Abstract
Challenge of the airways of sensitized guinea pigs with aerosolized ovalbumin resulted in an early phase of microvascular protein leakage and a delayed phase of eosinophil accumulation in the airway lumen, as measured using bronchoalveolar lavage (BAL). Immunoreactive eotaxin levels rose in airway tissue and BAL fluid to a peak at 6 h falling to low levels by 12 h. Eosinophil numbers in the tissue correlated with eotaxin levels until 6 h but eosinophils persisted until the last measurement time point at 24 h. In contrast, few eosinophils appeared in BAL over the first 12 h, major trafficking through the airway epithelium occurring at 12–24 h when eotaxin levels were low. Constitutive eotaxin was present in BAL fluid. Both constitutive and allergen-induced eosinophil chemoattractant activity in BAL fluid was neutralized by an antibody to eotaxin. Allergen-induced eotaxin appeared to be mainly in airway epithelium and macrophages, as detected by immunostaining. Allergen challenge of the lung resulted in a rapid release of bone marrow eosinophils into the blood. An antibody to IL-5 suppressed bone marrow eosinophil release and lung eosinophilia, without affecting lung eotaxin levels. Thus, IL-5 and eotaxin appear to cooperate in mediating a rapid transfer of eosinophils from the bone marrow to the lung in response to allergen challenge.
Allergen provocation of allergic asthmatic patients results in an early phase of bronchoconstriction associated with cross-linking of IgE bound to FcεRI receptors on mast cells inducing degranulation, and a late phase bronchoconstriction associated with the appearance of eosinophils in the airways (1, 2). There is increasing evidence linking the presence of eosinophils with airway damage and dysfunction, but this remains controversial. The numbers of eosinophils detected in bronchoalveolar lavage (BAL)1 and airway mucosa correlate with the degree of lung dysfunction in asthmatics (3, 4). Moreover, eosinophils contain chemicals as part of the host defense system against helminths which, if misdirected, can damage airway mucosa and lung tissue (5, 6). Some of these products, major basic protein, eosinophil peroxidase (EPO), and eosinophil cationic protein, have been detected in BAL fluid from asthmatics, a consequence of eosinophil degranulation in the lung (6–8). Blood microvessels in the lung have an important role, both as the route for entry of inflammatory cells including T lymphocytes (particularly of the Th2 subset) and eosinophils, and in terms of the extravascular supply of plasma proteins through opening of interendothelial cell junctions. The latter is manifest as increasing plasma albumin concentrations detectable in BAL fluid after allergen challenge (9–11).
Knowledge concerning the mechanisms underlying allergic inflammation in the asthmatic lung has grown through observations made in patients and patient-derived samples, together with observations made in animal systems which model certain important features of the human condition. Notable among the latter are studies in ovalbumin-sensitized guinea pigs challenged with aerosolized allergen (12–15). On allergen challenge, guinea pigs demonstrate immediate bronchoconstriction associated with mast cell degranulation resulting in histamine release and peptidoleukotriene secretion, and a late response associated with eosinophil accumulation in the airways.
Recently we identified a small protein in BAL fluid from ovalbumin-challenged, sensitized guinea pigs which is a powerful and selective eosinophil chemoattractant. This protein was purified, sequenced, and found to be a 73–amino acid CC chemokine which we called eotaxin (16). Murine (17, 18) and human (19, 20) homologues have now been identified, and have been found to act through an eotaxin receptor, CCR3, present in high numbers on eosinophils (21, 22). Allergen challenge of sensitized animals has been shown to be associated with increased mRNA for eotaxin in the lungs of both guinea pigs (23, 24) and mice (18).
We have now developed an immunoassay for guinea pig eotaxin and this paper describes its use to analyze the relationship between the appearance of eotaxin protein and eosinophil accumulation in the guinea pig allergic airways model. Further, we have demonstrated recently a potentially important interaction between eotaxin and IL-5 using exogenous agents in vivo (25). This paper delineates the relationship between endogenous eotaxin and IL-5 in the context of the allergic reaction in the lung.
Materials and Methods
Animals.
Male Dunkin Hartley guinea pigs (Charles River Laboratories, Margate, Kent, UK) were used for all the in vivo procedures described. Female exbreeder guinea pigs from the same source were used as eosinophil donors.
Materials.
Ovalbumin (grade V), Pyrilamine, BSA, hexadecyltrimethylammonium bromide, o-phenylenediamine, hydrogen peroxide, dexamethasone 21-phosphate, n-octanoic acid, Freund's adjuvants, and control rat IgG were purchased from Sigma Chemical Co. (Poole, Dorset, UK). Aluminum hydroxide, low viscosity fluid gel was from Wilfred Smith Ltd. (Middlesex, UK). HBSS and Hepes were from Life Technologies (Paisley, UK). Iodogen reagent was from Pierce and Warriner (Chester, UK). Na125I and 111InCl3 were from Amersham International (Buckinghamshire, UK). Goat anti–rabbit IgG second antibody for RIA was from Nordic Immunological Laboratories (Tilburg, The Netherlands). mAbs and substrate for immunohistochemistry were from Dako (High Wycombe, Buckinghamshire, UK). C18 reversed phase SepPak cartridges were from Millipore (Watford, UK). Percoll and protein A–Sepharose were from Pharmacia Biotech (St Albans, Hertfordshire, UK).
Eotaxin for use in skin bioassays was purified from the BAL fluid of allergen-challenged guinea pigs as described previously (16). TRFK5 mAb was a gift from Dr. Paul Hiss (Glaxo-Wellcome, Stevenage, Hertfordshire, UK).
Sensitization and In Vivo Allergen Challenge of Guinea Pigs.
Guinea pigs (200–250 g) were sensitized with an i.p. injection of ovalbumin (10 μg) mixed with aluminum hydroxide (2 mg) in 1 ml of saline on days 1 and 15. On day 28, animals were pretreated with pyrilamine (10 mg/kg, i.p.), to prevent acute fatality, and exposed to aerosolized ovalbumin (1%) or saline for 20 min. At different times after allergen challenge, animals were treated with atropine (0.06 mg/kg, i.p.), to prevent bronchoconstriction, and killed with a barbiturate overdose. BAL was performed with 15 ml of saline (containing EDTA and Hepes, each at 10 mM) and the fluid centrifuged at 4°C for 10 min at 400 g. BAL cells were resuspended in HBSS (containing EDTA, Hepes, and 0.25% BSA) and total cell counts determined using Kimura stain. The 400 g supernatant was centrifuged again (3,470 g, 20 min at 4°C) and the resulting supernatant stored at −20°C until assayed for eotaxin. Differential cell counts were performed on eosin and methylene blue–stained cytospin preparations. 500 cells were counted per slide. After collection of BAL fluid, the lungs were excised, the pulmonary artery cannulated, and the lungs flushed with 20 ml of saline containing 10 mM EDTA. Lung tissue was chopped and weighed, and ∼50% was processed immediately for EPO measurement while the remainder was stored at −80°C for subsequent determination of lung eotaxin.
Modulation of the Inflammatory Response by TRFK5 and Dexamethasone.
TRFK5 mAb or control rat IgG (0.3 mg/kg) was injected i.v. 45 min before saline or allergen challenge. Dexamethasone (40 mg/kg at each time point) or saline was given i.p. at 24 and 1 h before saline or ovalbumin challenge. Animals were killed at 6 and 24 h after aerosol challenge.
Determination of Eosinophil Numbers in Bone Marrow.
The right femur was isolated, the femoral head and condyles were removed, and the displaceable cells were recovered by flushing the lumen of the femur shaft with 5 ml HBSS (containing 30 mM Hepes, 0.25% BSA, and 10 U/ml heparin). The bone marrow suspension was passed through a 19 gauge needle to create a single cell suspension, and total nucleated cells were counted using Kimura stain. Differential cell counts were performed on May-Grunwald-Giemsa–stained cytospin preparations to determine the percentage of eosinophils. 400 cells were counted per slide.
Determination of Albumin in BAL Fluid.
Albumin was measured using a fluorogenic substrate (26). BAL fluid was incubated with 8-anilino-1-naphthalenesulfonic acid sodium salt (2 × 10−4 M, in 0.25 M sucrose/0.05 M Tris-HCl, pH 7.4) at room temperature for 30 min and the fluorescence measured at 360 nm emission and 460 nm excitation. Fluorescence was converted to albumin concentration by reference to a guinea pig albumin standard (0.015–0.5 mg/ml).
