Abstract
We recently demonstrated that gene-targeted disruption of the C5a anaphylatoxin receptor prevented lung injury in immune complex–mediated inflammation. In this study, we compare the effect of C5aR deficiency in immune complex–induced inflammation in the peritoneal cavity and skin with the results derived from our immune complex alveolitis model. C5aR- deficient mice exhibit decreased migration of neutrophils and decreased levels of TNF-α and interleukin 6 in the peritoneal reverse passive Arthus reaction compared to their wild-type littermates. In the reverse passive Arthus reaction in the skin the C5aR was also required for the full expression of neutrophil influx and edema formation; C5aR-deficient mice showed reduced neutrophil migration and microvascular permeability changes. In contrast to our studies in immune complex–induced lung inflammation, C5aR deficiency does not completely prevent injury in the peritoneal cavity and skin. These data indicate a dominant role for the C5aR and its ligand in the reverse passive Arthus reaction in the lung and a synergistic role together with other inflammatory mediators in immune complex–mediated peritonitis and skin injury.
The formation of immune complexes and their local deposition induces an acute inflammatory response. Diseases such as SLE, RA, immune glomerulonephritis, certain vasculitides, and Goodpasture's syndrome are examples where immune complexes are injurious to the host. The cascade of events leading to tissue injury has been studied for nearly a century (1). The Arthus response is now a classical immune complex model.
The activation of humoral complement has long been associated with immune complex injury and the Arthus response (2). Activation of the complement system via the classical or alternative pathways results in formation of the cell–lytic complex C5b-9 as well as the generation of the anaphylatoxins C3a, C4a, and C5a (3). Complement activation products cause degranulation of phagocytic cells, mast cells, and basophils, smooth muscle contraction, and increases in vascular permeability (4, 5). The anaphylatoxin C5a has the most diverse activity profile, including promotion of leukocyte chemotaxis and activation, enhancement of neutrophil–endothelial cell adhesion, induction of granule secretion in phagocytes, as well as induction and release of several cytokines (i.e., IL-1, IL-6, IL-8, and TNF-α) from leukocytes (6–10). Several studies in complement-depleted animals or in mice genetically deficient for C5 have been effective in demonstrating reduced injury in anaphylaxis, in the Arthus reaction, and in other complement-dependent inflammatory models (11).
Investigations in the peritoneal reverse passive Arthus reaction have shown that activated complement and mast cell mediators play a key role in the initiation of neutrophil recruitment (12, 13). Mast cell degranulation as well as the release of TNF-α during the initiation of the inflammatory process were blocked by decomplementation and C5 deficiency.
Recent studies have attempted to redefine the sequence of events leading to the dermal injury in the Arthus reaction. A central role for dermal mast cells and other resident myeloid cells initiating the inflammation via Fc receptor signaling was demonstrated using FcRγ-deficient mice (14) in comparison to complement-depleted animals (15, 16) and just recently in comparison to C3-, C4-, and C5-deficient mice (17). Thus, it has been proposed that the immune complex-mediated injury is complement independent and Fc receptor dependent. However, other studies in complement sufficient and complement depleted FcγRIII-deficient mice suggested that the IgG immune complex–mediated Arthus reaction in the skin can be induced via a complement-dependent and a complement-independent pathway, and that the complement-independent pathway depends exclusively on FcγRIII activation of effector cells (18).
Based on these studies the relative role of IgG Fc receptors and complement in IgG immune complex–mediated injury remains controversial. However, the earlier studies are complicated by the fact that C5-deficient animals are deficient in both the lytic complex C5b–C9 as well as the mediator C5a. Furthermore, there is an assumption that immune complex–mediated inflammation occurs via a single common mechanism. To address these issues throughout the tissues of the body, we analyzed C5aR-deficient mice in three models of immune complex injury.
In this study, we define the role of the C5a receptor for the inflammatory response in cutaneous and peritoneal Arthus reactions in comparison to our studies in immune complex–induced lung injury. We demonstrated that mice which are genetically deficient in the C5aR showed nearly complete protection against inflammation in immune complex–mediated alveolitis. However, we found quantitatively less reduction of the inflammatory response in immune complex–induced peritonitis or skin injury. These studies provide strong evidence that the C5aR plays a key role in the initiation of IgG-mediated hypersensitivity reaction in the lung whereas in the skin and peritoneum the C5aR acts synergistically with other mediators. Furthermore, the data support a critical role for Fc receptors in immune complex–mediated peritonitis and skin injury. Reconciliation of these data with those obtained with C3- and C5-deficient mice may imply that ligands for the C5aR other than C5a exist.
Materials and Methods
Mice.
C5aR-deficient mice were generated using the method of homologous recombination as previously described (19). Receptor-deficient mice and their normal littermates were used at the age of 12–14 wk, and were age- and sex-matched for each experiment.
Peritoneal Reverse Passive Arthus Reaction.
The reverse passive Arthus reaction was initiated in the peritoneal cavity by the i.v. injection of chicken egg albumin, 20 mg/kg, followed by the i.p. instillation of 800 μg/mouse rabbit anti–chicken egg albumin Ab, IgG fraction (Organon Teknika, Inc., Durham, NC). Mice treated with Ab i.p., but without i.v. injection of albumin, served as Ab controls. Ag controls received PBS i.p. after pretreatment i.v. with chicken egg albumin. At times indicated after challenge, mice were killed and their peritoneal cavity lavaged with 2 ml of ice-cold PBS, 0.1% BSA. The recovered peritoneal lavage fluid was assessed for differential cell counts and cytokine measurements.
Cytokine ELISAs.
Levels of soluble murine TNF-α were measured in appropriately diluted peritoneal lavage samples. The TNF-α concentration was determined with a mouse TNF-α ELISA kit as described in the manufacturer's instructions (Genzyme Corp., Cambridge, MA).
