Abstract
Estrogen deficiency causes bone loss, which can be prevented by estrogen replacement therapy. Using a recently developed technique for isolation of highly purified mammalian osteoclasts, we showed that 17 β-estradiol (E2) was able to directly inhibit osteoclastic bone resorption. At concentrations effective for inhibiting bone resorption, E2 also directly induced osteoclast apoptosis in a dose- and time-dependent manner. ICI164,384 and tamoxifen, as pure and partial antagonists, respectively, completely or partially blocked the effect of E2 on both inhibition of osteoclastic bone resorption and induction of osteoclast apoptosis. These data suggest that the protective effects of estrogen against postmenopausal osteoporosis are mediated in part by the direct induction of apoptosis of the bone-resorbing osteoclasts by an estrogen receptor– mediated mechanism.
Estrogen deficiency, caused by either menopause or ovariectomy, results in pathological bone loss, which can be prevented by estrogen replacement therapy (1, 2). Although it is believed that estrogen's main action in preventing bone loss is through inhibition of osteoclastic bone resorption, the precise mechanism of such effects is not clear, largely due to technical difficulties in obtaining purified functional osteoclasts (3, 4). Osteoclasts are terminally differentiated multinucleate cells the main function of which is to dissolve bone matrix and minerals in the resorption phase of bone remodeling (5). Recruitment, differentiation, and activity of osteoclasts are tightly controlled by systemic and local factors. For instance, vitamin D3, prostaglandins, TGF-β, IL-1, IL-6, and TNF-α stimulate osteoclast differentiation and activity via direct or indirect mechanisms, whereas calcitonin directly inhibits osteoclast activity (6–10). The fate of osteoclasts after bone resorption is largely unknown. Certain factors, such as calcitonin, inactivate osteoclasts without induction of cell death, whereas other factors, such as bisphosphonates and vitamin K2, are suggested to induce apoptosis of osteoclasts (11–13). Apoptosis, or programmed cell death, is characterized by nucleosomal DNA fragmentation and grossly changed morphology of the nuclei without a change in the morphology of the intracellular organelles (14, 15).
Estrogen effects on osteoclasts are thought to be mediated indirectly through nonosteoclastic cells. For instance, loss of estrogen at menopause or by ovariectomy is associated with increased secretion of IL-1, IL-6, and TNF-α from the peripheral blood monocytes, bone marrow stromal cells, or osteoblasts, and decreased expression of TGF-β in bone (16–19). Elevated levels of these factors result in increased osteoclastogenesis (20). Moreover, Hughes et al. (21) recently reported that estrogen promoted apoptosis of murine osteoclast–like cells mediated by TGF-β in a mixed cell population in culture. Using a recently developed technique for isolation of highly purified authentic osteoclasts, we showed in this study the direct effects of estrogen on osteoclastic bone-resorbing activity and osteoclast apoptosis. These estrogen effects on osteoclasts were blocked by treatment with antiestrogens. Moreover, Northern blot analysis demonstrated abundant estrogen receptor α (ERα)1 mRNA expression in isolated osteoclasts, suggesting that estrogen may also have a direct impact on osteoclasts (22– 24). The data presented here indicate that estrogen inhibits osteoclastic bone resorption activity in part by targeting osteoclasts directly to undergo apoptosis through ER-mediated mechanisms.
Materials and Methods
Osteoclast Preparation.
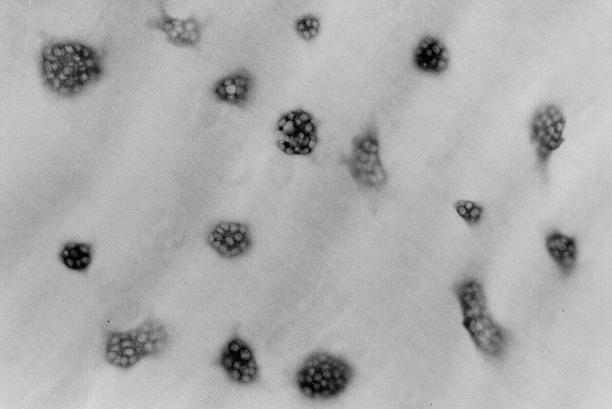
Purified rabbit osteoclasts were prepared by the method of Kakudo et al. (25) from unfractionated bone cells obtained according to the procedure described by Takada et al. (26). Briefly, cell suspensions obtained from minced long bones of 10-d-old rabbits (Japan White; Saitama Experimental Animals Supply Co., Saitama, Japan) were agitated by vortexing and plated in 10-cm tissue culture dishes (Becton Dickinson Labware, Lincoln Park, NJ) coated with 0.24% collagen gel (Nitta Gelatin Co., Tokyo, Japan). After a 3-h incubation, adherent nonosteoclast cells were removed from the collagen gel by sequential treatment with 0.001% pronase E and 0.01% collagenase (Wako Pure Chemical Industries, Osaka, Japan). The remaining osteoclasts were then collected by 0.1% collagenase solution treatment and replated. When these cell suspensions were seeded onto tissue culture dishes, osteoclasts attached and spread out on the dishes. By staining these cells for tartrate-resistant acid phosphatase (TRAcP, a marker of osteoclasts) activity using a leukocyte acid phosphatase kit (Sigma Chemical Co., St. Louis, MO) after a 2-h incubation, we estimated that the purity of the TRAcP-positive multinucleate cells (>3 nuclei) was >99% (Fig. 1). When the cells harvested from collagen gels were cultured on dentine slices, they formed resorption pits as judged by scanning electron microscopic observation (25). We used these pure osteoclast cell suspensions for all experiments.
Figure 1.
Purified osteoclasts harvested from collagen gels. 2,000 osteoclasts were plated on a dentine slice and stained for TRAcP activity after a 2-h incubation. (×100).
