Abstract
To investigate the role of T cell–mediated, perforin-dependent cytotoxicity in autoimmune diabetes, perforin-deficient mice were backcrossed with the nonobese diabetes mouse strain. It was found that the incidence of spontaneous diabetes over a 1 yr period was reduced from 77% in perforin +/+ control to 16% in perforin-deficient mice. Also, the disease onset was markedly delayed (median onset of 39.5 versus 19 wk) in the latter. Insulitis with infiltration of CD4+ and CD8+ T cells occurred similarly in both groups of animals. Lower incidence and delayed disease onset were also evident in perforin-deficient mice when diabetes was induced by cyclophosphamide injection. Thus, perforin-dependent cytotoxicity is a crucial effector mechanism for β cell elimination by cytotoxic T cells in autoimmune diabetes. However, in the absence of perforin chronic inflammation of the islets can lead to diabetogenic β cell loss by less efficient secondary effector mechanisms.
Insulin-dependent diabetes mellitus (IDDM)1 is an autoimmune disease characterized by the loss of insulin-producing pancreatic β cells. In its early and clinically silent phase T cells and other inflammatory cells infiltrate into the islets causing a progressive loss of β cells. When a majority of β cells has disappeared, the lack of insulin secretion leads to a failure of blood glucose homeostasis and diabetes. While there is a consensus that IDDM is caused by autoreactive T cells, many other aspects of the disease are still poorly understood. These include the breakdown of tolerance against islet cell antigens, the failure of mechanisms controlling self-reactive T cells, genetic and environmental susceptibility factors, and the molecular effector mechanisms that are responsible for the elimination of β cells.
In the past it has been attempted to address this last point by defining the role of the CD4+ (helper) T cells versus the CD8+ (cytotoxic) T cell subset. In these studies the nonobese diabetic (NOD) mouse strain has proved useful because it models the spontaneous initiation and the chronic progressive course of the disease and the polygenic inheritance of susceptibility genes quite well (1). Several studies have shown that CD4+ and CD8+ primary T cells are required to adoptively transfer diabetes (2, 3). However, cloned islet cell–reactive NOD CD4+ T cells were able to induce diabetes in NOD-SCID mice in the absence of CD8+ T cells (4, 5). At the time, these findings were taken as evidence that both T cell subsets are required for the transfer of diabetes with polyclonal primary T cells but that cloned CD4+ T cells are able to induce diabetes independently of CD8+ T cells, given high numbers and specificity.
On the other hand, a cytofluorometric study of islet-infiltrating leukocytes has shown that CD8+ T cells infiltrated into the pancreas of young, prediabetic NOD mice earlier than CD4+ T and B cells (6). Similarly, in a pancreas from a human patient who had died only a month after diagnosis of diabetes the islet-infiltrating T cells consisted mainly of the CD8+ subset (7). Several recent studies further supported the crucial role of CD8+ T cells in diabetes of NOD mice: β2-microglobulin–negative and hence CD8+ T cell–deficient NOD mice developed neither insulitis nor diabetes (8–11). Also, depletion of CD8+ T cells by antibody treatment at 2–5-wk after birth prevents insulitis development and also abrogates the ability of CD4+ T cells to induce insulitis (12). Finally, CD8+ T cell clones from NOD mice that were generated by restimulation with transgenic islet cells expressing the costimulatory molecule B7.1 were able to transfer diabetes to irradiated NOD and NOD-SCID mice (13). These findings clearly demonstrated that CD8+ T cells are not only responsible for the lysis of β cells in the late effector phase, but that they also may have a role in the early induction phase by affecting the properties of autoreactive CD4+ T cells. Perforin-deficient mice lack a major pathway of T cell–mediated cytotoxicity and NK cell– mediated cytotoxicity (14–18). Since perforin-deficient mice have no defect in activation and proliferation of T cells and generate normal B cell responses (14), they are well suited to directly address the role of cytotoxicity in vivo. We have previously crossed perforin-deficient mice with transgenic mice expressing glycoprotein (GP) of lymphocytic choriomeningitis virus (LCMV) in the pancreas. Infection with LCMV triggers an acute virus-specific immune response which induces insulitis and diabetes in perforin-expressing transgenic mice by day 10 after infection (19). In contrast, LCMV-GP transgenic perforin-deficient mice did not develop diabetes, although they developed marked insulitis (20). These findings indicated that perforin-dependent cytotoxicity is not required for the initiation of insulitis but is crucial for the destruction of β cells in the later effector phase. However, there was the possibility that these findings were specific to this model system, since LCMV induces a very strong cytotoxic immune response and, unlike human diabetes, the diabetes develops very acutely without chronic long-term insulitis. It was therefore of interest to test the role of perforin-dependent cytotoxicity in the NOD mouse model, where the spontaneous onset and the chronic inflammation of the pancreatic islets are more similar to the human disease. In addition, we surmised that the slower course of diabetes in the NOD mouse may reveal additional perforin-independent effector mechanisms, which may be masked during the very acute progression of diabetes in the LCMV-GP transgenic model.
We report here that diabetes developed only with greatly reduced incidence and delayed onset in perforin-deficient NOD mice. This shows that perforin-dependent cytotoxicity is the main effector mechanism accounting for β cell loss in the NOD mouse but also indicates that one or several perforin-independent mechanisms, possibly involving Fas, can cause diabetes with reduced efficiency.
MATERIALS AND METHODS
Breeding of Perforin-deficient NOD Mice.
