Abstract
Interactions between lymphocyte surface receptors and their ligands on vascular endothelial cells regulate the exit of lymphocytes from the circulation. Distinct subsets of mononuclear cells bind to high endothelial venules (HEVs) in different lymphoid organs to a different extent, but the molecular mechanisms behind this selectivity have remained poorly characterized. Here we show that vascular adhesion protein-1 (VAP-1) mediates subtype-specific binding of CD8-positive T cells and natural killer cells to human endothelium. VAP-1–dependent, oligosaccharide-dependent peripheral lymph node (PLN) HEV adhesion under shear was independent of L-selectin, P-selectin glycoprotein ligand 1, and α4 integrins, the known lymphocyte receptors involved in the initial recognition of endothelial cells. PLN HEV adhesion was also critically dependent on peripheral lymph node vascular addressins (PNAds), but lymphocyte L-selectin was absolutely required for PNAd binding. Most lymphocytes relied on both PNAd and VAP-1 in HEV binding. The overlapping function of L-selectin ligands and VAP-1 in PLN introduces a new control point into the lymphocyte extravasation process. Finally, intravital microscopy revealed that VAP-1 is involved in initial interactions between human lymphocytes and endothelial cells in inflamed rabbit mesenterial venules in vivo. In conclusion, VAP-1 is a novel contact-initiating ligand that discriminates between different subpopulations of mononuclear cells and is an appealing target for selective modulation of adhesion of CD8- and CD16-positive effector cells.
Naive lymphocytes travel continuously throughout the body in search of antigens. Blood-borne lymphocytes leave the circulation by binding to the endothelium of specialized postcapillary high endothelial venules (HEVs)1 in lymph nodes, percolate through the tissue stroma, and eventually return to the circulation via efferent lymphatics (1). Virgin lymphocytes can freely traffic through both peripheral lymph nodes (PLNs) and mucosa-associated lymphatic tissues, which represent two functionally distinct recirculatory systems. It has been long known that T cells bind and immigrate 2–5 times better to PLN than do B cells. Conversely, B cells adhere 2–3 times more efficiently than T cells to HEVs in mucosal lymphatic organs. Moreover, regardless of the source, CD8-positive cells bind 1.5– 2.0 times better than CD4-positive cells to PLN HEVs (2– 6). When naive lymphocytes engage their cognate antigen, the migratory properties of the activated lymphocytes change dramatically. They no longer randomly patrol through different lymphatic organs but, instead, selectively extravasate at sites of the original antigenic insult and in related lymphoid tissues (7, 8).
Several classes of adhesion molecules are involved in the extravasation process that is thought to encompass at least three sequential but overlapping steps (7–12). The first stage of lymphocyte extravasation is mediated by the interaction of selectins (C type lectin-like molecules) with their oligosaccharide-based ligands, and it results in tethering and rolling of the lymphocyte along the endothelial cell lining. Reversible contacts and reduction of lymphocyte speed allows local activation of lymphocytes, which leads to triggering of stable adhesion and diapedesis, mainly via integrin-mediated pathways. However, no endothelial (or lymphocyte) determinant has been defined so far that could explain the observed in vitro and in vivo HEV-binding selectivity of different T and B cell subpopulations.
Vascular adhesion protein-1 (VAP-1) is an endothelial molecule that supports lymphocyte binding to HEVs in humans under nonstatic conditions (13). VAP-1 is a dimeric 170-kD sialoglycoprotein preferentially expressed on PLN HEVs as opposed to mucosal ones. Since an anti–VAP-1 mAb inhibits 50–75% of PBL binding to PLN HEVs but never completely abolishes the binding, we addressed the possibility that VAP-1 would only support the HEV adhesion of some leukocyte subtypes. Moreover, because VAP-1 shares many characteristics in expression, function, and structure with the prototype peripheral lymph node vascular addressin (PNAd; 14, 15), we directly compared the PLN adhesion pathways mediated by VAP-1 and PNAd. Finally, we wanted to confirm in a physiological model that VAP-1 functions under shear conditions that are relevant in vivo.
We show here that VAP-1 can bind mononuclear leukocytes in a subtype-selective manner and independent of the known lymphocyte surface receptors mediating initial recognition of endothelial cells in PLN HEVs under nonstatic conditions. We also show in an in vivo rabbit model that blocking of VAP-1 inhibits by 30% the number of initial contacts between unselected human lymphocytes and VAP-1 expressing mesenteric venules. Our results suggest that endothelial binding of L-selectin negative, CD8-positive effector lymphocytes can be selectively interfered by blocking the function of endothelial VAP-1.
Materials and Methods
Antibodies, Cells, and Tissues.
Function-inhibiting mAbs 1B2 against VAP-1 (13), MECA-79 against PNAd (14), WAPS12.2 against P-selectin (16), 1.2B6 against E-selectin, 1G11 against VCAM-1 (17), Dreg-56 against L-selectin (18), HP2/1 against α4 integrin (19), and PL1 against P-selectin glycoprotein ligand-1 (PSGL-1; 20) have been described. A new mouse mAb, 5B11, against human VAP-1 was produced as will be described in detail elsewhere. FITC-conjugated mAbs against CD4, CD8, and CD14 and PE-conjugated mAbs against CD16 and CD19 were purchased from Becton Dickinson (San Jose, CA). Isotype-matched control mAbs were 3G6 against chicken T cells, 2C8 against human CD31, HB116 against human HLA ABC, TIB146 against mouse B220 (all described in reference 21), 4D7 against v6 isoform of human CD44 (produced in our laboratory), FITC-conjugated 3G6, and PE-conjugated L3T4 (PharMingen, San Diego, CA).
Human tonsils were from tonsillectomies and PLN were unaffected nodes removed during surgical operations performed for diagnostic purposes. Normal synovia were from bone donors, and inflamed synovia from rheumatoid arthritis patients.
Normal PBL (either from whole blood or from buffy coats) from healthy adults were isolated by density-gradient centrifugations over Ficoll-Hypaque. PLN-derived IL-2–activated T cell lines were established and cultured as described (22). Tonsil lymphocytes were isolated by mincing the tissue and gently squeezing the lymphocytes through a metal screen. For intravital microscopy, tonsillar lymphocytes were labeled with a membrane-permeable fluorescent dye Calcein–acetoxymethyl ester (Molecular Probes Inc., Eugene, OR, 5 μg/107 cells in RPMI for 30 min at room temperature) and washed twice.
Isolation of Subfractions of Peripheral Blood Mononuclear Cells.
Ficoll-isolated blood mononuclear cells from buffy coats were used as a starting material (1–5 × 108 cells/subtype) for purification of CD4- and CD8- positive T cells, CD19-positive B cells, CD16-positive NK cells, and CD14-positive monocytes. Cells were separated with superparamagnetic beads directly coupled with mAbs against these different leukocyte subtype markers (CD4, CD8, CD14, CD16, and CD19 magnetic-activated cell sorting [MACS] beads, Miltenyi Biotec Inc., Sunnyvale, CA) according to the protocol suggested by the manufacturer. In each case, 20 μl specific beads in 80 μl PBS containing 2% BSA were used for 107 cells. To isolate L-selectin–positive cells, PBL were first reacted with mAb Leu-8 (a nonblocking anti–L-selectin antibody) and then with mouse IgG2a+2b–specific microbeads. Washed cells were subjected to MACS isolation in BS type columns, and the bound cells were collected. For certain experiments, negatively selected (in flow-through) cell populations depleted of CD4- or CD8-positive PBL were isolated. L-selectin negative PBL were isolated similarly using saturating amounts of anti–L-selectin mAb Dreg-56 (2.5 × 108 cells, 500 μg mAb) and rat anti–mouse IgG1 superparamagnetic microbeads. The specificity of the selection was confirmed with immunofluorescence stainings using FITC- or PE-conjugated mAbs against different epitopes as reporters. The purity of every population used for the functional binding assays was at least 95% and the viability of cells was >95%.
HEV-binding Assays.
An in vitro frozen section assay was used since it reproduces the in vivo homing specificity of normal and malignant lymphoid cells (2, 23, 24) and allows studies to be performed under conditions of low shear (rotation) with VAP-1 (and PNAd) displayed in a natural array and context on endothelial cells. The human PLNs used in these studies were histopathologically noninflamed and their HEVs expressed high levels of VAP-1 and PNAd, and low levels of ICAM-1, whereas only a negligible number of E-selectin–positive (1–2%), surface P-selectin–positive (1–2%), and VCAM-1–positive (0-1%) HEVs were encountered. When appropriate, lymphocyte binding to inflamed tonsil and to normal and inflamed synovial samples was measured.