Assay for Osteoclastic Bone-resorbing Activity.
In an assay to evaluate osteoclastic bone-resorbing activity, resorption pits formed by osteoclasts on dentine slices were measured for area, and their number was counted. Briefly, purified osteoclasts were replated onto dentine slices (circular, 6 mm in diameter) in each well of 96-well plates (Becton Dickinson) at 150 cells/well and cultured for 24 h in phenol red–free α-MEM (Life Technologies, Inc., Grand Island, NY) supplemented with 0.1% BSA (Sigma Chemical Co.). Dentine slices were then brushed with a rubber policeman to remove cells after observation under a fluorescence microscope, and stained with acid hematoxylin (Sigma Chemical Co.) for 5 min. Total number of pits (reflecting active osteoclast number) was counted under a light microscope, and total area of resorption pits (reflecting bone-resorbing activity) was quantified by densitometric analysis of images of the whole area of dentine slices put into a computer. 17β-Estradiol (E2) and ICI164,384 (ICI) were provided by Shionogi Pharmaceuticals Inc. (Osaka, Japan) and Dr. A.E. Wakeling (ZENECA Pharmaceuticals, Cheshire, UK), respectively. Tamoxifen (TAM) was obtained from Sigma Chemical Co. At the point of replating, agents were added to the cultures at the desired concentrations. At the end of the culture period, osteoclasts were fixed with formalin–acetone solution (37% formalin: acetone: 18 mM citric acid/19 mM Na Citrate/ 12 mM NaCl; 7:65:25) and stained for TRAcP activity to determine the osteoclast number on the dentine slices.
Osteoclast Apoptosis.
The method used to detect osteoclast apoptosis by fluorescence microscopy was described previously (27). After treatment with E2, recombinant human TGF-β1 (R&D Systems, Minneapolis, MN) or salmon calcitonin (CT; 28), cells were fixed with 2% glutaraldehyde solution (Wako Pure Chemical Co.) for 10 min, and stained with 0.2 mM Hoechst 33258 for visualization of chromatin condensation under a fluorescence microscope (VANOX AHB-LB, Olympus Co., Tokyo, Japan). Transmission electron microscopy was performed as follows. After fixation of osteoclasts on dentine slices with 2% glutaraldehyde solution for 1 h, the cells were immersed in 0.16 M EDTA (pH 7.2) and 0.2 M sucrose at 4°C for 2 wk to decalcify the dentine slices (29), and postfixed in 1% osmium tetraoxide solution (Electron Microscopy Science, Fort Washington, PA) for 1 h. After dehydration in graded ethanol solutions, the cells were embedded in epoxy resin. Sections were cut, stained with 4% uranyl acetate, and examined under a transmission electron microscope (JEM-100CX, JEOL).
Northern Blot Analysis.
For assessment of the mRNA expression of osteoclast-specific genes (cathepsin K [Cat K] and carbonic anhydrase II [ECA II]), total RNA was extracted by the AGPC method (30) from osteoclasts cultured on dentine slices in phenol red–free α-MEM supplemented with 0.1% BSA with or without 0.1 nM E2 for 6 or 24 h, and for examination of the mRNA expression of ERs, osteoclasts isolated by the treatment with 0.001% pronase E solution (31) were cultured on tissue culture dishes for 3 h in phenol red–free α-MEM supplemented with 0.1% BSA, and then total RNA was extracted by the same method. The RNA was blotted onto a nitrocellulose membrane after formaldehyde agarose gel electrophoresis, and Northern blotting was carried out. 32P-labeled radioactive probes were prepared by the random primer labeling procedure, and the blot was hybridized in 50% formamide/5× SSPE/5× Denhart's reagent/0.2 mg/ml salmon sperm DNA/labeled probe. After hybridization, the membrane was washed under stringent washing conditions (0.1× SSPE/0.1% NaPPi/1% SDS) at 65°C before autoradiography. The hybridization probes were rabbit Cat K cDNA (30), rabbit CA II cDNA, and human ERα cDNA, all of which were obtained from American Type Culture Collection (Rockville, MD). A rat ERβ cDNA was cloned by RT-PCR method from rat testes. A human β-actin cDNA probe was used as a reference.
TUNEL Assay.
To detect in situ DNA fragmentation of apoptotic osteoclasts, we employed the TUNEL (TdT-mediated dUTP-biotin nick end-labeling) assay by using a TACS Blue Label™ kit (Trevigen, Inc., Gaithersburg, MD) according to the procedure recommended by the manufacturer.
Results and Discussion
Estrogen Directly Impacts Osteoclast Function.
