Abstract
Isolation of large numbers of surface IgD+CD38− naive and surface IgD−CD38− memory B cells allowed us to study the intrinsic differences between these two populations. Upon in vitro culture with IL-2 and IL-10, human CD40–activated memory B cells undergo terminal differentiation into plasma cells more readily than do naive B cells, as they give rise to five- to eightfold more plasma cells and three- to fourfold more secreted immunoglobulins. By contrast, naive B cells give rise to a larger number of nondifferentiated B blasts. Saturating concentrations of CD40 ligand, which fully inhibit naive B cell differentiation, only partially affect that of memory B cells. The propensity of memory B cells to undergo terminal plasma cell differentiation may explain the extensive extra follicular plasma cell reaction and the limited germinal center reaction observed in vivo after secondary immunizations, which contrast with primary responses in carrier-primed animals. This unique feature of memory B cells may confer two important capacities to the immune system: (a) the rapid generation of a large number of effector cells to efficiently eliminate the pathogens; and (b) the prevention of the overexpansion and chronic accumulation of one particular memory B cell clone that would freeze the available peripheral repertoire.
Memory B lymphocytes are mainly generated in the germinal centers (GCs)1 of secondary lymphoid organs (1–8). Within these structures, proliferating B blasts can increase the affinity of their surface Igs through somatic mutation of their Ig variable region genes and positive selection of high affinity mutants (9–16). Isotype switch of the Igs can also occur during GC reaction (17–19). After leaving the GCs, memory B cells either join the recirculating pool of lymphocytes, or home to antigen draining sites such as the marginal zone of the spleen (20). Memory B cells display several intrinsic differences with naive B cells: (a) lower threshold for activation, (b) ability to directly present antigen to helper T cells, and (c) longer life span (21–27). Although these features of memory B cells are essential for the immune system to make a robust secondary antibody response, they may lead to an overexpansion of a particular memory B cell clone, which would overload the immune system during chronic infections or antigenic stimulations (26). Several hypotheses can be put forward to explain how the immune system controls the size of memory cell clones: (a) decreasing potential hypothesis: memory (T and B) cells generated after each round of stimulation acquire a decreased potential to generate new memory cells and an increased potential to undergo terminal differentiation into effector cells (26, 27), (b) growth factor/costimulatory signal starvation hypothesis: at the late stage of immune responses, the clonally expanded memory blasts (T and B) undergo apoptosis in the absence of growth factors/ costimulating molecules (26, 27), and (c) hypothesis of Fas ligand–mediated apoptosis: T cells can undergo autocrine Fas ligand–mediated apoptosis (28–30), and B cells can be sensitized to Fas ligand–mediated apoptosis by CD40 triggering (31).
Recently, large amounts of human memory B cells have been purified from human tonsils and blood, based on their IgD−CD38− or IgM+IgD− or IgA1+ phenotypes (32–36). These cells contained somatically mutated IgV genes, an indication of their germinal center origin. The isolation of these memory B cells allowed us to directly test the decreasing potential hypothesis by culturing memory and naive B cells in vitro. Herein, we describe a novel important feature of memory B cells: their bias towards terminal plasma cell differentiation.
Materials and Methods
Antibodies and Reagents.
The mouse mAbs used for the phenotypic studies were FITC-conjugated anti-CD20 (IOB20; Immunotech, Marseille, France) and PE-conjugated anti-CD38 (Leu17; Becton Dickinson Monoclonal Center, Mountain View, CA). Antibodies used for cell purification and cell culture were anti-CD4 (Q4120) and biotinylated anti-IgD (HJ9) purchased from Sigma Chemical Co. (St. Louis, MO), anti-CD38 (T16), anti-Igκ (6E1), and anti-Igλ (C4) purchased from Immunotech, and anti-CD2, -CD3, -CD8 ascites produced in our own laboratory using the OKT hybridomas obtained from American Type Culture Collection (Rockville, MD). Antibodies used for immunoenzymatic stainings are described in the corresponding section. Anti-CD40 ligand (LL48) -blocking mAb and CD40 ligand (CD40L) -transfected murine fibroblasts were produced in our laboratory (31).
Recombinant human IL-2 was purchased from Amgen Biologicals (Thousand Oaks, CA) and recombinant human IL-10 is from Schering-Plough Research Institute (Kenilworth, NJ). IL-2 was used at 10 U/ml and IL-10 at 100 ng/ml in cultures.
Giemsa-Gurr and Mayer's hematoxylin staining solutions were purchased from BDH Laboratory Supplies (Poole, England) and Sigma Chemical Co., respectively.
Purification of B Cell Populations.
