autoreactive B cells in the periphery? and (c) What is the mechanism in follicles that makes B cells become long lived?
Figure 1.
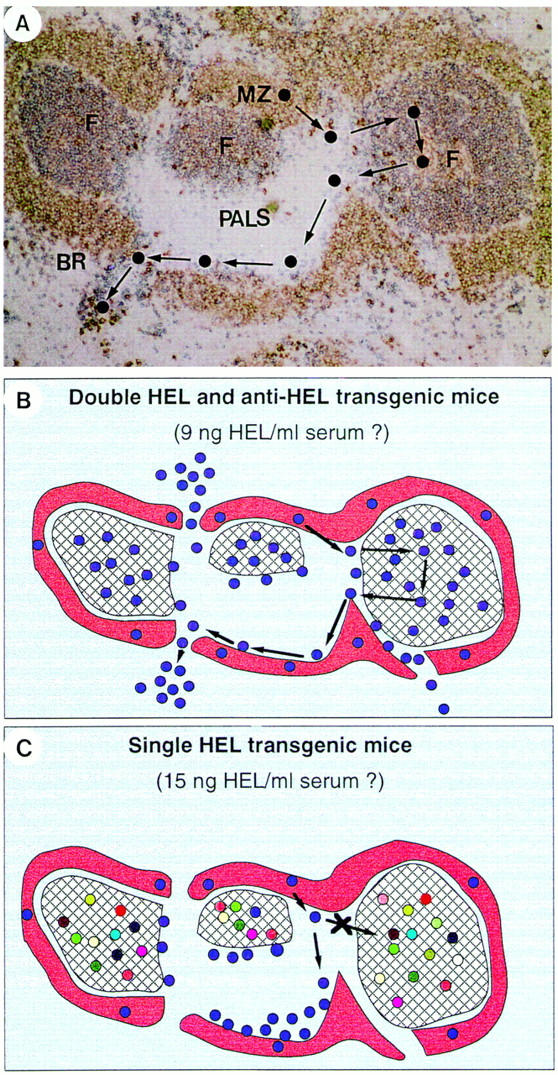
The structure of splenic white pulp and the migration pathways of B lymphocytes. (A) Double anti-IgM (red) and anti-IgD (blue) staining of a splenic section of a nonimmunized rat. Although nonrecirculating IgM+IgD− marginal zone B cells are stained in red, recirculating IgM+IgD++ B cells are stained in blue. The black circles show the proposed migration pathway of a recirculating B lymphocyte. It enters the spleen marginal zone (MZ) through a terminal branch of a central arteriole. It crosses the marginal zone sinus and migrates into a primary follicle. After some hours, it leaves the follicle, migrates along the outer PALS, and finally reaches the red pulp through a bridging channel. (B) The migration pathway of HEL-specific B cells after transfer into HEL and anti-HEL double transgenic mice. (C) The migration pathway of HEL-specific B cells after transfer into HEL single transgenic mice (27–32).
Recently, the molecular mechanism underlying B cell migration from the outer PALS towards follicles was partially revealed by a series of mice lacking TNF-α (14), lymphotoxin α (15), lymphotoxin β (16), TNFR1 (15), and a putative chemokine receptor Burkitt's lymphoma receptor 1 (BLR1) (17). In all these mice, IgD+ B cells were retained within the outer PALS. Importantly, BLR1 was shown to be selectively expressed on mature recirculating B cells, but not on newly produced B cells. This suggests that BLR1 is the navigator that directs the migration of B cells from the outer PALS to the follicle. It will be interesting to know (a) if the regulation of BLR1 expression is by TNF family members or by antigen receptor complex triggering; and (b) if follicular dendritic cells or germinal center dendritic cells (DCs; 18) produce the BLR1 ligand(s).
Outer PALS, the Initial Site of Antigen-specific B Cell Accumulation and Activation in Response to All Types of Antigens.