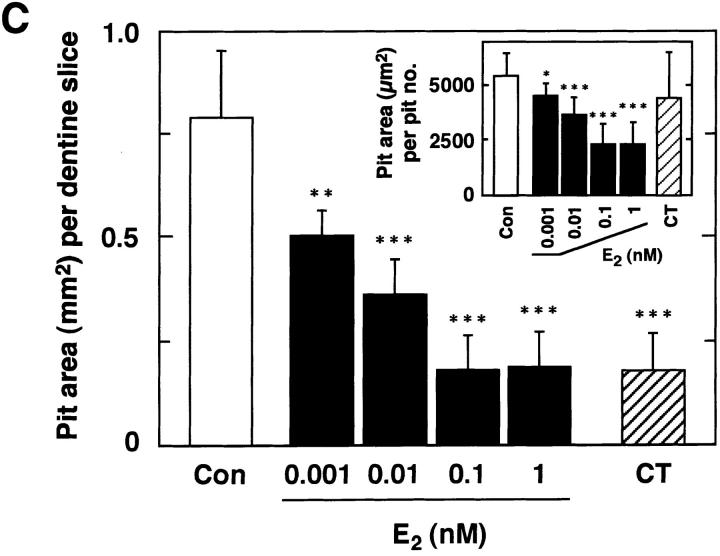
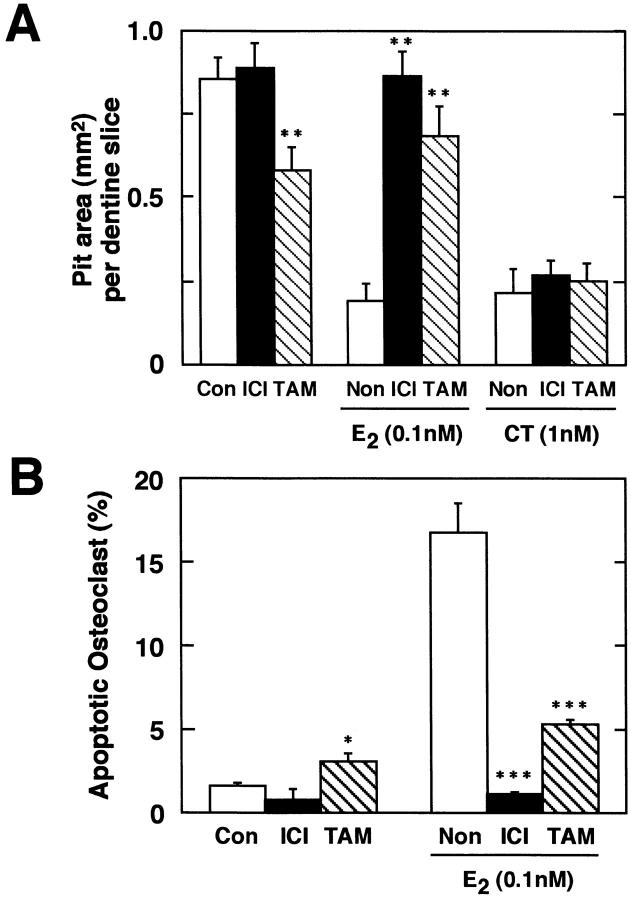
Direct reduction of osteoclastic bone resorption by E2 started within 6 h of treatment (data not shown), and great reduction was observed by 24 h (Fig. 2, A–C). The ratio of pit area per pit number was reduced by the treatment with E2, indicating that E2 inhibition of bone resorption occurred at the level of individual osteoclast activity (Fig. 2 C, inset). The level of inhibition of osteoclastic bone resorption by E2 was comparable to that obtained for calcitonin (Fig. 2 C).
Figure 2.
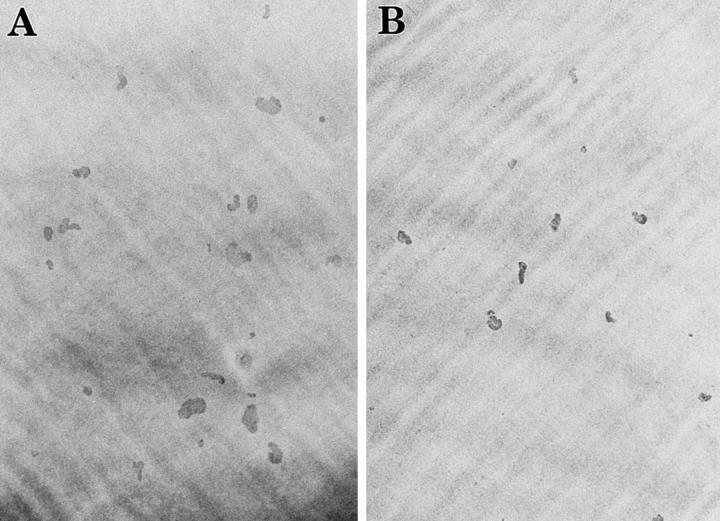
Estrogen inhibition of bone resorption by purified rabbit osteoclasts. Purified osteoclasts were incubated with medium (A) lacking or (B) containing 0.1 nM E2 for 24 h on dentine slices (×40). After removal of osteoclasts, resorption pits formed by osteoclasts were stained with acid hematoxylin and observed under a light microscope. (C) Inhibitory effects of E2 on osteoclast-mediated bone resorption. Purified osteoclasts were cultured on dentine slices (150 cells/slice) in medium (Con) or in medium containing 0.001–1 nM E2 (E2) or 1 nM salmon calcitonin (CT). Osteoclastic bone resorption activity was measured in terms of pit area formed by purified osteoclasts after 24 h of incubation. Pit area per pit number is also indicated (inset). Values are means ± SD, n = 4. *P <0.05, **P <0.01, ***P <0.005 compared with control groups. Data are representatives of those obtained in three additional independent experiments.
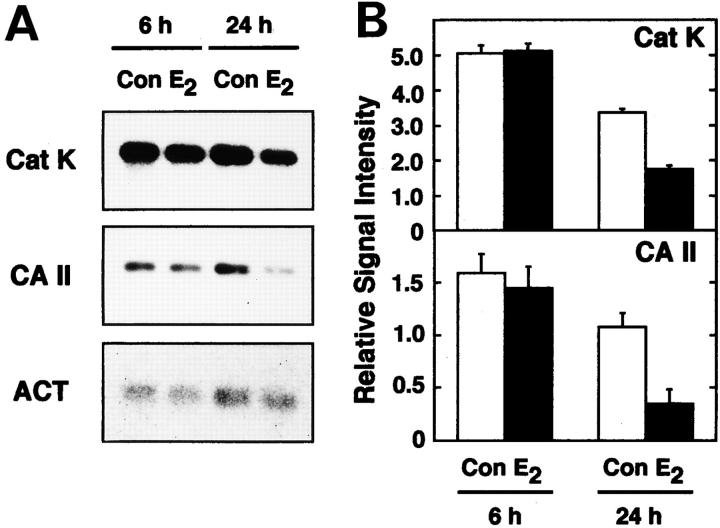
To verify the molecular mechanisms of estrogen-inhibited osteoclastic bone-resorbing activity, we examined whether E2 would affect the mRNA of Cat K and CA II, enzymes involved in the bone-resorbing activity of osteoclasts. E2 reduced mRNA levels of both Cat K and CA II in a time-dependent manner (Fig. 3, A and B). Such reduction was correlated with E2 inhibition of formation of resorption pits by the osteoclasts (data not shown). These data corresponded to the results that E2 inhibition of bone resorption occurred at the level of individual osteoclast activity (Fig. 2 C, inset).