Naive and memory B cells were purified from human tonsils obtained from children undergoing routine tonsillectomy, as previously described (33). In brief, tonsils were finely minced in RPMI 1640 (GIBCO BRL, Paisley, UK). Cell suspension was washed twice and T cells were depleted by sheep RBC rosetting and centrifugation at room temperature on ditrizoate-ficoll (density = 1,077; Eurobio, Les Ulis, France). B cells were then incubated with biotinylated anti–human IgD antibodies. For naive cell purification, two rounds of positive selection were performed with a magnetic activated cell sorter (MACS®; Miltenyi Biotec, Bergisch Gladbach, Germany). For memory cell preparation, two rounds of negative magnetic beads depletion (Streptavidin-coated Dynabeads; Dynal, Oslo, Norway) were performed. Both resulting IgD+ and IgD− populations were further depleted of T cells and CD38+ (i.e., GC) B cells by incubation with anti-CD2, -CD3, -CD4, -CD8, and -CD38 antibodies followed by two rounds of depletion with anti–mouse IgG-coated magnetic beads (Dynal). This procedure lead to 98–99.5% pure naive and 95–99.5% pure memory B cell populations.
Proliferation Assays.
For DNA synthesis, 2.5 × 104 B cells were cultured together with 5 × 103, 75 Gy–irradiated, CD40L– transfected fibroblasts in 200 μl Iscove medium (GIBCO) complemented with 5% FCS (GIBCO) for 12 d in the presence of IL-2 and IL-10. DNA synthesis was assessed by incubation with 1 μCi of tritiated thymidine (Amersham, Les Ulis, France) during the last 8 h of culture. For cellular expansion, 1.5 × 105 B cells were cultured with 5 × 104 CD40L–transfected fibroblasts for 12 d in the presence of IL-2 and IL-10. Cells were harvested and counted in tripan blue (GIBCO) to exclude dead cells.
Two-step Cell Cultures.
1.5–2 × 107 purified naive or memory B cells were cultured for 3 d in 20 ml Iscove medium complemented with 5% FCS in the presence of IL-2, IL-10, and CD40L–transfected fibroblasts (5:1, B cells/fibroblast). Cells were then harvested, washed, and recultured with or without CD40L. In another set of experiments, anti-Igλ and Igκ antibodies were used to trigger B cell receptor (BCR) at 2 μg/ml final concentration. Secondary cultures consisted of 1.5 × 105 B cells in 1 ml Iscove medium containing IL-2 and IL-10, together with 5 × 104 irradiated murine fibroblasts. Murine fibroblasts were either CD40L–transfected cells or nontransfected cells together with anti-CD40L–blocking antibody at 2 μg/ml to block the signals given by CD40L–transfected cells that could have been harvested from the primary cultures. All secondary cultures were done in triplicate. After 4 d, cultures were harvested, supernatants frozen for antibody titer assays, and cells kept for analysis.
Quantitation of CD40L Molecules on Murine Fibroblasts. The number of CD40L molecules expressed on transfected fibroblasts was estimated using a Qifikit® system (Dako, Goldstrup, Denmark) immediately before establishment of cultures. In brief, cells were incubated at saturation with either an anti-CD40L mAb (IgG1 isotype) or a nonrelevant control-matched antibody for 20 min on ice. After two washes, they were incubated with FITC-conjugated sheep anti–mouse immunoglobulins at the same time as different beads suspensions, coated with a known number of mouse Igs. Cells and beads were then analyzed using a FACScan® (Becton Dickinson, Sunnyvale, CA). Means of fluorescence intensity were then plotted against the number of mouse Igs on beads and linear regression was calculated (r2 ⩾0.998 in all experiments). The number of recognized molecules (CD40L) on stained fibroblasts was calculated using the linear regression and the fluorescence intensity of these cells, after taking account of the fluorescence of the cells stained with the control-matched antibody.
Cell Cultures with Progressive Triggering of CD40.
To assess the effect of progressive triggering of CD40 antigen on naive and memory B cells, a second two-step culture was established. Cells were grown in primary cultures as in the previous two-step culture system. After 3 d, cells were recultured under seven different conditions. As the number of CD40L molecules on transfected fibroblasts varies from one experiment to another, a fixed cell ratio, rather than a fixed number of molecules, was chosen to avoid differences in the fibroblast feeder effects. Therefore, 1.5 × 105 B cells were cultured for 4 d in 1 ml Iscove medium containing IL-2 and IL-10, together with 5 × 104 irradiated fibroblasts. One culture condition was established with CD40L-transfected cells whose CD40L molecules number has been determined. These cells are then diluted with nontransfected irradiated fibroblasts for other culture conditions at the ratios of 1/2, 1/4, 1/8, and 1/16. Two other cultures were also set using parental cells, with or without anti-CD40L antibody at 2 μg/ml. All secondary cultures were set in triplicates and designed as the number of CD40L molecules present in the culture per B cell.
Ig Secretion Assays.
IgA, IgG, and IgM concentrations in culture supernatants were measured using ELISA. Total Ig levels are given as the summation of these values.
Cell Sorting.