The development of immunohistological techniques for detecting antigen-specific plasma cells (19) and antigen-specific B cells (20) have permitted an analysis of the microenvironments for antigen-specific B cell responses. A common picture appearing early in spleens during immune responses to all types of antigens is the accumulation of antigen-specific proliferating B cells within the outer PALS (Fig. 2; 19–23). In response to T cell–dependent antigen (TD; protein), the proliferating B cells within the outer PALS either migrate into the follicles to initiate the germinal center reaction, or undergo plasma cell differentiation within the PALS (Fig. 2, A and B). In response to T cell–independent type 1 antigen (TI-1; lipopolysaccharide), even though there is an impressive plasma cell reaction within the outer PALS and red pulp, follicular B cell proliferation is moderate (Fig. 2 C). In response to TI-2 (polysaccharide), most proliferating B cells differentiate into plasma cells (Fig. 2 D). In secondary TD responses, there is a robust B cell proliferation and plasma cell differentiation within the outer PALS. However, the follicular B cell proliferation is less extensive compared to that of primary responses (22, 24).
Figure 2.
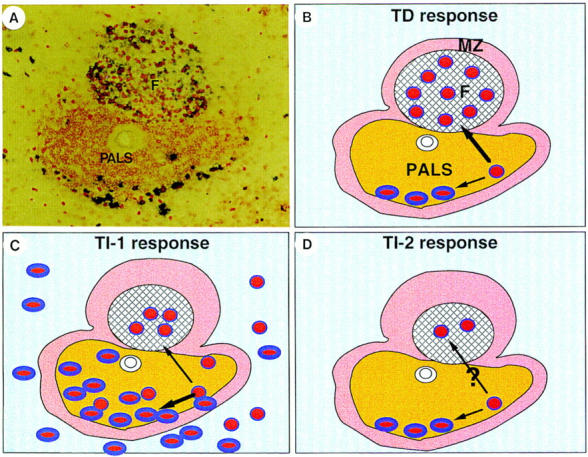
Outer PALS arrest of anti-DNP–specific B cells in T cell–dependent and –independent antibody responses. (A) 48 h after immunization with DNP protein antigen in the rat, antigen-specific proliferating plasma blasts and B blasts can be respectively observed within the outer PALS and follicle of spleen. Blue stains DNP-binding cells; red stains BrdU; brown stains T cells. (B) Schematic representation of A. (C) Schematic representation of splenic B cell response to TI-1 antigen (DNP-LPS). It shows a very impressive antigen-specific B cell proliferation and differentiation within the outer PALS and red pulp. In contrast, follicular B cell proliferation is moderate. (D) Schematic representation of splenic B cell response to TI-2 antigen (DNP-Ficoll). It shows that most proliferating B cells within the outer PALS differentiate into plasma cells.
Outer PALS, the Site of Autoreactive B Cell Inactivation and Elimination.
Double transgenic mice coexpressing self-antigen and anti–self-antibody have revolutionized our understanding of the mechanisms underlying immunological tolerance (25, 26). A striking phenomenon observed by Cyster and co-workers was that within the splenic outer PALS, self-reactive B cells were stopped from migrating into the B cell follicles and they died within the outer PALS (27, 28). This antigen receptor–mediated inability of follicular homing was suggested to be most closely linked to the failure of self-reactive B cells to compete with other B cells for the follicular niches in the presence of self-antigen (Fig. 1, B and C). A similar phenomenon was observed by Fulcher and Basten (29). However, they suggested that the inability of follicular homing was caused by a strong antigen receptor engagment, which was independent of other B cells (29). These different views might be due to the different interpretations of available self-antigen (hen egg lysozyme; HEL) concentration in double transgenic mice expressing both anti–HEL antibody and HEL antigen and in single transgenic mice expressing only HEL antigen. Fulcher and Basten estimated that although single transgenic mice contained 15 ng/ml sera HEL antigen, double transgenic mice contained 9 ng/ml sera HEL antigen, possibly due to the fixation of HEL antigen by anti–HEL antibodies on B cells and in secreted form. Accordingly, when anti–HEL B cells were transferred into double transgenic mice, the 9 ng/ml sera HEL presumably triggered only 26% of the antigen receptors on B cells, which may be under the threshold needed to arrest B cells within the outer PALS and to prevent their entry into the follicles (Fig. 1 B). In contrast, the 15 ng/ml sera HEL antigen in single transgenic mice presumably triggered 47% of the antigen receptors on B cells, which may be well above the threshold (Fig. 1 C). This interpretation was questioned by the accuracy of serum antigen measurement (30) and by the uncertainty that this relatively small difference in degree of receptor engagement (26 versus 47%) could exert such a major difference in the capacity of cell homing to follicles (31). To clarify this issue, it will be necessary to measure the intracellular biochemical signals within HEL-specific B cells after incubation with sera from double and single transgenic mice, respectively.