Figure 3.
Negative regulation of mRNA levels for Cat K and CA II by estrogen. (A) Northern blot analysis. Total RNAs (3 μg) from ∼25,000 purified osteoclasts cultured on dentine slices (150 cells/dentine slices) with (E2) or without 0.1 nM E2 (Con) for 6 or 24 h were used as samples. (B) Relative abundance of Cat K and CA II mRNAs. The relative abundance of Cat K and CA II mRNAs on Northern blotting was evaluated from the values of densitometric scanning. The values shown were normalized with respect to the abundance of β-actin (ACT), and expressed as means ± SD of samples from three other independent experiments.
Estrogen Directly Promotes Osteoclast Apoptosis.
To understand the mechanism of estrogen-induced osteoclast inactivation, we evaluated the morphological changes in osteoclasts upon estrogen treatment at the subcellular level. Apoptosis is quite different from necrosis, which is accompanied by cell disintegration, and provides an organized means of cell death to minimize damage to the surrounding cells (32). To examine direct effects of estrogen on osteoclast apoptosis, we observed pure authentic osteoclasts attached to dentine slices in this study, as >90% of the osteoclasts plated remained attached to the dentine slices after 24 h of cultures. Moreover, we confirmed by video monitoring that osteoclasts did not detach easily even during the morphological change to the apoptotic appearance, especially up to 24 h (data not shown). After 24 h of E2 treatment, osteoclasts displayed characteristics of apoptosis, including chromatin condensation and grossly changed cellular and nuclear morphology as detected by fluorescence microscopy (Fig. 4 A). These observations were further confirmed by transmission electron microscopy (Fig. 4 C). E2 treatment did not change the morphology of intracellular organelles of osteoclasts, and cell membranes remained intact (Fig. 4 C). Such features are characteristic of apoptosis, and clearly indicated that the cause of cell death was not necrosis, which is accompanied by cellular disintegration (14, 15). Furthermore, in situ DNA fragmentation in these cells was detected by the TUNEL method (Fig. 4 B). The number of DNA fragment–bearing osteoclasts (TUNEL-positive cells) was correlated with the number of morphological apoptotic osteoclasts detected by Hoechst 33258 staining. In unfractionated bone cells, E2 also induced osteoclast apoptosis without causing changes in nonosteoclastic cells (data not shown).
Figure 4.
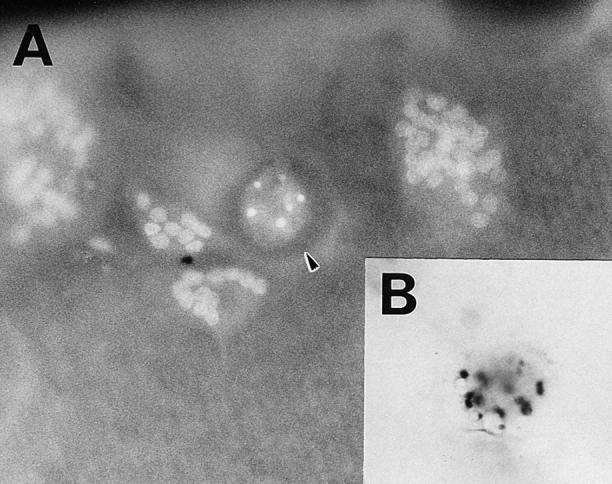
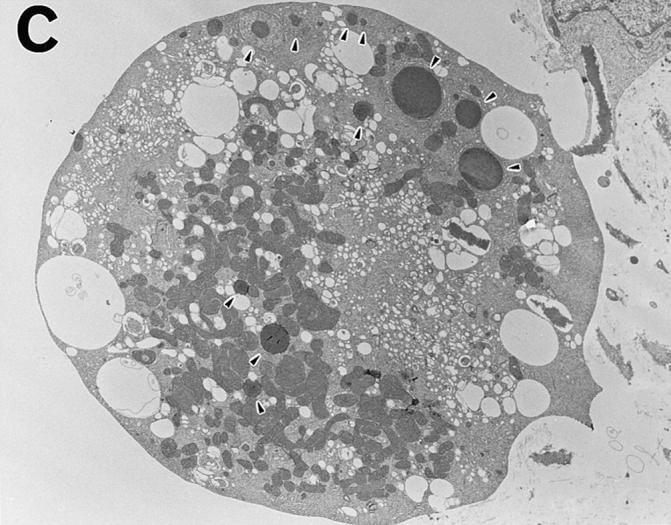
Estrogen-induced osteoclast apoptosis. Osteoclasts were cultured on dentine slices with 0.1 nM E2 for 24 h. (A) A fluorescence micrograph shows normal and apoptotic osteoclasts (arrowhead) attached to a dentine slice in a 24-h culture. ×250. (B) The TUNEL assay indicates DNA fragmentation in an apoptotic osteoclast. ×250. (C) This micrograph, obtained by electron transmission microscopy, demonstrates the gross morphological changes in an apoptotic osteoclast. Nuclear fragments are indicated by the arrowheads. ×2,000.