Murine IL-6 was quantitated in appropriately diluted peritoneal lavage samples using a double-ligand ELISA, specific for murine IL-6. Briefly, flat-bottomed 96-well microtiter plates (Marsh Biomedical Products, Inc., Rochester, NY) were coated with 50 μl/well of rat anti–IL-6 Ab (PharMingen, San Diego, CA) (2 μg/ml in 50 mM carbonate buffer, pH 9.6) for 16 h at 4°C, and then blocked with 2% BSA in PBS and incubated for 2 h at 37°C. Plates were washed four times with PBS, pH 7.5, 0.05% Tween 20 (wash buffer). Cell-free supernatants (100 μl) in duplicate were added, followed by incubation for 4 h at room temperature (RT).1 Plates were washed six times, followed by the addition of 50 μl/well of biotinylated rat anti–IL-6 (PharMingen) (2 μg/ml in PBS, pH 7.5, 0.05% Tween 20, and 2% FCS), and plates were incubated for 45 min at RT. Plates were washed six times, streptavidin-peroxidase conjugate (Sigma Chemical Co., St. Louis, MO) was added, and the plates were incubated for 30 min at RT. Plates were washed eight times and 3,3′,5,5′-tetramethylbenzidine peroxidase substrate (Kirkegaard & Perry Labs., Inc., Gaithersburg, MD) was added (100 μl/well) and the reaction was terminated with 100 μl/well of 1:7 (vol/vol) sulfuric acid:H2O solution. Plates were read at 450 nm in an ELISA reader. Standards were 1:2 log dilutions of recombinant murine IL-6 from 2,000 to 7.8 pg/ml.
Reverse Arthus Reaction in the Skin.
Mice were anesthetized by inhalation of methoxyflurane and their basolateral sides were shaved. Varying amounts of rabbit IgG anti–chicken egg albumin (ranging from 25 to 100 μg/25 μl per injection site) or 25 μl sterile PBS were injected intradermally (i.d.), followed immediately thereafter by i.v. injection of 100 μl chicken egg albumin (20 mg/kg). The i.d. injection of the Ab followed by i.v. instillation of 100 μl sterile PBS served as an Ab control. The animals were killed 3, 4, or 8 h later, and 1 cm2 injected area of skin was removed, weighed, and processed for quantitation of edema, hemorrhage, and neutrophil infiltration.
Quantitation of Edema and Hemorrhage.
At times indicated after challenge, 100 μl of Evans blue dye (6.25 mg/ml) was administered via the tail vein to assess permeability changes and subsequent edema. Edema was measured by determining the wet weight of the skin sections at 3 h after challenge. Specific edema was assessed by subtracting the weight of the control area (PBS injection) from that injected with anti–chicken egg albumin IgG.
In addition, edema was quantitated by measuring the absorbance of formamide extracts of Evans blue dye at 610 nm with a spectrophotometer. To extract extravasated Evans blue dye, tissue samples were incubated in 1 ml formamide at RT for 24 h.
Quantitation of Neutrophil Infiltration.
Mice were killed at 8 h, 1 cm2 injected areas of skin were removed, and neutrophil content was assessed by quantitation of myeloperoxidase (MPO). MPO was extracted from injected areas of skin and colorimetrically measured as described (20).
Statistical Analysis.
Continuous data wild-type and C5aR (−/−) groups were considered statistically significant when P <0.05, as determined by the unpaired Student's t test.
Results
Complement-Mediated Leukocyte Elicitation in the Peritoneal Reverse Passive Arthus Reaction.
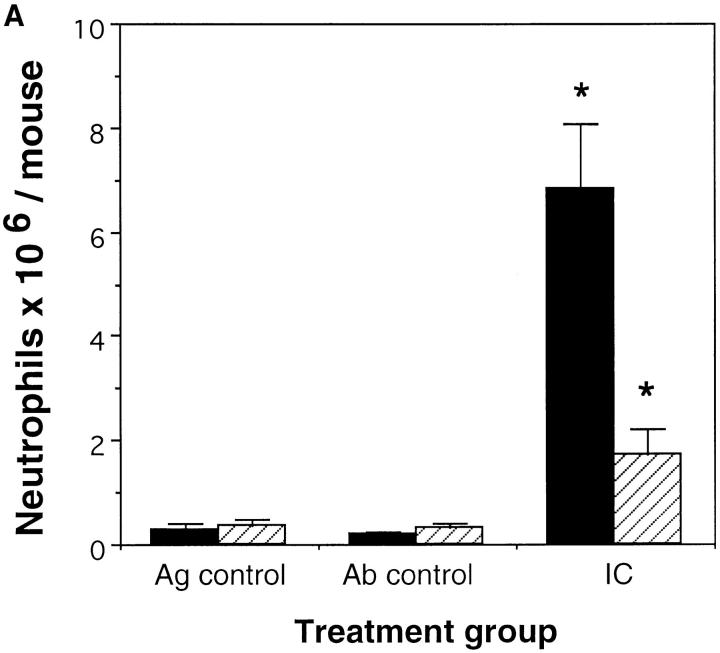
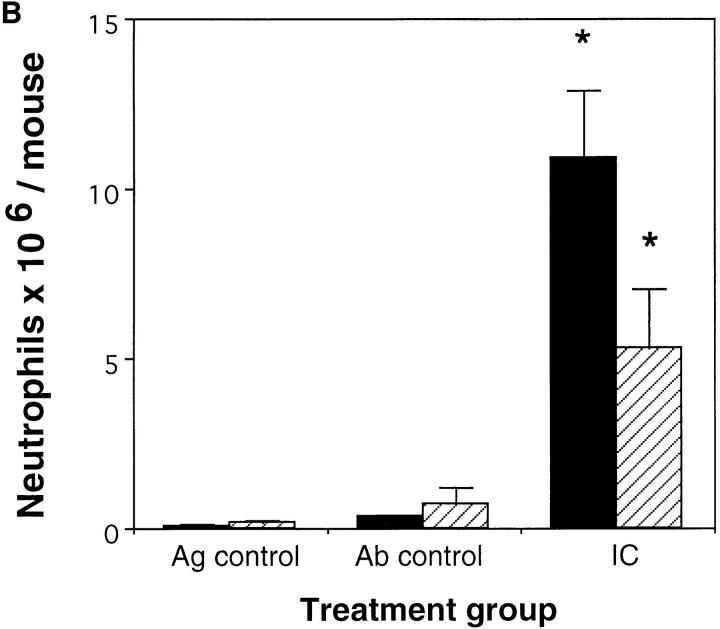
Previously we demonstrated the critical role for complement in immune complex– mediated acute lung injury (21) by challenging the C5aR (−/−) mice, selectively eliminating contributions of the C5a anaphylatoxin. In this study we investigated the role of the C5a receptor in the recruitment and activation of acute inflammatory leukocytes in the peritoneal reverse passive Arthus reaction. Administration of rabbit anti–chicken egg albumin Ab in the peritoneal cavity after i.v. injection of chicken egg albumin caused neutrophil influx into the peritoneal cavity as well as secretion of diverse inflammatory cytokines. C5aR-deficient mice showed significantly reduced neutrophil migration compared to their wild-type controls at 4 h (Fig. 1 A) and at 8 h (Fig. 1 B) of the reverse passive Arthus reaction. Neutrophil accumulation reached a maximum at 8 h in both sets of mice. However, the decrease in leukocyte influx in C5aR (−/−) mice compared to their wild-type controls was more profound at 4 h (74%) than at 8 h (51%) suggesting that C5a might contribute more to the initiation of the Arthus reaction rather than in the subsequent recruitment and activation steps.