In vitro frozen section–binding assays were modified from a protocol described earlier (25). In brief, 8 μm frozen sections were pretreated with saturating amounts of mAbs against endothelial cell adhesion molecules or with class-matched control mAbs in a 100 μl volume (there is no difference in HEV adhesion of lymphocytes when the tissue has been pretreated with a binding or a nonbinding control; reference 26) for 30 min at 7°C on an orbital shaker operating at 60 rpm. The HEV binding of lymphoid cells was analyzed in three types of assays. (a) Different subpopulations of mononuclear cells isolated with MACS (see above) were washed and filtered. 3 × 106 cells in 50 μl RPMI 1640 medium containing 10% FCS and 10 mM Hepes were applied onto tissue sections. The assay was continued for another 30 min at 7°C with constant rotation. Thereafter, the nonbinding cells were gently tilted off and the adherent cells were fixed to sections overnight in cold PBS containing 1% glutaraldehyde. The number of cells adhering to HEVs was counted from coded samples under dark field microscopy. (b) Ficoll-isolated PBL were labeled with FITC-conjugated anti-CD4 or anti-CD8 mAb for 15 min in RPMI 1640 medium containing 5% FCS, and washed. Thereafter, the HEV-binding assay was performed as described above. The number of FITC-labeled cells was scored from each HEV under epiillumination. (c) When the role of different lymphocyte receptors as a VAP-1 counter structure was evaluated, the cells to be tested were washed and treated with function-blocking mAbs against L-selectin (Dreg-56), PSGL-1 (PL1), α4 integrin (HP2/1), or with control mAbs. In certain experiments, PBL were first treated with O-sialoglycoprotease (2.5 × 107 cells, 50 μl enzyme [from Cedarlane Labs. Ltd., Hornby, Canada] in RPMI 1640, 30 min, 37°C), which destroys mucin-type molecules including PSGL-1 (20, 27, 28). After washings, the cells were used for the HEV-binding assays, in which the tissue sections had been pretreated with anti–VAP-1 and control mAbs as described above. An aliquot of original and O-sialoglycoprotease–treated PBL were used for control immunofluorescence stainings, which revealed the expected loss of sialomucins from the enzyme-treated cells.
All adhesion assays in a–c have been performed 2–5 times using PLN, tonsil, and synovia from at least two different donors. At least 120 HEVs/sample were scored. The results are expressed as percentage of control binding (the number of cells adherent to HEVs in the presence of an appropriate control mAb defines 100% adherence).
L-selectin Transfectants and Ig Chimeras.
Non–HEV-binding mouse pre–B cell L1-2 lymphoma cells stably transfected with a full-length human L-selectin cDNA in a eukaryotic expression vector pMRB101 have been described (15). Purified L-selectin Ig chimera containing the extracellular part of human L-selectin (lectin, epidermal growth factor, and two complement repeat-like domains) fused to human IgG1 hinge, constant heavy 2 and 3 regions, and a control CD4-Ig chimera have been described (29, 30).
Immunostainings.
Surface expression of VAP-1 in inflamed rabbit mesenterial venules was studied by injecting the cross-reacting anti–VAP-1 mAb 5B11 intravenously, killing the animal after 15 min, making frozen sections from the rabbit organs, and subjecting them to a second-stage peroxidase-conjugated rabbit anti–mouse Ig in vitro. The reaction was developed by diaminobenzidine. Thus, a positive reaction is only seen if the mAb has bound to its antigen exposed on the lumenal surface of the vessel in vivo.
Cell suspensions were stained for flow cytometry analyses as described earlier (22). 10,000 cells were analyzed using FACScan® and Lysys II software (Becton Dickinson).
Immunoblottings and Precipitations.
L-selectin and CD4 chimeras were loaded on protein G–beads (100 μg/100 μl beads) overnight at 4°C. After washings, these beads were used to immunoprecipitate antigens from tonsil stromal 1% NP-40 lysates containing 2 mM CaCl2, 2 mM MgCl2, and 2 mM MnCl2 from which Ig were depleted by protein G–Sepharose preincubations. The resultant supernatants were used for SDS-PAGE and immunoblotting using enhanced chemiluminescence. The intensity of the bands was quantified using Microcomputer Imaging Device (MCID; Imaging Res. Inc., Ontario, Canada).
Intravital Microscopy.
New Zealand white rabbits (2.5–2.6 kg) were injected intraperitoneally with 2,000 IU recombinant human IL-1 in 5 ml PBS to induce inflammation and premedicated with loperamide (Imodium, 2 mg, peroral; Janssen Pharmaceutica, Beerse, Belgium) to reduce gut motility 3 h before the surgery. Surgical anesthesia was induced with intramuscular injections of midazolam (Dormicum; 2 mg/kg; Hoffmann-La Roche, Basel, Switzerland) and fentanyl-fluanisone (Hypnorm; 0.3 ml/kg; Janssen Pharmaceutica). Marginal ear vein was cannulated for continuous infusion of physiological saline (6 ml/kg/h by syringe pump, model 22; Harvard Apparatus, S. Natick, MA) and for the maintenance of surgical anesthesia with 10% Hypnorm in aqua (3 ml/kg/h). The animals were mechanically ventilated via a tracheal tube.
Technique and procedures of intravital microscopy were adapted from von Andrian et al. (31) and Ley et al. (32). In brief, the mesentery was gently exteriorized, and a region near the distal ileum and appendix was spread over a special microscope stage. All exposed parts of the gut were covered with saline-soaked gauze and the temperature of the preparate was adjusted to 37°C by a heating lamp. The mesentery was kept moist by continuous superfusion of thermocontrolled 37°C bicarbonate-buffered salt solution (32). Appropriate venules were visualized through a microscope (BX50WI, equipped with water immersion objectives × 10/0.3 and ×20/0.5 UMPlanFl, 3.3 mm working distance, and a FITC/tetramethylrhodamine isothiocyanate filter block; Olympus Corp., Lake Success, NY). After a 15-min stabilization period, a bolus of an isotype-matched negative control mAb 4D7 was given (3.2 mg/kg) and allowed to circulate for 15 min. Thereafter, 5–15 × 108 Calcein-acetoxymethyl ester–labeled human tonsillar lymphocytes were filtered through a 44-μm silk mesh and infused via marginal ear vein. Cells were observed with stroboscopic (50 s−1 Strobex 11360; Chadwick Helmuth, Mountain View, CA) epiillumination and low-level background light and the data were collected with a charge-coupled device camera (model C5405; Hamamatsu Corp., Bridgewater, NJ) using digital video cassette recorder (DHR10000NP; Sony Corp., Montvale, NJ). Thereafter, 3.2 mg/kg of an anti–VAP-1 mAb 5B11 was administered and allowed to bind for 15 min. Then an equal number of fluorescently labeled tonsil lymphocytes were freshly filtered and infused to the animal, and the same vessel segment was recorded again.
The data were analyzed off-line using a slow or frame-by-frame playback mode to assign each labeled lymphocyte either as noninteracting (freely flowing) or interacting. Each video frame was digitized with a custom-made frame grabber and analysis program operating under Windows. The velocities of >50 consecutive lymphocytes were determined by measuring the distance traveled between two or more successive video frames, and the highest velocity was taken as a conservative estimate of centerline blood velocities (VCL; reference 33). The mean diameter of the vessels (dvessel) was measured from images in 10 different locations along the vessel. The Newtonian wall shear rate (γ) was counted as described (31): γ = VCL/1.6 × 1/dvessel × 8 (1/s). The shear stress (τ) acting on interacting lymphocytes in venules was approximated using an estimate of 0.025 Poise for the viscosity (ρ) of blood as: τ = γ × ρ (dyn/cm2; reference 31).
Statistical Analyses.
The HEV-binding results are given as mean ± SEM of all experiments. The statistical significance of the inhibitions seen in HEV-binding assays and in intravital microscopy were analyzed by two-tailed Student's t test.
Results
VAP-1 Selectively Binds CD8-positive T Cells.