Lung Tissue Eosinophil Content Measured as EPO Activity.
Eosinophil numbers in lung tissue were quantitated as previously described (27). Briefly, the tissue was homogenized to give a 5% wt/vol suspension in HBSS and centrifuged (1,950 g, 10 min). After red blood cell lysis, the pellet was homogenized to give a 5% suspension in HBSS containing 0.5% hexadecyltrimethylammonium bromide. The suspension was freeze/thawed three times using liquid nitrogen before being stored at −20°C until assayed. After centrifugation, the lung extract was placed (75 μl/well, in duplicate) in a 96-well plate followed by addition of 150 μl of substrate (1.5 mM o-phenylenediamine and 6.6 mM hydrogen peroxide in 0.05 M Tris-HCl, pH 8.0). After 30 min at room temperature, the reaction was stopped by the addition of 75 μl of 4 M sulfuric acid and the absorbance read at 492 nm. A standard curve was prepared using guinea pig peritoneal eosinophils (see below) after red blood cell lysis. The cross-reactivity with guinea pig neutrophil myeloperoxidase was <1%. Results are expressed as eosinophils × 106/g lung tissue.
Production of Antieotaxin Antibodies.
Recombinant guinea pig eotaxin was expressed in the bacterial host Escherichia coli as a fusion protein under the control of the T7 polymerase system and purified from inclusion bodies as previously described for RANTES (28). Two rabbits were immunized with the recombinant eotaxin (60 μg/rabbit) in Freund's complete adjuvant and boosted (100 μg/rabbit, in Freund's incomplete adjuvant) 3 and 6 wk later. The animals were bled out 11 d after the final boost.
Diluted serum was used for the radioimmunoassays. For neutralization studies, the IgG fraction was prepared by use of n-octanoic acid to precipitate unwanted proteins (29) followed by precipitation of the IgG with 50% ammonium sulfate. For immunohistochemistry, the IgG fraction was prepared by binding to protein A–Sepharose CL-4B and elution with 0.1 M glycine-HCl, pH 3.0 (30). In both cases the IgG was made up to the original volume of serum, dialyzed against saline, filtered, and stored at −20°C in aliquots.
Lung Tissue Extraction for Eotaxin Measurement.
Frozen lung samples were homogenized to give a 10% wt/vol suspension in 0.2% TFA and centrifuged at 4°C (3,470 g, 20 min). The supernatant was neutralized by adding an equal volume of 0.01 M NaOH in 0.29 M NaCl/0.1 M sodium phosphate buffer, pH 7.4, and centrifuged in a microfuge. The supernatant was then diluted with an equal volume of PBS/0.05% sodium azide/22% polyethylene glycol 6,000/1% protamine sulfate. After a second microcentrifugation, the supernatant (equivalent to 2.5% lung homogenate) was assayed for eotaxin.
RIA for Guinea Pig Eotaxin.
Guinea pig eotaxin (2.5 μg) was iodinated (31) using iodogen reagent and Na125I (18.5 MBq). The competitive binding assay protocol was similar to that described previously for complement C5a (32). BAL fluid (100 μl, mixed with 100 μl of PBS/azide/11% polyethylene glycol/0.5% protamine sulfate) or lung tissue extract (100 μl, mixed with 100 μl of the lavage medium) was incubated with 125I-ligand (50 μl, 30 fmol) and antiserum (50 μl of 1:700 dilution) (both in 0.2 M sodium phosphate buffer, pH 7.4, containing 0.3% BSA/10 mM EDTA/azide). After 20–24 h at room temperature, 25 μl of goat anti–rabbit IgG was added and the samples incubated for a further 8–16 h. Addition of 1 ml of PBS/azide was rapidly followed by centrifugation (5,420 g, 10 min) and aspiration of the supernatant. Ab-bound radioactivity in the pellet was counted in a gamma counter and concentration calculated by comparison with a guinea pig eotaxin standard curve. All samples were assayed in duplicate. Nonspecific binding in the absence of antieotaxin was 3.06 ± 0.11% (mean±SEM, n = 48 assays). The limit of adequate measurement (i.e., the concentration required to inhibit binding of 125I-ligand by 20%) was 0.32 ± 0.01 nM and 50% inhibition of binding was achieved with 1.27 ± 0.03 nM eotaxin (n = 53). Using frozen aliquots of a sample of pooled BAL fluid, the interassay coefficient of variation was 4.5% (n = 23 assays) and the intraassay coefficient of variation was 3.4% (six measurements in one assay). Cross-reactivity of guinea pig MCP-1, RANTES and IL-8, human eotaxin, MCP 1, 2, 3, 4, MIP-1α, β, RANTES, and murine eotaxin (all assayed at 100 nM) was <1%.
Bioassay of Eosinophil Chemoattractant Activity in Naive Guinea Pig Skin.
Eosinophils, harvested from the peritoneal cavity of female exbreeder guinea pigs (800–900 g) given repeated i.p. injections of horse serum, were purified (>95%) over discontinuous Percoll gradients and radiolabeled with 111In as previously described (33). 111In-eosinophils (5 × 106) were injected i.v. via an ear vein and, 5 min later, test agents were injected intradermally (i.d.) (0.1 ml) into the shaved dorsal skin of naive recipient guinea pigs (400 g). To determine the neutralizing capacity of antieotaxin IgG, eotaxin (1.5 pmol/site), purified from guinea pig BAL fluid (16), was coinjected i.d. with various amounts of antieotaxin (0, 1, 3, 10, and 30 μl) or control IgG (30 μl). To investigate eosinophil chemoattractant activity in BAL fluid, obtained 6 h after aerosolized saline or ovalbumin, samples were concentrated by use of C18 reversed phase SepPak cartridges, eluted with acetonitrile in 0.1% trifluoroacetic acid, lyophilized, and redissolved in HBSS/0.1% BSA (<0.1 ng endotoxin/mg albumin). Samples were then coinjected i.d. with 50 μl antieotaxin or control IgG such that the final concentration factor of the BAL fluid was 18-fold. After 4 h, the animals were killed, the dorsal skin removed, and the injection sites punched out (17 mm diameter) for gamma counting. Radiolabeled cell accumulation is expressed as the number of 111In-eosinophils per skin site.
Immunohistochemistry.
Immediately after killing and without performing BAL, the lungs were inflated via the trachea with 4% paraformaldehyde in PBS, and fixed by immersion for 4 h at 4°C, transferred into 15% sucrose in PBS, and processed as paraffin blocks. 6-μm sections were cut and mounted on poly l-lysine– coated slides. Hematoxylin and eosin–stained sections were used to assess the morphological changes associated with sensitization and allergen challenge. For the immunohistological localization of eotaxin the slides were treated in 0.2% H2O2 in absolute ethanol to block endogenous staining and the primary polyclonal antibody (1:100 dilution) was added and incubated for 1 h at room temperature. After washing in Tris-buffered saline, a second layer mouse anti–rabbit IgG mAb (1:50 dilution) was added, followed by a third layer rabbit anti–mouse antibody conjugated with horseradish peroxidase (1:50 dilution). The substrate solution (3-amino-9-ethyl carbazole) was used to produce a dark red precipitate wherever the primary antibody detected eotaxin. Normal guinea pig IgG (1:100 dilution) was substituted for the primary antibody as negative control.
Statistical Analysis.
Results are presented as the mean±SEM of n animals. Analysis of skin bioassay responses was carried out on log10-transformed data using repeated measures ANOVA. All other data were analyzed on untransformed data using an unpaired two-tailed Student's t test. P <0.05 was considered to be statistically significant.
Results
Relationship between the Kinetics of Albumin and Eosinophil Accumulation in BAL.