Figure 1.
Role of C5a in neutrophil elicitation in immune complex–induced peritonitis. The reverse passive Arthus reaction was allowed to proceed for 4 h (A) or 8 h (B) in C5aR (−/−) mice (striped bars) and their wild-type littermates (black bars). Total cell numbers (data not shown) and percentage of neutrophils in the peritoneal fluid were determined as described previously (13). Ab controls (Ab control) received Ab to chicken egg albumin i.p. without i.v. injection of chicken egg albumin. Mice treated with PBS i.p. followed by i.v. chicken egg albumin served as Ag controls (Ag control). Data are represented as mean ± SEM, n = 7–8 mice *P <0.004 (A), and n = 8–11 mice *P <0.05 (B).
TNF-α Content during IgG Immune Complex Peritonitis.
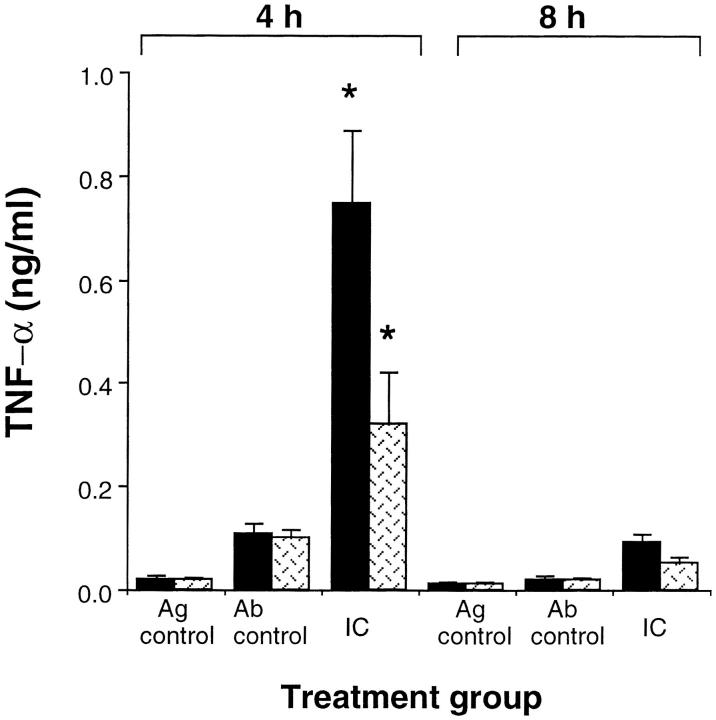
Ag–Ab complexes have been shown to stimulate the release and production of TNF-α, IL-1β (22), and the IL-1 receptor antagonist (23) from human monocytes in vitro. We stimulated murine peritoneal macrophages with preformed immune complexes for 8 or 24 h and assayed the cell supernatant for TNF-α and IL-6 content. At both time points we were able to detect significant secretion of both cytokines in vitro (data not shown). The response to immune complexes in vivo has also been reported to depend on the synthesis and release of TNF-α from mast cells, neutrophils, and mononuclear phagocytes. The release of TNF-α during the initiation of inflammation was blocked by decomplementation and C5 deficiency (12). This prompted us to compare the release of TNF-α in our immune complex–mediated peritonitis model in C5aR-deficient mice versus their wild-type littermates. At 4 h wild-type and heterozygous animals contained 745 ± 140 pg/ml TNF-α (mean ± SEM) (Fig. 2) while C5aR (−/−) animals showed significantly decreased TNF-α levels of 321 ± 98.9 pg/ml (mean ± SEM) (Fig. 2). At 8 h wild-type and heterozygous animals contained 91.3 ± 19.1 pg/ml TNF-α (mean ± SEM) (Fig. 2), while C5aR (−/−) animals showed TNF-α levels of 51.1 ± 11.3 pg/ml (mean ± SEM) (Fig. 2) peritoneal lavage fluid. TNF-α was undetectable in the lavage fluids of Ag control animals. These findings indicate that the C5a receptor contributes to the secretion of TNF-α from neutrophils, macrophages, or from mast cells during the course of immune complex–mediated inflammation in the peritoneum. Although neutrophil influx reached a maximum 8 h after challenge, TNF-α levels had already peaked at 4 h and showed significantly lower levels at 8 h after challenge. This suggests that later on in the inflammatory response other inflammatory mediators such as IL-8 (in the mouse: analogs KC, MIP-2) might be responsible for the recruitment of neutrophils.