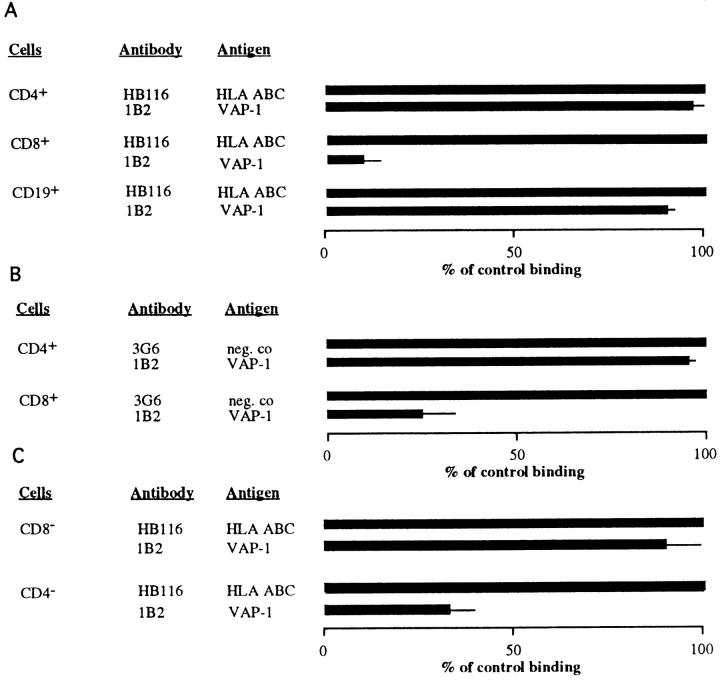
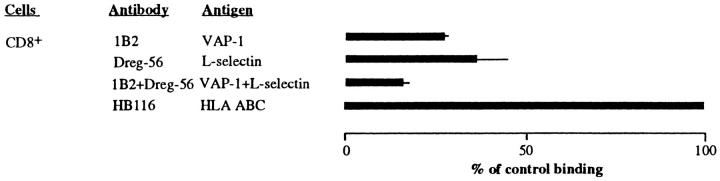
To study the contribution of VAP-1–dependent adhesion among different lymphocyte subpopulations, we immunomagnetically purified CD4- and CD8-positive T cells and CD19-positive B cells and analyzed their PLN HEV adhesion. Each positively selected population was 95–100% pure when analyzed by immunofluorescence stainings and FACS® (data not shown). Moreover, the presence of the minute (50-nm diameter, 8 × 10−6 volume of a lymphocyte) paramagnetic beads on the cells did not functionally interfere with their HEV adherence, since each purified subpopulation bound well to PLN HEVs in the sections pretreated with the negative control mAb. Preincubation of the tissue sections with an anti–VAP-1 mAb 1B2 displayed strikingly different effects on the HEV binding of different lymphocyte subpopulations (Fig. 1 A). Blocking of VAP-1 had no effect on the capacity of CD4-positive T cells or CD19-positive B cells to adhere to PLN HEVs. In contrast, inhibition of VAP-1 function practically abolished all HEV binding of CD8-positive T cells. Thus, among PBL, only CD8-positive T lymphocytes use VAP-1 in PLN HEV adhesion.
Figure 1.
VAP-1 selectively mediates HEV adhesion of CD8-positive lymphocytes. (A) CD4-, CD8-, and CD19-positive PBL were isolated with MACS, and their adherence to PLN HEVs pretreated with a control or an anti–VAP-1 mAb was tested as detailed in Materials and Methods. (B) PBL were labeled with FITC-conjugated anti-CD4 or -CD8 mAbs, and the number of labeled cells adherent to PLN HEVs in the presence of control and anti–VAP-1 mAbs was determined. (C) PBL were subjected to negative selection (removal of CD8-positive cells yielding a CD8-negative population for analysis (CD8 −), or removal of CD4-positive cells yielding a CD4-negative population (CD4 −) and the effect of anti–VAP-1 and control mAb pretreatments on HEV adhesion was determined.
To confirm these results with an independent method, we separately labeled freshly isolated PBL with FITC-conjugated mAbs against CD4 and CD8, and evaluated the HEV binding of the labeled cells (Fig. 1 B). Although CD4-positive cells always dominated in the starting populations, half of the PLN HEV–bound cells were CD8-positive when the target tissue was pretreated with an irrelevant control antibody. When an anti–VAP-1 mAb 1B2 was used, binding of CD8-positive cells was efficiently abrogated, whereas this pretreatment had no effect on the HEV adhesion of CD4-positive PBL. Moreover, binding of negatively selected PBL depleted of CD8-positive cells to PLN HEVs was only marginally affected (10% inhibition) by anti–VAP-1 mAb 1B2, whereas adhesion of PBL depleted of CD4-positive cells was inhibited ∼70% by mAb 1B2 (Fig. 1 C). Thus, analyses with negatively selected PBL confirm the results obtained with positively selected T cells.
VAP-1 Mediates Binding of NK Cells, but Not That of Monocytes.
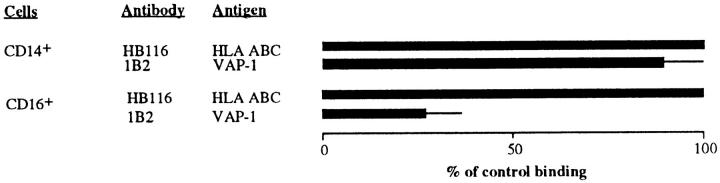
To analyze the behavior of other mononuclear cells, we isolated NK cells and monocytes from blood and analyzed their HEV adhesion. These binding assays were done using inflamed tonsil rather than noninflamed PLNs as the target tissue, since these two leukocyte types are known to extravasate mainly to areas of inflammation. Anti–VAP-1 treatment markedly reduced tonsil HEV adhesion of NK cells, but had no effect on the binding of monocytes (Fig. 2). CD16-positive cells did not adhere at all to vessels in noninflamed nonlymphatic tissue (only two adherent CD16-positive cells were found in 200 vessels in normal synovium), whereas these cells interacted effectively with inflamed vessels in inflamed synovia. Blocking of VAP-1 function abrogated 63 ± 2% of binding of CD16-positive cells to HEVs in inflamed synovia. Thus, only two distinct peripheral blood leukocyte populations, CD8 T cells and NK cells, use VAP-1 as their endothelial ligand.
Figure 2.
NK, but not monocytes, use VAP-1 for endothelial binding. CD14- and CD16-positive PBL were isolated with MACS and their binding to tonsil HEVs pretreated with a control or anti–VAP-1 mAb was tested.
VAP-1 Mediates L-selectin–independent PLN HEV Adhesion.
We have shown earlier that one L-selectin–negative IL-2–activated T cell line can adhere to tonsil HEVs in a VAP-1–dependent manner. However, the applicability of this finding to PBL and the question whether L-selectin can also function as a VAP-1 receptor have not been elucidated. It is also unclear whether VAP-1 mediates L-selectin–independent binding also in PLNs, since HEV adhesion in that organ has been thought to be solely dependent on the function of L-selectin (15).
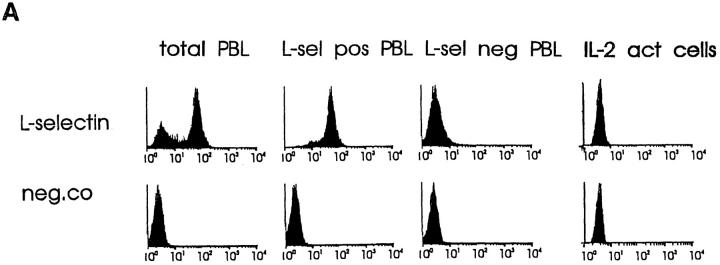
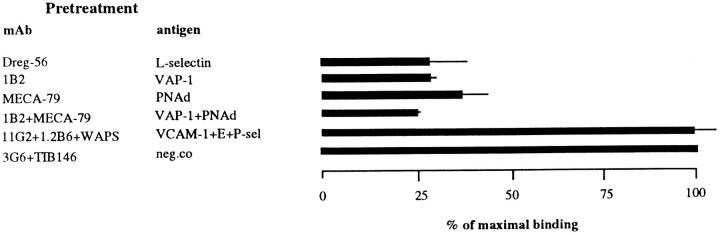
To address these questions, we first used PLN-derived IL-2–dependent human immunoblasts that express no detectable surface L-selectin (Fig. 3 A, last column) and are LFA-1high, α4intermediate, β7intermediate, and CD44high. Intriguingly, these activated T cells bound 1.4 times better than PBL to PLN HEVs (Fig. 3 B and data not shown). As expected, no inhibition of binding was obtained by pretreating the immunoblasts with a function-blocking anti–L-selectin mAb Dreg-56 (Fig. 3 C). mAb MECA-79 pretreatment of PLNs also had essentially no effect on adhesion, confirming the absence of the L-selectin–PNAd pathway in this model. In contrast, mAb 1B2 pretreatment of target tissue abolished >50% of immunoblast binding (Fig. 3 C). Thus, L-selectin is not a prerequisite for adherence of all types of lymphoid cells to PLN HEVs under conditions of low shear, since cells can directly bind to VAP-1.