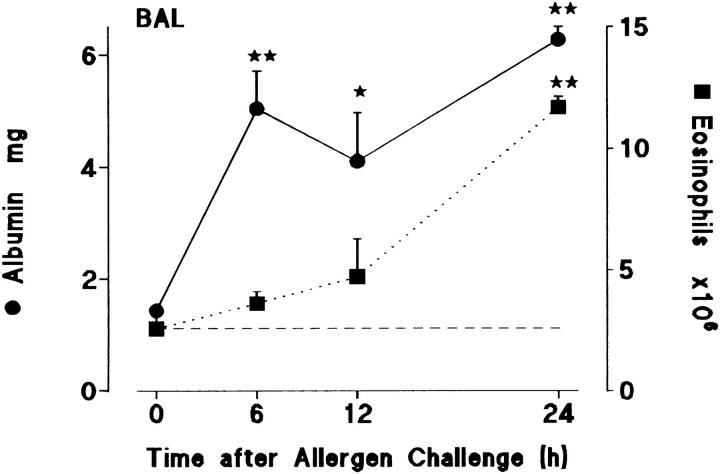
Sensitized guinea pigs were challenged with aerosolized ovalbumin and BAL samples were collected at intervals up to 24 h later. Groups of 5–11 animals were used to generate data at each time point. As shown in Fig. 1 plasma albumin concentrations rose rapidly during an early phase from 0–6 h and remained high up to 24 h. In contrast, there was a protracted delay before significant eosinophil numbers appeared in the BAL: only low numbers were present at 12 h, but accumulation was rapid between 12 and 24 h. Thus, microvascular leakage correlated mainly with an early phase of the allergic response whereas eosinophils appeared in BAL mainly in a late phase after an apparent protracted latent period.
Figure 1.
Time course of albumin extravasation (•, solid line) and eosinophil accumulation (▪, dotted line) in BAL after allergen challenge. Sensitized guinea pigs were exposed to aerosolized ovalbumin and killed at various times after challenge. Results are presented as a mean±SEM of 5–11 animals/time point. 0 h represents sensitized/nonchallenged animals (n = 5). Significant differences from 0 h are indicated as *P <0.02 and **P <0.005. Basal eosinophil numbers in BAL are shown by the dashed line.
Correlation between the Kinetics of Eosinophil Infiltration and the Appearance of Immunoreactive Eotaxin.
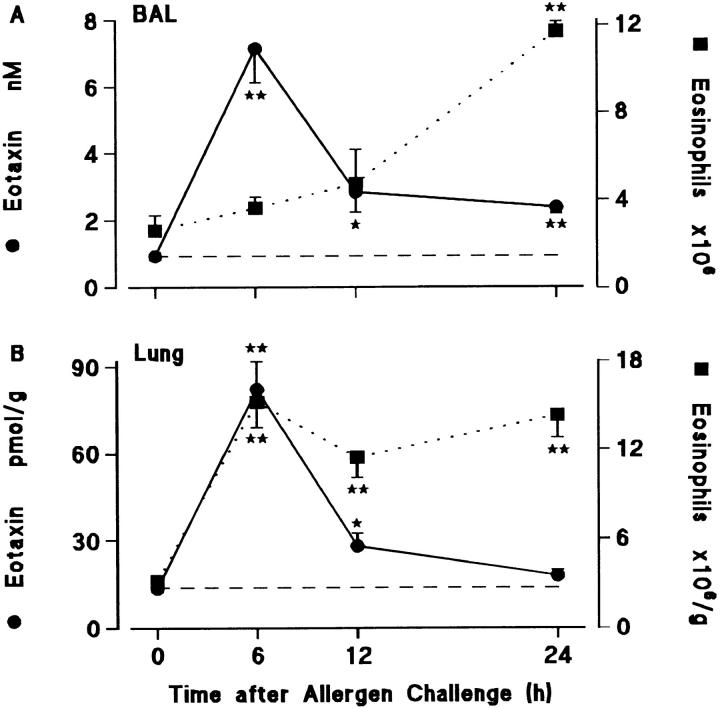
Fig. 2 A shows that there was a dissociation between eosinophil accumulation and the presence of immunoreactive eotaxin when measured in BAL samples. Eotaxin rose to high levels at 6 h at a time when eosinophil numbers were not significantly elevated, but fell to low levels by 12 h, at a time when eosinophil accumulation was beginning to accelerate.
Figure 2.
Time course of eotaxin generation (•, solid line) and eosinophil accumulation (▪, dotted line) in (A) BAL and (B) lung tissue of sensitized guinea pigs after allergen challenge. Results are presented as a mean±SEM (n = 5–9 animals/time point). 0 h represents sensitized/ nonchallenged animals (n = 5). Significant differences from 0 h are indicated as *P <0.05 and **P <0.005. Constitutive levels of eotaxin in BAL fluid and lung tissue are shown by the dashed lines.
In contrast, there was a good correlation between the appearance of eotaxin and eosinophil accumulation when measured by EPO assay in lung tissue extracts. However, levels of the chemokine again fell by 12 h, whereas the eosinophil numbers remained high up to 24 h (Fig. 2 B). Most previous studies have determined kinetics of eosinophil accumulation from BAL samples which exhibit a clear delayed response in terms of eosinophil accumulation, as seen in Fig. 2 A. Results from this model suggest that eosinophil accumulation in lung tissue occurs much earlier. Thus, eosinophil recruitment through the endothelium of airway blood microvessels appears to be rapid, whereas extensive traffic through the airway epithelium is considerably delayed.
Neutralization of Eosinophil Chemoattractant Activity in BAL Fluid.
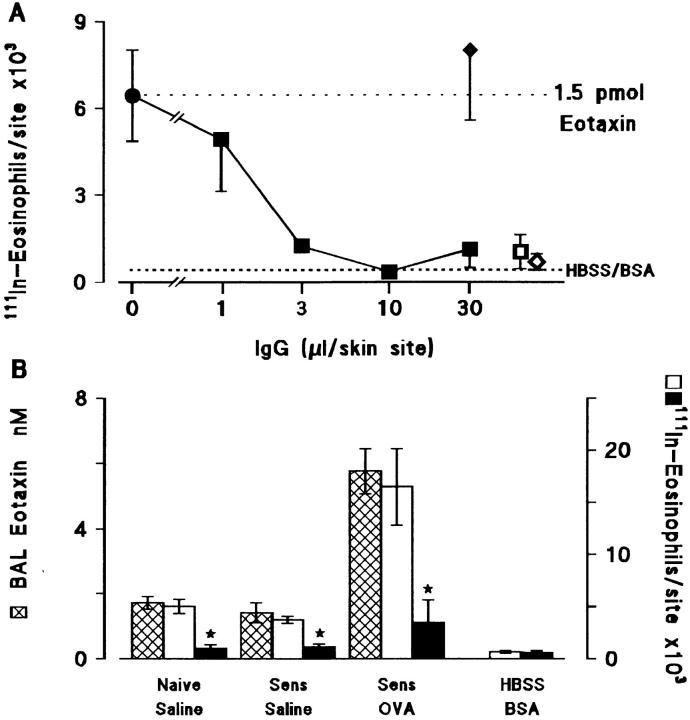
Fig. 3 A shows that a polyclonal antibody generated to recombinant guinea pig eotaxin completely blocked 111In-eosinophil accumulation when mixed with eotaxin and injected into guinea pig skin. The neutralizing antibody was then used to investigate the contribution of eotaxin to eosinophil chemoattractant activity in BAL fluid tested in the skin. Addition of the antieotaxin antibody virtually abolished the chemoattractant activity in BAL fluid from allergen challenged animals as well as the lesser amount of bioactivity in fluid from the control groups of naive and sensitized animals exposed to saline (Fig. 3 B). The eosinophil chemoattractant activity in BAL fluid correlated closely with the level of immunoreactive eotaxin (Fig. 3 B). As shown for eotaxin mRNA in the lung (23, 24), there was significant eotaxin protein in the fluid from naive/saline- and sensitized/saline-exposed animals which was markedly elevated in the sensitized/ovalbumin-challenged animals (Fig. 3 B). Data for eotaxin levels in the lung tissue from these animals are given in the legend to Fig. 3 and demonstrate that, in terms of total eotaxin per guinea pig, there was five to six times more in the lung tissue than in BAL fluid in all three groups.
Figure 3.