Figure 2.
C5a-dependent secretion of TNF-α. The reverse passive Arthus reaction (IC) was allowed to proceed for 4 or 8 h in C5aR (−/−) (dotted bars) mice and their wild-type littermates (black bars). TNF-α content in the peritoneal fluid was determined by a TNF-α–specific ELISA. Ab controls (Ab control) received Ab to chicken egg albumin i.p. without i.v. injection of chicken egg albumin. Mice treated with PBS i.p. followed by i.v. chicken egg albumin served as Ag controls (Ag control). Data are represented as mean ± SEM, n = 14–17 mice (4 h) and n = 7–9 mice (8 h). *P <0.025.
IL-6 Content During IgG Immune Complex Peritonitis.
It has been shown that immune complexes also induce the secretion of IL-6 and IL-10 from human monocytes (24). To test the role of the C5aR and its ligand in regulating IL-6 synthesis in immune complex peritonitis we measured IL-6 levels in the peritoneal lavage samples of C5aR (−/−) and wild-type animals 4 and 8 h after challenge. As demonstrated in Fig. 3, remarkable IL-6 levels were measurable at 4 h (wild type: 16.43 ± 3.62 ng/ml IL-6 [mean ± SEM]; C5aR [−/−]: 5.72 ± 2.42 ng/ml IL-6 [mean ± SEM]) and to a lesser extent at 8 h (wild type: 1.88 ± 0.74 IL-6 [mean ± SEM]; C5aR [−/−]: 0.97 ± 0.18 ng/ml IL-6 [mean ± SEM]). No detectable levels of IL-6 were found in Ag controls. In the exudate from C5aR (−/−) mice the IL-6 levels were significantly reduced compared to their wild-type controls at 4 h (65.2%), whereas the decrease in IL-6 levels in the C5aR (−/−) mice at 8 h (34.8%) did not reach statistical significance. These results suggest that C5a enhances IL-6 secretion mainly in the earlier phase of immune complex–induced inflammation in the peritoneum.
Figure 3.
C5a-dependent secretion of IL-6. The reverse passive Arthus reaction (IC) was allowed to proceed for 4 or 8 h in C5aR (−/−) mice (dotted bars) and their wild-type littermates (black bars). IL-6 content in the peritoneal fluid was determined by an IL-6–specific ELISA. Ab controls (Ab control) received Ab to chicken egg albumin i.p. without i.v. injection of chicken egg albumin. Mice treated with PBS i.p. followed by i.v. chicken egg albumin served as Ag controls (Ag control). Data are represented as mean ± SEM, n = 14–17 mice (4 h) and n = 7–9 mice (8 h). *P <0.004.
Reverse Passive Arthus Reaction in the Skin Attenuated by C5a and its Receptor.
Reverse passive Arthus reaction in the skin caused dramatic PMN infiltration at sites containing the anti–chicken egg albumin Ab accompanied by severe edema and hemorrhage in wild-type animals. These responses were significantly, but not completely, attenuated in C5aR deficient mice.
Neutrophil Influx.
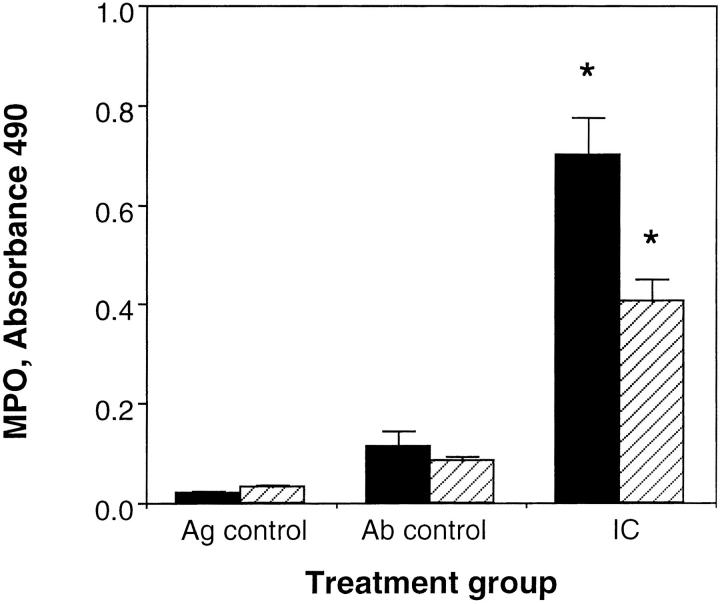
Leukocyte influx in the reverse Arthus reaction in the skin reaches a maximum at 8 h after challenge (25). Therefore, we evaluated neutrophil influx at 8 h. The margination of neutrophils was quantitated by using the myeloid specific enzyme MPO as an indicator of neutrophil accumulation. With a dose of 100 μg Ab/site, a dramatic PMN influx was observed at 8 h. As demonstrated in Fig. 4 the MPO activity in C5aR-deficient mice was significantly lower (43.8%) than in wild-type littermates. No MPO activity was detected in skin which was treated only with PBS and only very weak MPO activity was observed when mice were treated with 100 μg Ab/site without application of Ag.
Figure 4.
PMN infiltration in the reverse Arthus reaction in the skin. Extraction of MPO from skin of C5aR (−/−) (striped bars) mice and their wild-type littermates (black bars) was performed 8 h after initiation of the Arthus reaction. Ab controls (Ab control) received Ab to chicken egg albumin intratracheally without i.v. injection of chicken egg albumin. Mice treated with PBS intratracheally followed by i.v. chicken egg albumin served as Ag controls (Ag control). Data are represented as mean ± SEM, n = 12–13 animals in each group. *P <0.003.
Edema and Hemorrhage.
