Abstract
Interleukin-5 (IL-5) regulates the growth and function of eosinophils. It induces rapid tyrosine phosphorylation of Lyn and Jak2 tyrosine kinases. The role of tyrosine phosphatases in IL-5 signal transduction has not been investigated. In this study, we provide first evidence that SH2 protein tyrosine phosphatase 2 (SHPTP2) phosphotyrosine phosphatase plays a key role in prevention of eosinophil death by IL-5. We found that IL-5 produced a rapid activation and tyrosine phosphorylation of SHPTP2 within 1 min. The tyrosine phosphorylated SHPTP2 was complexed with the adapter protein Grb2 in IL-5–stimulated eosinophils. Furthermore, SHPTP2 appeared to physically associate with β common (βc) chain of the IL-5 receptor (IL-5βcR). The association of SHPTP2 with IL-5βcR was reconstituted using a synthetic phosphotyrosine-containing peptide, βc 605–624, encompassing tyrosine (Y)612. The binding to the phosphotyrosine-containing peptide increased the phosphatase activity of SHPTP2, whereas the same peptide with the phosphorylated Y612→ F mutation did not activate SHPTP2. Only SHPTP2 antisense oligonucleotides, but not sense SHPTP2, could inhibit tyrosine phosphorylation of microtubule-associated protein kinase, and reverse the eosinophil survival advantage provided by IL-5. Therefore, we conclude that the physical association of SHPTP2 with the phosphorylated βc receptor and Grb2 and its early activation are required for the coupling of the receptor to the Ras signaling pathway and for prevention of eosinophil death by IL-5.
Eosinophils play an integral role in the pathogenesis of allergic and parasitic disorders (1). IL-5 is a cytokine that primarily promotes the differentiation of eosinophils from the stem cells and stimulates the survival and function of mature eosinophils (2). This diverse effect of IL-5 on eosinophils has been proposed as the key mechanism for the development of blood and tissue eosinophilia in the course of allergic inflammation.
IL-5R is a member of the hematopoietic receptor superfamily and is composed of a ligand-specific α subunit and shared with IL-3 and GM-CSF β common (βc) subunit (3). Although neither of the receptor subunits contain a kinase-like catalytic domain, IL-5 does induce a rapid and reversible tyrosine phosphorylation of various cellular proteins (4). Recent findings indicate that for IL-5R type, the binding of the ligand results in the activation of cytoplasmic tyrosine kinases of the Jak/Tyk and Src type families (5, 6). Indeed, we have shown that the stimulation of eosinophils with IL-5 results in phosphorylation and activation of the receptor-bound Lyn and Jak2 kinases. The consequence of activation of these tyrosine kinases is the propagation of signal through the ras-raf-1-MEK-MAP (MAP, microtubule-associated protein, MEK, MAP or Erk kinase) kinase pathway and the Jak-STAT pathway. These observations clearly support a major role of protein tyrosine phosphorylation in IL-5–mediated signaling.
Little is known about the function of protein tyrosine phosphatases (PTPs)1 in the signaling process initiated by the receptors of the cytokine receptor superfamily. The PTP Src homology (SH)PTP2, recently designated Src homology 2 phosphatase 2 (SHP2) and also called PTP1D or Syp, is one member of a small family of Src homology 2 (SH2) domain–containing PTPs, which also includes Corkscrew (Csw) and PTP-1C (also called SH-PTP1 or HCP) (7, 8). SHPTP2 contains two SH2 domains and a single catalytic domain. This phosphatase is ubiquitously expressed and found to be tyrosine phosphorylated and activated in response to erythropoietin, platelet-derived growth factor (PDGF), prolactin, IL-3, and GM-CSF (9–12). Upon specific stimulation, SHPTP2 binds to the receptor for PDGF and Epo. Once phosphorylated on tyrosine, SHPTP2 generates a binding site for the adapter protein, Grb2, which in turn may lead to activation of the Ras-signaling pathway (13, 14). Although it has been postulated that PTPs function as the negative regulators of the signal generated by protein tyrosine kinases, several recent studies show that the inactivation of SHPTP2 leads to a decrease in mitogenesis in response to epidermal growth factor, PDGF, and insulin, indicating that this phosphatase may, in some instances, act as a positive signal transmitter (11, 15, 16).
The role of SHPTP2 in the IL-5 signal transduction in eosinophils has not been investigated. In this study we investigated the phosphorylation state, association with other proteins, and catalytic activity of this phosphatase after triggering of the IL-5R. Furthermore, using specific antisense oligodeoxynucleotides, we investigated the role of SHPTP2 in IL-5–mediated prolongation of eosinophil survival.
Materials and Methods
Reagents.
Percoll was purchased from Pharmacia, Inc. (Piscataway, NJ). The mAb against antiphosphotyrosine (clone 4G10) was obtained from Upstate Biotechnology Inc. (Lake Placid, NY). Rabbit polyclonal anti-SHPTP2, anti-Grb2, anti-Erk 2, and monoclonal anti–IL-5βR antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Enhanced chemiluminescence detection system was purchased from Amersham Corp. (Arlington Heights, IL).
Eosinophil Purification.
Peripheral blood for eosinophil purification was obtained from subjects with mild to moderate eosinophilia (6–12%). Eosinophils were isolated by sedimentation with 3% hydroxyethyl starch, followed by centrifugation on discontinuous Percoll gradients according to the method of Gartner, as described previously (17). The cells were further purified by negative selection using anti-CD16 immunomagnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Eosinophils (>99% purity) were then suspended in RPMI 1640 in tubes coated with 3% human serum albumin.
Preparation of Cytosolic Cell Extracts and Immunoprecipitation.
Eosinophils (2–4 × 106/ml) were incubated with IL-5 at a concentration of 10−10 M or medium at 37°C for 5 min. When kinetic studies were performed, IL-5 was added to samples at 0, 25, and 29 min, and all samples were harvested at 30 min, i.e., eosinophils were stimulated for 30, 5, and 1 min, respectively. This way of stimulation allowed us to use one control sample, and all samples could be prepared simultaneously, under the same conditions. The stimulation was terminated by addition of 1 vol of ice-cold PBS containing 1 mM Na3VO4. The cells were pelleted by centrifugation, washed rapidly with PBS, and lysed in a buffer containing 50 mM Tris HCl, pH 7.4, 150 mM NaCl, 1 mM EGTA, 0.25% sodium deoxycholate, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, 0.5% Triton X-100, and 1 μg/ml of aprotinin, leupeptin, and pepstatin. For study of phosphatase activity, Na3VO4 was excluded from PBS and lysis buffer. After an incubation on ice for 10 min, the lysates were passaged several times through a 26-gauge needle, and detergent insoluble materials were removed by centrifugation at 4°C at 12,000 g. The protein concentration was determined using bicinchoninic acid assay (Pierce Chem. Co., Rockford, IL).