Figure 3.
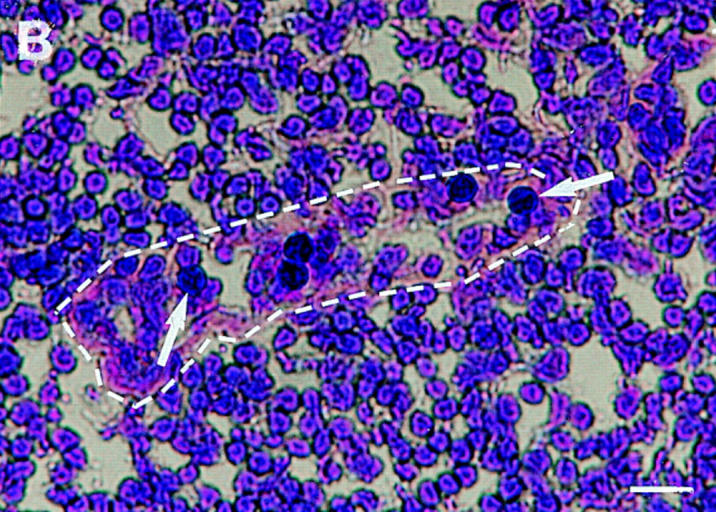
VAP-1 mediates binding of both L-selectin–positive and –negative lymphocytes to PLN HEV. (A) Expression of L-selectin on different lymphocyte populations. Total PBL, immunomagnetically separated L-selectin–positive and –negative subpopulations of PBL, and IL-2–activated T cells were stained for L-selectin expression and analyzed using FACScan®. (B) L-selectin–negative T cells specifically recognize PLN HEVs. Five immunoblasts (black round cells, two pointed out by arrows) binding to a HEV (lilac basement membrane outlined by a dashed line) are shown in the micrograph. Bar, 20 μm. (C) Binding of these different lymphocytes to PLN HEVs after pretreatments with the indicated mAbs was analyzed.
VAP-1 also supported binding of normal, nonactivated, L-selectin–negative PBL to PLN HEVs. These cells (Fig. 3 A) bound to PLN HEVs ∼3 times less efficiently than unseparated PBL. Importantly, mAb 1B2 inhibited >55% of this adhesion (Fig. 3 C). In contrast, mAb MECA-79 had no effects. Thus, a VAP-1–dependent pathway mediates the adherence of L-selectin negative (activated and memory) PBL to PLN HEVs under nonstatic conditions.
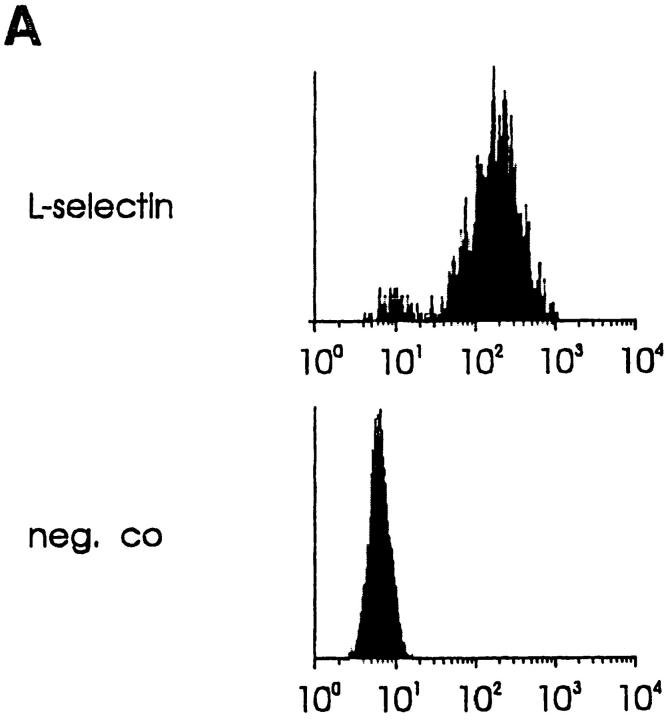
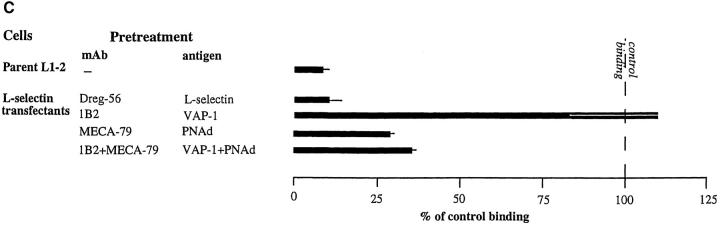
Binding of an immunomagnetically separated L-selectin– positive subpopulation of PBL to PLNs was also efficiently blocked by mAb 1B2 pretreatment (Fig. 3 C). Therefore, we determined whether L-selectin, although not necessary for lymphocyte binding to VAP-1, can also function as a receptor of VAP-1. The high level of surface expression of human L-selectin on mouse pre–B cell lymphoma L1-2 transfectants (Fig. 4 A) was enough to confer on these transfectants a strong ability to bind to human PLN HEVs (Fig. 4 B). This adherence was completely inhibitable by anti–L-selectin mAb Dreg-56 pretreatment of lymphocytes and >70% was also abrogated by mAb MECA-79 pretreatment of the endothelial cells (Fig. 4 C). In contrast, the binding of L-selectin transfectants was completely unaffected by mAb 1B2 treatment (Fig. 4 C). Hence, L-selectin expression is sufficient to mediate binding of lymphoma cells to PLN HEVs, but this binding is entirely independent of VAP-1 function.
Figure 4.
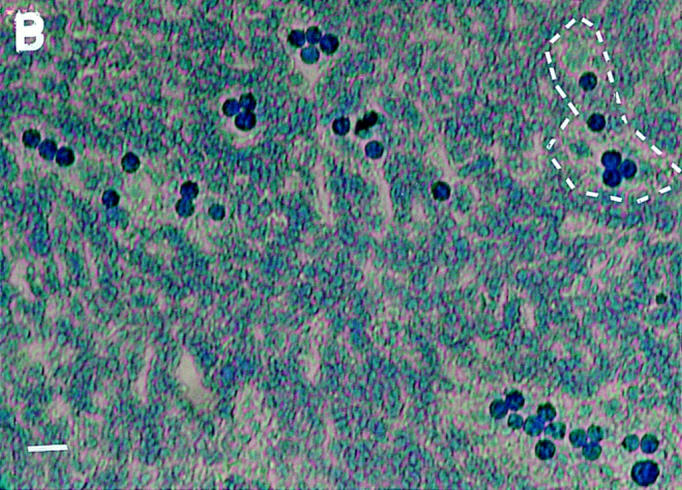
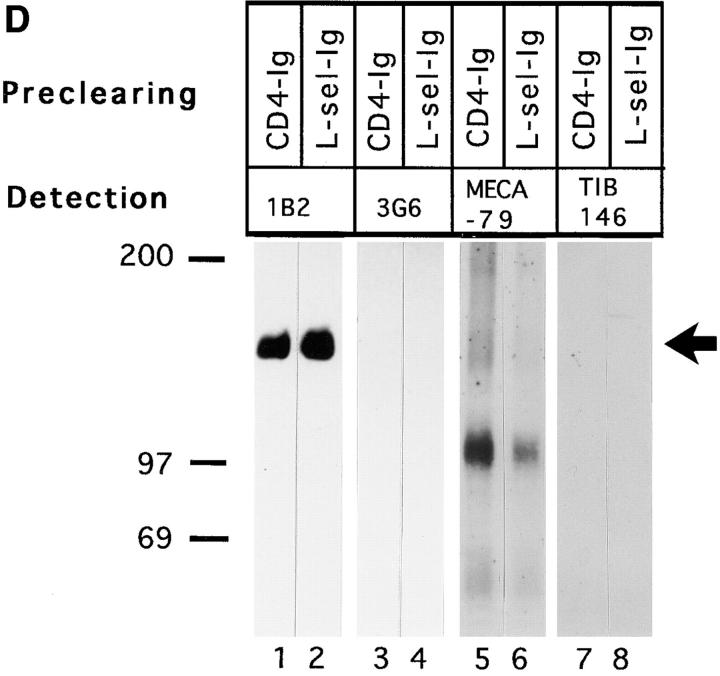
VAP-1 does not recognize L-selectin. (A) Expression of human L-selectin on mouse L1-2 L-selectin transfectants. (B) L1-2 L-selectin transfectants (dark blue spots) efficiently bind to human PLN HEVs (seven different-sized HEV, one outlined by a dashed line, can be seen as light blue structures). (C) L1-2 L-selectin transfectant binding to HEV in human PLNs is independent of VAP-1. Transfectants were pretreated with Dreg-56 and PLN tissue with 1B2 and/or MECA-79, and the HEV adhesion was determined. (D) L-selectin chimera binds PNAd, but not VAP-1. The Ig-chimeras were used to deplete tonsil lysate and the nonprecipitated molecules were analyzed using immunoblotting and the mAbs indicated.