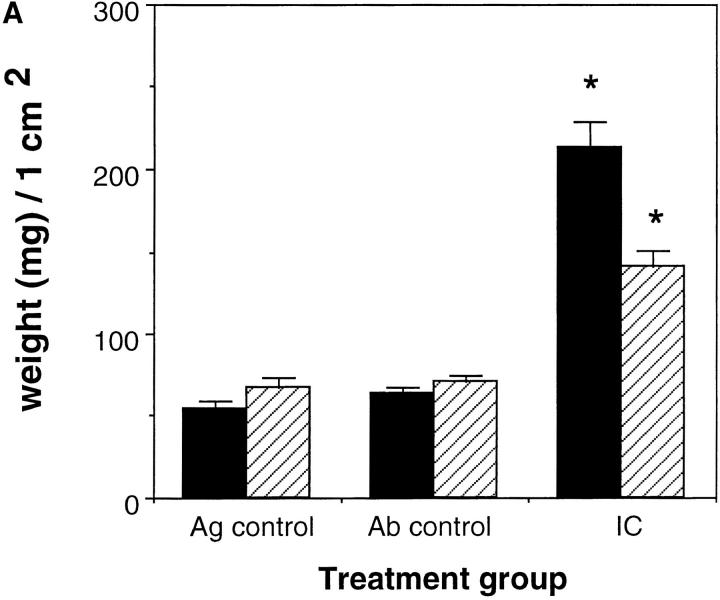
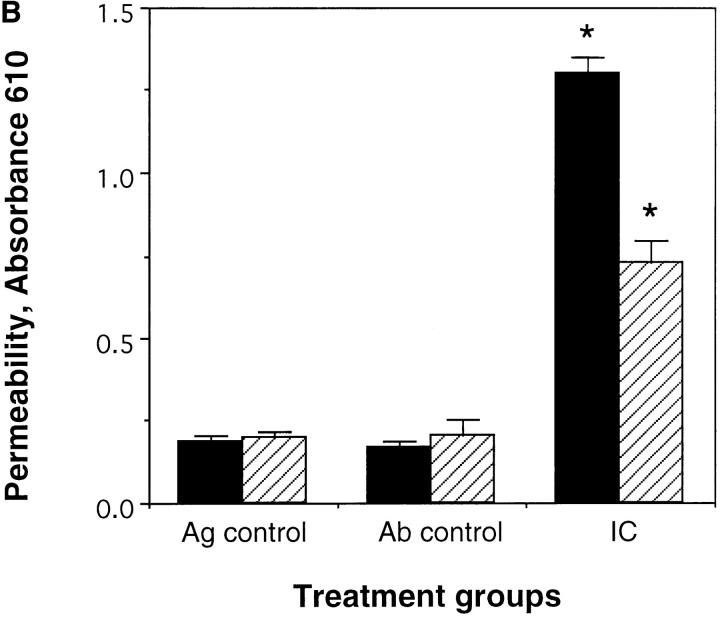
Edema was measured by determining the wet weight of standardized skin punches at 3 h after treatment. In addition, alterations in vascular permeability were determined by i.v. injection of Evans blue dye 1 h after treatment and quantifying the amount of Evans blue dye in the skin biopsy. Evans blue dye binds to serum proteins and thus can be used to quantify alterations in vascular permeability. The skins of wild-type mice and C5aR-deficient mice were harvested at 3 h after initiation of the Arthus reaction with 25 μg Ab/site. As demonstrated in Fig. 5, A and B, edema was evaluated as weight of skin biopsies (Fig. 5 A) or as Evans blue dye extravasation (Fig. 5 B). No edema was observed in Ag or Ab controls. In both wild-type mice and C5aR-deficient mice profound edema was noticed. However, there was significantly less edema as determined by Evans blue dye extravasation and wet weight of skin biopsies in the C5aR-deficient mice compared to their wild-type littermates (Fig. 5, A and B) 3 h after challenge (permeability changes: 54.7%; weight: 48.1%).
Figure 5.
Edema formation in the reverse Arthus reaction in the skin. 25 μg of rabbit anti–chicken egg albumin IgG was injected i.d. followed by 20 mg/kg body weight i.v. chicken egg albumin together with 100 μl Evans blue dye (6.25 mg/ml). Dorsal skins were harvested from C5aR (−/−) mice (striped bars) and their wild-type littermates (black bars) after 3 h and edema was evaluated as weight of 1 cm2 skin sections (A). In addition, edema was quantitated as enhanced microvascular permeability by extraction of extravasated Evan's blue dye with formamide from injected skin sections (B). Ab controls (Ab control) received Ab to chicken egg albumin intratracheally without i.v. injection of chicken egg albumin. Mice treated with PBS intratracheally followed by i.v. chicken egg albumin served as Ag controls (Ag control). Data are represented as mean ± SEM, n = 8–12 animals in each group. *P <0.001 (A), *P <0.0001 (B).
Discussion
Inflammatory injury initiated by immune complexes, as modeled by the reverse Arthus reaction, demonstrates the toxic effects of the inflammatory response (26). With complement activation in immune complex–induced inflammation complement fragments C3a (C3a des Arg), C5a (C5a des Arg), and C5b-9 are generated, which lead to neutrophil accumulation and stimulation as well as mast cell degranulation at sites of inflammation. Earlier studies in cobra venom factor (CVF)-treated animals (27, 28) and in C5-deficient mice (29) have suggested that some components of complement are central to the main inflammatory reaction in immune complex–mediated lung injury. Parameters of permeability and hemorrhage decreased towards control values in complement-depleted rats after treatment with preformed immune complexes (27). C5-deficient mice showed a significant decrease in cell migration, pulmonary histologic finding, and wet/dry weight ratios of lungs after immune complex–mediated lung inflammation in comparison to wild-type animals (29). Because CVF-treated animals or C5-deficient mice lack multiple mediators in addition to C5a, we previously isolated the central contribution of the C5aR in immune complex–mediated lung injury (21). However, the role of complement, and by inference, C5aR in other models of immune complex– induced inflammation has been questioned.