(A) Inhibition of eotaxin-induced 111In-eosinophil accumulation by antieotaxin antibody. Purified eotaxin (1.5 pmol) was coinjected with antieotaxin IgG (▪, 1–30 μl), control IgG (♦, 30 μl) or without IgG (•, upper dotted line) into the skin of naive guinea pigs that had received an i.v. injection of 111In-eosinophils. Results are presented as a mean±SEM of 111In-eosinophils/skin site after 4 h (n = 4–6 bioassay animals). Responses to the intradermal vehicle (HBSS/0.25% BSA) alone are shown by the lower dotted line. Antieotaxin (□, 30 μl) or control IgG (⋄, 30 μl) added to the HBSS/BSA did not induce significant 111In-eosinophil accumulation. (B) Inhibition of 111In-eosinophil chemoattractant activity in 6 h BAL fluid by antieotaxin antibody. BAL fluid obtained 6 h after exposure of naive or sensitized guinea pigs to aerosolized saline or ovalbumin was concentrated by C18 SepPak chromatography (see Materials and Methods) and coinjected with 50 μl control IgG (open bars) or antieotaxin IgG (solid bars) into the skin of naive guinea pigs which had received an i.v. injection of 111In-eosinophils. Responses to HBSS/BSA + control IgG or antieotaxin antibodies were also determined. Results are presented as a mean±SEM (n = 4 bioassay animals) and significant differences between control IgG and antieotaxin IgG treated samples are indicated as *P <0.01. Eotaxin concentrations in unconcentrated BAL fluid (cross hatched bars) are also shown in B. Eotaxin in lung tissue was 28.6 ± 2.5, 24.9 ± 2.1, and 89.6 ± 3.0 pmol/g (mean±SEM, n = 5) in the naive/saline, sensitized/saline, and sensitized/ovalbumin groups, respectively. When these results are expressed as total eotaxin per guinea pig lung, there was approximately five to six times more eotaxin in lung tissue (114 ± 8, 115 ± 16, and 379 ± 14 pmol) than in BAL fluid (23 ± 3, 18 ± 4, and 82 ± 10 pmol, respectively).
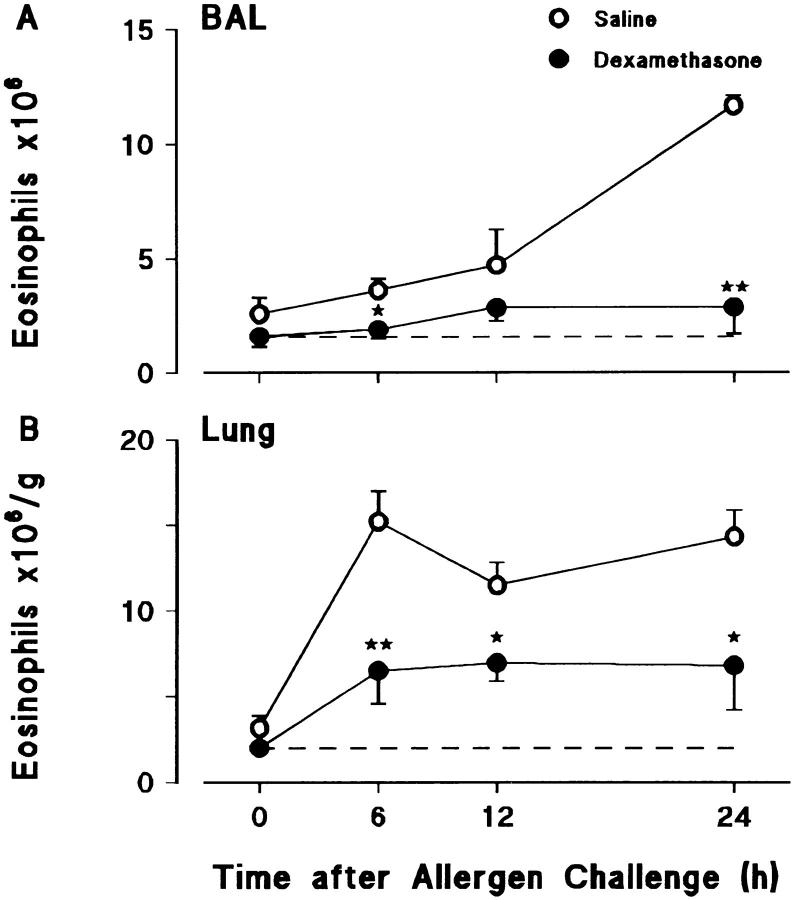
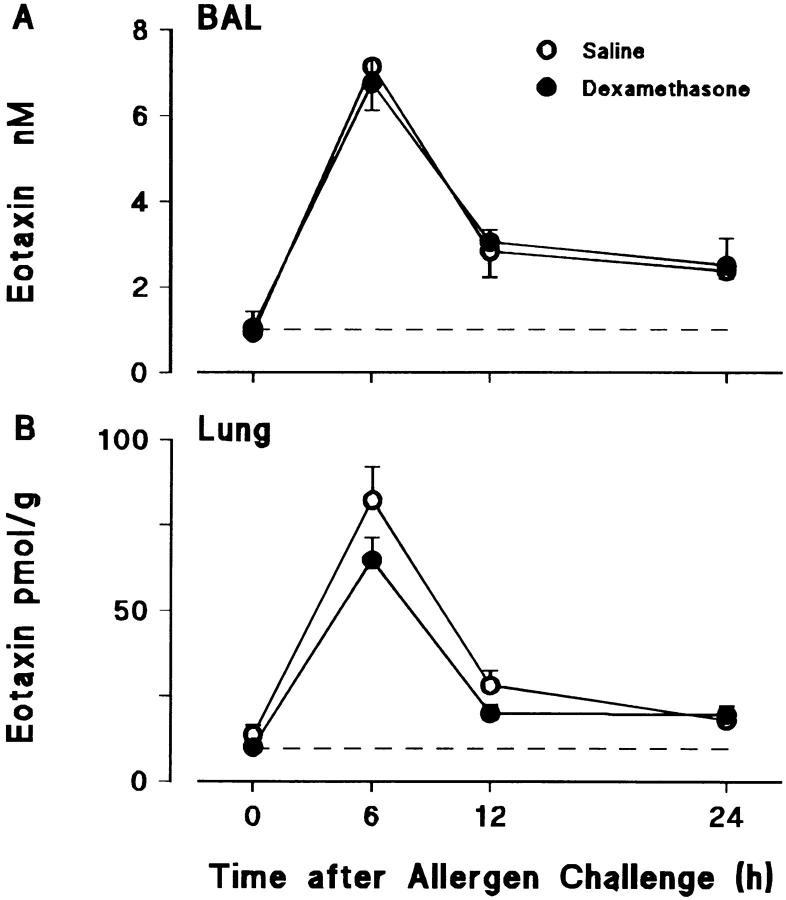
Effects of Dexamethasone on Allergen-induced Eosinophil Accumulation and Immunoreactive Eotaxin Levels in BAL Fluid and Lung Tissue.
Fig. 4 shows the effects of dexamethasone pretreatment on eosinophil accumulation in the lungs of sensitized/ovalbumin-challenged animals. The glucocorticoid markedly suppressed eosinophil accumulation in lung tissue and virtually eliminated their appearance in the BAL. However, as shown in Fig. 5, this effect was not the result of suppression of eotaxin generation, dexamethasone having no significant effect on levels of immunoreactive eotaxin.
Figure 4.
Effect of dexamethasone on allergen-induced eosinophil accumulation in (A) BAL and (B) lung tissue. Sensitized guinea pigs were injected i.p. with dexamethasone (40 mg/kg) or saline at 24 and 1 h before allergen challenge. 0 h represents sensitized/nonchallenged animals (n = 5). Results are presented as mean±SEM (n = 5–11 allergen-challenged animals/time point) and significant differences between dexamethasone- and saline-treated groups are indicated as *P <0.05 and **P <0.005. Basal eosinophil numbers in BAL and lung tissue are shown by the dashed lines.
Figure 5.
Effect of dexamethasone on allergen-induced eotaxin generation in (A) BAL fluid and (B) lung tissue. Sensitized guinea pigs were injected i.p. with dexamethasone (40 mg/kg) or saline at 24 and 1 h before allergen challenge. 0 h represents sensitized/nonchallenged animals (n = 5). Results are presented as mean±SEM (n = 5–11 allergen-challenged animals/time point). Constitutive levels of eotaxin in BAL and lung tissue are shown by the dashed lines.
Effect of a Neutralizing Antibody to IL-5 on Allergen-induced Eosinophil Accumulation and Immunoreactive Eotaxin Levels in BAL Fluid and Lung Tissue.