Naive and memory cells were cultured for 3 d in the presence of IL-2, IL-10, and CD40L-transfected fibroblasts. They were then harvested and recultured for an additional 4 d with IL-2, IL-10, and parental fibroblasts. After harvesting, debris and dead cells were depleted from the cultures by centrifugation on ditrizoate-ficoll (Eurobio). Cells were then stained with FITC-conjugated anti-CD20 and PE-conjugated anti-CD38 antibodies. Both CD20−/lowCD38high and CD20+CD38− populations were sorted using a FACStar® (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Giemsa and Immunoenzymatic Stainings.
7 × 104 sorted cells were cytocentrifuged on microscope slides. Some slides were stained with Giemsa-Gurr solution, whereas others were kept for immunoenzymatic staining. Human Igs were revealed by anti– human κ and λ light chain antibodies (A8B5 and N10/2 clones, respectively, IgG1 isotypes; Dako), whereas IgM isotype Igs were revealed by anti–human IgM mAb (145-8, IgG1 isotype; Becton Dickinson Monoclonal Center). Enzymatic activity was developed with Fast Red substrate (Dako). All immunoenzymatically colored slides were lightly counterstained with Mayer's hematoxylin solution.
Results
Memory B Cells Undergo Prompt Differentiation into Plasma Cells upon Activation.
Using a two-step culture system, we previously demonstrated that continuous triggering of CD40 antigen on GC cells inhibits their terminal differentiation into plasma cells (PC; 37). To determine the influence of CD40L on the capacity of memory and naive B cells to generate PCs, similar culture conditions were used. Both populations were cultured for 3 d over CD40L-transfected fibroblasts in the presence of IL-2 and IL-10. Activated B cell blasts were then recultured for 4 d with nontransfected fibroblasts, IL-2, IL-10, and an anti-CD40L–blocking antibody to block the CD40L-transfected fibroblasts carried over from the primary culture. Although naive B cells yielded 16.4 ± 6.6% CD20−/lowCD38high plasma cells (mean ± SD, n = 7; Fig. 1 B; Table 1), memory B cells yielded 62.4 ± 11.9% plasma cells (mean ± SD, n = 4; Fig. 1 D; Table 1). Accordingly, naive B cells yielded three times more nondifferentiated CD20+CD38low B blasts than did memory cells. Addition of CD40L during the secondary culture (Fig. 1, A and C) considerably inhibited the plasma cell differentiation of B cell blasts, generated from both naive and memory cells (Table 1).
Figure 1.
Naive and memory cells differentiation at the end of secondary cultures, followed by CD20 and CD38 expression. PCs are CD20−/lowCD38high and nondifferentiated B blasts (BLASTS) are CD20+CD38low (37). (A) Naive B cells cultured with IL-2 and IL-10 over CD40L-transfected L cells. (B) Naive B cells cultured with IL-2 and IL-10 over parental nontransfected L cells together with an anti-CD40L– blocking antibody at 2 μg/ml. (C) Memory B cells cultured with IL-2 and IL-10 over CD40L-transfected L cells. (D) Memory B cells cultured IL-2 and IL-10 over nontransfected L cells together with an anti-CD40L– blocking antibody at 2 μg/ml.
Table 1.
Memory B Cells Promptly Differentiate into PC
| Percentage of CD20−/lowCD38high cells generated from | ||||||||
|---|---|---|---|---|---|---|---|---|
| Secondary cultures | Naive cells | Memory cells | ||||||
| CD40L-transfected fibroblasts | 3.7 ± 1.8 | [1.1–6.7] | 23.8 ± 7.8 | [15–31] | ||||
| Parental fibroblasts + anti-CD40L mAb | 16.4 ± 6.6 | [5.4–25] | 62.4 ± 11.9 | [50.8–79] | ||||
Mean, standard deviation, and range (brackets) of percentages of CD20−/low CD38high cells generated from naive and memory B cells from seven and four experiments, respectively. Cells were cultured for 3 d in the presence of IL-2, IL-10, and CD40L-transfected fibroblasts before being seeded for 4 d in the secondary cultures together with IL-2, IL-10, and fibroblasts. The fibroblasts used in secondary cultures are listed in the table.
FACS®-sorted CD20−/lowCD38high cells generated from both naive and memory B blasts display the morphology of terminally differentiated PCs (Fig. 2 A), as well as an intense Igκ and Igλ light chain staining (Fig. 2 C). In contrast, CD20+CD38low populations display the morphology of blasts with a weak surface Ig expression (Fig. 2, B and D). Although 50% of plasma cells generated from naive B cells contain intracytoplasmic IgM (Fig. 2 E), only 20% of plasma cells generated from memory B cells expressed IgM (Fig. 2 F).
Figure 2.
Morphology and intracellular Ig content. Giemsa staining of sorted CD20−/lowCD38high PC (A, original magnification: 1,000) and CD20+CD38low B blasts (B, original magnification: 1,000). Red anti-Igκ + λ light chain staining of sorted CD20−/lowCD38high PCs (C, original magnification: 1,000) and CD20+CD38low B blasts (D, original magnification: 1,000). Red anti-IgM staining of sorted CD20−/lowCD38high PCs derived from naive B cells (E, original magnification: 400) and from memory B cells (F, original magnification: 400).