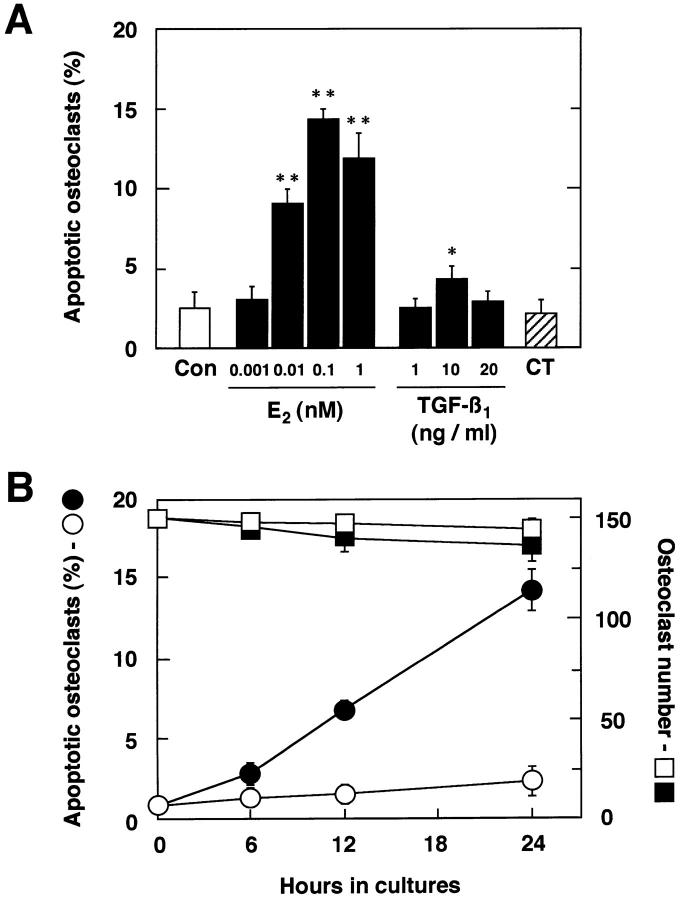
Quantification of E2-induced osteoclast apoptosis showed a dose-dependent increase (Fig. 5 A), which correlated with the dose range of E2 for inhibition of bone resorption (Fig. 2 B). In contrast to estrogen, calcitonin, which inhibits osteoclast activity directly through its receptors on osteoclasts, did not cause osteoclasts to undergo apoptosis, suggesting that inhibition of osteoclastic bone resorption by E2 is mediated by a distinctively different mechanism than that used by calcitonin. As for detached cells, significant cellular disintegration prevented us from detecting osteoclast apoptosis in them in 24-h and older cultures. E2-induced osteoclast apoptosis was also time dependent (Fig. 5 B). E2 (0.1 nM) inhibition of formation of resorption pits by osteoclasts was correlated with a reduction in the expression of mRNAs for Ca K and CA II and with an increased induction of osteoclast apoptosis in a time course study. These findings suggest that E2 directly acts on osteoclasts and inhibits osteoclastic bone resorption by causing osteoclast inactivation partially due to apoptosis. A recent report indicated that estrogen promoted TGF-β–mediated apoptosis of in vitro–developed murine osteoclast-like cells in mixed cell cultures (21). TGF-β1 (10 ng/ml) induces apoptosis of our pure authentic osteoclasts. However, the incidence was lower than that by E2 treatment in our mature cells or that of TGF-β–induced apoptosis in murine mixed cell cultures (Fig. 5 A, reference 21). The causes of this discrepancy might be partially due to their different species; however, these results suggest that estrogen-enhanced osteoclast apoptosis mediated by TGF-β might occur in an indirect manner. The direct induction of osteoclast apoptosis by estrogen besides indirect induction might exist.
Figure 5.
Dose- and time-dependent manner of estrogen-induced osteoclast apoptosis. (A) Purified osteoclasts were cultured on dentine slices (150 cells/slice) for 24 h in medium (Con) or in medium containing 0.001–1 nM E2, 1–20 ng/ml TGF-β1, or 1 nM CT. Apoptotic osteoclasts were quantified under a fluorescence microscope. (B) Time-dependent effects of E2 on osteoclast apoptosis and osteoclast number. Under the same culture conditions, purified osteoclasts were incubated in medium without 0.1 nM E2 (apoptosis: ○; cell number: □) or with 0.1 nM E2 (apoptosis: •, cell number: ▪) for 6, 12, or 24 h. Apoptotic osteoclasts are expressed as a percentage of total number of adherent osteoclasts. Values are means ± SD, n = 4. *P <0.05, **P <0.005 compared with time = 0 groups. Data are representative of those of three additional independent experiments.
Estrogen Inhibits Osteoclastic Bone-resorbing Activity and Promotes Osteoclast Apoptosis Through ER-mediated Mechanisms.
Using a pure or a partial antagonist which can block estrogen effects by binding to the ER, we examined whether these estrogen effects on osteoclasts were ER mediated or not. ICI, a pure antiestrogen, had no effect on pit formation when expressed in terms of pit area, whereas TAM, a partial estrogen antagonist, partially inhibited the resorption activity of purified osteoclasts (Fig. 6 A). When combined with E2, both ICI and TAM reversed the inhibitory effect of E2, indicating that E2 inhibited osteoclastic bone-resorbing activity through ER. In contrast, the inhibitory effect of calcitonin was not affected by either ICI or TAM (Fig. 6 A). Consistent with their lack of, or partial, ability to inhibit pit formation, ICI (0.001–100 nM) did not induce apoptosis of osteoclasts, whereas TAM induced osteoclast apoptosis partially at 1 nM , but not in a statistically significant manner, at 0.001–0.1 nM or at 10–100 nM (Fig. 6 B, data not shown). These contradictory effects of antiestrogens might be due to variety of ERs and coregulators (33, 34). When combined with E2, ICI and TAM blocked E2-induced osteoclast apoptosis as a pure or partial antagonist, respectively (Fig. 6 B, actual number of attached osteoclasts, plated at 150 cells, onto dentine slice after 24 h of culture: control, 143.5 ± 5.2; 1 nM ICI, 139.5 ± 7.3; 1 nM TAM, 141.0 ± 7.8; 0.1 nM E2, 137.0 ± 10.9; 0.1 nM E2 + 1 nM ICI, 141.0 ± 7.2; 0.1 nM E2 + 1 nM TAM, 136.9 ± 7.8). These results suggest that estrogen effects on osteoclasts might be mediated by the ER and not be a consequence of toxicity. Only a high dose of TAM was reported to be able to induce osteoclast apoptosis in rat osteoclasts as well as E2 treatment (35). In this culture system, however, the optimal concentration of TAM for both inducing osteoclast apoptosis and rescuing osteoclasts from undergoing apoptosis by the treatment with E2 was 1 nM, which might be due to partial mimicking of estrogen effects. TAM may act on osteoclasts as a partial agonist rather than as a partial antagonist.