Perforin-deficient mice of the C57BL/6 strain have been previously described (14). These mice were backcrossed for seven generations with NOD mice (provided by Hans Acha-Orbea, Institute of Biochemistry, University of Lausanne, Epalinges, Switzerland). At each backcross generation, heterozygous mice were identified by PCR analysis for further breeding. In addition, the H-2g7 complex, which contains the I-Ag7 locus and is strongly associated with diabetes susceptibility in the NOD strain, was enriched by screening at the second backcross generation for the absence of the Kb allele by flow cytofluorometry of blood cells with the antibody B8-24-3. At the third generation, the second strongest susceptibility locus Idd3 was enriched by PCR screening as previously described for the microsatellite marker D3Nds1 (21). In brief, the microsatellite marker was amplified by PCR from genomic DNA with primers 5′-GGA TCT GGC ACC TCC AGG G-3′ and 5′-TAT GTT GCC TTG GCA AAT AGA TG-3′. The reaction product was resolved on a 5% Nusieve agarose gel (fragment size: NOD> C57BL/6; FMC BioProducts, Rockland, ME). Littermate controls were used in all experiments.
Genotyping of the Perforin Allele.
The perforin genotype was determined with PCR and two different primer pairs on DNA prepared from tail biopsies. The first pair (5′-TTT TTG AGA CCC TGT AGA CCC A-3′, 5′-GCA TCG CCT TCT ATC GCC TTC T-3′) yields a band of 665 bp for the mutated and is negative for the wild-type allele. The second pair (5′-CCG GTC CTG AAC TCC TGG CCA A-3′, 5′-CCC CTG CAC ACA TTA CTG GAA G-3′) yields a 300-bp fragment for the wild-type and a 1,300-bp fragment for the mutated allele.
Cytofluorometry.
T lymphocyte marker expression was analyzed by incubating spleen cell suspensions with PE-conjugated CD8-specific and FITC-conjugated CD4-specific antibodies (PharMingen, San Diego, CA). After washing, the cell suspensions were analyzed on a FACScan® flow cytometer (Becton Dickinson, Mountain View, CA) using logarithmic scales. Viable lymphocytes were gated for by a combination of forward light scatter and 90° side scatter.
Measurement of Blood Glucose.
The glucose concentration in blood obtained from a tail vein was measured using Haemo-Glucotest strips (Boehringer Mannheim, Mannheim, Germany).
Induction of Diabetes by Injection of Cyclophosphamide.
6 mg of cyclophosphamide (Sigma Chemical Co., St. Louis, MO) was injected i.p. into 10–12-wk-old mice on day 0. If the first injection failed to produce diabetes, 6 mg of cyclophosphamide was again injected on day 14.
Immunohistochemistry.
Pancreata were immersed in HBSS and snap-frozen in liquid nitrogen. Cryostat sections (5 μm) of tissue were cut and fixed in cold acetone. Sections were incubated with rat anti–mouse monoclonal antibodies YTS191.1 (anti-CD4) and YTS169.4.2 (anti-CD8) (22). Alkaline phosphatase–labeled goat anti– rat immunoglobulin antibodies, followed by alkaline phosphatase– labeled donkey anti–goat immunoglobulin antibodies (Tago, Inc., Burlingame, CA) were used as secondary reagents. The substrate for the red color reaction was naphtol AS-BI phosphate/New Fuchsin.
Insulin was detected on paraffin-embedded formalin-fixed tissues with an insulin-specific guinea pig antiserum (Dako A/S, Glostrup, Denmark). Primary antibodies were detected by an indirect immunoenzymatic staining procedure with biotinylated antiimmunoglobulin antibodies (Zymed, San Francisco, CA) and streptavidin-conjugated horseradish peroxidase (Dako A/S). The red color signal was obtained by developing with aminoethylcarbazole (Sigma Chemical Co.).
Measurement of the Volume Density of Islets in the Pancreas.
Islets are more frequent in the head of the pancreas than in the tail. Thus, the head of the pancreas was fixed in formaldehyde and cut in sections at eight consecutive levels each 100 μm apart. The volume density was determined according to the Delesse principle by calculating the ratio of the sum of the islet areas to the sum of the section areas (23). Islet and section areas were determined by computer-assisted morphometry on a microscope equipped with a video camera. The results are given as the mean of the volume density from several individual mice together with the SEM.
Statistical Analysis.
The time course of spontaneous diabetes onset in normal control, heterozygous, and perforin-deficient NOD mice was analyzed using Kaplan-Meier curves. The curves were tested for significance by log-rank tests. All statistical procedures were performed with the lifetest program of the SAS statistical software package (SAS Institute, Inc., Cary, NC).
RESULTS
Frequency of CD4+ and CD8+ T Cells in Perforin-deficient NOD Mice.
We have previously shown that T cells from perforin-deficient C57BL/6 mice have no defect in maturation, activation, or proliferation (14). To exclude the possibility that backcrossing with the NOD strain revealed a defect in T lymphocyte maturation, we checked for the presence of CD4+ and CD8+ T cells in the spleens of 8-wk-old NOD mice. It was found that CD4- and CD8-expressing T lymphocytes were present at comparable percentages in normal control, heterozygous, and perforin-deficient NOD mice, indicating that the lack of perforin did not affect T lymphocyte development in the NOD strain (data not shown).
Similar Development of Insulitis in Control and Perforin-deficient NOD Mice.