High Concentrations of CD40L Do Not Completely Block the Terminal Differentiation of Memory B Cells.
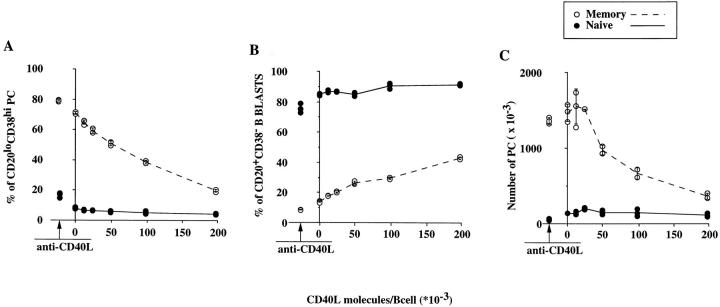
To further understand the propensity of memory B cells to undergo plasma cell differentiation, secondary cultures of naive and memory blasts were set up in the presence of increasing density of CD40L. For that purpose, absolute numbers of CD40L molecules per fibroblast were estimated using quantitative flow cytometry and CD40L-transfected fibroblasts were gradually diluted with their parental cells (see Material and Methods). As shown in Fig. 3 A, increased CD40 ligation of memory cells results in a decreased production of plasma cells and a concommitant increase of B blasts (Fig. 3 B). In fact, there is a linear correlation between the log (1/ CD40L available per memory blast) and the percentage of generated plasma cells (r2 = 0.945, 0.966, and 0.983 from three experiments). Note that CD40L-transfected fibroblasts were indeed carried over from the primary cultures, as the addition of anti-CD40L antibody to the cultures with nontransfected fibroblasts further enhanced the plasma cell generation. As shown in Fig. 3 C, in the absence of CD40L in the secondary culture, memory cells can generate up to eight times more PCs than do naive cells. Note that very high amounts of CD40L molecules in the secondary cultures do not completely inhibit the generation of PCs from memory cells, since up to 2 × 105 CD40L molecules/B blast led to the generation of 3.6 × 105 PCs from an initial input of 1.5 × 105 blasts (Fig. 3 C).
Figure 3.
CD40L inhibits B cell differentiation during secondary cultures in a dose-dependent fashion. (A) Percentages of CD20−/lowCD38high PCs derived from naive (closed circles) and memory (open circles) B cells. (B) Percentages of CD20+CD38low nondifferentiated B blasts derived from naive (closed circles) and memory (open circles) B cells. (C) Total numbers of CD20−/lowCD38high PCs derived from naive (closed circles) and memory (open circles) B cells. The number of CD40L molecules per B cell in secondary cultures was measured and used as x-axis (a negative value artificially represents cultures with parental fibroblasts in the presence of a blocking antibody to CD40L at 2 μg/ml). Cell input was 1.5 × 105 at the beginning of secondary cultures. Each circle represents an individual value. Mean values are linked (plain and dotted lines represent naive and memory cell cultures, respectively). Standard deviations are vertical bars.
Increasing the number of CD40L molecules available in the cultures not only inhibited the plasma cell generation, but also the secretion of Igs (Fig. 4 A). Furthermore, in all culture conditions, memory B cells produced more total Igs than naive B cells (Fig. 4 A). With regard to secreted isotype, although naive and memory B cells produced comparable levels of IgM (Fig. 4 D), memory cells, as expected, produced considerably more IgG and IgA (Fig. 4, B and C).
Figure 4.
. CD40L inhibits Ig production during secondary cultures in a dose-dependent fashion. The culture conditions are the same as described in Fig. 3. (A) Total IgG + IgA + IgM production from 106 cells of naive (closed circles) and of memory B cell cultures (open circles). (B) IgA production. (C) IgG production. (D) IgM production. Each circle (closed and open correspond to naive and memory cell cultures, respectively) represents an individual value. Mean values are linked (plain and dotted lines represent naive and memory cell cultures, respectively). Standard deviations are vertical bars.
Naive and Memory B Cells Proliferate Equally Well. We then questioned whether the poor differentiation capacity of naive B cells, as compared to that of memory B cells, may indeed reflect a reduced activation and proliferation capacity. Thus, purified naive and memory B cells were cultured over CD40L-transfected fibroblasts with IL-2 and IL-10, and proliferation was assessed by measuring thymidine incorporation, as well as viable cell numbers. As shown in Fig. 5, naive B cells proliferate at least as much as memory B cells do.
Figure 5.
Naive and memory B cells undergo comparable proliferation and expansion during 12 d of cultures with IL-2 and IL-10 over CD40L-transfected L cells. (A) [3H]thymidine uptake by cultured naive (closed circles) and memory (open circles) B cells. (B) Cell numbers of cultured naive (closed circles) and memory (open circles) B cells. Initial cell inputs were 2.5 × 104 for [3H]thymidine uptake and 1.5 × 105 for the viable cell numbers.