Figure 6.
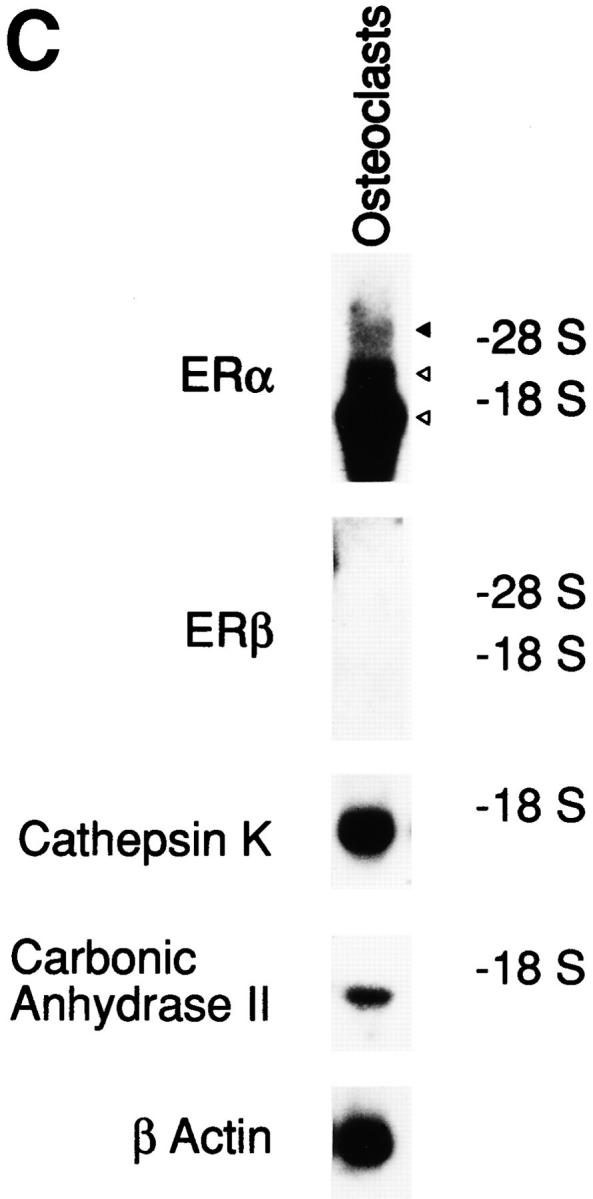
Estrogen affects osteoclasts through ER-mediated mechanisms. (A) Antiestrogens recovered E2-inhibited osteoclastic bone resorption. ICI at 1 nM or 1 nM TAM was added to osteoclast cultures in the presence or absence of 0.1 nM E2 or 1 nM salmon CT. 24 h later, osteoclastic bone resorption activity was measured in terms of pit area. (B) Antiestrogens blocked E2-induced osteoclast apoptosis. Under the same culture conditions, purified osteoclasts were incubated in medium with or without 1 nM ICI or 1 nM TAM in the presence or absence of 0.1 nM E2, and osteoclast apoptosis was measured after a 24-h treatment. Apoptotic osteoclasts are expressed as a percentage of total number of adherent osteoclasts. (C) Northern blot analysis of ERα and ERβ mRNA expression in osteoclasts. Total RNA (50 μg) from ∼5 × 105 purified osteoclasts cultured on tissue culture dishes with 0.1 nM E2 for 6 h were used as samples. Values are means ± SD, n = 4. *P <0.05, **P <0.01, ***P <0.005 compared with control or estrogen-treated groups for A and B. Data are representative of those of three additional independent experiments.
Several isoforms of ERα and one subtype of ER, called ERβ, have been reported (36–38). In rabbit osteoclasts, we previously demonstrated the expression of mRNAs (∼1.5, 2, and 6 kb with the 6-kb mRNA considered to be putative ERα mRNA) which hybridized with ERα cDNA in Northern blots (22). In this study, Northern blot analysis revealed that osteoclasts expressed ERα mRNA, but ERβ mRNA was undetectable (Fig. 6 C). ER cDNA-hybridizing mRNAs besides putative ERα in pure osteoclasts might be isoforms of ERα mRNA or some other mRNA but not ERβ mRNA. These data suggest that rabbit osteoclasts might be controlled by estrogen through ERαs.
In conclusion, the data presented in this study demonstrate that in addition to the indirect effects of estrogen on bone resorption mediated by soluble factors secreted from nonosteoclastic cells and cell-to-cell contact with nonosteoclastic cells, estrogen can function directly on osteoclasts to inhibit their bone-resorbing activity. The ability of estrogen to induce apoptosis of osteoclasts at correlative concentrations effective for inhibition of bone resorption may be the mechanism underlying such effects. The effects of the antiestrogens also suggest that osteoclast apoptosis induced by estrogen is mediated by the ER. These findings, therefore, may shed new light on our understanding of the cellular mechanism by which estrogen provides protective effects on the skeleton, and support the validity of using estrogen replacement therapy for treating postmenopausal osteoporosis.