A hallmark of the NOD mouse model system for IDDM is the progressive infiltration of mononuclear cells into the islets. Inflammatory islet-infiltrating mononuclear cells start to appear at the age of 5 wk in most NOD mice. Histologic analysis in 8-wk-old heterozygous and perforin-deficient mice revealed varying degrees of insulitis but mostly periinsulitis in both types of mice with little islet cell damage detectable in heterozygous and perforin-deficient mice (see Fig. 2 A). At 55 wk of age, islets of perforin-expressing NOD mice which had not succumbed to diabetes were strongly infiltrated by mononuclear cells and often displayed little or no endocrine tissue. Since diabetes only develops when >90% of islet tissue is lost, the mice were still normoglycemic. In nondiabetic perforin-deficient mice severe insulitis had developed as well, but more frequently than in control NOD mice the infiltrates were associated with patches of endocrine tissue. Sections that were stained by immunohistochemistry for the lymphocyte surface markers CD4 and CD8 revealed that the mononuclear infiltrate in both groups of mice contained similar proportions of CD4+ and CD8+ T cells, with CD4+ outnumbering the CD8+ cells in both groups (see Fig. 2 A).
Figure 2.

Histological analysis of pancreatic islets. (A) Comparable degree of insulitis in pancreata of perforin-competent (+/0, top) and perforin-deficient (0/0, bottom) NOD mice. Pancreas sections from young (8-wk-old) and adult (55-wk-old) mice were stained with either hematoxylin and eosin (HE) or used for immunohistochemistry with CD4- or CD8-specific antibodies. Antibody binding resulted in red staining. (B) Presence of insulin-containing β cells in the islets of diabetic perforin-deficient mice. Sections from a 30-wk-old heterozygous and a 41-wk-old perforin-deficient diabetic NOD mouse were stained either with hematoxylin and eosin (HE) or with insulin-specific antibodies (red staining). Arrowheads in the left panel indicate reddish, eosinophilic dying islet cells. (C) Pancreatic islets of diabetic heterozygous and perforin-deficient NOD mice (both 7–10 wk old) after injection with cyclophosphamide. Diabetes occurred in the heterozygous mice 13 d after the first injection with 6 mg cyclophosphamide and in the perforin-deficient mouse 16 d after the second injection. Pancreas sections were either stained with hematoxylin and eosin (HE) or antiinsulin antibodies (red staining). Note the presence of insulin-containing β cells in the diabetic perforin-deficient NOD mouse and their lower intensity of insulin staining compared to islets from a healthy, untreated control C57BL/6 mouse.
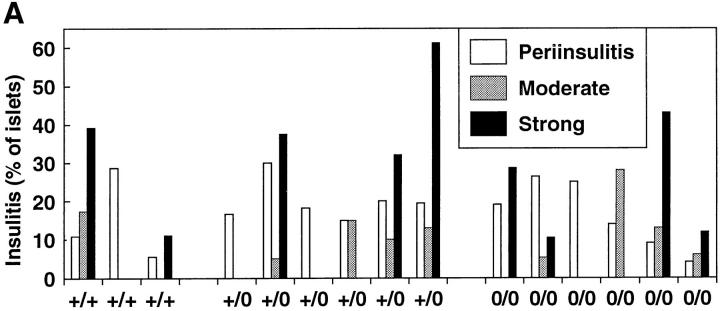
To quantitate the development of insulitis, 20–40 islets from individual 9–12-wk-old female NOD mice were assessed for the severity of insulitis. We found considerable variability in the degree of insulitis in individual mice with no significant difference between normal control, heterozygous, and perforin-deficient mice (Fig. 1 A). Thus, perforin-dependent cytotoxicity is not required for the breakdown of self tolerance nor ignorance in NOD mice, which causes the infiltration of T cells into the islets.
Figure 1.

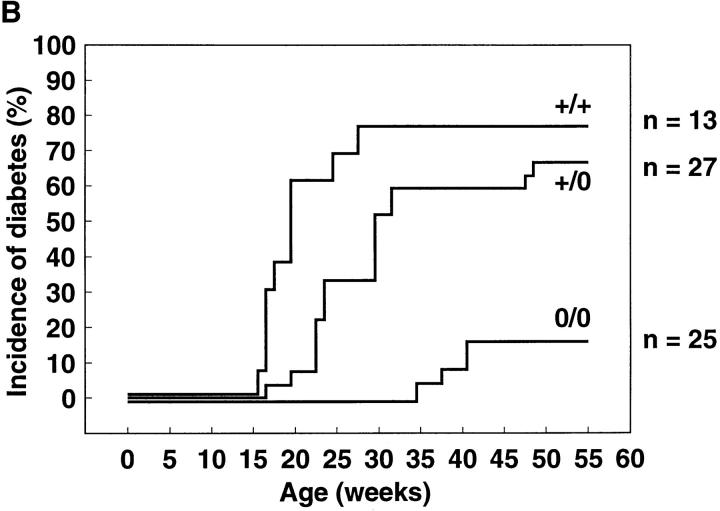
Development of insulitis and diabetes. (A) Semiquantitative assessment of insulitis in 9–12-wk-old normal control (+/+), heterozygous (+/0), or perforin-deficient (0/0) NOD mice. 20–40 randomly chosen islets/mouse were assessed on pancreas sections stained with hematoxylin and eosin. Insulitis was classified as either periinsulitis, moderate insulitis, or strong insulitis. Periinsulitis indicates a weak peripheral inflammatory infiltrate that does not penetrate the islet tissue, moderate insulitis indicates an infiltrate <50% of the islet area, and strong insulitis indicates an infiltrate >50% of the islet area. The results are given as percentage of islets per total islets scored for each mouse. Sections from three perforin +/+, six perforin +/0, and six perforin 0/0 mice were assessed. (B) Delayed onset and reduced incidence of spontaneous diabetes in perforin-deficient mice. Normal control (+/+, n = 13), heterozygous (+/0, n = 27), and perforin-deficient (0/0, n = 25) female NOD mice (seventh generation backcross, littermates) were observed over a period of 55 wk. Animals were considered diabetic if two successive blood glucose measurements at least 1 d apart yielded values >17 mM. (C) Analysis of spontaneous diabetes in perforin-deficient mice. Insulin dependence in perforin-expressing and perforin-deficient diabetes mice. Newly diagnosed diabetic mice were injected with 1 IU of human recombinant insulin s.c. into the flank and blood glucose levels were monitored subsequently. Normal NOD and heterozygous mice were 25 and 33 wk old, respectively, whereas diabetic perforin-deficient mice were both 41 wk old. Blood glucose levels from individual mice are shown. The experiment was repeated twice with similar results.