Memory, but Not Naive, B Cells Undergo Rapid PC Differentiation in Cultures even after Anti-BCR Triggering.
Anti-Igs were shown to prevent B cell differentiation (38). Since naive B cells, but not memory B cells, were isolated by positive selection using anti-IgD, we questioned whether the difference in the differentiation capacity between naive and memory B cells could be due to the BCR triggering. Accordingly, in the first 3 d of primary cultures, 2 μg/ml of anti-Igκ and 2 μg/ml of anti-Igλ antibodies were added into the cultures in the presence of CD40L, IL-2, and IL-10. At the end of the culture, cells were washed and seeded in a 4 d secondary culture with IL-2, IL-10, and different concentrations of CD40L. Fig. 6 shows that in the presence of three different CD40L concentrations (9.6 × 104/cell, 4.8 × 104/cell, no CD40L), 3, 6, and 13% of CD38+ CD20− plasma cells were generated from the naive B cells. In the same culture conditions, 21, 29, and 43% of CD38+ CD20− plasma cells were generated from the memory B cells. This experiment indicates that memory B cells, but not naive B cells, preferentially undergo plasma cell differentiation even after BCR triggering.
Figure 6.

Memory, but not naive, B cells preferentially undergo plasma cell differentiation even after BCR triggering. Naive and memory B cells were cultured for 3 d with anti-Igκ and anti-Igλ antibodies, together with IL-2, IL-10, and CD40L. After washing, cells were recultured with IL-2, IL-10, and different concentrations of CD40L for 4 d. (A) CD38+CD20− plasma cells and CD38lowCD20+ undifferentiated B cells generated from naive B cells. (B) CD38+CD20− plasma cells and CD38lowCD20+ undifferentiated B cells generated from memory B cells. This is one representative of two experiments.
Discussion
This paper describes the striking differentiation ability of memory versus that of naive B cells. This correlates with previous histophysiological observations in vivo showing that secondary antigenic challenge in carrier-primed rats leads to a massive extrafollicular PC reaction and a poor follicular GC reaction in the spleen. In contrast, only small extrafollicular PC reactions, but large GC reactions develop upon primary immunization (Fig. 7; 39, 40). Likewise, in mice infected with reoviruses, adoptively transferred memory B cells give rise to a large extrafollicular PC reaction, but a small GC reaction; in contrast, transferred naive cells generate a large GC reaction (41). Thus, the differences in the capacity of memory versus naive B cells to differentiate is an intrinsic property of the B cells, rather than of the microenvironments. The propensity of memory B cells to undergo rapid differentiation into effector cells may confer two important properties to the immune system. First, it allows the rapid generation of large numbers of effector cells, whose products (antibodies) efficiently eliminate pathogens. This novel feature of memory B cells, together with their low threshold for activation, and their ability to home to the antigen draining sites and to directly present antigen to T cells, may all contribute to the velocity of secondary antibody responses. Second, it prevents the overexpansion and accumulation of a particular memory B cell clone that would otherwise overload the immune system and freeze the available Ig repertoire (26). Since PCs have a relatively short lifespan and do not proliferate in response to further stimulations (42–44), the majority of memory B cells will undergo clonal exhaustion by differentiating into effector cells during secondary immune responses. Interestingly, T memory cells show a similar tendency not to expand and overload the whole immune system, as towards the end of a primary immune response specific T blasts are rapidly eliminated (45–48).
Figure 7.

Memory B cells are biased towards terminal plasma cell differentiation in vivo and in vitro. Naive B cells predominantly give rise to germinal center reaction within a rat spleen after primary immunization with DNP-KLH in KLH-primed animals (A original magnification: 40; B original magnification: 200). The rats were given BrdU in their drinking water for 48 h before they were killed. Red stains BrdU, blue stains DNP-binding cells, brown stains total B cells. MZ, marginal zone; PALS, periarteriolar lymphoid sheath. Consistent with this in vivo finding, human naive B cells predominantly give rise to proliferating B blasts upon activation in vitro (C). Memory B cells predominantly give rise to plasma cell reaction along the outer edges of the periarteriolar lymphoid sheath and within the red pulp of a rat spleen after 2 d of secondary immunization with DNP-KLH (D and E). The rats have received BrdU in their drinking water for 48 h before killing. DNP-specific plasma blasts are cells with strong blue cytoplasmic staining. Consistent with this in vivo finding, human memory B cells predominantly give rise to plasma cells upon activation in vitro (F). The figures on immunohistology are derived from Y.-J. Liu and I.C.M. MacLennan (40).
The finding that CD40L inhibits the differentiation of both activated memory and naive B cells, complements the previous observations made with GC B cells (37) or total B cells isolated from blood and tonsils (49, 50). Thus, CD40L represents a differentiation suppressor during not only the GC, but also the extrafollicular reactions (51). Indeed, CD40L-expressing T cells have been reported by immunohistology, both within the GCs and the extrafollicular T zones (52–54).