In this issue of The Journal of Experimental Medicine, Cook et al. report a series of experiments analyzing several factors that may control the follicular homing of B cells (32): (a) dose and duration of antigen triggering; (b) the naive versus tolerant state of anti–HEL B cells; (c) the percentage of anti-HEL B cells relative to the total B cell population; and (d) the nature of the competing follicular B cells within the recipient mice, being either monoclonal syngenec naive B cells, polyclonal naive B cells, or monoclonal HEL-specific tolerated B cells. The results allow them to conclude that (a) B cells undergo arrest in the outer PALS in response to ligation of a critical number of BCRs; (b) this phenomenon is not related to the state of B cells, being either naive or tolerant; and (c) the results appear to be independent of the composition of follicular B cells within the recipient mice.
In summary, outer PALS arrest seems to be an intrinsic property of all types of B cells in response to BCR triggering by all types of antigens. These together with the facts that (a) both normal B cells and tolerant B cells require a similar dose of antigen to be arrested within the outer PALS and (b) tolerant B cells rapidly increase in their size after transfer into HEL transgenic mice (29) suggest that the tolerant B cells undergo antigen-driven abortive activation within the outer PALS, which prevent their further migration into the follicle.
An important conclusion derived from the elegant studies using antigen and antigen receptor transgenic mice is that the accumulation of antigen-specific B cells within the outer PALS in the first few days of immune response is critical for their encounter with rare antigen-specific T cells (for review see reference 30). However, the fate of B cells arrested in the outer PALS depends on not only T cell help, but also the types of immunizing antigen and the state of B cell differentiation and tolerance. In the absence of T cell help, B cells die in a TD response, but they differentiate into plasma cells within the outer PALS in a TI-2 response (19–21). This may be explained by a TI-2 antigen (a) having reiterative epitopes that strongly cross-link antigen receptors; (b) being presented by specialized cells such as marginal zone macrophages; (c) triggering special B cells such as B1 cells or marginal zone B cells (33). In a TD response, the naive B cells and tolerant B cells that accumulate in the outer PALS behave differently with respect to helper T cells. Although helper T cells allow naive B cells entering the follicle to initiate the germinal center reaction or to differentiate into plasma cells within the outer PALS, these T cells kill tolerant B cells upon encounters (28, 32, 34). This is because the Fas ligand possibly expressed by these helper T cells kills B cells when their antigen receptors are either not engaged or do not have a normal intracellular signal transduction pathway (35–37). Naive B cells and memory B cells that have accumulated in the outer PALS also respond differently to helper T cells in TD responses. Although naive B cells preferentially migrate into the follicle to initiate the GC reaction, memory B cells preferentially undergo terminal plasma cell differentiation within the outer PALS (21).
In conclusion, the splenic outer PALS represents a critical site where B cells undergo antigen-driven selection, activation, and deletion. It will be important to further analyze the cellular composition and cellular trafficking in this important anatomical site. For example, it appears that the outer PALS has reduced numbers of DCs, the key antigen-presenting cells in the initiation of primary TD responses (38). But a study by De Smedt et al. showed that a population of marginal zone DCs rapidly migrated into the outer PALS after administration of LPS into mice (39). Thus, the outer PALS represents a site where antigen-specific T and B cells as well as DCs meet after immunization. The recent discovery of chemokine receptors as HIV coreceptors has boosted the discovery of large numbers of chemokines and chemokine receptors by genomic programs. A collection of mice lacking these molecules will be expected to be generated during the next few years. These mice, together with those lacking TNF members/TNF receptors (for reviews see references 40–42) will help us to further understand the mechanisms of cellular migration and interaction within secondary lymphoid tissues during morphogenesis, immune response, and immune tolerance.