Lack of Perforin Leads to Reduced Incidence and Delayed Onset of Diabetes.
The role of perforin-dependent cytotoxicity in the diabetes disease process was evaluated by observing the incidence of spontaneous diabetes in female mice over a period of 55 wk (Fig. 1 B). In normal control NOD mice diabetes progressively developed between 15 and 30 wk (median: 19 wk) of age, and after that the incidence plateaued at 77% (n = 13). Heterozygous mice displayed a slightly delayed pattern of disease onset (median: 27 wk) with an incidence of diabetes at the end of the observation period of 67% (n = 27), possibly indicating a weak gene dosage effect (P = 4.75%). In contrast, in perforin-deficient mice, disease incidence was reduced to 16% (n = 21, P = 0.01%) and diabetes occurred only between 35 and 41 wk (median: 39.5 wk) of age. In all diabetic perforin-deficient mice, blood sugar rose to values comparable to values of diabetic control mice (28–44 mM) and did not spontaneously revert to lower values. Diabetes incidence in male perforin-expressing control mice was low (<20%). No diabetes was observed in perforin-deficient male NOD mice (data not shown).
To test whether the diabetes observed in perforin-deficient mice was insulin-dependent, spontaneously diabetic NOD mice were injected with 1 IU of insulin (Fig. 1 C). In perforin-expressing as well as perforin-deficient NOD mice insulin administration drastically lowered blood sugar levels 15 min after s.c. injection and normoglycemia was reached within 60 min after injection. Thus, diabetes in perforin-deficient mice is caused by a lack of insulin, either due to the loss of β cells or an inability of β cells to secrete sufficient amounts of insulin.
Histological Analysis of Pancreata from Diabetic Mice.
Histological analysis of pancreatic sections from diabetic animals revealed that in contrast to diabetic perforin-competent NOD mice, in which β cells were not detected, hematoxylin and eosin–stained sections from diabetic perforin-deficient NOD mice showed endocrine tissue which contained insulin-expressing β cells (Fig. 2 B). To investigate the question whether these β cells represent a low or a high percentage of the β cells present in healthy control mice, i.e., whether perforin-deficient mice were able to eliminate β cells, we compared the amount of endocrine tissue between young NOD mice and diabetic perforin-deficient mice. Representative sections were prepared from the head of the pancreas, where islets are more numerous than in the tail, and the volume density of endocrine islet tissue was calculated. The volume density of young 7-wk-old NOD mice was 1.18 ± 0.10% (n = 2). In contrast, perforin-deficient diabetic mice had a drastically reduced volume density of 0.010 ± 0.002% (n = 3). Thus, diabetes in perforin-deficient mice was caused by a loss of β cells, indicating that alternative mechanism can cause β cell damage in the absence of perforin-dependent cytotoxicity. This notion was also supported by the observation of occasional cells in islets from diabetic perforin-deficient mice with a prominent eosinophilic cytoplasm and condensed nuclei. The prominent cytoplasms indicated that these cells were β cells whereas eosinophilic staining and condensed nuclei are histological signs of cell death (Fig. 2 B).
Induction of Diabetes by Injection with Cyclophosphamide.
Injection of cyclophosphamide induces diabetes in NOD mice (24). The underlying mechanism of this effect is not completely clear, but it has been found that a single injection of cyclophosphamide induces a temporary reduction of CD4+ and CD8+ T lymphocytes (25). After recovery of the T cell compartment diabetes develops in a high percentage of young NOD mice of both sexes, but not in other nondiabetes–prone strains (1). Diabetes induced by cyclophosphamide is dependent on T cells (26, 27) and is probably caused by a yet poorly understood deregulation of the T cell compartment.