In a previous study (16) we found no evidence of eosinophil chemoattractant activity corresponding to IL-5 in BAL fluid from sensitized/ovalbumin-challenged guinea pigs. However, pretreatment of animals with a neutralizing antibody to IL-5, TRFK5, markedly suppressed allergen-induced eosinophil accumulation in lung tissue and BAL in sensitized animals (Fig. 6, A and B). The antibody also significantly reduced eosinophil numbers in the lung tissue of sensitized animals 6 h after aerosolized saline. TRFK5 had no significant effect on the numbers of mononuclear cells or neutrophils in BAL (data not shown) or on the levels of eotaxin in BAL fluid and lung tissue (Fig. 6, C and D).
Figure 6.
Effect of an anti–IL-5 antibody, TRFK5, on eosinophil numbers in (A) BAL and (B) lung tissue, and eotaxin levels in (C) BAL fluid and (D) lung tissue after allergen challenge. Sensitized guinea pigs were injected i.v. with control rat IgG or TRFK5 (0.3 mg/kg) 30 min before aerosol exposure to saline or allergen. Results are presented as mean±SEM (n = 5–6 animals/group) and significant differences between control IgG and TRFK5-treated groups are indicated as *P <0.05 and **P <0.005.
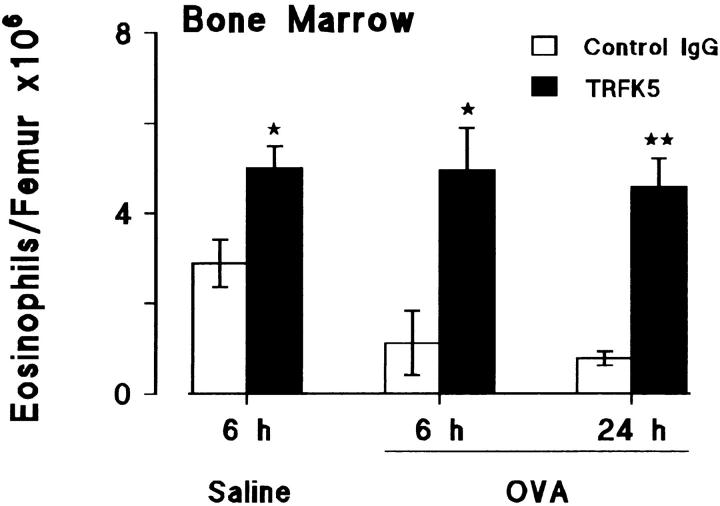
Effect of a Neutralizing Antibody to IL-5 on Numbers of Eosinophils in the Bone Marrow After Allergen Challenge.
In the same experiment as described above using TRFK5, the bone marrow in the femur was used to detect possible changes in the eosinophil population after allergen challenge. Fig. 7 (open bars) shows the marked reduction in bone marrow eosinophils in sensitized guinea pigs at 6 and 24 h after allergen challenge, compared to saline challenge at 6 h. This reduction was blocked by the antibody to IL-5. In fact, in all cases the antibody treatment increased bone marrow eosinophils when compared with numbers in the samples taken 6 h after saline (Fig. 7, closed bars) indicating that basal release of eosinophils was blocked as well as allergen-induced release.
Figure 7.
Effect of an anti–IL-5 antibody, TRFK5, on eosinophil numbers in bone marrow after allergen challenge. Sensitized guinea pigs were injected i.v. with control rat IgG or TRFK5 (0.3 mg/kg) 30 min before aerosol exposure to saline or allergen. Results are presented as mean±SEM (n = 5–6 animals/group) and significant differences between control and TRFK5-treated groups are indicated as *P <0.02 and **P <0.005.
Evidence for a Rapid Movement of Eosinophils from the Bone Marrow to the Lung in Response to Allergen Challenge.
Sensitized guinea pigs were challenged with aerosolized ovalbumin and the numbers of eosinophils monitored at intervals up to 6 h in the bone marrow of the femur, peripheral blood, and lung tissue. Allergen challenge resulted in a rapid and progressive reduction in bone marrow eosinophils which was mirrored by a marked rise in peripheral blood eosinophils (Fig. 8, A and B). The results presented in Fig. 7 indicate that this mobilization of eosinophils was mediated by endogenous IL-5. As shown in Fig. 8 C, eosinophils were rapidly recruited into the lung tissue where the eosinophil numbers correlated with increased eotaxin levels at 3 and 6 h. The speed of eosinophil recruitment into lung tissue contrasts with their slow migration into the airway lumen as evidenced by their late appearance in BAL samples (as shown in Fig. 1).
Figure 8.
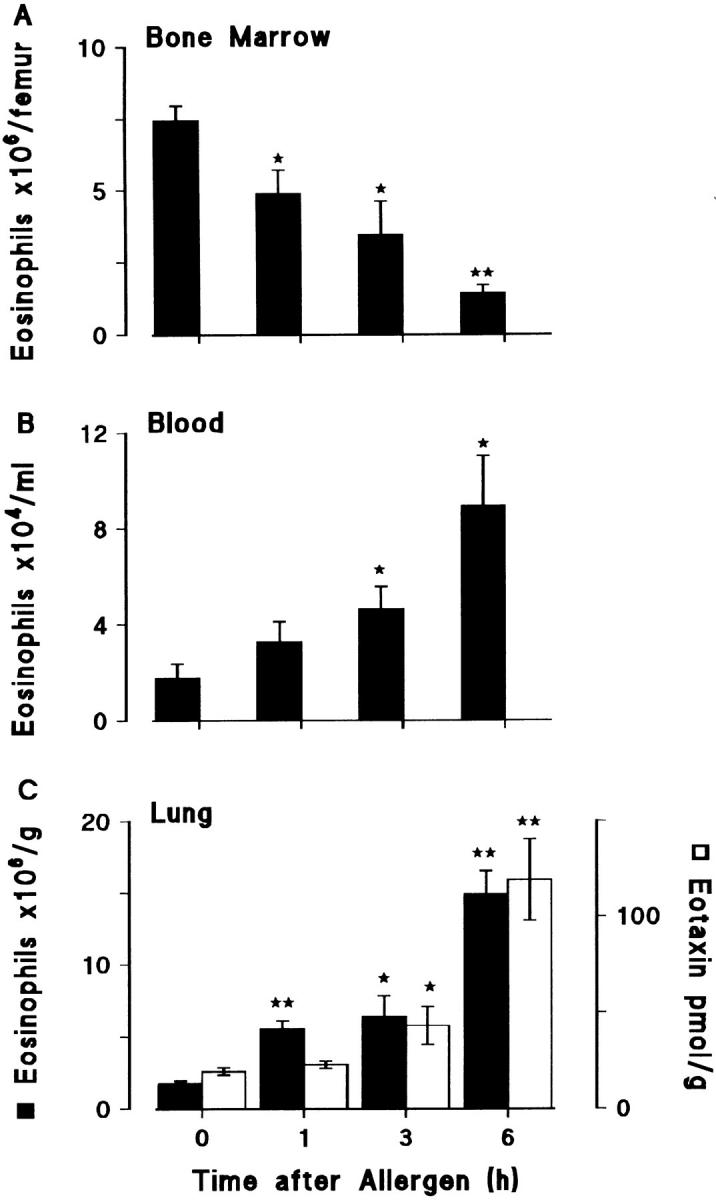
Eosinophil numbers (solid bars) in (A) femur bone marrow, (B) blood, and (C) lung tissue after allergen challenge. Sensitized guinea pigs were exposed to aerosolized ovalbumin and killed at various times after challenge. 0 h represents sensitized/nonchallenged animals. Eotaxin levels in lung tissue are shown as the open bars. Results are presented as mean±SEM (n = 4–5 animals/time point) and significant differences from 0 h are indicated as *P <0.03 and **P <0.001.
Morphological Changes and Cellular Localization of Eotaxin.
In comparison with the sensitized/nonchallenged animals, sensitized/ovalbumin-challenged guinea pig lungs showed pronounced eosinophil infiltration of airway epithelium and surrounding interstitial tissues (Fig. 9 B). The eosinophilic infiltrate was associated with both airways and pulmonary arteries. In the edematous interstitial tissues between large airways and pulmonary artery, there were also neutrophils and mononuclear cells. Goblet cell hyperplasia was evident in large intrapulmonary airways. In small (distal) airways, changes included marked epithelial hyperplasia and epithelial folding associated with airways contraction and obstruction of the airway lumina. The parenchyma of the lung was congested and macrophages in alveoli surrounding the airways were increased in number.
Figure 9.