The cell lysates were precleared by incubation with 20 μl of the protein A/G agarose Plus (Santa Cruz Biotechnology) for 2 h. After removal of the beads, the lysates were incubated with appropriate Ab (1–5 μg for each sample) and 25 μl of protein A/G Agarose Plus for 4 to 6 h at 4°C. The immunoprecipitates were washed three times with the cold lysis buffer and for SDS electrophoresis, boiled in twofold concentrated Laemmli reducing buffer for 4 min.
Immune Complex Phosphatase Assay.
Immunoprecipitates of SHPTP2 were prepared as described above and washed sequentially 3 times with the cold lysis buffer (without Na3VO4) and 3 times with the phosphatase buffer (50 mM Hepes, pH 7.4, 60 mM NaCl, 60 mM KCl, 0.1 mM PMSF, 1 μM pepstatin, 5 μg/ml aprotinin, 1 μg/ml leupeptin) (15, 18). Phosphatase activity was assayed by resuspending the final pellet in a total volume of 80 μl of reaction buffer (phosphatase buffer, pH 5.5, containing 1 mg/ml bovine serum albumin, 5 mM EDTA, 10 mM dithiothreitol). The reaction was initiated by the addition of para-nitrophenyl phosphate (10 nM, final concentration) for 30 min at 30°C. The reaction was stopped by the addition of 0.9 ml of 1 N NaOH, and the absorbance of the samples was measured at 410 nm.
Peptide Binding Assay.
A biotinylated and tyrosine phosphorylated peptide corresponding to the amino acid residues 605–624 of IL-5βR (biotin-PPPGSLE phosphorylated tyrosine (pY)612LCLPAGGQVQLV-NH2) was synthesized by the Quality Controlled Biochemicals, Inc. (Hopkinton, MA). Two control peptides were also synthesized. One control peptide had the same sequence except pY612 was replaced with F. SHPTP2 is likely to bind βc through its SH2 domain. Since SH2 domain binds to pY only but not to Y residues, we used a second control peptide derived from βc 450–465 which contained two nonphosphorylated Y residues: biotin-YGYRLRRKWEEKIPNP. All peptides had a COOH-terminal amide. The peptides were purified by HPLC to >95% purity and evaluated by mass spectrometry for correct molecular weight. The lyophilized peptides were reconstituted in distilled water and then diluted in 100 mM Hepes, pH 7.4. For peptide binding assay we used lysates from promyelocytic human cell line HL-60, which expresses SHPTP2 (CCL 240; American Type Culture Collection, Rockville, MD). These nondifferentiated leukemic cells were maintained in RPMI 1640 with 10% FCS. SHPTP2 immunoprecipitated from HL-60 has no detectable phosphotyrosine and its basal phosphatase activity is comparable to that obtained from nonstimulated eosinophils. HL-60 cell lysates were prepared as described above and precleared with avidin-conjugated agarose beads (Sigma Chemical Co., St. Louis, MO) for 1 h. Aliquots of cell lysates (equivalent to 107 cells) were then incubated with biotinylated peptides (50 μM) for 2–4 h, and subsequently with avidin-conjugated agarose beads (50 μl) for 2 h at 4°C. After the last incubation, the beads were washed five times with the lysis buffer. The beads were suspended in 50 μl of 2 times concentrated electrophoresis buffer, boiled for 4 min, and subjected to electrophoresis and Western blotting with anti-SHPTP2 Ab.
In another set of experiments, HL-60 cell lysates (equivalent of 2 × 107 cells) were incubated with appropriate peptides (100 μM) for 2 h at 4°C, and then immunoprecipitated with anti-SHPTP2 and subjected for phosphatase activity assay as described above.
Gel Electrophoresis and Immunoblotting.
SDS–polyacrylamide gels were prepared according to the Laemmli protocol and used for immunoblotting. The concentration of polyacrylamide was either 7 or 12%, depending on the molecular weight range of the proteins studied. Gels were blotted onto Hybond membranes for Western blotting using the enhanced chemiluminescence system (Amersham Corp.). Blots were incubated in blocking buffer containing 5% BSA in TBST buffer (20 mM Tris base, 137 mM NaCl, made to pH 7.6, and 0.05% Tween 20) for 1 h followed by incubation with the primary Ab (0.1 μg/ml) for 1 h. After washing five times in TBST buffer, blots were incubated for 30 min with a horseradish peroxidase conjugated secondary antibody (0.1 μg/ml) directed against the primary Ab. The blots were developed with the enhanced chemiluminescence substrate according to manufacturer's protocol. In some experiments, blots were reprobed with another Ab after stripping in a buffer of 62.5 mM Tris-HCl (pH 6.7), 100 mM 2-mercaptoheptanol, and 2% SDS at 50°C for 30 min.
Antisense Oligodeoxynucleotides.
Three 20-mer oligodeoxynucleotides (ODNs) were synthesized by Operon Technologies (Alameda, CA) based on sequence information provided by H.E. Broxmeyer (Indiana University Medical Center, Indianapolis, IN). Sequences used were as follows: an antisense (CTCCGCGATGTCATGTTCCT) of SHPTP2 199–219 nucleotides, a sense (GAGGAACATGACATCGCGGA) for the same region, and a nonsense (TGGGTGTGTCCAAGAGAACT). The ODNs were phosphorothioate modified and resuspended in a sterile H2O at 100 μM concentration.
Eosinophil Survival Assay.
Purified eosinophils were suspended at 106/ml in RPMI 1640 without FCS and cultured in duplicate in the presence of ODNs at concentration of 7.5 μM. After 6 h, eosinophils were counted and cultured with IL-5 (10−10 M) for 2 h. At this time, duplicates were lysed and subjected to immunoblotting for SHPTP2 expression. After treatment with IL-5, cells were washed and resuspended in RPMI 1640 without IL-5, FCS, and ODNs and cultured for another 24 h. The viability of the cultured eosinophils was assessed immediately before and 24 h after IL-5 stimulation. The relative amounts of dead cells were determined by uptake of 5 μM propidium iodide which was analyzed under a fluorescence microscope. In some experiments, cells were lysed after stimulation with IL-5 for 10 min and subjected to immunoprecipitation with an anti–Erk-2 antibody followed by Western blotting with antiphosphotyrosine antibody.
Results
Activation of SHPTP2.
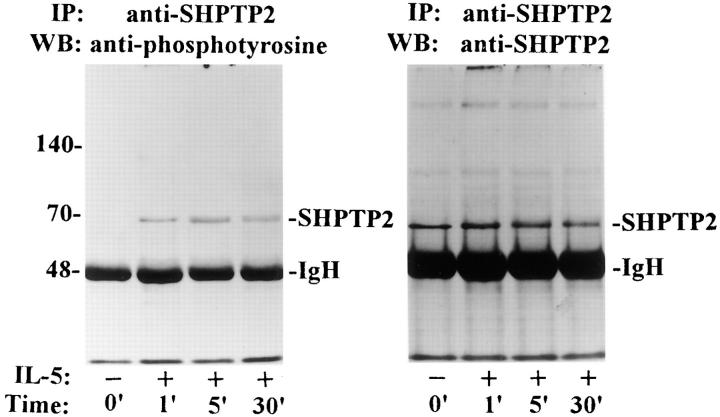
It has been reported that the tyrosine phosphorylation of SHPTP2 is associated with its increased phosphatase activity (19). Therefore, we first tested whether IL-5 induced tyrosine phosphorylation of SHPTP2. In this analysis, eosinophils were stimulated with IL-5 for various time periods and then lysed and immunoprecipitated with a polyclonal anti-SHPTP2 antibody. Western blotting with an antiphosphotyrosine antibody revealed phosphorylation of SHPTP2 as early as 1 min, reaching the highest level 5 min after the stimulation (Fig. 1). This suggests a relatively early involvement of SHPTP2 in the IL-5 signaling pathway. Reprobing the membrane with the anti-SHPTP2 antibody confirmed the presence of equal amounts of the immunoprecipitated protein.