Acknowledgments
We thank Ms. Yoko Katagiri and Ms. Sachiko Ishii for technical assistance and preparation of the manuscript, respectively. We are also grateful to Ms. Yumiko Kanda for her assistance with the transmission electron microscopy.
Footnotes
Dr. T. Kameda and Dr. H. Mano contributed equally to this work.
Abbreviations used in this paper: CA II, carbonic anhydrase II; Cat K, cathepsin K; CT, calcitonin; E2, 17β-estradiol; ER, estrogen receptor; ICI, ICI164,384; TAM, tamoxifen; TRAcP, tartrate-resistant acid phosphatase; TUNEL, TdT-mediated dUTP-biotin nick end-labeling.
References
- 1.Lindsay R, Hart DM, Aitken JM, MacDonald EB, Anderson JB, Clarke AC. Long-term prevention of postmenopausal osteoporosis by oestrogen. Evidence for an increased bone mass after delayed onset of oestrogen treatment. Lancet. 1976;1:1038–1041. doi: 10.1016/s0140-6736(76)92217-0. [DOI] [PubMed] [Google Scholar]
- 2.Ettinger B, Genant HK, Cann CE. Long-term estrogen replacement therapy prevents bone loss and fractures. Ann Intern Med. 1985;102:319–324. doi: 10.7326/0003-4819-102-3-319. [DOI] [PubMed] [Google Scholar]
- 3.Heaney RP, Recker RR, Saville PD. Menopausal changes in bone remodeling. J Lab Clin Med. 1978;92:964–970. [PubMed] [Google Scholar]
- 4.Selby PL, Peacock M, Barkworth SA, Brown WB, Taylor GA. Early effects of ethinyloestradiol and norethisterone treatment in post-menopausal women on bone resorption and calcium regulating hormones. Clin Sci (Lond) 1985;69:265–271. doi: 10.1042/cs0690265. [DOI] [PubMed] [Google Scholar]
- 5.Vaes G. Cellular biology and biochemical mechanism of bone resorption. A review of recent developments on the formation, activation, and mode of action of osteoclasts. Clin Orthop Relat Res. 1988;231:239–271. [PubMed] [Google Scholar]
- 6.Dewhirst FE, Stashenko PP, Mole JE, Tsurumachi T. Purification and partial sequence of human osteoclast-activating factor: identity with interleukin 1 beta. J Immunol. 1985;135:2562–2568. [PubMed] [Google Scholar]
- 7.Pfeilschifter J, Chenu C, Bird A, Mundy GR, Roodman GD. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclast-like cells in vitro. J Bone Miner Res. 1989;4:113–118. doi: 10.1002/jbmr.5650040116. [DOI] [PubMed] [Google Scholar]
- 8.Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature (Lond) 1986;319:516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- 9.Lowik CW, van der Pluijm G, Bloys H, Hoekman K, Bijvoet OL, Aarden LA, Papapoulos SE. Parathyroid hormone (PTH) and PTH-like protein (PLP) stimulate interleukin-6 production by osteogenic cells: a possible role of interleukin-6 in osteoclastogenesis. Biochem Biophys Res Commun. 1989;162:1546–1552. doi: 10.1016/0006-291x(89)90851-6. [DOI] [PubMed] [Google Scholar]
- 10.Chambers TJ, Athanasou NA, Fuller K. Effect of parathyroid hormone and calcitonin on the cytoplasmic spreading of isolated osteoclasts. J Endocrinol. 1984;102:281–286. doi: 10.1677/joe.0.1020281. [DOI] [PubMed] [Google Scholar]
- 11.Zaidi M, Datta HK, Moonga BS, MacIntyre I. Evidence that the action of calcitonin on rat osteoclasts is mediated by two G proteins acting via separate post-receptor pathways. J Endocrinol. 1990;126:473–481. doi: 10.1677/joe.0.1260473. [DOI] [PubMed] [Google Scholar]
- 12.Kameda T, Miyazawa K, Mori Y, Yuasa T, Shiokawa M, Nakamaru Y, Mano H, Hakeda Y, Kameda A, Kumegawa M. Vitamin K2inhibits osteoclastic bone resorption by inducing osteoclast apoptosis. Biochem Biophys Res Commun. 1996;220:515–519. doi: 10.1006/bbrc.1996.0436. [DOI] [PubMed] [Google Scholar]
- 13.Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF. Bisphosphonates promote apoptosis in murine osteoclasts in vivo and in vitro. J Bone Miner Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- 14.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;6:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 15.Wyllie AH, Morris RG, Smith AL, Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984;142:67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- 16.Pacifici R, Rifas L, Teitelbaum S, Slatopolsky E, McCracken R, Bergfeld M, Lee W, Avioli LV, Peck WA. Spontaneous release of interleukin 1 from human blood monocytes reflects bone formation in idiopathic osteoporosis. Proc Natl Acad Sci USA. 1987;84:4616–4620. doi: 10.1073/pnas.84.13.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen-Solal ME, Graulet AM, Denne MA, Gueris J, Baylink D, de Vernejoul MC. Peripheral monocyte culture supernatants of menopausal women can induce bone resorption: involvement of cytokines. J Clin Endocrinol Metab. 1993;77:1648–1653. doi: 10.1210/jcem.77.6.8263153. [DOI] [PubMed] [Google Scholar]