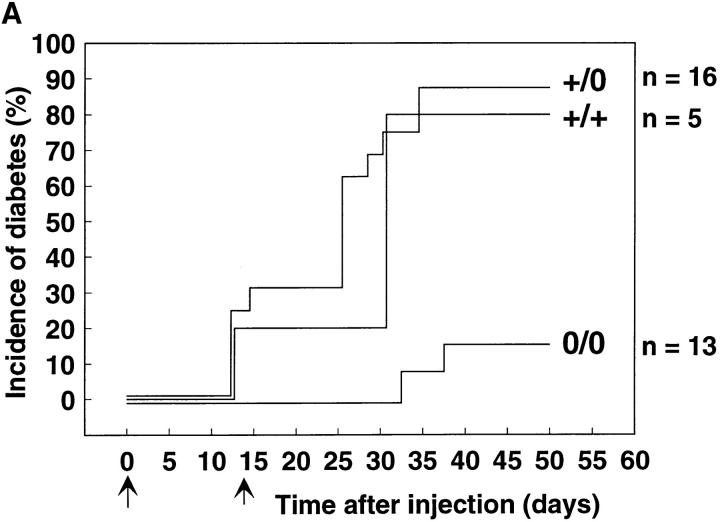
Cyclophosphamide was injected into 8–12-wk-old control or perforin-deficient NOD mice, with the first injection on day 0 and the second on day 14. Some of the normal control and heterozygous mice developed diabetes on days 12–14 before the second injection, but most became diabetic only after the second injection of cyclophosphamide between days 25 and 30, with incidences of 80% for normal control and 90% for heterozygous mice at 50 d after the first injection (Fig. 3 A). However, in perforin-deficient mice diabetes occurred only between days 32 and 38 and the incidence was reduced to 18%. Whereas blood glucose values in all diabetic perforin-expressing mice quickly rose to values of 44 mM, often followed by death from ketoacidosis, perforin-deficient mice displayed only temporary hyperglycemia with intermediate blood glucose values of 17 or 28 mM and eventually returned to normoglycemia (Fig. 3 B). Histological analysis of pancreas sections from diabetic perforin-expressing mice showed pronounced insulitis and complete elimination of islet cells. In contrast, in diabetic perforin-deficient mice secretory islet cells rich in cytoplasm were still present despite a marked mononuclear infiltrate. Antiinsulin immunohistochemical staining confirmed the absence of β cells from pancreata of diabetic perforin-expressing mice whereas diabetic perforin-deficient mice retained some insulin-containing β cells (Fig. 2 C). Thus the reduced incidence and later onset of diabetes in perforin-deficient NOD mice in the presence of marked insulitis confirm the finding with spontaneous diabetes and indicate that perforin plays a crucial role in the development of diabetes.
Figure 3.
Induction of diabetes by injection of cyclophosphamide. (A) Incidence of diabetes after i.p. injection of 6 mg of cyclophosphamide on days 0 and 14 into 8–12-wk-old normal control (n = 5), heterozygous (n = 16), and perforin-deficient (n = 13) mice. The experiment was repeated twice with similar results. (B) Blood glucose values in four representative heterozygous or perforin-deficient mice from the experiment shown in A.
DISCUSSION
The results of this study show that inactivation of perforin in NOD mice results in delayed onset and reduced incidence of diabetes. Perforin-dependent cytotoxicity is generally exerted by CD8+ T and NK cells (14, 18). In pancreatic islets of NOD mice, only a few NK cells have been found; perforin expression is thus confined to infiltrating CD8+ T cells (28). That perforin-dependent cytotoxicity mediated by CD8+ T cells is crucial in the late effector phase of diabetes in NOD mice is supported by the following evidence. First, the incidence of spontaneous and cyclophosphamide-induced diabetes is markedly reduced in perforin-deficient NOD mice. Second, the low percentage of perforin-deficient mice that developed diabetes showed a delayed disease process with significantly later onset. Third, in cyclophosphamide-induced diabetes, the blood sugar levels in perforin-deficient mice only temporarily reached diabetic levels. Finally, perforin-deficient NOD mice developed infiltration of CD4+ and CD8+ T cells into the islets comparable to those in control mice. This last result indicates that perforin-dependent cytotoxicity is not involved in the breakdown of tolerance and/or ignorance towards islet antigens in the CD4+ or in the CD8+ T cell subset which results in insulitis in the early stages of the disease. Therefore, the reduced incidence of diabetes in perforin-deficient mice is not explained by a correspondingly reduced presence of inflammatory cells in the islets, but rather reflects a defect at the level of the diabetogenic effector mechanism.
Backcrossing perforin-deficient mice with the NOD strain and screening for the disrupted, C57BL/6-derived perforin allele results in coselection of C57BL/6-derived chromosomal regions linked to the perforin gene on chromosome 10 (29). These regions may carry a diabetes susceptibility gene in the NOD mouse. Theoretically, the reduced incidence of diabetes in perforin-deficient mice may be explained by the absence of a diabetes susceptibility gene linked to the perforin locus. However, since none of the known 13 susceptibility markers has been mapped to chromosome 10 (30) and since in a backcross analysis no association with diabetes was found for a marker on chromosome 10 (21), this possibility is unlikely. A weak and only barely significant (P = 4.75%) gene dosage effect between perforin +/+ and +/0 mice was observed in diabetes incidence. During the functional analysis of perforin-deficient mice, gene dosage effects were observed in other experiments as well, e.g., in the cytotoxic activity of peritoneal exudate lymphocytes against allogeneic fibroblast target cells and in the control of injected syngeneic fibrosarcoma tumor cells (14). This, together with the low abundance of perforin mRNA in vivo (31), indicates that the amount of perforin in CTLs may be a limiting factor for certain effector functions.
Recently, absence of insulitis has been reported in β2-microglobulin–deficient NOD mice (9–11) and in NOD mice that were treated during an age window of 2–5 wk after birth with CD8+ T cell–depleting antibodies (12). Transfer experiments indicated that CD8+ T cells are required during that period to induce the ability to infiltrate the pancreatic islets in CD4+ T cells. It has been suggested that β cell antigens released by CD8+ T cell–mediated lysis are required to trigger a CD4+ T cell response (32). However, in perforin-deficient mice CD4+ T cells infiltrate the pancreas to a similar degree as in control mice. Thus induction of insulitis by CD4+ T cells does not require the lysis of β cells by perforin-dependent cytotoxicity. This also indicates that perforin-dependent cytotoxicity is not involved in the early initiation phase but rather in the later effector phase, accounting for the lysis of β cells. Taken together, the current evidence favors a model where disease progresses in three phases. The first phase, which is required for the development of islet-reactive CD4+ T cells, is dependent on CD8+ T cells but does not require perforin. In the second phase, CD4+ T cell–dependent insulitis develops. In the third phase, diabetes is induced as CD8+ T cells lyse β cells via perforin-dependent cytotoxicity.