Figure 1.
IL-5 induces tyrosine phosphorylation of SHPTP2 in eosinophils. Eosinophils were incubated with IL-5 as indicated for 1, 5, and 30 min. Cells were then lysed, and the cell lysates were immunoprecipitated (IP) with anti-SHPTP2 antibody. The membranes were Western blotted (WB) with antiphosphotyrosine (left) and anti-SHPTP2 (right) antibodies. The left panel shows tyrosine-phosphorylated SHPTP2 bands in stimulated eosinophils. The right panel confirms the position and amount of SHPTP2. The bottom thick bands are due to the rabbit IgH in the immunoprecipitation.
Next, we assessed whether IL-5 stimulated the enzymatic activity of SHPTP2. SHPTP2 was immunoprecipitated from control or IL-5–treated eosinophils, and the phosphatase activity was measured. The results are shown in Fig. 2. The kinetics of phosphatase bioactivity was similar to the pattern of tyrosine phosphorylation, reaching the peak in 5 min. A significant activity was detectable as late as 30 min after stimulation. A two- to threefold increase in phosphatase activity was consistently observed in three independent experiments.
Figure 2.
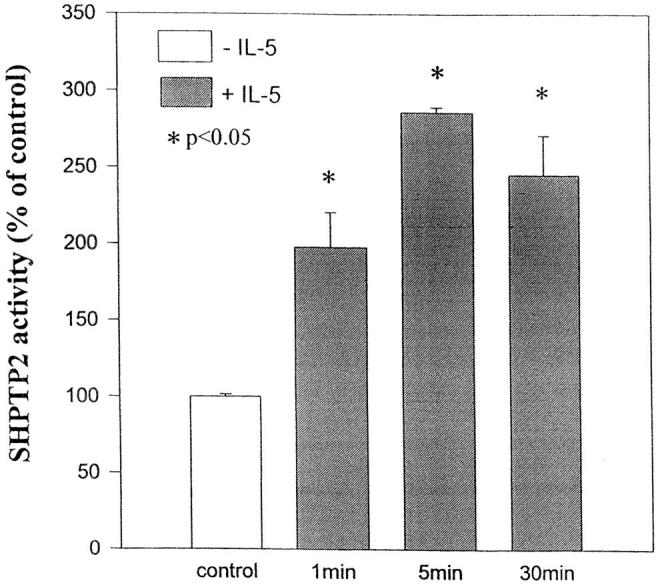
SHPTP2 activity in eosinophils stimulated with IL-5. Eosinophils were stimulated with IL-5 for 0, 1, 5, and 30 min at 37°C. Cell lysates were immunoprecipitated with anti-SHPTP2 antibody, and immunoprecipitates were assayed for phosphatase activity using para-nitrophenyl phosphate as a substrate. Results are the means ± SD of three experiments. *Significantly different from the control at P <0.05 (Student's t test).
Physical Association with Grb2.
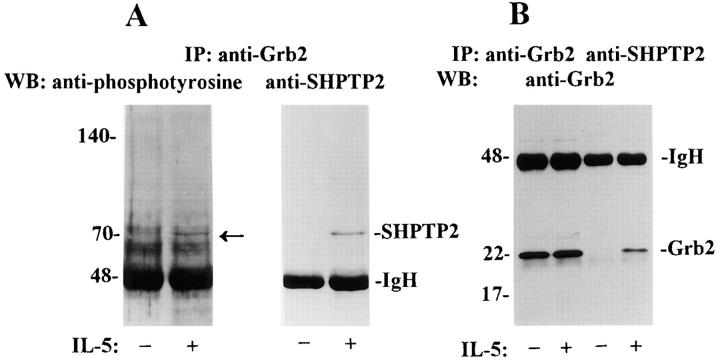
Once phosphorylated on tyrosine, SHPTP2 conforms to the consensus binding site, pYXNX, for the SH2 domain of Grb2 adapter protein (20). Since the binding of Grb2 to SHPTP2 might explain Ras activation after IL-5 stimulation, we asked whether SHPTP2 could bind Grb2 in stimulated eosinophils. To analyze the interaction between SHPTP2 and Grb2, first we performed immunoprecipitation of Grb2 from IL-5– stimulated and control cells followed by immunoblotting with antiphosphotyrosine and anti-SHPTP2 antibodies.
As shown in Fig. 3 A, we detected a band of a 70-kD tyrosine phosphorylated protein in the immunoprecipitate of Grb2 from IL-5–stimulated cells. Reprobing the same membrane with the anti-SHPTP2 antibody confirmed the presence of SHPTP2 in immunoprecipitate of Grb2 (Fig. 3 A). In a next set of experiments, we looked for the presence of Grb2 in the immunoprecipitates of SHPTP2. As shown in Fig. 3 B, Grb2 was detectable in the anti-SHPTP2 immunoprecipitates and predictably, in the anti-Grb2 immunoprecipitate from IL-5–stimulated eosinophils. These experiments suggest a physical association of SHPTP2 and Grb2 that occurs in an IL-5–dependent manner.
Figure 3.
Coimmunoprecipitation of SHPTP2 and Grb2 in IL-5–stimulated eosinophils. (A) Cell lysates from IL-5–stimulated and unstimulated eosinophils were immunoprecipitated (IP) with anti-Grb2 antibody. IL-5 (−) and (+) indicate cells incubated with medium or IL-5 for 5 min, respectively. Immunoblotting with antiphosphotyrosine antibody revealed a 70-kD protein, suggesting the presence of SHPTP2 in the immunoprecipitate of Grb2 (left). Western blotting with anti-SHPTP2 antibody confirmed the identity of the phosphatase. (B) Eosinophils lysates were immunoprecipitated with anti-Grb2 antibody and anti-SHPTP2 antibody. Western blotting with anti-Grb2 antibody revealed the presence of Grb2 in its own immunoprecipitates as well as in immunoprecipitates of SHPTP2 from IL-5–stimulated eosinophils.