Footnotes
I thank Drs. I. Fugier-Vivier, C. Mueller, E. Bates, and F. Brière for critical reading of the manuscript, and Mrs. S. Bonnet-Arnaud and Mrs. M. Vatan for editorial assistance. The study on site of B cell activation was done in 1985–1988 with I.C.M. MacLennan.
References
- 1.Nossal GJV, Lederberg J. Antibody production by a single cell. Nature (Lond) 1958;181:1419–1420. doi: 10.1038/1811419a0. [DOI] [PubMed] [Google Scholar]
- 2.MacLennan ICM, Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 3.Rajewsky K. Clonal selection and learning in the antibody system. Nature (Lond) 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 4.Sprent J. Lifespans of naive, memory and effector lymphocytes. Curr Opin Immunol. 1993;5:433–438. doi: 10.1016/0952-7915(93)90065-z. [DOI] [PubMed] [Google Scholar]
- 5.Opstelten D, Osmond DG. Pre-B cells in mouse bone marrow: immunofluorescence stathmokinetic studies of the proliferation of cytoplasmic μ-chain–bearing cells in normal mice. J Immunol. 1983;131:2635–2640. [PubMed] [Google Scholar]
- 6.Berek C, Milstein C. The dynamic nature of the antibody repertoire. Immunol Rev. 1988;105:1–26. doi: 10.1111/j.1600-065x.1988.tb00763.x. [DOI] [PubMed] [Google Scholar]
- 7.Gu H, Tarlington D, Müller W, Rajewsky K, Förster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991;173:1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freitas AA, Viale AC, Sundblad A, Heusser C, Coutinho A. Normal serum immunoglobulins participate in the selection of peripheral B-cell repertoires. Proc Natl Acad Sci USA. 1991;88:5640–5644. doi: 10.1073/pnas.88.13.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajewsky, K. 1989. Evolutionary and somatic immunological memory. In Progress in Immunology, VII. F. Melchers, editor. Springer Verlag, Berlin. 397–403.
- 10.Nieuwenhuis P, Ford WL. Comparative migration of B and T lymphocytes in the rat spleen and lymph nodes. Cell Immunol. 1976;23:254–267. doi: 10.1016/0008-8749(76)90191-x. [DOI] [PubMed] [Google Scholar]
- 11.Gowans JL, Knight J. The route of recirculation of lymphocytes in the rat. Proc R Soc Lond Ser B Biol Sci. 1964;159:257. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- 12.Lortan JE, Roobottom CA, Oldfield S, MacLennan ICM. Newly produced virgin B cells migrate to secondary lymphoid organs but their capacity to enter follicles is restricted. Eur J Immunol. 1987;17:1311–1316. doi: 10.1002/eji.1830170914. [DOI] [PubMed] [Google Scholar]
- 13.Chies JAB, Lembezat MP, Freitas AA. Entry of B lymphocytes into the persistent cell pool in non-immunized mice is not accompanied by somatic mutation of VHgenes. Eur J Immunol. 1994;24:1657–1664. doi: 10.1002/eji.1830240730. [DOI] [PubMed] [Google Scholar]
- 14.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNFα deficient mice: a critical requirement for TNFα in germinal center formation and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto M, Mariathasan S, Nahm MH, Baranyay F, Peschon JJ, Chaplin DD. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science (Wash DC) 1996;271:1289–1291. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- 16.Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins α and β revealed in lymphotoxin β-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 17.Förster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 18.Grouard G, Durand I, Filgueira L, Banchereau J, Liu YJ. Dendritic cells capable of stimulating T cells in germinal centers. Nature (Lond) 1996;384:364–367. doi: 10.1038/384364a0. [DOI] [PubMed] [Google Scholar]
- 19.Claassen E, Kors N, Dijkstra CD, Van Rooijen N. Marginal zone of the spleen and the development and localization of specific antibody forming cells against thymus-dependent and thymus-independent type-2 antigens. Immunology. 1986;57:399–403. [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y-J, Oldfield S, MacLennan ICM. Memory B cells in T cell–dependent antibody responses colonize the splenic marginal zones. Eur J Immunol. 1988;18:355–362. doi: 10.1002/eji.1830180306. [DOI] [PubMed] [Google Scholar]