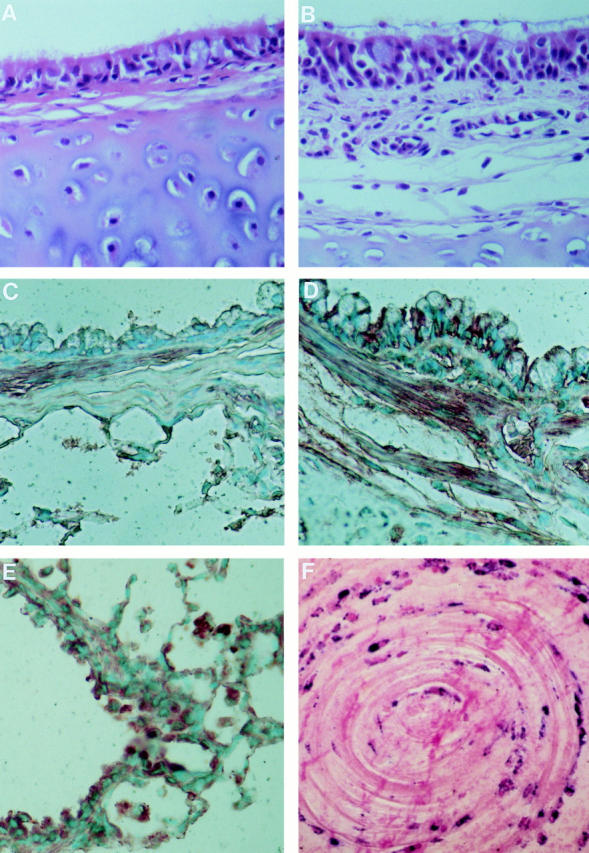
(A and B) Hematoxylin and eosin and (C–E) immunostaining for eotaxin in sections of hilar intrapulmonary airways from (A and C) sensitized/nonchallenged and (B and D) sensitized guinea pigs 3 h after allergen challenge. In the nonchallenged animals the epithelial and subepithelial tissues have few eosinophils while there is heavy infiltration of the mucosa by eosinophils after challenge. Immunolabeling with polyclonal antieotaxin IgG shows relatively weak staining in the absence of challenge compared with strong immunopositivity of epithelial cells, inflammatory cell infiltrate, and bronchial smooth muscle after challenge. The strong immunostaining of macrophages in the alveoli, after challenge, is demonstrated in E. (F) Immunohistology of an airway plug from a patient who had a fatal asthma attack. The sample was fixed in formalin, embedded in paraffin, immunostained with EG2, and counter-stained with nuclear fast red. There are concentric rings of activated eosinophils as shown by their immunopositivity for EG2 (41, 42). Magnification: A–D ×640, E ×320, F ×600.
Immunohistochemical staining with anti–guinea pig eotaxin was strongest and most abundant in the lungs of sensitized/ovalbumin-challenged animals (Fig. 9 D). In the large airways, eotaxin positivity was present in the airway epithelium and appeared to be confined to ciliated cells and the basal aspects of other epithelial cells. Bronchial smooth muscle immunostained strongly while vascular smooth muscle of large pulmonary vessels was negative. However, bronchial vessels in the lamina propria and the submucosa were positive. In bronchioli, the positive staining was localized to nonciliated epithelial (Clara) cells. Alveolar macrophages showed strong positivity only in the allergen-challenged animals (Fig. 9 E). There were also eotaxin-positive inflammatory cells in the tissue but the cell type could not be identified in this study. Sensitized/nonchallenged and naive animals showed constitutive staining particularly of bronchial smooth muscle and cartilage chondrocytes, and weak staining of apices of epithelial cells and, only occasionally, of alveolar macrophages. The negative control sections, in which the antieotaxin IgG was replaced by control IgG, showed very weak diffuse staining not localized to any tissue structure. A detailed light and electron microscopic study of guinea pig eotaxin immunolocalization is to be published separately.
Discussion
We have analyzed the kinetics of eosinophil accumulation in the lung using a model of allergic airways inflammation and have examined the association with levels of immunoreactive eotaxin. Previous extensive investigations in animals and humans have demonstrated that allergen challenge induces an immediate mast cell–mediated acute bronchospasm and a late bronchospasm associated with appearance of eosinophils. In these studies sampling by BAL revealed an apparent protracted latent period before the delayed accumulation of eosinophils (8, 11, 13–15, 34, 35). These results are consistent with those in Fig. 1, which shows two apparently distinct phases of the allergic response: an early phase of increased microvascular permeability as evidenced by increasing concentrations of albumin in BAL fluid, and a delayed phase of eosinophil accumulation in BAL from 12–24 h. This eosinophil influx into the airway lumen is apparently at variance with the kinetics of eotaxin levels: BAL and tissue eotaxin levels peaked at 6 h and fell to low levels by 12 h (Fig. 2).
Measurement of changes in lung tissue (Figs. 2 B and 8 C) throws light upon this apparent disassociation. We suggest the following sequence of events. Allergen challenge stimulates mast cell activation; degranulation products induce acute bronchoconstriction, and contribute to mucus secretion and increased microvascular permeability. Plasma proteins can readily pass through the airway epithelium and were detected in this study as increasing albumin concentrations over 0–6 h in BAL fluid (Fig. 1). Mast cell products may also mediate very early eosinophil accumulation: adherent eosinophils were seen histologically in microvessels within a few minutes of challenge in a previous study (12) and a significant increase in tissue eosinophils was detected at 1 h in our investigation (Fig. 8 C). Lung tissue eotaxin was significantly elevated at 3 h after allergen challenge, rising to a peak at 6 h and falling to low levels by 12 h (Fig. 2 B). Immunostaining suggested upregulation, particularly in airway epithelial cells and inflammatory cells such as macrophages (Fig. 9). Eosinophil levels in the tissue closely followed eotaxin levels up to 6 h, but the cells persisted until the last measurement time point at 24 h (Figs. 2 B and 8 C) probably influenced by factors increasing cell survival such as IL-5 (see below). Eotaxin generated in the tissue would be expected to pass from and through the airway epithelium into the lumen and indeed a similar eotaxin time course as in the tissue was seen in BAL fluid (Fig. 2 B). Close inspection of the eotaxin levels in BAL fluid and lung tissue (Fig. 2) suggests that eotaxin is cleared or metabolized faster from the lung tissue than from the lumen. Thus, while eotaxin is being generated in the lung tissue, a gradient between the tissue and the microvessel lumen will facilitate traffic across the vascular endothelium. However, during active chemokine secretion the eotaxin concentration in the fluid lining the airway will be lower than in the tissue and the direction of the gradient will inhibit migration into the airway lumen. Subsequently, when eotaxin secretion ceases, clearance and metabolism in the tissue may reverse the gradient across the epithelium, thus facilitating migration into the airway lumen over the 12–24 h period.
The immunoreactive eotaxin in the BAL fluid from ovalbumin-challenged and saline-challenged animals was active as a chemoattractant. Concentrated BAL fluid injected intradermally into bioassay animals induced 111In-eosinophil accumulation. This activity was virtually abolished when the sample was mixed with a neutralizing antibody to eotaxin (Fig. 3 B). It has been shown previously that the guinea pig lung has a constitutive eotaxin mRNA signal, suggesting that eotaxin may be responsible for maintaining the basal level of eosinophils found in the lung in this species (23, 24). This is supported by eotaxin protein levels measured by immunoassay and skin bioassay in samples from naive and sensitized/saline-exposed animals (Fig. 3 B). Immunostaining showed constitutive eotaxin particularly in cartilage chondrocytes and bronchial smooth muscle cells, the latter now recognized as a source of chemokines (36). Although the neutralizing antibody blocked the eosinophil chemoattractant activity in BAL fluid from sensitized/allergen-challenged animals, we were unable to suppress eosinophil accumulation in the lung when the antibody was administered intravenously (Humbles, A.A., unpublished data). The reason for this is unclear, but the results could reflect a contribution from other (possibly unstable) chemoattractants. Alternatively, eotaxin bound to extracelluar matrix in the lung may act as a sink for antibody. Higher affinity antibodies may be required for effective systemic treatment.