The observation that β cell loss and diabetes develop in perforin-deficient NOD mice argues for the existence of one or several perforin-independent effector mechanisms. However, since the increase of blood sugar in cyclophosphamide-induced diabetes was only temporary and since spontaneous diabetes developed only with delayed and reduced incidence in the absence of perforin, these alternative mechanisms appear to be of lower efficiency. They may act more slowly and therefore are able to induce diabetes only in chronic situations, in which diabetes develops from insulitis over the course of several weeks. We recently reported the complete absence of diabetes in perforin-deficient transgenic mice expressing LCMV-GP in β cells of the pancreas (20) whereas diabetes develops in control mice 10 d after LCMV infection. This suggests that in a more acute situation, perforin-independent mechanisms are not able to induce diabetes, as observed in the LCMV-GP transgenic model, or induce only temporary diabetes, as in the cyclophosphamide-induced model. However, the more chronic inflammatory process which occurs spontaneously in the islets of NOD mice may allow sufficient time for alternative mechanisms with low efficiency to cause diabetogenic β cell damage.
We can only speculate on the molecular nature of the mechanisms causing diabetes in perforin-deficient mice. A possible secondary mechanism for elimination of β cells is the elimination of β cells via the perforin-independent Fas pathway of T cell–mediated cytotoxicity, which would require the expression of Fas by β cells. Fas expression associated with chronic inflammation has been reported in a number of other glandular tissues including the salivary gland and the prostate (33). Islets that are free of inflammatory infiltrates do not express Fas (33, 34); however, incubation with IL-1β induced Fas expression on cultured human β cells (34). Moreover, a recent publication has shown that β cells express Fas after transfer of a β cell–specific CD8+ T cell clone (35). From the failure of Fas-deficient NOD-lpr mice to develop diabetes, and the accelerated diabetes in transgenic mice expressing Fas ligand on β cells, this group postulated that Fas-mediated death of β cells may be the main pathogenic mechanism in autoimmune diabetes. However, since the lpr mutation leads to multiple manifestations in the immune system including lymphadenopathy, accumulation of CD4−, CD8−, B220+ T cells, and constitutive upregulation of Fas ligand on lymphocytes, the absence of diabetes in NOD-lpr mice could be explained by these factors and not by the lack of Fas expression on β cells. Nevertheless, it is conceivable that β cell elimination in perforin-deficient NOD mice is caused by upregulation of the Fas molecule on β cells in response to inflammatory cytokines produced by the infiltrate. Cognate interaction with Fas ligand–expressing β cell–specific CTLs could then induce β cell death. Noncognate interaction with any other Fas ligand–expressing cell population in the islets also is conceivable, as it has been suggested for the elimination of thyrocytes in Hashimoto's thyroiditis (36).
Alternatively, soluble factors secreted by T cells and other infiltrating leukocytes could account for β cell lysis. It has been shown in vitro that IL-1 induces the formation of nitric oxide selectively in β cells but not in α cells (37, 38). Thus the production of IL-1 by monocytes and macrophages recruited into the islets by T cell–derived chemoattractants may account for β cell elimination in perforin- deficient mice. The islet toxicity of IL-1 may be potentiated further by IFN-γ and TNF-α, which synergistically inhibit glucose-induced insulin release and cause islet cell disintegration in vitro (39, 40). Neutralization of TNF-α precludes the development of islet-reactive T cells in NOD mice, indicating that TNF-α has a critical role in the early development of the islet-specific autoimmune response (41). On the other hand, IFN-γ was shown to be essential for upregulation of class I and class II MHC molecules in the islets of LCMV-GP transgenic mice. Absence of IFN-γ completely prevented insulitis and diabetes in this model system for diabetes (42). However, in IFN-γ–deficient NOD mice absence of IFN-γ had less dramatic consequences and merely led to reduced incidence and delayed onset of diabetes, but had no consequence on insulitis (43). These findings show that TNF-α and IFN-γ seem to be involved in the modulation of the autoreactive T cell response and it remains to be seen whether these molecules also mediate β cell elimination.
In two systems, CD4+ T cells were found to induce diabetes in the absence of CD8+ T cells. Cloned islet-reactive CD4+ T cells were able to transfer diabetes to NOD-SCID recipients (4). Also, transgenic mice expressing a TCR from the same CD4+ T cell clone developed insulitis and diabetes even when they were rendered incapable of rearranging the endogenous TCR-α locus and therefore lacked CD8+ T cells (5). In both of these systems, the requirement for CD8+ T cells may be overcome by the high number of autoreactive T cells. Similar effector mechanisms may underlie both the induction of β cell loss in the absence of perforin and diabetes induced by cloned or TCR transgenic CD4+ T cells.
In conclusion, we have confirmed the important role of perforin-dependent cytotoxicity in the destruction of β cells leading to diabetes. However, our findings also show that this role is not exclusive and that perforin-independent mechanisms acting in situations of chronic inflammation can cause diabetes with a slower time course and lower incidence.
Acknowledgments
We thank Hans Acha-Orbea for helpful discussions and providing NOD mice, Kajsa Karlsson and Jolanda Bretscher for excellent technical assistance with genotyping mutant mice, Christine Quarrington and Rudolf Jörg for maintaining the colony of specific pathogen-free NOD mice, James Ho for immunohistological analysis of insulin expression, and Lisa Martin for help with the statistical analysis. We appreciate the scientific editorial assistance of Mary Saunders.