Although CD40L inhibits the PC differentiation of naive, GC, and memory B cells, a fraction of the memory cell subset seems to be resistant to this effect. Differential effects of CD40L on mature B cell subsets have already been noticed. For instance, CD40 triggering is an important survival but a minor proliferative signal for GC cells (55–57), whereas it provides a strong and long-term proliferative signal to resting naive and memory B cells (58–61). The molecular mechanisms underlying the propensity of memory B cells to undergo terminal differentiation are still unknown. CD40 triggering on human GCs and resting mature B cells results in the activation of different protein kinases (62, 63). Further comparative studies of CD40 signaling pathways in naive, GC, and memory B cells should now be carried on to explain how mature B cells change their responses to CD40 triggering at different stages of their immunopoiesis.
Acknowledgments
The authors wish to thank S. Bonnet-Arnaud and M. Vatan for superb editorial assistance; I. Durand for flow cytometry; Dr. J. Chiller for supporting this work; and Drs. F. Brière, I. Fugier-Vivier, and C. Mueller for critical reading of the manuscript.
Footnotes
C. Arpin is the recipient of a grant from Fondation Mérieux.
Abbreviations used in this paper: BCR, B cell receptor; CD40L, CD40 ligand; GC, germinal center; PC, plasma cell.
References
- 1.Kroese, F.G.M., W. Timens, and P. Nieuwenhuis. 1990. Germinal center reaction and B lymphocytes: morphology and function. In Current Topics in Pathology. Reaction Pattern of the Lymph Node. Springer Verlag, Berlin. 103–148. [DOI] [PubMed]
- 2.Liu YJ, Johnson GD, Gordon J, MacLennan ICM. Germinal centers in T-cell–dependent antibody responses. Immunol Today. 1992;13:17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- 3.Nossal GJV. The molecular and cellular basis of affinity maturation in the antibody response. Cell. 1992;68:1–3. doi: 10.1016/0092-8674(92)90198-l. [DOI] [PubMed] [Google Scholar]
- 4.Gray D. Immunological memory. Annu Rev Immunol. 1993;11:49–77. doi: 10.1146/annurev.iy.11.040193.000405. [DOI] [PubMed] [Google Scholar]
- 5.MacLennan ICM. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 6.Weissman IL. Developmental switches in the immune system. Cell. 1994;76:207–218. doi: 10.1016/0092-8674(94)90329-8. [DOI] [PubMed] [Google Scholar]
- 7.Kelsoe G. In situ studies of the germinal center reaction. Adv Immunol. 1995;60:267–288. doi: 10.1016/s0065-2776(08)60587-8. [DOI] [PubMed] [Google Scholar]
- 8.Thorbecke GJ, Amin AR, Tsiagbe VK. Biology of germinal centers in lymphoid tissue. FASEB J. 1994;8:832–840. doi: 10.1096/fasebj.8.11.8070632. [DOI] [PubMed] [Google Scholar]
- 9.Manser T, Wysocki LJ, Gridley T, Near RI, Gefter ML. The molecular evolution of the immune response. Immunol Today. 1985;6:94–101. doi: 10.1016/0167-5699(85)90024-6. [DOI] [PubMed] [Google Scholar]
- 10.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 11.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature (Lond) 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 12.Küppers R, Zhao M, Hansmann M-L, Rajewsky K. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO (Eur Mol Biol Organ) J. 1993;12:4955–4967. doi: 10.1002/j.1460-2075.1993.tb06189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McHeyzer-Williams MG, McLean MJ, Lalor PA, Nossal GJV. Antigen-driven B cell differentiation in vivo. J Exp Med. 1993;178:295–307. doi: 10.1084/jem.178.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein U, Küppers R, Rajewsky K. Variable region gene analysis of B cell subsets derived from a 4-year-old child: somatically mutated memory B cells accumulate in the peripheral blood already at young age. J Exp Med. 1994;180:1383–1393. doi: 10.1084/jem.180.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pascual V, Liu YJ, Magalski A, de Bouteiller O, Banchereau J, Capra JD. Analysis of somatic mutation in five B cell subsets of human tonsil. J Exp Med. 1994;180:329–339. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajewsky K. Clonal selection and learning in the antibody system. Nature (Lond) 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 17.Kraal G, Weissman IL, Butcher EC. Germinal centre B cells: antigen specificity and charges in heavy chain class expression. Nature (Lond) 1982;298:377–379. doi: 10.1038/298377a0. [DOI] [PubMed] [Google Scholar]
- 18.Liu YJ, Malisan F, de Bouteiller O, Guret C, Lebecque S, Banchereau J, Mills FC, Max EE, Martinez-Valdez H. Within germinal centers isotype switching of immunoglobulin genes occurs after onset of somatic mutation. Immunity. 1996;4:241–250. doi: 10.1016/s1074-7613(00)80432-x. [DOI] [PubMed] [Google Scholar]
- 19.Toellner KM, Gulbranson-Judge A, Taylor DR, Man-Yuen D, MacLennan ICM. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. J Exp Med. 1996;183:2303–2312. doi: 10.1084/jem.183.5.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y-J, Oldfield S, MacLennan ICM. Memory B cells in T cell–dependent antibody responses colonize the splenic marginal zones. Eur J Immunol. 1988;18:355–362. doi: 10.1002/eji.1830180306. [DOI] [PubMed] [Google Scholar]
- 21.MacLennan ICM, Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 22.Rajewsky, K. 1989. Evolutionary and somatic immunological memory. In Progress in Immunology, VII. F. Melchers, editor. Springer Verlag, Berlin. 397–403.