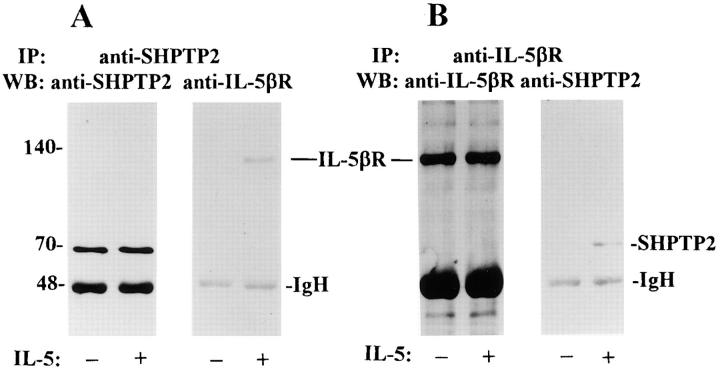
Physical Association with IL-5βcR.
The next set of coimmunoprecipitation experiments was carried out to investigate the physical association of SHPTP2 with IL-5βcR. For this purpose, eosinophil lysates were immunoprecipitated with anti-SHPTP2 antibody and immunoblotted with antireceptor IL-5βc antibody. We found the presence of IL-5βcR in immunoprecipitate of SHPTP2 obtained from IL-5–stimulated cells (Fig. 4 A). In the next set of experiments we looked for the presence of SHPTP2 in immunoprecipitates of IL-5βcR. Western blotting with anti-SHPTP2 antibody revealed the presence of the phosphatase in immunoprecipitate of IL-5βcR (Fig. 4 B). The association between SHPTP2 and receptor appears to be present only in stimulated eosinophils.
Figure 4.
Coimmunoprecipitation of SHPTP2 and IL-5βcR in IL-5– stimulated eosinophils. (A) Eosinophil lysates were immunoprecipitated with a polyclonal anti-SHPTP2 antibody and subjected to Western blot analysis with anti-SHPTP2 (left) and anti–IL-5βcR (right) antibodies. The band of IL-5βcR was present in the immunoprecipitate of SHPTP2 obtained from IL-5–stimulated cells. (B) Anti–IL-5βcR was used to immunoprecipitate lysates from eosinophils treated with (+) and without (−) IL-5 (5 min). The resulting immunoblot was screened with anti–IL-5βcR antibody showing the presence of equal amounts of the receptor and with anti-SHPTP2 antibody revealing the presence of phosphatase in immunoprecipitate of IL-5βcR from stimulated eosinophils. Different intensity of IgH is due to different isotypes of Abs used for immunoprecipitation reacting with Western blotting secondary Ab.
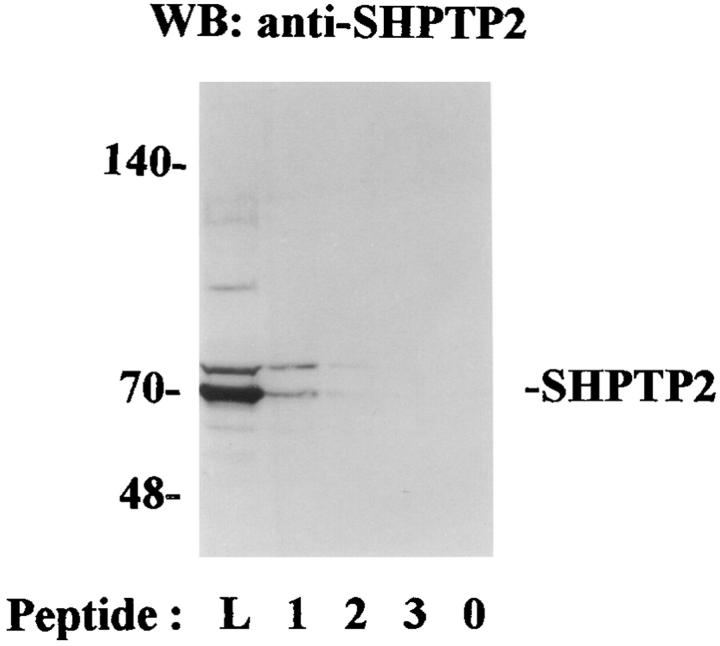
The binding of SHPTP2 with IL-5βcR only in stimulated cells suggests that the physical association occurs through the SH2 domains of SHPTP2. The SH2 domain of SHPTP2 is predicted to bind to a consensus sequence of SXXpYXXL (21, 22). The βc subunit of IL-5R has seven tyrosine residues in cytosolic region and one of the tyrosine residues at position 612 conforms best to the tyrosine binding motif of protein tyrosine phosphatases. We synthesized a tyrosine phosphorylated (pY612) peptide encompassing the residues 605–624 of IL-5βcR. Two peptides served as controls. The first control peptide had the same sequence except the replacement of pY612 with F. Since SH2 domain binds to phosphorylated Y and not to nonphosphorylated Y residues, we used a second control peptide, βc450–465 that contained two nonphosphorylated Y residues. All peptides were biotinylated. For peptide binding experiments, we used lysates from HL-60 cells instead of eosinophils. HL-60 cells constitutively express SHPTP2 and served as a readily available source of the phosphatase. The peptides were incubated with HL-60 cell lysates and the precipitated proteins were blotted with anti-SHPTP2 antibody. As shown in Fig. 5, only the phosphotyrosine-containing peptide bound SHPTP2. This experiment shows that the phosphorylated β receptor can directly bind to SHPTP2 phosphatase.
Figure 5.
The binding of SHPTP2 to pY612 peptide. We synthesized a phosphotyrosine-based ITIM motif-containing peptide (peptide 1) derived from IL-5βcR (residues 605–624). The controls were a nonphosphorylated F612 peptide (peptide 2) and a βc 450–465 peptide (peptide 3). The peptides were biotinylated and used in a peptide binding assay using the HL-60 cell lysate. The bound proteins were precipitated with streptavidin-agarose, followed by immunoblotting with anti-SHPTP2. The first lane, (L, cell lysate) shows the position of the 70-kD SHPTP2. The identity of the upper band is unknown. The buffer control is shown in the last lane (0).
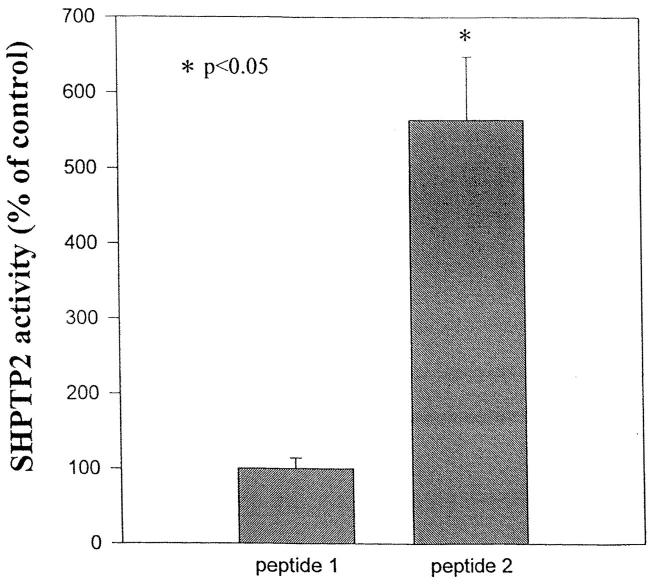
To examine the potential effect of the IL-5βcR binding to SHPTP2, we asked whether addition of the phosphopeptide, βc 605–624 affected SHPTP2 activity. SHPTP2 activity was assayed in the absence or presence of the phosphopeptide. The mutated peptide was used as the control. The addition of the phosphopeptide βc 605–624 strongly stimulated SHPTP2 activity by more than fivefold (Fig. 6) suggesting that binding of the phosphatase alone is responsible for its activation. The mutated βc 605–624 peptide did not activate the phosphatase.