This work was supported by the Swiss National Science Foundation.
Footnotes
Abbreviations used in this paper: GP, glycoprotein; IDDM, insulin-dependent diabetes mellitus; LCMV, lymphocytic choriomeningitis virus; NOD, nonobese diabetic.
REFERENCES
- 1.Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Breeding of a non-obese diabetic strain of mice. Exp Anim (Tokyo) 1980;29:1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac A, Carnaud C, Boitard C, Bach JF. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates: requirement for both L3T4+ and Lyt-2+T cells. J Exp Med. 1987;166:823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller BJ, Appel MC, O'Neil JJ, Wicker LS. Both the Lyt-2+ and L3T4+T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988;140:52–58. [PubMed] [Google Scholar]
- 4.Bradley BJ, Haskins K, La FG, Rosa, Lafferty KJ. CD8 T cells are not required for islet destruction induced by a CD4-positive islet-specific T cell clone. Diabetes. 1992;41:1603–1608. doi: 10.2337/diab.41.12.1603. [DOI] [PubMed] [Google Scholar]
- 5.Katz JD, Benoist C. T helper cell subsets in insulin-dependent diabetes. Science (Wash DC) 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 6.Jarpe AJ, Hickman MR, Anderson JT, Winter WE, Peck AB. Flow cytometric enumeration of mononuclear cell populations infiltrating the islets of Langerhans in prediabetic NOD mice: development of a model of autoimmune insulitis for type I diabetes. Reg Immunol. 1991;3:305–317. [PubMed] [Google Scholar]
- 7.Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PGF, Gamble DR. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985;313:353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- 8.Katz J, Benoist C, Mathis D. Major histocompatibility complex class I molecules are required for the development of insulitis in non-obese diabetic mice. Eur J Immunol. 1993;23:3358–3360. doi: 10.1002/eji.1830231244. [DOI] [PubMed] [Google Scholar]
- 9.Wicker LS, Leiter EH, Todd JA, Renjilian RJ, Peterson E, Fischer PA, Podolin PL, Zijlstra M, Jaenisch R, Peterson LB. β2-microglobulin-deficient mice do not develop insulitis or diabetes. Diabetes. 1994;43:500–504. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- 10.Sumida T, Furukawa M, Sakamoto A, Namekawa T, Maeda T, Zijlstra M, Iwamoto I, Koike T, Yoshida S, Tomioka H, Taniguchi M. Prevention of insulitis and diabetes in β2-microglobulin–deficient non-obese diabetic mice. Int Immunol. 1994;6:1445–1449. doi: 10.1093/intimm/6.9.1445. [DOI] [PubMed] [Google Scholar]
- 11.Serreze DV, Leiter E, Christianson J, Greiner D, Roopenian DC. Major histocompatibility complex class I-deficient NOD-B2m nullmice are diabetes and insulitis resistant. Diabetes. 1994;43:505–509. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Gonzalez A, Benoist C, Mathis D. The role of CD8+T cells in the initiation of insulin-dependent diabetes mellitus. Eur J Immunol. 1996;26:1762–1769. doi: 10.1002/eji.1830260815. [DOI] [PubMed] [Google Scholar]
- 13.Wong FS, Visintin I, Wen L, Flavell RA, Janeway CA. CD8 T cell clones from young nonobese diabetic (NOD) islets can transfer rapid onset of diabetes in NOD mice in the absence of CD4 cells. J Exp Med. 1996;183:67–76. doi: 10.1084/jem.183.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen KJ, Podack E, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature (Lond) 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 15.Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science (Wash DC) 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 16.Kojima H, Shinohara N, Hanaoka S, Someya-Shirota Y, Takagaki Y, Ohno H, Saito T, Katayama T, Yagita H, Okumura K, et al. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994;1:357–364. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 17.Lowin B, Beermann F, Schmidt A, Tschopp J. A null mutation in the perforin gene impairs cytolytic T lymphocyte- and natural killer cell-mediated cytotoxicity. Proc Natl Acad Sci USA. 1994;91:11571–11575. doi: 10.1073/pnas.91.24.11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh CM, Matloubian M, Liu C-C, Ueda R, Kurahara CG, Christensen JL, Huang MTF, Young JD-E, Ahmed R, Clark WR. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohashi PS, Oehen S, Bürki K, Pircher HP, Ohashi CT, Odermatt B, Malissen B, Zinkernagel R, Hengartner H. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 20.Kägi D, Odermatt B, Ohashi PS, Zinkernagel RM, Hengartner H. Development of insulitis without diabetes in transgenic mice lacking perforin-dependent cytotoxicity. J Exp Med. 1996;183:2143–2152. doi: 10.1084/jem.183.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Todd JA, Aitman TJ, Cornall RJ, Ghosh S, Hall JR, Hearne CM, Knight AM, Love JM, McAleer MA, Prins JB. Genetic analysis of autoimmune type 1 diabetes mellitus in mice. Nature (Lond) 1991;351:542–547. doi: 10.1038/351542a0. [DOI] [PubMed] [Google Scholar]
- 22.Cobbold SP, Jayasuriya A, Nash A, Prospero TD, Waldmann H. Therapy with monoclonal antibodies by elimination of T cell subsets in vivo. Nature (Lond) 1984;312:548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 23.Weibel, E.R. 1973. Stereological techniques for electron microscopy. In Principles and Techniques of Electron Microscopy. Biological Applications. Vol.3. M.A. Hayat, editor. Van Nostrand Reinhold Company, New York. 237–296.