- 21.Matsuno K, Ezaki T, Kotani M. Splenic outer periarterial lymphoid sheath (PALS): an immunoproliferative microenvironment constituted by antigen-laden marginal metallophils and ED2-positive macrophages in the rat. Cell Tissue Res. 1989;257:459–470. doi: 10.1007/BF00221456. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y-J, Zhang J, Lane PJL, Chan EY-T, MacLennan ICM. Sites of specific B cell activation in primary and secondary responses to T cell–dependent and T cell–independent antigens. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 23.Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl) acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu YJ, Arpin C. Germinal center development. Immunol Rev. 1997;156:111–126. doi: 10.1111/j.1600-065x.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 25.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature (Lond) 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 26.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature (Lond) 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 27.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature (Lond) 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 28.Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995;3:691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 29.Fulcher DA, Lyons AB, Korn SL, Cook MC, Koleda C, Parish C, Fazekas de St B, Groth, Basten A. The fate of self-reactive B cells depends primarily on the degree of antigen receptor engagement and availability of T cell help. J Exp Med. 1996;183:2313–2328. doi: 10.1084/jem.183.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cyster JG. Signaling thresholds and interclonal competition in preimmune B-cell selection. Immunol Rev. 1997;156:87–101. doi: 10.1111/j.1600-065x.1997.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 31.Nossal GJV. Clonal anergy of B cells: a flexible, reversible, and quantitative concept. J Exp Med. 1996;183:1953–1956. doi: 10.1084/jem.183.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook MC, Basten A, Fazekas de St B, Groth Outer periarteriolar lymphoid sheath arrest and subsequent differentiation of both naive and tolerant immunoglobulin transgenic B cells is determined by B cell receptor occupancy. J Exp Med. 1997;186:631–643. doi: 10.1084/jem.186.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacLennan ICM, Gray D, Kumararatne DS, Bazi H. The lymphocytes of the splenic marginal zones: a distinct B-cell lineage. Immunol Today. 1982;3:305–307. doi: 10.1016/0167-5699(82)90032-9. [DOI] [PubMed] [Google Scholar]
- 34.Rathmell JC, Cooke MP, Ho WY, Grein J, Townsend SE, Davis MM, Goodnow CC. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature (Lond) 1995;376:181–184. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- 35.Rothstein TL, Wang JKM, Panka DJ, Foote LC, Wang Z, Stanger B, Cui H, Ju S-T, Marshak-Rothstein A. Protection against Fas-dependent Th1-mediated apoptosis by antigen receptor engagement in B cells. Nature (Lond) 1995;374:163–165. doi: 10.1038/374163a0. [DOI] [PubMed] [Google Scholar]
- 36.Garrone P, Neidhardt EM, Garcia E, Galibert L, van Kooten C, Banchereau J. Fas ligation induces apoptosis of CD40-activated human B lymphocytes. J Exp Med. 1995;182:1265–1273. doi: 10.1084/jem.182.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rathmell JC, Townsend SE, Xu JC, Flavell RA, Goodnow CC. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligand modulated by the B cell antigen receptor. Cell. 1996;87:319–329. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- 38.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 39.De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, De Baetselier P, Urbain J, Leo O, Moser M. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu YJ, Banchereau J. Mutant mice without B lymphocyte follicles. J Exp Med. 1996;184:1207–1211. doi: 10.1084/jem.184.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kosco-Vilbois MH, Bonnefoy JY, Chvatchko Y. The physiology of murine germinal center reactions. Immunol Rev. 1997;156:127–136. doi: 10.1111/j.1600-065x.1997.tb00964.x. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto M, Fu YX, Molina H, Chaplin DD. Lymphotoxin-α–deficient and TNF receptor-I–deficient mice define developmental and functional characteristics of germinal centers. Immunol Rev. 1997;156:137–144. doi: 10.1111/j.1600-065x.1997.tb00965.x. [DOI] [PubMed] [Google Scholar]