In this study, taking advantage of transgenic mice genetically deficient in the C5aR, we demonstrate an involvement of this receptor in several models of immune complex injury. Assessment of the reverse Arthus reaction in the lung, peritoneum, and in the skin demonstrates that the complement C5a receptor is implicated in the Arthus reaction, but quantitatively to different degrees in the various tissues. In the lung, immune complex–mediated activation of complement and subsequently the release of the anaphylatoxin C5a has been shown to be the main event triggering the IgG-mediated hypersensitivity reaction (21). The lung tissues of the immune complex–challenged C5aR (−/−) mice appear to be indifferent from those of the controls challenged with Ab or saline, as quantitated by measuring extravasation of Evans blue dye into alveolar space and exudation of neutrophils into the airways and lung alveoli. Thus, we conclude that C5a acting through its specific heterotrimeric GTP-binding protein–coupled receptor is essential for the formation of the full injury seen in this model of immune complex–mediated lung injury. However, when C5aR-deficient mice were assayed in the immune complex–mediated peritonitis and skin injury, the C5aR was less central to the inflammatory response. Our data show that while C5aR deficiency significantly reduces the inflammatory response in both immune complex– mediated peritonitis and dermal reverse Arthus reaction, C5aR-independent pathways occur.
In the peritoneal reverse passive Arthus reaction, we observed significantly reduced neutrophil infiltration in the C5aR-deficient mice compared to their wild-type controls at 4 h (74%; Fig. 1 A) and also at 8 h after challenge (51%; Fig. 1 B). The decrease in neutrophil infiltration was more profound after 4 h than after 8 h, suggesting that C5a is important for neutrophil recruitment in the early phase of the inflammatory response. Studies in rabbits investigating the generation of neutrophil chemoattractants in ischemic myocardium reperfusion showed that C5a was generated within 5 min whereas other neutrophil chemoattractants such as IL-8 were released after a delay (30). I.d. injections of the same chemoattractants into the skin of guinea pigs demonstrated C5a-induced eosinophil accumulation of i.v. administered eosinophils within the first hour. In contrast, IL-8 showed a delayed induction of eosinophil accumulation compared to C5a (31). The parallel reduction of TNF-α levels in the C5aR-deficient mice (Fig. 2) suggests that the decrease in neutrophil migration in the peritoneal cavity in the C5aR (−/−) mice may be partially due to the decrease in TNF-α secretion from macrophages and mast cells. The reduction of neutrophil infiltration in the peritoneal cavity in C5aR-deficient mice of 50–70% is similar to the findings in recently published data (13), which were aimed to elucidate the role of complement in mast cell activation and neutrophil recruitment in immune complex– induced peritonitis in mast cell–deficient, C5-deficient, and decomplemented mice. In all three deficiencies a reduced neutrophil influx of 40–60% and a reduced TNF-α release of 40–70% were observed. Complete inhibition of both parameters was observed in decomplemented mast cell–deficient mice, suggesting a close link in vivo between complement and mast cell activation and neutrophil recruitment in the peritoneal reverse passive Arthus reaction. As well as decreased TNF-α levels, we observed a significant decrease in IL-6 levels in the peritoneal lavage samples of C5aR (−/−) animals compared to their wild-type controls 4 and 8 h after challenge (Fig. 3). IL-6 is a proinflammatory cytokine which induces the synthesis and release of acute-phase proteins by hepatocytes and serves as a growth factor for activated B cells. IL-6 is detectable in the circulation following gram-negative bacterial infection or TNF-α infusion. Recent publications have shown that C5a enhances LPS- induced IL-6 release in vitro and in vivo (9, 32). However, IL-6 may also function as an antiinflammatory mediator causing inhibition of acute neutrophilic efflux and LPS- induced TNF-α release (33).
Here, we demonstrate decreased IL-6 levels in C5aR-deficient mice during the course of immune complex– induced inflammation. These data provide direct in vivo evidence for an immune regulatory role of the C5aR and its ligand through enhancement of IL-6 secretion, a cytokine which may function as a proinflammatory as well as an antiinflammatory mediator.
In the reverse passive Arthus reaction in the skin the absence of the C5aR-reduced neutrophil infiltration by ∼40% and permeability changes with associated edema by ∼55%. Thus, in comparison to our IgG-mediated Arthus reaction in the lung, where we observed essentially quantitative inhibition, the inflammatory reaction in the skin showed partial abrogation through disruption of the C5aR gene. Our data in the dermal Arthus reaction are in agreement with studies in FcγRIII-deficient mice, which showed that variation in the extravasation intensity in FcγRIII (−/−) mice correlated with fluctuation in serum complement levels (18). After complement depletion with CVF complete abrogation of extravasation in all FcγRIII (−/−) mice was observed, while wild-type mice remained unaffected by CVF treatment. These data suggest that IgG immune complex–mediated Arthus reaction in the skin can be induced via a complement-dependent and a complement-independent pathway. In contrast, Sylvestre and Ravetch (15) showed that the Arthus reaction in the skin is markedly attenuated in animals deficient in the FcRγ chain and not significantly altered by treatment of both wild-type and FcRγ (−/−) animals with CVF. Therefore the authors conclude that complement has only a secondary role by amplifying the FcR-triggered response. More recently, Sylvestre et al. studied IgG-mediated inflammatory response in the skin in C3-, C4-, and C5-deficient mice (17). In their view, the activation of cell based FcγR receptors, but not complement, is required for Ab-triggered murine inflammatory responses.
To demonstrate partial abrogation of a dramatic inflammatory response, as we observed in the C5aR (−/−) mice in the IgG-mediated Arthus reaction in the skin, sensitive and quantitative measurements are required. Therefore we complemented our histologic observations with quantitative measurement of neutrophil migration (MPO data; Fig. 4) and quantitative measurement of permeability changes (Evans blue dye extravasation; Fig. 5 B). By simple microscopic interpretation through histological scoring of hemorrhage, edema, and neutrophil infiltration, one may overlook partial contribution of other inflammatory mediators such as complement in the IgG-mediated Arthus reaction in the skin.