We were interested in possible effects of an antiinflammatory compound on eotaxin generation in the allergen challenge model. Dexamethasone suppressed eosinophil accumulation in the lung tissue and airways but had no significant effect on eotaxin levels (Figs. 4 and 5). This suggests that the glucocorticoid inhibited eosinophil migration through the microvascular endothelium by some mechanism independent of eotaxin release in this model. This may at least be partly due to inhibition of the release or action of IL-5, which is important in this model (see below). Inhibition of eosinophil accumulation into the airway lumen was even greater than into tissue, indicating a further action of dexamethasone on eosinophil migration through the airway epithelium. The reported inhibitory effects of glucocorticoids on eosinophil survival (37, 38) may contribute to the latter effect. An incidental observation from these experiments is that eosinophils themselves are unlikely to be a major source of lung eotaxin over the time period studied because marked suppression of eosinophil accumulation had no detectable effect on eotaxin levels.
IL-5 has an important role in eosinophil accumulation in allergen-challenged lungs. As shown in Fig. 6, an IL-5 neutralizing antibody, TRFK5, effectively suppressed eosinophil accumulation in the lung tissue and BAL after allergen challenge. Other laboratories have taken such results as evidence that IL-5 is an important eosinophil chemoattractant. However, we found no evidence of IL-5 eosinophil chemoattractant activity in HPLC fractions of BAL fluid from sensitized/challenged guinea pigs in previous studies (16, 39). Further, endogenous IL-5 does not appear to regulate eotaxin generation as TRFK5 had no effect on eotaxin levels in the lung (Fig. 6). We have shown recently that intravenous IL-5 rapidly releases eosinophils from the bone marrow into the blood of naive guinea pigs and that this potentiates eosinophil recruitment into skin sites injected with eotaxin (25). The results presented here suggest that this phenomenon extrapolates to the allergic inflammation model, as allergen challenge of the lung resulted in a decrease in bone marrow eosinophils (Figs. 7 and 8). The eosinophils progressively moved out of the bone marrow, beginning within 1 h of aerosolized allergen challenge, and were seen in higher numbers in the blood and lung within 3 h. This release of eosinophils appears to be mediated by IL-5 since it was inhibited by TRFK5. In fact, TRFK5 increased the retention of bone marrow eosinophils with or without allergen challenge (Fig. 7) suggesting that constitutive IL-5 regulates basal release of eosinophils. IL-5 will also have effects other than causing acute release of a preformed pool of eosinophils, e.g., in the short term priming eosinophils for recruitment, in the longer term prolonging eosinophil survival in the tissue and inducing proliferation and differentiation of bone marrow eosinophils. Our results using TRFK5 are consistent with those of Foster et al. (40) who have shown that the eosinophilia, lung damage, and airways hyperresponsiveness resulting from allergen challenge were abolished in IL-5–deficient mice.
The results presented here suggest that allergen provocation of the lung in the sensitized guinea pig stimulates a rapid movement of eosinophils from the bone marrow via the blood to the lung (Fig. 8) which involves the cooperative effort of IL-5 and eotaxin. We propose that allergen stimulates the production of IL-5 and eotaxin in the lung. IL-5 is carried by the blood to the bone marrow where it mobilizes eosinophils into the circulation. Eotaxin then has the role of recruiting eosinophils from the microvasculature. These effects begin surprisingly rapidly after allergen challenge and the onset of eosinophil recruitment occurs during the early phase of microvascular protein leakage and increased mucus secretion. When sampling from the airway lumen there is an apparent wide separation between early and late inflammatory components of the allergic response, as shown in Fig. 1. We suggest that this is largely artifactual: protein leaking from blood microvessels moves rapidly through the airway epithelium, whereas the movement of eosinophils into the airway lumen is delayed, giving the late cellular response. The numbers of activated eosinophils in the airway wall would be expected to be most relevant to changes in the lung function. Indeed, experimentally and clinically the onset of the late bronchoconstrictor response occurs at 4–12 h, corresponding to the phase of high tissue eosinophilia in our study.
If similar events occur in the lungs of asthmatic patients it is not difficult to imagine repeated allergen provocation giving rise to waves of infiltration of eosinophils and increasing damage to lung tissue. Asynchronous phases of protein and eosinophil movement into the airway lumen would be predicted by analogy with the results in Fig. 1. This could give rise to the concentric rings of protein and eosinophils seen in airway plugs of fatal asthma as graphically illustrated in Fig. 9 F.
We suggest that eotaxin and related chemokines acting through the eotaxin CCR3 receptor may play a major role in eosinophil recruitment into the asthmatic lung and thus offer an attractive target for therapeutic intervention.
Acknowledgments
We are indebted to Dr. Christine Power and Dr. Bernard Allet (Glaxo-Wellcome Molecular, Geneva, Switzerland) for their help in producing recombinant eotaxin and to Dr. Paul Hiss (Glaxo-Wellcome, Stevenage, United Kingdom) for the gift of TRFK5.
We are indebted to the Wellcome Trust, the National Asthma Campaign, Allen & Hanburys, the European Commission, and Glaxo-Wellcome Molecular, Geneva, for generously supporting this research. Dr. S. Marleau was a visiting fellow from the Faculty of Pharmacy, University of Montreal, Montreal, Quebec, Canada.
Footnotes
Abbreviations used in this paper: BAL, bronchoalveolar lavage; EPO, eosinophil peroxidase; i.d., intradermally.
References
- 1.Metzger WJ, Zavala D, Richerson HB, Moseley P, Iwamota P, Monick M, Sjoerdsma K, Hunninghake GW. Local allergen challenge and bronchoalveolar lavage of allergic asthmatic lungs. Am Rev Respir Dis. 1987;135:433–440. doi: 10.1164/arrd.1987.135.2.433. [DOI] [PubMed] [Google Scholar]
- 2.de Monchy JGR, Kauffman HF, Venge P, Koeter GH, Jansen HM, Sluiter HJ, de Vries K. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am Rev Respir Dis. 1985;131:373–376. doi: 10.1164/arrd.1985.131.3.373. [DOI] [PubMed] [Google Scholar]
- 3.Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian MN, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, Michel P-B. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 4.Bradley BL, Azzawi M, Jacobson M, Assoufi B, Collins JV, Irani A-MA, Schwartz LB, Durham SR, Jeffery PK, Kay AB. Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J Allergy Clin Immunol. 1991;88:661–674. doi: 10.1016/0091-6749(91)90160-p. [DOI] [PubMed] [Google Scholar]
- 5.Gleich GJ, Flavahan NA, Fujisawa T, Vanhoutte PM. The eosinophil as a mediator of damage to respiratory epithelium: a model for bronchial hyperreactivity. J Allergy Clin Immunol. 1988;81:776–781. doi: 10.1016/0091-6749(88)90931-1. [DOI] [PubMed] [Google Scholar]
- 6.Wardlaw AJ, Dunnette S, Gleich GJ, Collins JV, Kay AB. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am Rev Respir Dis. 1988;137:62–69. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]
- 7.Dahl R, Venge P, Olsson I. Variations of blood eosinophils and eosinophil cationic protein in serum in patients with bronchial asthma. Allergy. 1978;33:211–215. doi: 10.1111/j.1398-9995.1978.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 8.Sedgwick JB, Calhoun WJ, Gleich GJ, Kita H, Abrams JS, Schwartz LB, Volovitz B, Ben-Yaakov M, Busse WW. Immediate and late airway response of allergic rhinitis patients to segmental antigen challenge. Am Rev Respir Dis. 1991;144:1274–1281. doi: 10.1164/ajrccm/144.6.1274. [DOI] [PubMed] [Google Scholar]
- 9.Erjefalt I, Persson CGA. Inflammatory passage of plasma macromolecules into airway wall and lumen. Pulm Pharmacol. 1989;2:93–102. doi: 10.1016/0952-0600(89)90030-6. [DOI] [PubMed] [Google Scholar]