Figure 6.
SHPTP2 activity is stimulated by pY612 peptide. HL-60 cell lysates were incubated with the control F612 (peptide 1) and phosphorylated pY612 peptide (peptide 2), and then immunoprecipitated with anti-SHPTP2 and subjected for phosphatase assay. Shown are the means ± SD of six independent determinations from two separate experiments. *Significantly different from the control (peptide 1) at P <0.05 (Student's t test).
Survival of Eosinophils Treated with SHPTP2 Antisense ODNs.
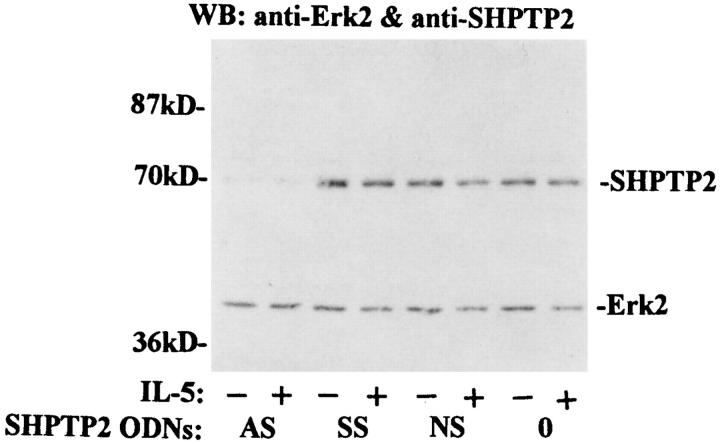
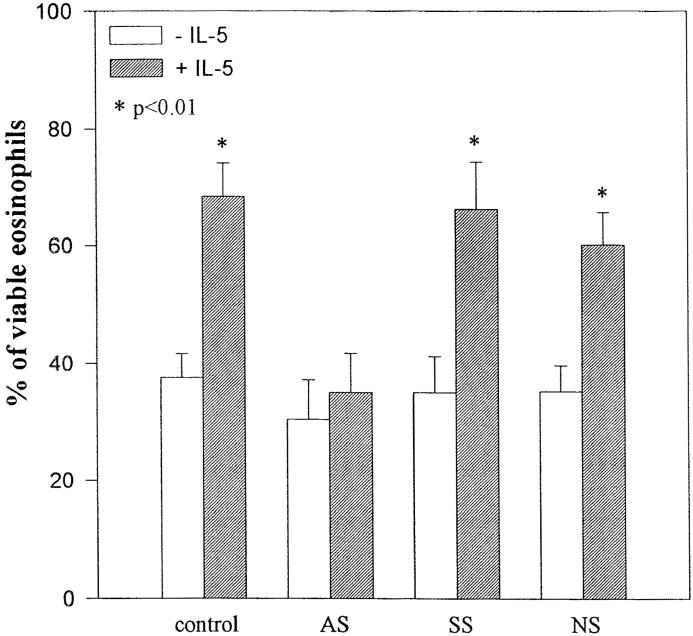
Since eosinophils are terminally differentiated cells with life spans of ∼4 d, the use of antisense oligodeoxynucleotides is the most practical method to specifically alter expression of SHPTP2. Eosinophils were incubated with ODNs without FCS to protect stability of ODNs. As demonstrated in Fig. 7, eosinophils exposed to 7.5 μM antisense ODN for 6 h expressed little or no detectable SHPTP2, whereas sense or nonsense ODNs did not alter SHPTP2 level. Antisense ODNs used in our assay did not alter expression of a closely related phosphatase SHPTP1 (data not shown). The viability of eosinophils assessed at this time (immediately before stimulation with IL-5) always exceeded 90% and was not different from control samples, indicating that at concentration of 7.5 μM the ODNs were not toxic to the cells. After 2 h of stimulation, eosinophils were resuspended in medium without IL-5, ODNs, and FCS. We excluded FCS from this stage of experiment, since in a previous study by Inhorn et al., serum appeared to alleviate the requirement for the Ras pathway for GM-CSF–dependent viability and proliferation in mutant cells with β receptor lacking the 626–763 amino acid residues (23). Viability of the cells cultured without serum was always higher than 55% as assessed 24 h after stimulation with IL-5. In contrast to IL-5–stimulated cells, there was usually <40% viable control (not treated with IL-5) eosinophils at the same time. However, as shown in Fig. 8, SHPTP2 antisense oligonucleotides blocked the ability of IL-5 to prevent eosinophil death (35.0 ± 6.8 versus 66.2 ± 8.0 versus 60.2 ± 5.4% for antisense, sense, and nonsense ODN– treated cells, respectively, P <0.05) indicating critical role of SHPTP2 phosphatase in IL-5–induced survival of eosinophils.
Figure 7.
Effect of SHPTP2 oligodeoxynucleotides on SHPTP2 and Erk2 expression. Whole cell lysates were prepared from eosinophils treated with 7.5 μM SHPTP2 antisense (AS), sense (SS), and nonsense (NS) ODNs or medium (0 ) for 6 h. The lysates with 10 μg of protein/lane were resolved by SDS-PAGE. Anti-SHPTP2 and anti–Erk-2 Abs were used in Western blot analysis to assess expression of proteins. Pretreatment with SHPTP2 antisense ODN, but not with SS or NS, significantly decreased expression of SHPTP2 in eosinophils. Lower p42 band shows equal amounts of Erk2 kinases not affected by treatment with ODNs.
Figure 8.
SHPTP2 antisense oligonucleotides block the survival promoting effect of IL-5. Eosinophils were cultured in the presence of 7.5 μM SHPTP2 antisense (AS), sense (SS), and nonsense (NS) ODNs or medium for 6 h. Cells were then further incubated with or without IL-5 (10−10 M) for 2 h, and then washed and incubated without ODNs and IL-5 for an additional 24 h. No inhibition of eosinophil death by IL-5 was observed in SHPTP2 AS-treated cells. In contrast, SHPTP2 SS- and NS-treated cells demonstrated a significant prolongation of eosinophil survival by IL-5. None of the ODNs at 7.5 μM concentration was toxic to eosinophils. Shown are the means ± SD of five independent experiments. *Significantly different from the cells not treated with IL-5 at P <0.05 (Student's t test).