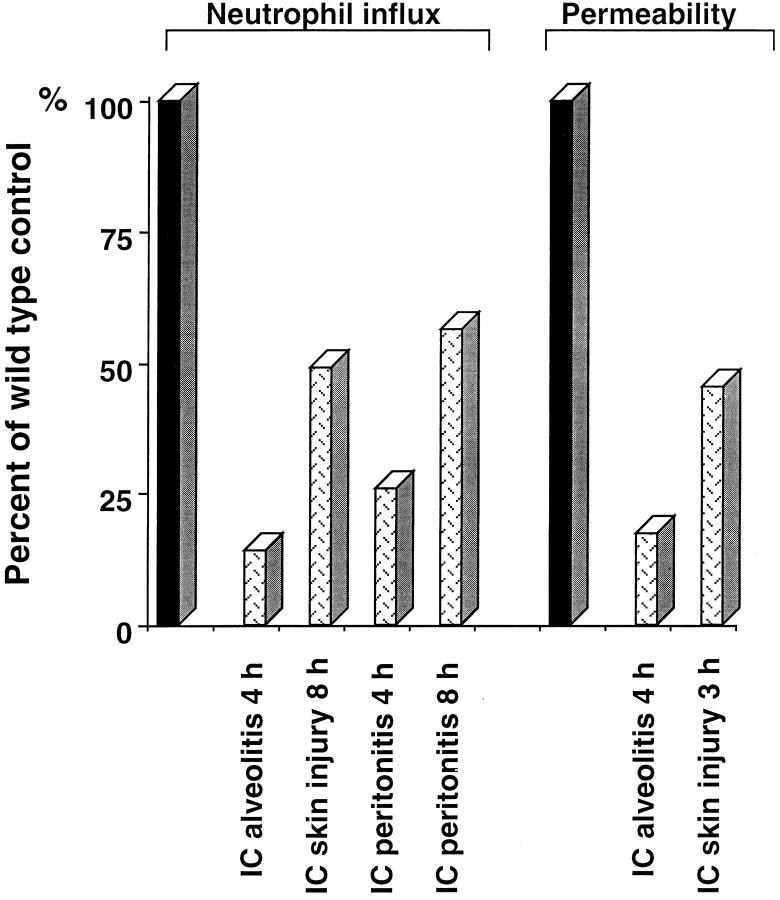
These data support a model where immune complex– mediated injury has multiple, redundant components. The degree of dependence on the complement C5aR, FcR, and mast cells appears to be tissue specific. With regard to the C5aR, the contribution is lung > peritoneum > skin (Fig. 6). Additionally, a kinetic contribution occurs, with C5aR more important in early inflammation in peritoneum and skin.
Figure 6.
Effect of C5aR deficiency in immune complex–induced neutrophil influx and permeability changes. The decrease in neutrophil influx and permeability changes in C5aR (−/−) (dotted bars) is shown as percentage of wild-type controls (black bars; normalized to 100%). Results are expressed as means, n = 7–13 animals in each group.
In summary, the data suggest that the mechanisms of immune complex injury are more complex than currently appreciated, and that local environments may utilize distinct inflammatory pathways to the same end point.
Finally, we acknowledge that C5aR deficiency is not synonymous with C5a deficiency, because the C5aR may theoretically interact with other ligands to promote inflammation. This latter issue might explain differences between C3- and C5-deficient mice versus C5aR (−/−) mice in the dermal reverse Arthus reaction. Should another ligand exist for the C5aR, its presence in C3- and C5-deficient animals would obscure the role of the C5aR reported here.
Acknowledgments
The authors would like to thank Dr. A. Rehm for reading the manuscript.
This work was supported in part by National Institutes of Health grants HL-36162 and HL-51366 and the Rubenstein-Cable Cystic Fibrosis Research Fund at the Perlmutter Laboratory.
Footnotes
Abbreviations used in this paper: CVF, cobra venom factor; i.d., intradermally; MPO, myeloperoxidase; RT, room temperature.
References
- 1.Arthus M. Injections répétées de sérum de cheval chez le lapin. Soc Biol. 1903;55:817–825. [Google Scholar]
- 2.Cochrane, C.G., and A. Janoff. 1974. Chapter 3. In The Inflammatory Process, Vol. 3. B.W. Zweifach, L. Grant, and R.T. McCluskey, editors. Academic Press, New York.
- 3.Gerard C, Gerard NP. C5a anaphylatoxin and its seven transmembrane-segment receptor. Annu Rev Immunol. 1994;12:775–808. doi: 10.1146/annurev.iy.12.040194.004015. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein, I.M. 1988. Complement: biologically active products. In Inflammation: Basic Principles and Clinical Correlates. J.I. Gallin, I.M. Goldstein, and R. Snyderman, editors. Raven Press, New York. 55.