- 23.Gray D, Sprent J. Immunological memory. Curr Top Microbiol Immunol. 1990;159:V–VII. [PubMed] [Google Scholar]
- 24.Vitetta ES, Berton MT, Burger C, Kepron M, Lee WT, Yin XM. Memory B and T cells. Annu Rev Immunol. 1991;9:193–217. doi: 10.1146/annurev.iy.09.040191.001205. [DOI] [PubMed] [Google Scholar]
- 25.Mackay CR. Immunological memory. Adv Immunol. 1993;53:217–265. doi: 10.1016/s0065-2776(08)60501-5. [DOI] [PubMed] [Google Scholar]
- 26.Sprent J. T and B memory cells. Cell. 1994;76:315–322. doi: 10.1016/0092-8674(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science (Wash DC) 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 28.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green DR. Cell-autonomous Fas (CD95)/ Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature (Lond) 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 29.Dhein J, Walczak H, Bäumler C, Debatin K-M, Krammer PH. Autocrine T-cell suicide mediated by APO-1(Fas/CD95) Nature (Lond) 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 30.Ju S-T, Panka DJ, Cui H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature (Lond) 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 31.Garrone P, Neidhardt EM, Garcia E, Galibert L, van Kooten C, Banchereau J. Fas ligation induces apoptosis of CD40-activated human B lymphocytes. J Exp Med. 1995;182:1265–1273. doi: 10.1084/jem.182.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagresle C, Bella C, Defrance T. Phenotypic and functional heterogeneity of the IgD−B cell compartment: identification of two major tonsillar B cell subsets. Int Immunol. 1993;5:1259–1268. doi: 10.1093/intimm/5.10.1259. [DOI] [PubMed] [Google Scholar]
- 33.Liu YJ, Barthélémy C, de Bouteiller O, Arpin C, Durand I, Banchereau J. Memory B cells from human tonsils colonize mucosal epithelium and directly present antigen to T cells by rapid upregulation of B7.1 and B7.2. Immunity. 1995;2:238–248. doi: 10.1016/1074-7613(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 34.Casamayor-Palleja M, Feuillard J, Ball J, Drew M, MacLennan ICM. Centrocytes rapidly adopt a memory B cell phenotype on co-culture with autologous germinal centre T cell–enriched preparations. Int Immunol. 1996;8:737–744. doi: 10.1093/intimm/8.5.737. [DOI] [PubMed] [Google Scholar]
- 35.Irsch J, Irlenbusch S, Radl J, Burrows PD, Cooper MD, Radbruch AH. Switch recombination in normal IgA1+B lymphocytes. Proc Natl Acad Sci USA. 1994;91:1323–1327. doi: 10.1073/pnas.91.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein U, Küppers R, Rajewsky K. Evidence for a large compartment of IgM-expressing memory B cells in humans. Blood. 1997;89:1288–1298. [PubMed] [Google Scholar]
- 37.Arpin C, Déchanet J, van Kooten C, Merville P, Grouard G, Brière F, Banchereau J, Liu YJ. Generation of memory B cells and plasma cells in vitro. Science (Wash DC) 1995;268:720–722. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- 38.Lawton AR, Asofsky R, Hylton MB, Cooper MD. Suppression of immunoglobulin class synthesis in mice. I. Effects of treatment with antibody to μ-chain. J Exp Med. 1972;135:277–282. doi: 10.1084/jem.135.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroese FGM, Seijen HG, Nieuwenhuis P. The initiation of germinal centre reactivity. Res Immunol. 1991;142:249–252. doi: 10.1016/0923-2494(91)90069-u. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y-J, Zhang J, Lane PJL, Chan EY-T, MacLennan ICM. Sites of specific B cell activation in primary and secondary responses to T cell–dependent and T cell–independent antigens. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 41.Cebra JJ, Schrader CE, Shroff KE, Weinstein PD. Are Peyer's patch germinal centre reactions different from those occuring in other lymphoid tissues? . Res Immunol. 1991;142:222–226. doi: 10.1016/0923-2494(91)90063-o. [DOI] [PubMed] [Google Scholar]
- 42.Benner R, Hijmans W, Haaijman JJ. The bone marrow: the major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin Exp Immunol. 1981;46:1–8. [PMC free article] [PubMed] [Google Scholar]
- 43.Ho F, Lortan JE, MacLennan ICM, Khan M. Distinct short-lived and long-lived antibody-producing cell populations. Eur J Immunol. 1986;16:1297–1301. doi: 10.1002/eji.1830161018. [DOI] [PubMed] [Google Scholar]