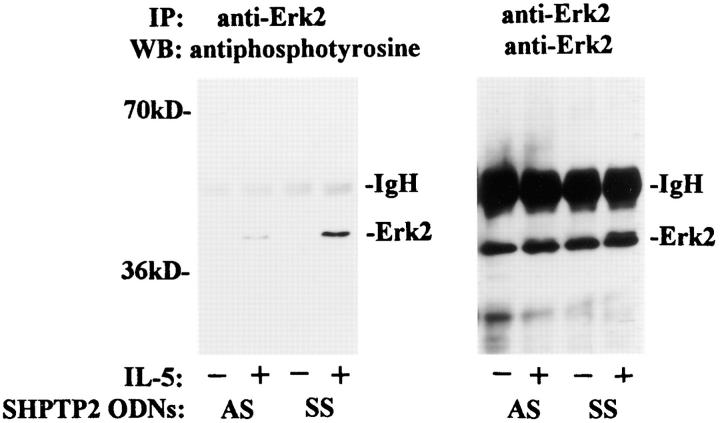
Next we addressed the question of requirements of SHPTP2 phosphatase in IL-5–induced phosphorylation of MAP/Erk2 kinase. As shown in Fig. 9, there was significant reduction in MAP kinase tyrosine phosphorylation in eosinophils that were treated with the antisense ODNs. Since MAP kinase is considered a downstream molecule of the Ras signaling pathway, these results suggest that SHPTP2 plays a positive role in IL-5–induced activation of the Ras-MAP kinase pathway.
Figure 9.
SHPTP2 antisense oligodeoxynucleotides inhibit IL-5– induced tyrosine phosphorylation of MAP/Erk2 kinase. Eosinophils were cultured in the presence of 7.5 μM SHPTP2 antisense (AS) or sense (SS) ODNs for 6 h, and then stimulated with IL-5 for 10 min. The cell lysates were immunoprecipitated with anti-Erk2 Ab, resolved by SDS-PAGE, and immunoblotted by an antiphosphotyrosine mAb (left) followed by reprobing with anti-Erk-2 Ab (right). An increase in tyrosine phosphorylation of Erk2 was observed in eosinophils pretreated with sense ODN, whereas pretreatment with antisense SHPTP2 significantly inhibited IL-5–induced phosphorylation of MAP/Erk2 kinase. The blot is representative of three independent experiments.
Discussion
Previously, we have demonstrated that IL-5 stimulates the phosphorylation and activation of the receptor-bound tyrosine kinases in eosinophils. In this report, we show that the IL-5 stimulation results in the phosphorylation and activation of the protein tyrosine phosphatase, SHPTP2. Others have reported the tyrosine phosphorylation of SHPTP2 in IL-3 and GM-CSF–stimulated murine myeloid cell lines (12). Our data extend these findings by showing that SHPTP2 binds to the βc chain of the IL-5R in human eosinophils. In additional studies, we have demonstrated that the tyrosine phosphorylated βc 605–624 peptide encompassing pY612 binds and activates SHPTP2. There was no binding of SHPTP2 to the same peptide when phosphotyrosine (pY612) was replaced with phenylalanine. This is the first evidence that SHPTP2 directly interacts with the native fragment of βc chain of IL-5R. Finally, we demonstrate the requirement of the SHPTP2 for IL-5–induced phosphorylation of MAP/Erk2 kinase and prolongation of eosinophil survival.
Specific domains within the βc receptor may contain information necessary to initiate distinct events such as proliferation or differentiation through propagation of distinct signaling pathways. Previous studies using a series of truncated mutants revealed that the cytoplasmic domain of βc contained two functional regions required for signaling (24). The membrane proximal region (amino acid residues 456– 517) was shown to be essential for proliferation, activation of Jak2 and pim-1, and induction of c-myc. A second domain (amino acid residues 627–763) was found to be necessary for activation of Shc, p21 Ras, Raf-1, and MAP kinase as well as for induction of c-fos and c-jun. It is noteworthy that site-directed mutagenesis of tyrosine 750 to phenylalanine revealed a viability defect of mutated cells comparable to deletion of the entire 627–763 domain (23). In a recent work, Itoh et al. attempted to find the functional region of the βc responsible for activation of Ras and induction of c-fos promoter (25). They found that GM-CSF–induced tyrosine phosphorylation of two signaling molecules, Shc and SHPTP2, functioning in Ras activation. As tyrosine phosphorylation of Shc was abrogated by the single substitution at Y577 of the full-length βc receptor, Y577 has an essential role in Shc activation. In contrast to Shc, both β589 truncated and β wild with Y577→ F mutation, were capable of inducing tyrosine phosphorylation of SHPTP2, indicating that activation of the phosphatase is mediated by multiple sites, including Y577 as well as by other functional sites located COOH-terminal to amino acid 589.
In our study, we found that βc coprecipitates with SHPTP2, suggesting that physical association of these molecules occurs in IL-5–stimulated eosinophils. The direct association of SHPTP2 and βc seems reasonable since the synthetic 20-mer peptide, βc605–624 containing a phosphorylated tyrosine (Y612), was able to bind SHPTP2. However, the exact binding site for SHPTP2 on βc receptor remains to be clarified. There are four tyrosine residues (Y612, Y750, Y806, and Y869) located COOH-terminal to amino acid 589 on βc receptor surrounded by amino acid residues partially matching a consensus sequence of Y(I/V)Xaa(V/I/L/P) that favors the binding of the NH2-terminal SH2 domain of SHPTP2 (21). We speculate that Y612 and/or Y750 are likely binding sites for SHPTP2, since the sequence surrounding Y612 and Y750 human IL-5βcR conform to the so-called immunoreceptor tyrosine-based inhibitory motif (ITIM) sequence motif of (T/S)XX(pY)XXL (22). Synthetic phosphopeptides representing the ITIM sequence have been shown to bind tyrosine phosphatases SHPTP1 and SHPTP2 and, what is of much interest, this binding results in dramatic increase in their phosphatase activity (26). Indeed, we have shown that addition of a phosphotyrosine peptide comprising the region around pY612 stimulates SHPTP2 activity four- to sixfold, whereas the nonphosphorylated peptide has no stimulatory effect. The phosphorylated βc 612–624 reported here is the first native peptide derived from βc that potently stimulates SHPTP2 phosphatase activity.