- 5.Johnson AR, Hugli TE, Müller-Eberhard HJ. Release of histamine from rat mast cells by complement peptides C3a and C5a. Immunology. 1975;28:1067–1080. [PMC free article] [PubMed] [Google Scholar]
- 6.Okusawa S, Dinarello CA, Yancey KB, Endres S, Lawley TJ, Frank MM, Burke JF, Gelfand JA. C5a induction of human interleukin 1. Synergistic effect with endotoxin or interferon-gamma. J Immunol. 1987;139:2635–2640. [PubMed] [Google Scholar]
- 7.Okusawa S, Yancey KB, van der Meer JWU, Endres S, Lonnemann G, Hefter K, Frank MM, Burke JF, Dinarello CA, Gelfand JA. C5a stimulates secretion of tumor necrosis factor from human mononuclear cells in vitro. Comparison with secretion of interleukin 1β and interleukin 1α. J Exp Med. 1988;168:443–448. doi: 10.1084/jem.168.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scholz W, McClurg MR, Cordenas GJ, Smith M, Noonan DJ, Hugli TE, Morgan EL. C5a-mediated release of interleukin-6 by human monocytes. Clin Immunol Immunopathol. 1990;57:297–307. doi: 10.1016/0090-1229(90)90043-p. [DOI] [PubMed] [Google Scholar]
- 9.Montz H, Koch K-C, Zierz R, Götze O. The role of C5a in interleukin-6 production induced by lipopolysaccharide or interleukin-1. Immunology. 1991;74:373–379. [PMC free article] [PubMed] [Google Scholar]
- 10.Ember JA, Sanderson SD, Hugli TE, Morgan EL. Induction of interleukin-8 synthesis from monocytes by human C5a anaphylatoxin. Am J Pathol. 1994;144:393–403. [PMC free article] [PubMed] [Google Scholar]
- 11.Colten HR. Immunology. Drawing a double-edged sword. Nature (Lond) 1994;371:474–475. doi: 10.1038/371474a0. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Ramos BF, Jakschik BA. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science (Wash DC) 1992;258:1957–1959. doi: 10.1126/science.1470922. [DOI] [PubMed] [Google Scholar]
- 13.Ramos BF, Zhang Y, Jakschik BA. Neutrophil elicitation in the reverse passive Arthus reaction. J Immunol. 1994;152:1380–1384. [PubMed] [Google Scholar]
- 14.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcRγ chain deletion results in pleiotropic effector cell defect. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 15.Sylvestre DL, Ravetch JV. Fc receptors initiate the Arthus reaction: redefining the inflammatory cascade. Science (Wash DC) 1994;265:1095–1098. doi: 10.1126/science.8066448. [DOI] [PubMed] [Google Scholar]
- 16.Ravetch JV. Fc receptors: rubor redux. Cell. 1994;78:553–560. doi: 10.1016/0092-8674(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 17.Sylvestre D, Clynes R, Minga M, Warren H, Carroll MC, Ravetch JV. Immunoglobulin G–mediated inflammatory responses develop normally in complement-deficient mice. J Exp Med. 1996;184:2385–2392. doi: 10.1084/jem.184.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazenbos WLW, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, Schmidt RE, Sandor M, Capel PJ, Daëron M, et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in FC gamma RIII (CD16) deficient mice. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 19.Höpken UE, Lu B, Gerard NP, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature (Lond) 1996;383:86–89. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- 20.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–213. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 21.Bozic CR, Lu B, Höpken UE, Gerard C, Gerard NP. Neurogenic amplification of immune complex inflammation. Science (Wash DC) 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- 22.Polat GL, Laufer J, Fabian I, Passwell JH. Cross-linking of monocyte plasma membrane Fc alpha, Fc gamma or mannose receptors induces TNF production. Immunology. 1993;80:287–292. [PMC free article] [PubMed] [Google Scholar]
- 23.Arend WP, Joslin FG, Massoni RJ. Effects of immune complexes on production by human monocytes of interleukin 1 or an interleukin 1 inhibitor. J Immunol. 1985;134:3868–3875. [PubMed] [Google Scholar]
- 24.Berger S, Balló H, Stutte HJ. Immune complex–induced interleukin-6, interleukin-10 and prostaglandin secretion by human monocytes: a network of pro- and anti-inflammatory cytokines dependent on the antigen:antibody ratio. Eur J Immunol. 1996;26:1297–1301. doi: 10.1002/eji.1830260618. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Ramos BF, Jakschik BA. Augmentation of reverse Arthus reaction by mast cells in mice. J Clin Invest. 1991;88:841–846. doi: 10.1172/JCI115385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniele RP, Henson PM, Fantone JC, Ward PA, Dreisin RB. Immune complex injury of the lung. Symposium held at the 74th annual meeting of the American Thoracic Society, Las Vegas, Nevada, May 1979. Am Rev Respir Dis. 1981;124:738–755. [PubMed] [Google Scholar]
- 27.Sherzer H, Ward PA. Lung and dermal vascular injury produced by preformed immune complexes. Am Rev Respir Dis. 1978;117:551–557. doi: 10.1164/arrd.1978.117.3.551. [DOI] [PubMed] [Google Scholar]
- 28.Roska AKB, Garancis JC, Moore VL, Abramoff P. Immune complex disease in guinea pig lungs. Clin Immunol Immunopathol. 1977;8:213–224. doi: 10.1016/0090-1229(77)90111-8. [DOI] [PubMed] [Google Scholar]
- 29.Larsen GL, Mitchell BC, Henson PM. The pulmonary response of C5 sufficient and deficient mice to immune complexes. Am Rev Respir Dis. 1981;123:434–439. doi: 10.1164/arrd.1981.123.4.434. [DOI] [PubMed] [Google Scholar]
- 30.Ivey CL, Williams FM, Collins PD, Jose PJ, Williams TJ. Neutrophil chemoattractants generated in two phases during reperfusion of ischemic myocardium in the rabbit. Evidence for a role for C5a and interleukin-8. J Clin Invest. 1995;95:2720–2728. doi: 10.1172/JCI117974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins PD, Weg VB, Faccioli LH, Watson ML, Moqbel R, Williams TJ. Eosinophil accumulation induced by human interleukin-8 in the guinea-pig in vivo. Immunology. 1993;79:312–318. [PMC free article] [PubMed] [Google Scholar]
- 32.Höpken UE, Mohr M, Strüber A, Montz H, Burchardi H, Götze O, Oppermann M. Inhibition of interleukin-6 synthesis in an animal model of septic shock by anti-C5a monoclonal antibodies. Eur J Immunol. 1996;26:1103–1109. doi: 10.1002/eji.1830260522. [DOI] [PubMed] [Google Scholar]
- 33.Akira S, Taga T, Kishimoto A. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]