An L-selectin chimera was used to confirm that VAP-1 does not recognize L-selectin. In these experiments, tonsil lysates were precleared with chimeric molecules containing the extracellular domains of L-selectin or CD4 joined to the Fc tail of IgG. As shown in Fig. 4 D, the L-selectin chimera did not remove VAP-1 reactivity from lysates (lane 2), but effectively depleted most mAb MECA-79 reactivity (lane 6). Image analyses revealed 78, 56, and 62% reduction of the 170-, 105-, and 50-kD forms of PNAd, respectively, when compared to precipitations with a control CD4 chimera. Hence, all three detectable species of MECA-79 antigens bound to L-selectin, whereas L-selectin did not interact with VAP-1.
VAP-1 Binds to a Novel Contact-initiating Receptor on Lymphocytes.
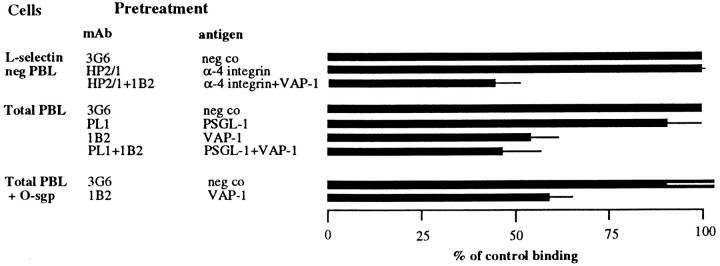
In addition to L-selectin, α4 integrins and PSGL-1 can mediate tethering and rolling of lymphocytes on vascular endothelium or isolated endothelial ligands (28, 33–36). To address whether α4 integrins can function as lymphocyte ligands of VAP-1, we analyzed the role of α4 integrin in PLN HEV adhesion of L-selectin–negative PBL (Fig. 5). A function-inhibiting anti-α4 mAb, HP2/1, which detects both α4β1 and α4β7 heterodimers, had no effect on adherence of these cells to HEVs under rotatory conditions. Moreover, mAb 1B2 still efficiently inhibited PLN HEV adhesion of α4-blocked PBL, indicating that functional α4 is not needed to support VAP-1–dependent binding. VAP-1 function was independent of the lymphocyte PSGL-1 as well. When PBL were pretreated with a function-blocking anti–PSGL-1 mAb PL1, ∼10% inhibition in lymphocyte binding to tonsil HEV was seen. When the anti–PSGL-1 mAb–treated PBL were added to anti–VAP-1 mAb–treated tonsil sections, 10% additive inhibitory effect on the adhesion was seen when compared to the effect of the anti– VAP-1 mAb alone. To assess the role of other sialomucins as potential counterreceptors for VAP-1, we treated lymphocytes with the sialomucin-degrading enzyme O-sialoglycoprotease, and then analyzed the VAP-1 dependence of their tonsil HEV binding. This treatment had a marginal effect on PBL adhesion to HEV, but the binding of O-sialoglycoprotease–treated cells was still efficiently blocked by mAb 1B2 pretreatment of the target tissue indicating that an O-sialoglycoprotease–sensitive molecule is not at least the principal lymphocyte ligand of VAP-1. Thus, none of the currently known lymphocyte adhesion molecules (L-selectin, α4 integrins, or PSGL-1) mediating rolling on endothelial cells are receptors for VAP-1.
Figure 5.
VAP-1 binds to a novel contact initiating lymphocyte counterreceptor. PBL were treated with the indicated mAbs (HP2/1 and PL1) against lymphocyte adhesion receptors or with O-sialoglycoprotease (O-sgp), and their binding to the target tissue treated with anti–VAP-1 or control mAbs was evaluated. Binding of PBL treated with anti–α4 integrin mAb was tested to PLN HEVs, whereas binding of PBL pretreated with anti– PSGL-1 mAb and O-sgp was evaluated using tonsil as a target tissue.
VAP-1 and PNAd Both Mediate PLN HEV Adhesion of PBL.
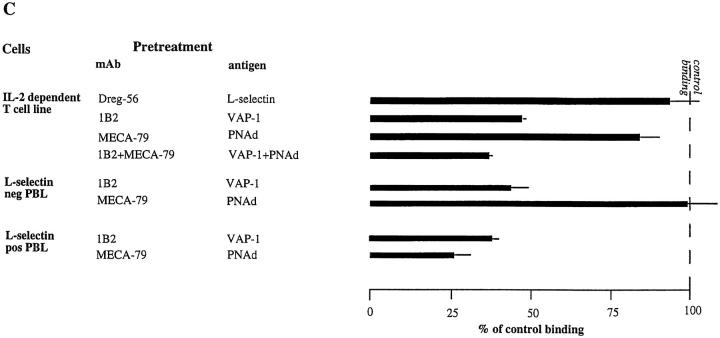
Next we wanted to see whether VAP-1 and PNAd represent two distinct adhesion routes to PLN HEVs. Binding of normal PBL, which contain 50–80% L-selectin positive cells, to PLN HEVs was reduced by ∼75% with separate anti-PNAd, anti–L-selectin, and anti–VAP-1 treatments in these assays (Fig. 6). In contrast, E-selectin, P-selectin, and VCAM-1 played no role in PBL binding to normal PLN. When the function of both VAP-1 and PNAd was blocked, we observed ∼10% more inhibition of HEV binding when compared to sections pretreated with mAb MECA-79 only. This additive inhibition was seen in every experiment performed, but it failed to reach statistical significance (P = 0.07 when comparing MECA-79 pretreatment to combined MECA-79 and 1B2 pretreatment). These data indicate that most PBL use both VAP-1 and PNAd to bind to PLN HEVs, but also reveal that a small PBL subpopulation may rely exclusively on VAP-1. L-selectin was also important for PLN HEV adhesion of CD8-positive PBL (Fig. 7). In support of additional inhibition of total PBL binding to PLN HEVs seen by combined blocking of PNAd and VAP-1, combined blocking of both L-selectin and VAP-1 also produced more marked inhibition in PLN HEV adhesion of CD8-positive PBL than blocking of either adhesion molecule separately.
Figure 6.
Most PBL use both PNAd and VAP-1 in PLN HEV binding. PBL were treated with anti–L-selectin mAb and PLNs with mAbs against different endothelial adhesion molecules alone or in combination, and the HEV adherence was determined.
Figure 7.
CD8-positive PBL use both L-selectin and VAP-1 to adhere to PLN HEVs. Positively selected CD8-positive cells (CD8 +) were treated with anti– L-selectin and control mAb, PLN tissue section was pretreated with anti–VAP-1 and control mAb, and the HEV binding was determined.
VAP-1 Mediates Initial Contacts Between Lymphocytes and Inflamed Endothelium In Vivo.
To confirm that VAP-1 could mediate initial interactions between PBL and endothelial lining under physiologically relevant conditions, we used intravital microscopy. Since mAb 1B2 does not cross-react with laboratory rodents, we screened a new panel of anti–human VAP-1 mAbs against different animal species and found that one mAb, 5B11, cross-reacts with rabbit. mAb 5B11 is specific against VAP-1 since it stains the same structures in human tissues as the prototype mAb 1B2, it reacts in immunoblotting with a 170-kD molecule that is absent from a lysate after preclearing with 1B2-coupled beads, and it reacts with VAP-1 transfectants (data not shown). mAb 5B11 inhibits human PBL binding to human tonsil HEVs in Stamper-Woodruff assay at least as efficiently as 1B2. Importantly, mAb 5B11 recognizes in rabbits an antigen with similar tissue and cell distribution to human VAP-1 (Fig. 8 a), and mAb 5B11 abrogated 75% of binding of rabbit mesenterial lymph node lymphocytes to rabbit mesenterial lymph node HEVs indicating conserved function of VAP-1 between humans and rabbits. This mAb also detects rabbit VAP-1 on the surface of inflamed mesenterial venules 4 h after IL-1–induced peritonitis (Fig. 8 c).