The current model of the IL-5/IL-3/GM-CSF signaling pathway predicts that the stimulation of the receptor by the ligand causes the activation of the receptor bound tyrosine kinases. The activated tyrosine kinases then tyrosine phosphorylate βc receptor. Tyrosine kinases bound to box 1 (W458–P465) are likely to be responsible for this event, since deletion of this motif results in loss of tyrosine phosphorylation of βc (25). Indeed, in mutant cells lacking Jak2 kinase, there was no phosphorylation of βc in response to GM-CSF. Moreover, these cells failed to phosphorylate SHPTP2 (27). We believe that one of the phosphorylated tyrosines on βc creates the binding site for the SH2 domain of SHPTP2. It is possible that SHPTP2 binds to more than one phosphorylated tyrosine residue, and which one is critical for SHPTP2 activation remains to be determined. The occupancy of SH2 domains results in an increase in phosphatase activity, presumably due to the induction of conformational changes in SHPTP2 (28). The role of increased phosphatase activity of SHPTP2, as well as its target molecule remains unclear. One possible target for activated SHPTP2 phosphatase is βc. Upon βc binding, activated SHPTP2 might attenuate the βc signal by dephosphorylating the receptor, receptor-associated proteins, or itself. However, the observation that SHPTP2 remains associated with the phosphorylated IL-5βcR might argue against this model. We did not observe dephosphorylation of βc, SHPTP2, Jak2, and Lyn immunoprecipitated from IL-5–stimulated cells at the time of maximal activation of SHPTP2 (data not shown). An alternative possibility is that SHPTP2 acts as a positive signal transducer by dephosphorylating inhibitory phosphotyrosine residues. Although we did not observe inhibition of Lyn and Jak2 phosphorylation in eosinophils lacking SHPTP2 phosphatase, we cannot exclude other tyrosine kinases as a target of the phosphatase. Feng et al. showed that SHPTP2 becomes constitutively tyrosine phosphorylated in v-Src–transformed cells (29). The authors suggested that SHPTP2 might dephosphorylate c-src on Tyr527, whose phosphorylation blocks tyrosine kinase activity. IL-5 has been shown to activate several tyrosine kinases including members of Src kinases family Lyn and Fyn and tyrosine kinase Syk (5, 30, 31). Moreover, SHPTP2 and βc have been shown to coprecipitate with PI-3 kinase (12). Whether SHPTP2 is involved in regulation of any of these kinases in response to IL-5 remains to be determined.
SHPTP2 is phosphorylated on tyrosine residues after IL-5 stimulation. However, the biological significance of this event as well as its relation to phosphatase activity is not yet clear. Our data predict that the binding to the receptor alone stimulates its catalytic activity. This result is consistent with a model in which IL-5 activates tyrosine kinases and tyrosine kinases phosphorylate several tyrosines within βc. SHPTP2 then binds, via its SH2, to one or two phosphorylated tyrosines, which leads to a conformational change in SHPTP2 and an increase of phosphatase activity. Once bound to the receptor, SHPTP2 becomes a target for receptor-associated tyrosine kinases, perhaps Jak2 or Lyn kinases. It has been postulated that tyrosine phosphorylation of SHPTP2 modulates its interaction with other SH2-containing signaling proteins such as Grb2. Grb2 tends to bind to YXNX motif which is present on SHPTP2 (e.g., Y542 and Y580). The binding of SHPTP2 to Grb2 is inhibited by phosphopeptide containing this motif (32). In our study, SHPTP2 and Grb2 were coimmunoprecipitated by anti-SHPTP2 and anti-Grb2 antibody, respectively. At this time we do not know whether Grb2 also binds to βc receptor. Grb2 is known to bind to nucleotide-exchange protein Sos, which activates Ras. Thus, tyrosine phosphorylation of SHPTP2 may result in recruitment of the Grb2–Sos complex to the membrane, where the substrate for Sos, Ras, is located (13).
Recently, it has been demonstrated that SHPTP2 functions as a positive growth regulator. Microinjection of anti-SHPTP2 antibody results in decreased insulin-mediated mitogenesis (16). Mutant inactive forms of SHPTP2 strongly inhibited the mitogenic signals induced by PDGF, α-thrombin (15), and IFN-α and -β (33). Further, negative mutations of SHPTP2 led to inhibition of prolactin-induced lactogenic signaling (11). Using antisense oligodeoxynucleotides against SHPTP2, we demonstrated a positive role of the phosphatase in IL-5–induced activation of eosinophils. Regardless of the target molecule for SHPTP2, this phosphatase appears to act upstream of Ras-Raf-MAP kinase pathway activated in response to IL-5. Further, an activation of SHPTP2 phosphatase and Ras-Raf-MAP pathway seems to be required for the delay of the death in eosinophils. In a recent paper, Yousefi et al. showed the requirement of Lyn and Syk tyrosine kinases for the prevention of apoptosis by IL-5 and GM-CSF in eosinophils (31). Our identification of SHPTP2 provides a further definition of intracellular signaling pathways activated by IL-5 by showing the requirement of the tyrosine phosphatase for eosinophil survival.
Acknowledgments
This work was supported by grants from National Institutes of Health (RO1 35713) and John Sealy Memorial Foundation. K. Pazdrak was supported by a McLaughlin Foundation Postdoctoral Fellowship, the President's Grant-In-Aid from the American Academy of Allergy, Asthma & Immunology, and the Texas Allergy and Immunology Society Memorial Foundation. T. Adachi is the recipient of a McLaughlin Postdoctoral Fellowship.
Footnotes
Abbreviations used in this paper: βc, β common; ITIM, immunoreceptor tyrosine-based inhibitory motif; MAP, microtubule-associated protein; ODN, oligodeoxynucleotide; PDGF, platelet-derived growth factor; PTP, protein tyrosine phosphatase; SH, Src homology.
References
- 1.Corrigan CJ, Kay AB. T cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992;13:501–503. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- 2.Owen, W.F., Jr., and K.F. Austen. 1994. Cytokine regulation of eosinophil-mediated inflammatory reactions by modulation of eosinophil programmed cell death and subsequent priming for augmented function. In Eosinophils in Allergy and Inflammation. G.J. Gleich and A.B. Kay, editors. Marcel Decker, New York. 239–253.