- 44.Merville P, Dechanet J, Desmoulière A, Durand I, de Bouteiller O, Garrone P, Banchereau J, Liu YJ. Bcl-2 positive tonsillar plasma cells are rescued from prompt apoptosis by bone marrow fibroblasts. J Exp Med. 1996;183:227–236. doi: 10.1084/jem.183.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 46.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science (Wash DC) 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 47.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells (erratum published 364:262) Nature (Lond) 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 48.Webb SR, Sprent J. Factors controlling the reactivity of immature and mature T cells to Mls antigens in vivo. Immunol Rev. 1993;131:169–188. doi: 10.1111/j.1600-065x.1993.tb01535.x. [DOI] [PubMed] [Google Scholar]
- 49.Callard RE, Herbert J, Smith SH, Armitage RJ, Costelloe KE. CD40 cross-linking inhibits specific antibody production by human B cells. Int Immunol. 1995;7:1809–1815. doi: 10.1093/intimm/7.11.1809. [DOI] [PubMed] [Google Scholar]
- 50.Lane P, Burdet C, McConnell F, Lanzavecchia A, Padovan E. CD40 ligand-independent B cell activation revealed by CD40 ligand-deficient T cell clones: evidence for distinct activation requirements for antibody formation and B cell proliferation. Eur J Immunol. 1995;25:1788–1793. doi: 10.1002/eji.1830250646. [DOI] [PubMed] [Google Scholar]
- 51.Noelle RJ. CD40 and its ligand in host defense. Immunity. 1996;4:415–419. doi: 10.1016/s1074-7613(00)80408-2. [DOI] [PubMed] [Google Scholar]
- 52.Lederman S, Yellin MJ, Inghirami G, Lee JJ, Knowles DM, Chess L. Molecular interactions mediating T–B lymphocyte collaboration in human lymphoid follicles. Roles of T cell–B cell–activating molecule (5c8 antigen) and CD40 in contact-dependent help. J Immunol. 1992;149:3817–3826. [PubMed] [Google Scholar]
- 53.van den Eetwegh AJM, Noëlle RJ, Roy M, Shepherd DM, Aruffo A, Ledbetter JA, Boersma WJA, Claassen E. In vivo CD40–gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T–B cell interactions. J Exp Med. 1993;178:1555–1565. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casamayor-Palleja M, Khan M, MacLennan ICM. A subset of CD4+memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. J Exp Med. 1995;181:1293–1301. doi: 10.1084/jem.181.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan ICM. Mechanisms of antigen-driven selection in germinal centers. Nature (Lond) 1989;342:929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- 56.Holder MJ, Wang H, Milner AE, Casamayor M, Armitage R, Spriggs MK, Fanslow WC, MacLennan ICM, Gregory CD, Gordon J. Suppression of apoptosis in normal and neoplastic human B lymphocytes by CD40 ligand is independent of Bcl-2 induction. Eur J Immunol. 1993;23:2368–2371. doi: 10.1002/eji.1830230948. [DOI] [PubMed] [Google Scholar]
- 57.Gray D, Siepmann K, van Essen D, Poudrier J, Wykes M, Jainandunsing S, Bergthorsdottir S, Dullforce P. B–T lymphocyte interactions in the generation and survival of memory cells. Immunol Rev. 1996;150:45–61. doi: 10.1111/j.1600-065x.1996.tb00695.x. [DOI] [PubMed] [Google Scholar]
- 58.Clark EA, Ledbetter JA. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci USA. 1986;83:4494–4498. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gordon J, Millsum MJ, Guy GR, Ledbetter JA. Synergistic interaction between interleukin 4 and anti-Bp50 (CDw40) revealed in a novel B cell restimulation assay. Eur J Immunol. 1987;17:1535–1538. doi: 10.1002/eji.1830171026. [DOI] [PubMed] [Google Scholar]
- 60.Spriggs MK, Armitage RJ, Strockbine L, Clifford KN, Macduff BM, Sato TA, Maliszewski CR, Fanslow WC. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J Exp Med. 1992;176:1543–1550. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banchereau J, Bazan F, Blanchard D, Brière F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its Ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 62.Uckun FM, Schieven GL, Dibirdik I, Chandan-Langlie M, Tuel-Ahlgren L, Ledbetter JA. Stimulation of protein tyrosine phosphorylation, phosphoinositide turnover, and multiple previously unidentified serine/threonine-specific protein kinases by the pan–B-cell receptor CD40/ Bp50 at discrete developmental stages of human B-cell ontogeny. J Biol Chem. 1991;266:17478–17485. [PubMed] [Google Scholar]
- 63.Knox KA, Gordon J. Protein tyrosine phosphorylation is mandatory for CD40-mediated rescue of germinal center B cells from apoptosis. Eur J Immunol. 1993;23:2578–2584. doi: 10.1002/eji.1830231030. [DOI] [PubMed] [Google Scholar]