Figure 8.
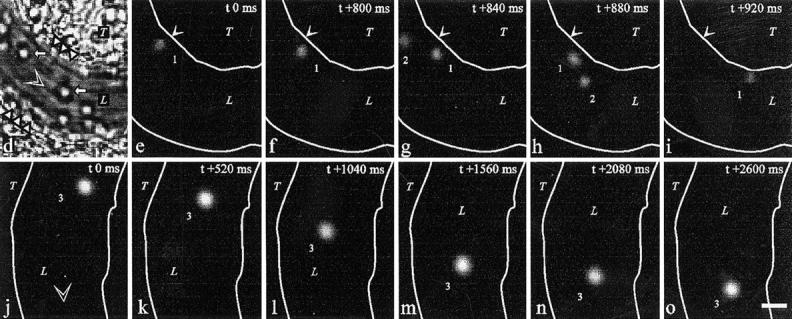
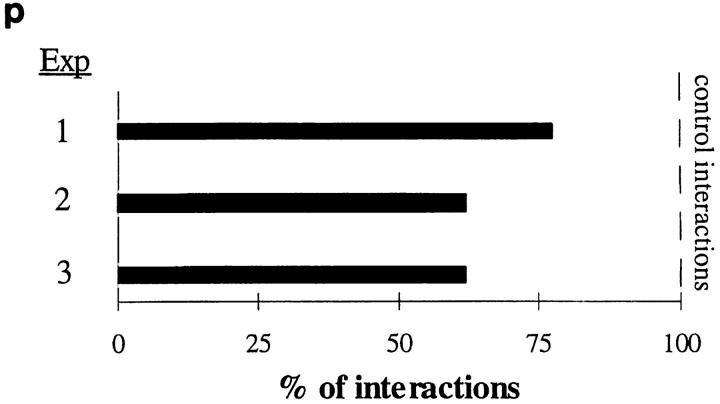
VAP-1 mediates initial interactions between human lymphocytes and inflamed vascular endothelium in vivo. (a) An anti–human VAP-1 mAb 5B11 recognizes VAP-1 in HEVs in frozen sections of rabbit mesenterial lymph node. (b) Negative control staining with mAb 4D7. An HEV is pointed out by an arrow. (c) Rabbit VAP-1 is expressed on the luminal surface of inflamed mesenterial vessel. After intravenous injection of mAb 5B11, the in vivo–bound mAb was immunohistochemically detected in the mesenterial section with a second-stage peroxidase-conjugated anti–mouse Ig. (d–o) Intravital microscopy was used to study the effect of a control mAb 4D7 and an anti–VAP-1 mAb 5B11 on binding of fluorescently labeled human tonsillar lymphocytes with inflamed mesenterial vessels in rabbits as detailed in Materials and Methods. (d) A micrograph of one segment of the vessel under study. L, lumen of the vessel; T, connective tissue of the mesenterium; white triangles, the walls of the vessel; white arrows, rolling rabbit granulocytes. (e–i) A tethering lymphocyte. The same segment as in d viewed under stroboscopic epiillumination after injecting fluorescently labeled human cells. A cell (1) docks to the vessel wall in e, moves less than 5 μm/s during the first 800 ms (e–f), speeds up in g, and detaches in h–i. A freely flowing cell (2; velocity 1,110 μm/s) is also seen. Arrows mark a reference point at the vessel wall. (j–o) A rolling lymphocyte. In another segment of a venule, a lymphocyte (3) that rolls along the bottom of the vessel for >2.5 s is seen (average velocity ∼24 μm/s). Blood flow (open arrowhead) is from left to right in d–i and from top to bottom in j–o. White lines, vessel walls. Time codes in the upper right corners indicate the time elapsed (in ms) from the first frame of the series. Bar, 10 μm. (p) Anti–VAP-1 mAb 5B11 reduces interactions between human lymphocytes and rabbit vessel wall when analyzed by intravital microscopy in three independent experiments. Number of control interactions was arbitrarily set at 100%.
In rabbits treated with an isotype-matched control mAb 4D7, which reacts to neither human lymphocytes nor rabbit endothelium, fluorescently labeled human tonsillar lymphocytes interacted with inflamed endothelium as observed by intravital microscopy. The estimated Newtonian wall shear stress was 4.0–7.8 dyn/cm2 in the venules (diameter 21–50 μm) analyzed. The majority of labeled cells was detected in the freely flowing fraction, but 14–26% of cells interacted with the endothelial cells. Most interacting cells typically displayed transient tethering to the endothelium, and few showed a rolling type movement along the endothelium (Fig. 8, d–o). Cell labeling did not adversely affect the rolling capacity of human lymphocytes in rabbits, since the same cells showed typical rolling in Peyer's patch HEVs (which, however, were VAP-1 surface negative). Notably, anti–VAP-1 mAb 5B11 pretreatment diminished the number of labeled cells interacting with VAP-1–positive mesenterial endothelial cells by 33 ± 5% (mean ± SEM of three independent assays with different rabbits, P = 0.0242 when compared to control mAb treatment periods; Fig. 8 p). The effect of anti–VAP-1 pretreatment was quite remarkable since only ∼10% of tonsillar cells were CD8 positive. Thus, VAP-1 does support initial contacts between human lymphocytes and vessel wall under physiologically relevant shear conditions.
Discussion
Our results show that VAP-1 mediates leukocyte subtype-specific recognition of HEVs under nonstatic conditions in humans, and hence introduces a new way of generation of the selectivity of leukocyte–endothelial interactions in the multistep adhesion cascade. VAP-1 is able to mediate PLN HEV adhesion of both L-selectin–positive, and importantly, L-selectin–negative lymphoid cells. Moreover, VAP-1 supports initial contacts (tethering and rolling) between lymphocytes and inflamed endothelium under physiologically relevant shear conditions in an animal model. Thus, VAP-1 extends the role of carbohydrate-dependent lymphocyte–endothelial cell interaction beyond the known selectins.
VAP-1 mediates HEV binding of lymphocytes, but not that of monocytes. Even more strikingly, only CD8-positive T cells and CD16-positive NK cells recognized VAP-1, whereas CD4-positive T cells and B cells adhered independently of VAP-1. Thus, VAP-1–dependent binding divides T cells dicotomically into two populations. Therefore, VAP-1 is the first defined endothelial adhesion molecule, which can explain the better binding of CD8- than that of CD4-positive T cells to PLN HEVs (5). Very recently it has been shown that Th1, but not Th2, CD4 cells bind to P- and E-selectin in mice (37). Together, these findings indicate that endothelial adhesion molecules may display greater selectivity toward defined lymphocyte subpopulations than previously anticipated.
VAP-1 mediates oligosaccharide-dependent adhesion to endothelial cells under nonstatic shear conditions (26), implying that it is involved relatively early in the adhesion cascade. Using intravital microscopy, we showed here that VAP-1 plays a role in the initial recognition of at least inflamed endothelium by human lymphocytes. Anti–VAP-1 mAb pretreatment diminished the number of lymphocytes tethering and rolling on the vessel wall by ∼30%. This is a notable reduction since only ∼10% of tonsil lymphocytes were CD8 positive and thus able to interact with VAP-1, and since all other adhesion pathways were left intact. These data suggest that VAP-1 functions quite early in the adhesion cascade, but we cannot rule out the possibility that it would play a role as an accessory molecule, e.g., as a part of a larger receptor complex or as a signaling molecule. On lymphocytes there are three known homing receptors, L-selectin, PSGL-1, and α4 integrins, mediating the initial tethering and rolling, and all of them recognize multiple endothelial and other ligands (7, 8). Therefore, it was of importance to see that none of these molecules served as the lymphocyte counterreceptor of VAP-1, although we cannot formally rule out the possibility that CD8- and CD16-positive cells would possess an L-selectin with unique posttranslational modifications that would render it capable of interacting with VAP-1. VAP-1–dependent binding is sialic acid dependent and VAP-1 contains both α2,3 and α2,6 sialic acid linkages (26). Thus, another lectin type molecule preferentially expressed on CD8-positive cells and NK cells would be an appealing candidate as a VAP-1 counterreceptor.