- 18.Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, Boyce B, Broxmeyer H, Manolagas SC. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science (Wash DC) 1992;257:88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda T, Shigeno C, Kasai R, Kohno H, Ohta S, Okumura H, Konishi J, Yamamuro T. Ovariectomy decreases the mRNA levels of transforming growth factor-beta 1 and increases the mRNA levels of osteocalcin in rat bone in vivo. Biochem Biophys Res Commun. 1993;194:1228–1233. doi: 10.1006/bbrc.1993.1954. [DOI] [PubMed] [Google Scholar]
- 20.Horowitz MC. Cytokines and estrogen in bone: anti-osteoporotic effects. Science (Wash DC) 1993;260:626–627. doi: 10.1126/science.8480174. [DOI] [PubMed] [Google Scholar]
- 21.Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-β. Nat Med. 1996;2:1132–1136. doi: 10.1038/nm1096-1132. [DOI] [PubMed] [Google Scholar]
- 22.Mano H, Yuasa T, Kameda T, Miyazawa K, Nakamaru Y, Shiokawa M, Mori Y, Yamada T, Miyata K, Shindo H, et al. Mammalian mature osteoclasts as estrogen target cells. Biochem Biophys Res Commun. 1996;223:637–642. doi: 10.1006/bbrc.1996.0947. [DOI] [PubMed] [Google Scholar]
- 23.Oursler MJ, Pederson L, Fitzpatrick L, Riggs BL, Spelsberg T. Human giant cell tumors of the bone (osteoclastomas) are estrogen target cells. Proc Natl Acad Sci USA. 1994;91:5227–5231. doi: 10.1073/pnas.91.12.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oursler MJ, Osdoby P, Pyfferoen J, Riggs BL, Spelsberg TC. Avian osteoclasts as estrogen target cells. Proc Natl Acad Sci USA. 1991;88:6613–6617. doi: 10.1073/pnas.88.15.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kakudo S, Miyazawa K, Kameda T, Mano H, Mori Y, Yuasa T, Nakamaru I, Shiokawa M, Nagahira K, Tokunaga S, et al. Isolation of highly enriched rabbit osteoclasts from collagen gels: a new assay system for bone-resorbing activity of mature osteoclasts. J Bone Miner Metab. 1996;14:129–136. [Google Scholar]
- 26.Takada Y, Kusuda M, Hiura K, Sato T, Mochizuki H, Nagao Y, Tomura M, Yahiro M, Hakeda Y, Kawashima H, Kumegawa M. A simple method to assess osteoclast-mediated bone resorption using unfractionated bone cells. Bone Miner. 1992;17:347–359. doi: 10.1016/0169-6009(92)90785-c. [DOI] [PubMed] [Google Scholar]
- 27.Kameda T, Ishikawa H, Tsutsui T. Detection and characterization of apoptosis in osteoclasts in vitro. Biochem Biophys Res Commun. 1995;207:753–760. doi: 10.1006/bbrc.1995.1251. [DOI] [PubMed] [Google Scholar]
- 28.Iida S, Kakudo S, Mori Y, Matsui M, Magota K, Kitajima Y, Nakamura N, Mano H, Hakeda Y, Azuma H, et al. Human calcitonin has the same inhibitory effect on osteoclastic bone resorption by human giant cell tumor cells as salmon calcitonin. Calcif Tissue Int. 1996;59:100–104. doi: 10.1007/s002239900094. [DOI] [PubMed] [Google Scholar]
- 29.Warshawsky H, Moore G. A technique for the fixation and decalcification of rat incisors for electron microscopy. J Histochem Cytochem. 1967;15:542–549. doi: 10.1177/15.9.542. [DOI] [PubMed] [Google Scholar]
- 30.Tezuka K, Tezuka Y, Maejima A, Sato T, Nemoto K, Kamioka H, Hakeda Y, Kumegawa M. Molecular cloning of a possible cysteine proteinase predominantly expressed in osteoclasts. J Biol Chem. 1994;269:1106–1109. [PubMed] [Google Scholar]
- 31.Tezuka K, Sato T, Kamioka H, Nijweide PJ, Tanaka K, Matsuo T, Ohta M, Kurihara N, Hakeda Y, Kumegawa M. Identification of osteopontin in isolated rabbit osteoclasts. Biochem Biophys Res Commun. 1992;186:911–917. doi: 10.1016/0006-291x(92)90832-6. [DOI] [PubMed] [Google Scholar]
- 32.Walker NI, Harmon BV, Gobe GC, Kerr JF. Patterns of cell death. Methods Achiev Exp Pathol. 1988;13:18–54. [PubMed] [Google Scholar]
- 33.Halachmi S, Marden E, Martin G, Mackay H, Abbondanza C, Brown M. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science (Wash DC) 1994;264:1455–1458. doi: 10.1126/science.8197458. [DOI] [PubMed] [Google Scholar]
- 34.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S-C, Heyman RA, Rose DW, Glass CK, Rosenfeld MG. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 35.Arnett TR, Lindsay R, Kilb JM, Moonga BS, Spowage M, Dempster DW. Selective toxic effects of tamoxifen on osteoclasts: comparison with the effects of oestrogen. J Endocrinol. 1996;149:503–508. doi: 10.1677/joe.0.1490503. [DOI] [PubMed] [Google Scholar]
- 36.Friend KE, Ang LW, Shupnik MA. Estrogen regulates the expression of several different estrogen receptor mRNA isoforms in rat pituitary. Proc Natl Acad Sci USA. 1995;92:4367–4371. doi: 10.1073/pnas.92.10.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inoue S, Hoshino S, Miyoshi H, Akishita M, Hosoi T, Orimo H, Ouchi Y. Identification of a novel isoform of estrogen receptor, a potential inhibitor of estrogen action, in vascular smooth muscle cells. Biochem Biophys Res Commun. 1996;219:766–772. doi: 10.1006/bbrc.1996.0308. [DOI] [PubMed] [Google Scholar]
- 38.Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-Å. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]