- 3.Tavernier J, Devos R, Cornelis S, Tuypens T, Van der Heyden J, Fiers W, Plaetnick G. A human high affinity interleukin-5 receptor (IL-5) is composed of an IL-5 specific α chain and a β chain shared with the receptor for GM-CSF. Cell. 1991;66:1175–1180. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- 4.Kanakura Y, Druker B, Connistra SA, Furukawa SA, Torinoko Y, Griffin JD. Signal transduction of the human granulocyte macrophage colony-stimulating factor and interleukin 3 receptors involves tyrosine phosphorylation of a common set of cytoplasmic proteins. Blood. 1990;76:706–711. [PubMed] [Google Scholar]
- 5.Pazdrak K, Schreiber D, Forsythe P, Justement L, Alam R. The signal transduction mechanism of IL-5 in eosinophils: the involvement of lyn tyrosine kinase and the ras-raf1-MEK-MAP kinase pathway. J Exp Med. 1995;181:1827–1834. doi: 10.1084/jem.181.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pazdrak K, Stafford S, Alam R. The activation of the Jak-STAT1 signaling pathway in eosinophils by IL-5. J Immunol. 1995;155:397–402. [PubMed] [Google Scholar]
- 7.Freeman RM, Jr, Plutzky J, Neel B G. Identification of a human src homology 2–containing protein-tyrosine-phosphatase: a putative homolog of Drosophilacorkscrew. Proc Natl Acad Sci USA. 1992;89:11239–11243. doi: 10.1073/pnas.89.23.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad S, Banville D, Zhao Z, Fisher EH, Shen S-H. A widely expressed human protein-tyrosine phosphatase containing src homology 2 domains. Proc Natl Acad Sci USA. 1993;90:2197–2201. doi: 10.1073/pnas.90.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tauchi T, Feng GS, Shen R, Hoatlin M, Bagby GC, Jr, Kabat D, Lu L, Broxmeyer HE. Involvement of SH2-containing phosphotyrosine phosphatase Syp in erythropoietin receptor signal transduction pathways. J Biol Chem. 1995;270:5631–5635. doi: 10.1074/jbc.270.10.5631. [DOI] [PubMed] [Google Scholar]
- 10.Lechleider RJ, Sugimoto S, Bennett AM, Kashishian AS, Cooper JA, Shoelson SE, Walsh CT, Neel BG. Activation of the SH2-containing phosphotyrosine phosphatase SH-PTP2 by its binding site, phosphotyrosine 1009, on the human platelet–derived growth factor receptor. J Biol Chem. 1993;268:21478–21481. [PubMed] [Google Scholar]
- 11.Ali S, Chen Z, Lebrun JJ, Vogel W, Kharitonenkov A, Kelly PA, Ulrich A. PTP1D is a positive regulator of the prolactin signal leading to β-casein promoter activation. EMBO (Eur Mol Biol Organ) J. 1996;15:135–142. [PMC free article] [PubMed] [Google Scholar]
- 12.Welham MJ, Dechert U, Leslie KB, Jirik F, Schrader JW. Interleukin (IL)-3 and granulocyte/macrophage colony-stimulating factor, but not IL-4, induce tyrosine phosphorylation, activation, and association of SHPTP2 with Grb2 and phosphatidylinositol 3′-kinase. J Biol Chem. 1994;269:23764–23768. [PubMed] [Google Scholar]
- 13.Bennet AM, Tang TL, Sugimoto S, Walsh CT, Neel BG. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor β to Ras. Proc Natl Acad Sci USA. 1994;91:7335–7339. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowenstein EJ, Daly RJ, Batzer AG, Li W, Margolis B, Lammers R, Urlich A, Schlesinger J. The SH2- and SH3-containing protein Grb2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70:431–437. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- 15.Rivard N, McKenzie FR, Brondello JM, Pouyssegur J. The phosphotyrosine phosphatase PTP1D, but not PTP1C, is an essential mediator of fibroblast proliferation induced by tyrosine kinase and G protein–coupled receptors. J Biol Chem. 1995;270:11017–11022. doi: 10.1074/jbc.270.18.11017. [DOI] [PubMed] [Google Scholar]
- 16.Xiao S, Rose DW, Sasaoka T, Maegawa H, Burke TR, Jr, Roller PP, Shoelson SE, Olefsky JM. Syp (SHPTP2) is a positive mediator of growth factor–stimulated mitogenic signal transduction. J Biol Chem. 1994;269:21244–21248. [PubMed] [Google Scholar]
- 17.Gartner I. Separation of human eosinophils in density gradients of polyvinylpyrrolidone-coated silica gel (Percoll) Immunology. 1980;40:133–136. [PMC free article] [PubMed] [Google Scholar]
- 18.Klinghoffer RA, Kazlauskas A. Identification of a putative Syp substrate, the PDGFβ receptor. J Biol Chem. 1995;270:22208–22214. doi: 10.1074/jbc.270.38.22208. [DOI] [PubMed] [Google Scholar]
- 19.Vogel W, Lammers R, Juang J, Ulrich A. Activation of phosphotyrosine phosphatase by tyrosine phosphorylation. Science (Wash DC) 1993;259:1611–1614. doi: 10.1126/science.7681217. [DOI] [PubMed] [Google Scholar]
- 20.Songyang Z, Shoelson SE, McGlade J, Olivier P, Pawson T, Bustelo X, Barbacid M, Sabe H, Hanafusa H, Yi T, et al. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, Grb2, HCP, SHC, Syk, and Vav. Mol Cell Biol. 1994;14:2777–2785. doi: 10.1128/mcb.14.4.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Songyang Z, Shoelson SS, Chaudhuri M, Gish G, Pawson T, Haser WG, King F, Roberts T, Ratnofsky S, Lechleider RJ, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 22.Thomas ML. Of ITAM and ITIMs: turning on and off the B cell antigen receptor. J Exp Med. 1995;181:1963–1966. doi: 10.1084/jem.181.6.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inhorn RC, Carlesso N, Durstin M, Frank DA, Griffin JD. Identification of a viability domain in the granulocyte/macrophage colony-stimulating factor receptor β-chain involving tyrosine-750. Proc Natl Acad Sci USA. 1995;92:8665–8669. doi: 10.1073/pnas.92.19.8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato N, Sakamaki K, Terada N, Arai K, Miyajima A. Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common β subunit responsible for different signaling. EMBO (Eur Mol Biol Organ) J. 1993;11:4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh T, Muto A, Watanabe S, Miyajima A, Yokota T, Arai K. Granulocyte-macrophage colony stimulating factor provokes ras activation and transcription of c-fosthrough different modes of signaling. J Biol Chem. 1996;271:7587–7592. doi: 10.1074/jbc.271.13.7587. [DOI] [PubMed] [Google Scholar]
- 26.Pei D, Lorenz U, Klingmuller U, Neel BG, Walsh CT. Intramolecular regulation of protein tyrosine SH-PTP1: a new function for Src homology domains. Biochemistry. 1994;33:15483–15488. doi: 10.1021/bi00255a030. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe S, Itoh T, Arai K. Jak2 is essential for activation of c-fos and c-mycpromoters and cell proliferation through the human granulocyte–macrophage colony-stimulating factor receptor in BA/F3 cells. J Biol Chem. 1996;271:12681–12686. doi: 10.1074/jbc.271.21.12681. [DOI] [PubMed] [Google Scholar]
- 28.Sugimoto S, Wandless TJ, Shoelson SE, Neel BG, Walsh CT. Activation of the SH2-containing protein tyrosine phosphatase, SHPTP2, by phosphotyrosine-containing peptides derived from insulin receptor substrate-1. J Biol Chem. 1994;269:13614–13622. [PubMed] [Google Scholar]
- 29.Feng GS, Hui CC, Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science (Wash DC) 1993;259:1607–1611. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- 30.Appleby MW, Kerner JD, Chien S, Maliszewski CR, Bondadaa S, Perlmutter RM. Involvement of p59fynT in interleukin-5 receptor signaling. J Exp Med. 1995;182:811–820. doi: 10.1084/jem.182.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yousefi S, Hoessli DC, Blaser K, Mills GB, Simon HU. Requirement of Lyn and Syk tyrosine kinases for the prevention of apoptosis by cytokines in human eosinophils. J Exp Med. 1996;183:1407–1414. doi: 10.1084/jem.183.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W, Nishimura R, Kashishian A, Batzer AG, Kim WJH, Cooper JA, Schlessinger J. A new function for a phosphotyrosine phosphatase: linking grb2-Sos to a receptor tyrosine kinase. Mol Cell Biol. 1994;14:509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.David M, Zhou G, Pine R, Dixon JE, Larner AC. The SH2 domain-containing tyrosine phosphatase PTP1D is required for interferon α/β-induced gene expression. J Biol Chem. 1996;271:15862–15865. doi: 10.1074/jbc.271.27.15862. [DOI] [PubMed] [Google Scholar]