The ability of VAP-1 to bind L-selectin–negative cells to PLNs deserves special emphasis. L-selectin is readily shed from the lymphocyte surface, and hence recently activated lymphocytes are L-selectin negative (18, 38, 39) and a large subpopulation of circulating CD45RO+RA− memory cells also lack L-selectin (40). It has remained enigmatic how these effector cell types can home to PLNs. We showed here that both totally L-selectin–negative activated immunoblasts and L-selectin–negative PBL can use VAP-1 in PLN HEV adhesion. These observations provide a molecular basis for those studies that have reported that L-selectin–negative cells nevertheless do bind to PLN HEVs. For example, we have shown earlier that L-selectin–negative cell lines derived from human tissues bind to PLN HEVs by unknown mechanisms (22). Moreover, L-selectin–negative T cell lines injected intravenously into mice have been reported to home into PLNs (41), and in antibody inhibition experiments, blockade of L-selectin only causes a 75% reduction of PLN homing in vivo (42). The putative mouse homologue of VAP-1 might well explain the residual lymphocyte trafficking seen in these animal models.
Our results appear to contradict those obtained in L-selectin knockout mice, in which 95–99% abrogation of HEV adhesion of mutant lymphocytes to PLN HEVs in Stamper- Woodruff assay has been reported, and in which no homing of lymphocytes into PLNs was detected (43, 44). We believe that there may be species-specific differences in the behavior of L-selectin–negative cells, and that in human L-selectin–independent PLN HEVs recognition may play a more prominent role. However, the other examples referred to above indicate that PLN HEVs homing of L-selectin– negative cells can also be detected in mice. The reasons for this discrepancy remain elusive, but may include strain differences, other aberrations in congenitally L-selectin–deficient animals (e.g., lack of maturational signals for the naive CD8-positive PBL deprived of lymph node microenvironment; see also reference 45), or subtle technical differences in the in vitro adhesion assay (Arbones et al. measured binding of mouse splenocytes from knockout mice to rat PLN HEVs after additional washes, counterstaining, and mounting; reference 43). It is also worth noting that many groups that have reported L-selectin to be absolutely necessary for PLN HEV binding use the original Stamper- Woodruff assay (23) in which the target tissue has been fixed with 3% glutaraldehyde before performing the binding assay. This treatment renders VAP-1, and possibly also some other adhesion molecules, completely nonfunctional, and hence excludes the possibility of detecting L-selectin– negative lymphocyte binding via VAP-1.
Our data from the in vitro functional studies show that at least two distinct pathways are operative in mediating lymphocyte–PLN HEV interaction under shear in humans: (a) L-selectin–PNAd and (b) non–L-selectin–VAP-1. At least in mice, binding of lymphocyte to PNAd in PLNs can also occur in vivo via a platelet bridge in which lymphocyte PSGL-1 binds to (presumably) P-selectin on activated platelets, and then other P-selectin molecules of the platelet interact with PNAd (46). CD44 is also involved in PLN HEV adhesion in humans (47), although in mouse models, CD44 may not be involved in PLN homing (48). In our experimental setup, P- and E-selectin, or interaction of α4 integrins with VCAM-1 and mucosal addressin cell adhesion molecule 1 do not play any role on PLN HEV adhesion. Most PLN type HEVs synthesize both VAP-1 and PNAd, but exclusive expression of VAP-1 or PNAd in certain HEVs offers a possibility to fine tune tissue-selective endothelial cell binding of lymphocytes by virtue of L-selectin expression status of the lymphocyte. Since combined VAP-1 and PNAd blockade was only minimally synergistic, the most likely possibility is that, for most lymphocytes, both molecules are involved and that they mediate sequential but overlapping events. For many L-selectin–positive lymphocytes, interaction through both PNAd and VAP-1 may occur, in parallel or sequentially. Involvement of VAP-1 and PNAd might be analogous, for example, to the roles of L-selectin and α4β7 integrin in lymphocyte homing to mucosa-associated Peyer's patch HEVs. In this site, L-selectin initiates the contact more avidly than α4β7; both molecules participate in rolling before lymphocyte activation and α4β7-mediated slowing of rolling appears essential for subsequent activation-dependent arrest involving β2 integrins (33, 35). Thus, if our in vitro observations reflect the situation in PLNs in vivo, L-selectin and PNAd might initiate contact more efficiently than VAP-1, but VAP-1 may be required for subsequent events before stable arrest. L-selectin–negative cells, on the other hand, can omit the PNAd binding and directly rely on VAP-1 in PLN HEV adhesion. Finally, results from our functional assays indicate a significant subpopulation of lymphoid cells (probably including many CD4-positive cells) adheres to PLN HEVs under nonstatic conditions by novel non–VAP-1–, non-PNAd–, and non–L-selectin–dependent mechanism(s).
VAP-1 selectively mediates PLN HEV adhesion of CD8-positive lymphocytes, and it can bind either L-selectin– negative or –positive cells. VAP-1 is the first endothelial adhesion molecule that mediates leukocyte–HEV binding in a subtype specific manner. Identification of VAP-1 as a new contact initiating ligand provides a unique molecular pathway for regulating the specificity and diversity of the initial steps of lymphocyte–endothelial cell interactions. Moreover, manipulation of VAP-1 opens new avenues for selective control of the trafficking of CD8-positive lymphocytes and NK cells. Selective blocking of the migration of these effector cells by VAP-1 antagonists should ablate much of the untoward inflammatory damage seen at different clinical conditions, and yet leave the other arms of the immune system intact.
Acknowledgments
We thank U. von Andrian (Harvard Medical School, Boston, MA) and K. Ley (University of Virginia, Charlottesville, VA) for introducing intravital microscopy to us and for their advice in setting up an intravital unit in Turku, R. Sara (Centre for Biotechnology, Turku University and Åbo Academi, Turku, Finland) and H. Sara (Centre for Biotechnology, Turku University and Åbo Academi, Turku, Finland) for programs for digital analyses of intravital data, R. Grenman (Turku University Hospital, Turku, Finland) for providing the tonsil and lymph node samples, S. Watson (Genetech Inc., South San Francisco, CA) for the L-selectin and CD4-Ig chimeras, D. Haskard (Hammersmith Hospital, London, UK) for donating mAbs 1.2B6 and 1G11, R. McEver (Medical University of Oklahoma, Oklahoma City, OK) and K. Moore (Medical University of Oklahoma, Oklahoma City, OK) for the PL1 mAb, D. Smith for critical reading of this manuscript, and R. Lehvonen and M. Iljamo for expert technical assistance.
This work was supported by the Finnish Academy, the Sigrid Juselius Foundation, Finnish Cancer Foundation, Farmos Research and Science Foundation, Paulo Foundation, and the Finnish Cultural Foundation.
Footnotes
Abbreviations used in this paper: HEV, high endothelial venule; MACS, magnetic-activated cell sorting; PLN, peripheral lymph node; PNAd, peripheral lymph node vascular addressin; PSGL-1, P-selectin glycoprotein ligand 1; VAP, vascular adhesion protein.