- 10.Chung KF, Rogers DF, Barnes PJ, Evans TW. The role of increased airway microvascular permeability and plasma exudation in asthma. Eur Respir J. 1990;3:329–337. [PubMed] [Google Scholar]
- 11.Liu MC, Hubbard WC, Proud D, Stealey BA, Galli SJ, Kagey-Sobotka A, Bleecker ER, Lichtenstein LM. Immediate and late inflammatory responses to ragweed antigen challenge of the peripheral airways in allergic asthmatics. Cellular, mediator, and permeability changes. Am Rev Respir Dis. 1991;144:51–58. doi: 10.1164/ajrccm/144.1.51. [DOI] [PubMed] [Google Scholar]
- 12.Dunn CJ, Elliott GA, Oostveen JA, Richards IM. Development of a prolonged eosinophil-rich inflammatory leukocyte infiltration in the guinea-pig asthmatic response to ovalbumin inhalation. Am Rev Respir Dis. 1988;137:541–547. doi: 10.1164/ajrccm/137.3.541. [DOI] [PubMed] [Google Scholar]
- 13.Huston PA, Church MK, Clay TP, Miller P, Holgate ST. Early and late-phase bronchoconstriction after allergen challenge of nonanesthetized guinea pigs. 1. The association of disordered airway physiology to leukocyte infiltration. Am Rev Respir Dis. 1988;137:548–557. doi: 10.1164/ajrccm/137.3.548. [DOI] [PubMed] [Google Scholar]
- 14.Sanjar S, Aoki S, Kristersson A, Smith D, Morley J. Antigen challenge induces pulmonary airway eosinophil accumulation and airway hyperreactivity in sensitized guinea-pigs: the effect of anti-asthma drugs. Br J Pharmacol. 1990;99:679–686. doi: 10.1111/j.1476-5381.1990.tb12989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boichot E, Lagente V, Carre C, Waltmann P, Mencia-Huerta JM, Braquet P. Bronchial hyperresponsiveness and cellular infiltration in the lung of guinea-pigs sensitized and challenged by aerosol. Clin Exp Allergy. 1991;21:67–76. doi: 10.1111/j.1365-2222.1991.tb00806.x. [DOI] [PubMed] [Google Scholar]
- 16.Jose PJ, Griffiths-Johnson DA, Collins PD, Walsh DT, Moqbel R, Totty NF, Truong O, Hsuan JJ, Williams TJ. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea-pig model of allergic airways inflammation. J Exp Med. 1994;179:881–887. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothenberg ME, Luster AD, Leder P. Murine eotaxin: an eosinophil chemoattractant inducible in endothelial cells and in interleukin 4-induced tumor suppression. Proc Natl Acad Sci USA. 1995;92:8960–8964. doi: 10.1073/pnas.92.19.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalo J-A, Jia G-Q, Aguirre V, Friend D, Coyle AJ, Jenkins NA, Lin G-S, Katz H, Lichtman A, Copeland N, et al. Mouse eotaxin expression parallels eosinophil accumulation during lung allergic inflammation but it is not restricted to a Th2-type response. Immunity. 1996;4:1–14. doi: 10.1016/s1074-7613(00)80293-9. [DOI] [PubMed] [Google Scholar]
- 19.Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam N, Smith H, Shi X, Gonzalo J-A, Newman W, et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–612. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med. 1996;2:449–456. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- 21.Daugherty BL, Siciliano SJ, DeMartino J, Malkowitz L, Sirontino A, Springer MS. Cloning, expression and characterization of the human eosinophil eotaxin receptor. J Exp Med. 1996;183:2349–2354. doi: 10.1084/jem.183.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponath PD, Qin S, Post TW, Wang J, Wu L, Gerard NP, Newman W, Gerard C, Mackay CR. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med. 1996;183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jose PJ, Adcock IM, Griffiths-Johnson DA, Berkman N, Wells TNC, Williams TJ, Power CA. Eotaxin: cloning of an eosinophil chemoattractant cytokine and increased mRNA expression in allergen-challenged guinea-pig lungs. Biochem Biophys Res Commun. 1994;205:788–794. doi: 10.1006/bbrc.1994.2734. [DOI] [PubMed] [Google Scholar]
- 24.Rothenberg ME, Luster AD, Lilly CM, Drazen JM, Leder P. Constitutive and allergen-induced expression of eotaxin mRNA in the guinea pig lung. J Exp Med. 1995;181:1211–1216. doi: 10.1084/jem.181.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins PD, Marleau S, Griffiths-Johnson DA, Jose PJ, Williams TJ. Cooperation between interleukin-5 and the chemokine, eotaxin, to induce eosinophil accumulation in vivo. J Exp Med. 1995;182:1169–1174. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takamatsu S, Horie T, Mishima T, Minamide Y, Hayashi M, Awazu S. Heterogeneity of rat plasma albumin and drug binding. Biochem Pharmacol. 1990;39:1205–1212. doi: 10.1016/0006-2952(90)90264-l. [DOI] [PubMed] [Google Scholar]
- 27.Das AM, Williams TJ, Lobb RR, Nourshargh S. Lung eosinophilia is dependent on IL-5, and the adhesion molecules CD18 and VLA-4 in a guinea-pig model. Immunology. 1995;84:41–46. [PMC free article] [PubMed] [Google Scholar]
- 28.Proudfoot AEI, Power CA, Hoogewerf A, Montjovent MO, Borlat F, Wells TNC. Characterisation of the RANTES/MIP-1 alpha receptor (CC CKR-1) stably transfected in HEK 293 cells and the recombinant ligands. FEBS (Fed Eur Biochem Soc) Lett. 1995;376:19–23. doi: 10.1016/0014-5793(95)01235-x. [DOI] [PubMed] [Google Scholar]
- 29.Steinbuch M, Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969;134:279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- 30.Hjelm H, Hjelm K, Sjoquist J. Protein A from Staphylococcus aureus.Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobins. FEBS (Fed Eur Biochem Soc) Lett. 1972;28:73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- 31.Collins PD, Jose PJ, Williams TJ. The sequential generation of neutrophil chemoattractant proteins in acute inflammation in the rabbit in vivo. Relationship between C5a and proteins with the characteristics of IL-8/neutrophil-activating protein 1. J Immunol. 1991;146:677–684. [PubMed] [Google Scholar]
- 32.Jose PJ, Moss IK, Maini RN, Williams TJ. Measurement of the chemotactic complement fragment C5a in rheumatoid synovial fluids by radioimmunoassay: role of C5a in the acute inflammatory phase. Ann Rheum Dis. 1990;49:747–752. doi: 10.1136/ard.49.10.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faccioli LH, Nourshargh S, Moqbel R, Williams FM, Sehmi R, Kay AB, Williams TJ. The accumulation of 111In-eosinophils induced by inflammatory mediators in vivo. Immunology. 1991;73:222–227. [PMC free article] [PubMed] [Google Scholar]
- 34.Lefort J, Nahori A-M, Ruffié C, Vargaftig BB, Pretolani M. In vivo neutralization of eosinophil-derived major basic protein inhibits antigen-induced bronchial hyperreactivity in sensitized guinea pigs. J Clin Invest. 1996;97:1117–1121. doi: 10.1172/JCI118505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalo J-A, Lloyd CM, Kremer L, Finger E, Martinez-A. C, Siegelman MH, Cybulsky MI, Gutierrez-Ramos J-C. Eosinophil recruitment to the lung in a murine model of allergic inflammation. The role of T cells, chemokines and adhesion receptors. J Clin Invest. 1996;98:2332–2345. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.John M, Hirst SJ, Jose PJ, Robichaud A, Berkman N, Witt C, Twort CHC, Barnes PJ, Fan K, Chung Human airway smooth muscle cells express and release RANTES in response to T helper 1 cytokines: regulation by T helper 2 cytokines and corticosteroids. J Immunol. 1997;158:1841–1847. [PubMed] [Google Scholar]
- 37.Wallen N, Kita H, Weiler D, Gleich GJ. Glucocorticoids inhibit cytokine-mediated eosinophil survival. J Immunol. 1991;147:3490–3495. [PubMed] [Google Scholar]
- 38.Meagher LC, Cousin JM, Seckl JR, Haslett C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol. 1996;156:4422–4428. [PubMed] [Google Scholar]
- 39.Griffiths-Johnson DA, Collins PD, Rossi AG, Jose PJ, Williams TJ. The chemokine, eotaxin, activates guinea-pig eosinophils in vitro, and causes their accumulation into the lung in vivo. Biochem Biophys Res Commun. 1993;197:1167–1172. doi: 10.1006/bbrc.1993.2599. [DOI] [PubMed] [Google Scholar]
- 40.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeffery PK. Pathology of asthma. Br Med Bull. 1992;48:23–39. doi: 10.1093/oxfordjournals.bmb.a072537. [DOI] [PubMed] [Google Scholar]
- 42.Jeffery, P.K. 1997. Airway pathology in asthma. In Asthma: Basic Mechanisms and Clinical Management, 3rd edition. P.J. Barnes, I.W. Rodger, and N.C. Thomson, editors. Academic Press, London. In press.