References
- 1.Gowans JL, Knight EJ. The route of re-circulation of lymphocytes in rat. Proc R Soc Lond Ser B. 1964;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- 2.Stevens SK, Weissman IL, Butcher EC. Differences in the migration of B and T lymphocytes: organ-selective localization in vivoand the role of lymphocyte– endothelial cell recognition. J Immunol. 1982;128:844–851. [PubMed] [Google Scholar]
- 3.Fossum S, Smith ME, Ford WL. The recirculation of T and B lymphocytes in the athymic, nude rat. Scand J Immunol. 1983;17:551–557. doi: 10.1111/j.1365-3083.1983.tb00823.x. [DOI] [PubMed] [Google Scholar]
- 4.van Dinther-Janssen ACHM, van Maarsseveen ACMT, DeGroot J, Scheper RJ. Comparative migration of T- and B-lymphocyte subpopulations into skin inflammatory sites. Immunology. 1983;48:519–527. [PMC free article] [PubMed] [Google Scholar]
- 5.Kraal G, Weissman IL, Butcher EC. Differences in in vivo distribution and homing of T cell subsets to mucosal vs.non-mucosal lymphoid organs. J Immunol. 1983;130:1097–1102. [PubMed] [Google Scholar]
- 6.Butcher EC. The regulation of lymphocyte traffic. Curr Top Microbiol Immunol. 1986;128:85–122. doi: 10.1007/978-3-642-71272-2_3. [DOI] [PubMed] [Google Scholar]
- 7.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science (Wash DC) 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 8.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu Y, Newman W, Tanaka Y, Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992;13:106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- 10.Carlos TM, Harlan JM. Leukocyte–endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 11.Ley K, Tedder TF. Leukocyte interactions with vascular endothelium. New insights into selectin-mediated attachment and rolling. J Immunol. 1995;155:525–528. [PubMed] [Google Scholar]
- 12.McEver RE, Moore KL, Cummings RD. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J Biol Chem. 1995;270:11025–11028. doi: 10.1074/jbc.270.19.11025. [DOI] [PubMed] [Google Scholar]
- 13.Salmi M, Jalkanen S. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science (Wash DC) 1992;257:1407–1409. doi: 10.1126/science.1529341. [DOI] [PubMed] [Google Scholar]
- 14.Streeter PR, Rouse BTN, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg EL, Robinson MK, Warnock RA, Butcher EC. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol. 1991;114:343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jutila MA, Bargatze RF, Kurk S, Warnock RA, Ehsani N, Watson SR, Walcheck B. Cell surface P- and E-selectin support shear-dependent rolling of bovine γ/δ T cells. J Immunol. 1994;153:3917–3928. [PubMed] [Google Scholar]
- 17.Wellicome SM, Thornhill MH, Pitzalis C, Thomas DS, Lanchbury JSS, Panayi GS, Haskard DO. A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumor necrosis factor, IL-1, or lipopolysaccharide. J Immunol. 1990;144:2558–2565. [PubMed] [Google Scholar]
- 18.Kishimoto TK, Jutila MA, Butcher EC. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated molecule. Proc Natl Acad Sci USA. 1990;87:2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sánchez-Madrid F, de Landázuri MO, Morago C, Cebrián M, Acevedo A, Bernabeu C. VLA-3: a novel polypeptide association within the VLA molecular complex: cell distribution and biochemical characterization. Eur J Immunol. 1986;16:1343–1349. doi: 10.1002/eji.1830161106. [DOI] [PubMed] [Google Scholar]
- 20.Moore KL, Patel KD, Bruehl RE, Li F, Johnson DA, Lichenstein HS, Cummings RD, Bainton DF, McEver RP. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arvilommi A-M, Salmi M, Kalimo K, Jalkanen S. Lymphocyte binding to vascular endothelium in inflamed skin revisited: a central role for vascular adhesion protein-1 (VAP-1) Eur J Immunol. 1996;26:825–833. doi: 10.1002/eji.1830260415. [DOI] [PubMed] [Google Scholar]
- 22.Salmi M, Granfors K, Leirisalo-Repo M, Hämäläinen M, MacDermott R, Leino R, Havia T, Jalkanen S. Selective endothelial binding of interleukin-2–dependent human T-cell lines derived from different tissues. Proc Natl Acad Sci USA. 1992;89:11436–11440. doi: 10.1073/pnas.89.23.11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stamper HB, Jr, Woodruff JJ. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976;144:828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butcher EC, Scollay RG, Weissman IL. Organ specificity of lymphocyte migration: mediation by highly selective lymphocyte interaction with organ-specific determinants on high endothelial venules. Eur J Immunol. 1980;10:556–561. doi: 10.1002/eji.1830100713. [DOI] [PubMed] [Google Scholar]
- 25.Jalkanen ST, Butcher EC. In vitro analysis of the homing properties of human lymphocytes: developmental regulation of functional receptors for high endothelial venules. Blood. 1985;66:577–582. [PubMed] [Google Scholar]
- 26.Salmi M, Jalkanen S. Human vascular adhesion protein–1 (VAP-1) is a unique sialoglycoprotein that mediates carbohydrate-dependent binding of lymphocytes to endothelial cells. J Exp Med. 1996;183:569–579. doi: 10.1084/jem.183.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutherland DR, Abdullah KM, Cyopick P, Mellors A. Cleavage of the cell-surface O-sialoglycoproteins CD34, CD43, CD44, and CD45 by a novel glycoprotease from Pasteurella haemolytica. . J Immunol. 1992;148:1458–1464. [PubMed] [Google Scholar]
- 28.Alon R, Rossiter H, Wang X, Springer TA, Kupper TS. Distinct cell surface ligands mediate T lymphocyte attachment and rolling on P and E selectin under physiological flow. J Cell Biol. 1995;127:1485–1495. doi: 10.1083/jcb.127.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson SR, Imai Y, Fennie C, Geoffrey JS, Rosen SD, Lasky LA. A homing receptor–IgG chimera as a probe for adhesive ligands of lymph node high endothelial venules. J Cell Biol. 1990;110:2221–2229. doi: 10.1083/jcb.110.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mebius RE, Watson SR. L- and E-selectin can recognize the same naturally occurring ligands on high endothelial venules. J Immunol. 1993;151:3252–3260. [PubMed] [Google Scholar]
- 31.von Andrian UH, Hansell P, Chambers JD, Berger EM, Torres-Filho I, Butcher EC, Arfors KE. L-selectin function is required for β2-integrin–mediated neutrophil adhesion at physiological shear rates in vivo. Am J Pathol. 1992;263:1034–1044. doi: 10.1152/ajpheart.1992.263.4.H1034. [DOI] [PubMed] [Google Scholar]
- 32.Ley K, Baker JB, Cybulsky MI, Gimbrone MA, Jr, Luscinskas FW. Intravenous interleukin-8 inhibits granulocyte emigration from rabbit mesenteric venules without altering L-selectin expression or leukocyte rolling. J Immunol. 1993;151:6347–6357. [PubMed] [Google Scholar]
- 33.Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 34.Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer's patch–HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 36.Norman KE, Moore KL, McEver RP, Ley K. Leukocyte rolling in vivo is mediated by P-selectin glycoprotein ligand-1. Blood. 1995;86:4417–4421. [PubMed] [Google Scholar]
- 37.Austrup F, Vestweber D, Borges E, Löhning M, Bräuer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P-and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature (Lond) 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 38.Hamann A, Jablonski-Westrich D, Scholz K-U, Duijvestijn A, Butcher EC, Thiele H-G. Regulation of lymphocyte homing. I. Alterations in homing receptor expression and organ-specific high endothelial venule binding of lymphocytes upon activation. J Immunol. 1988;140:737–743. [PubMed] [Google Scholar]
- 39.Jung TM, Gallatin WM, Weissman IL, Dailey MO. Down-regulation of homing receptors after T cell activation. J Immunol. 1988;141:4110–4117. [PubMed] [Google Scholar]
- 40.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Buck D, Terstappen LWMM. Control of lymphocyte recirculation in man. I. Differential regulation of the peripheral lymph node homing receptor L-selectin on T cells during the virgin to memory cell transition. J Immunol. 1993;150:1105–1121. [PubMed] [Google Scholar]
- 41.Bookman MA, Groves ES, Matis LA. Expression of MEL-14 antigen is not an absolute requirement for dissemination to lymph nodes after adoptive transfer of murine T lymphocyte clones. J Immunol. 1986;137:2107–2114. [PubMed] [Google Scholar]
- 42.Gallatin WM, Weissman IL, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature (Lond) 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 43.Arbonés ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin–deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 44.Steeber DA, Green NE, Sato S, Tedder TF. Lymphocyte migration in L-selectin–deficient mice. Altered subset migration and aging of the immune system. J Immunol. 1996;157:1096–1106. [PubMed] [Google Scholar]
- 45.Salmi M, Jalkanen S. How do lymphocytes know where to go: current concepts and enigmas of lymphocyte homing. Adv Immunol. 1996;64:139–218. doi: 10.1016/s0065-2776(08)60889-5. [DOI] [PubMed] [Google Scholar]
- 46.Diacovo TG, Puri KD, Warnock RA, Springer TA, von Andrian UH. Platelet-mediated lymphocyte delivery to high endothelial venules. Science (Wash DC) 1996;273:252–255. doi: 10.1126/science.273.5272.252. [DOI] [PubMed] [Google Scholar]
- 47.Pals ST, Hogervorst F, Keizer GD, Thepen T, Horst E, Figdor CC. Identification of a widely distributed 90-kDa glycoprotein that is homologous to the Hermes-1 human lymphocyte homing receptor. J Immunol. 1989;143:851–857. [PubMed] [Google Scholar]
- 48.Camp RL, Scheynius A, Johansson C, Puré E. CD44 is necessary for optimal contact allergic responses but is not required for normal leukocyte extravasation. J Exp Med. 1993;178:497–507. doi: 10.1084/jem.178.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]