- 24.Harada M, Makino S. Promotion of spontaneous diabetes in non-obese diabetic-prone mice by cyclophosphamide. Diabetologia. 1984;37:604–606. doi: 10.1007/BF00276978. [DOI] [PubMed] [Google Scholar]
- 25.Zhang ZL, Georgiou HM, Mandel TE. The effect of cyclophosphamide treatment on lymphocyte subsets in the nonobese diabetic mouse: a comparison of various lymphoid organs. Autoimmunity. 1993;15:1–10. doi: 10.3109/08916939309004833. [DOI] [PubMed] [Google Scholar]
- 26.Charlton B, Mandel TE. Progression from insulitis to β-cell destruction in NOD mice requires L3T4+lymphocytes. Diabetes. 1988;37:1108–1112. doi: 10.2337/diab.37.8.1108. [DOI] [PubMed] [Google Scholar]
- 27.Charlton B, Bacelj A, Mandel TE. Administration of silica particles or anti-Lyt2 antibody prevents β-cell destruction in NOD mice given cyclophosphamide. Diabetes. 1988;37:930–935. doi: 10.2337/diab.37.7.930. [DOI] [PubMed] [Google Scholar]
- 28.Young LH, Peterson LB, Wicker LS, Persechini PM, Young JD-E. In vivo expression of perforin by CD8+lymphocytes in autoimmune disease. Studies on spontaneous and adoptively transferred diabetes in nonobese diabetic mice. J Immunol. 1989;143:3994–3999. [PubMed] [Google Scholar]
- 29.Trapani JA, Kwon BY, Kozak CA, Chintamaneni C, Young JD-E, Dupont B. Genomic organization of the mouse pore-forming protein (perforin) gene and localization to chromosome 10: similarities to and differences from C9. J Exp Med. 1989;171:545–557. doi: 10.1084/jem.171.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wicker LS, Todd JA, Peterson LB. Genetic control of autoimmune diabetes in the NOD mouse. Annu Rev Immunol. 1995;13:179–200. doi: 10.1146/annurev.iy.13.040195.001143. [DOI] [PubMed] [Google Scholar]
- 31.Müller C, Kägi D, Aebischer T, Odermatt B, Held W, Podack ER, Zinkernagel RM, Hengartner H. Detection of perforin and granzyme A mRNAs in infiltrating cells during LCMV infection of mice. Eur J Immunol. 1989;19:1253–1259. doi: 10.1002/eji.1830190716. [DOI] [PubMed] [Google Scholar]
- 32.Shehadeh NN, Lafferty KJ. The role of T-cells in the development of autoimmune diabetes. Diabetes Rev. 1993;1:141–151. [Google Scholar]
- 33.Leithäuser F, Dhein J, Mechtersheimer G, Koretz K, Brüderlein S, Henne C, Schmidt A, Debatin K-M, Krammer PH, Möller P. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993;69:415–429. [PubMed] [Google Scholar]
- 34.Stassi G, Todaro M, Richiusa P, Giordano M, Mattina A, Sbriglia MS, Lo A, Monte, Buscemi G, Galluzo A, Giordano C. Expression of apoptosis-inducing CD95 (Fas/Apo-1) on human β-cells sorted by flow cytometry and cultured in vitro. Transplant Proc. 1995;27:3271–3275. [PubMed] [Google Scholar]
- 35.Chervonsky AV, Wang Y, Wong S, Visintin I, Flavell R, Janeway CA, Matis LA. The role of Fas in autoimmune diabetes. Cell. 1997;89:17–24. doi: 10.1016/s0092-8674(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 36.Giordano C, Stassi G, De Maria R, Todaro M, Richiusa P, Papoff G, Ruberti G, Bagnasco M, Testi R, Galluzzo A. Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto's thyroiditis. Science (Wash DC) 1997;275:960–963. doi: 10.1126/science.275.5302.960. [DOI] [PubMed] [Google Scholar]
- 37.Bendtzen K, Mandrup-Poulsen T, Nerup J, Nielsen JH, Dinarello CA, Svenson M. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science (Wash DC) 1986;232:1545–1547. doi: 10.1126/science.3086977. [DOI] [PubMed] [Google Scholar]
- 38.Corbett JA, McDaniel ML. Perspectives in diabetes: does nitric oxide mediate autoimmune destruction of β-cells? Possible therapeutic interventions in IDDM. Diabetes. 1992;41:897–903. doi: 10.2337/diab.41.8.897. [DOI] [PubMed] [Google Scholar]
- 39.Campbell IL, Iscaro A, Harrison LC. IFN-γ and tumor necrosis factor-α cytototoxicity to murine islets of Langerhans. J Immunol. 1988;141:2325–2329. [PubMed] [Google Scholar]
- 40.Mandrup-Poulsen T, Bendtzen K, Dinarello CA, Nerup J. Human necrosis factor potentiates human interleukin-1 mediated rat pancreatic β-cell cytotoxicity. J Immunol. 1987;139:4077–4082. [PubMed] [Google Scholar]
- 41.Yang X-D, Tisch R, Singer SM, Cao ZA, Liblau RS, Schreiber RD, McDevitt HO. Effect of tumor necrosis factor α on insulin-dependent diabetes mellitus in NOD mice. I. The early development of autoimmunity and the diabetogenic process. J Exp Med. 1994;180:995–1004. doi: 10.1084/jem.180.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Herrath MG, Oldstone MBA. Interferon γ is essential for destruction of β cells and development of insulin-dependent diabetes mellitus. J Exp Med. 1996;185:531–539. doi: 10.1084/jem.185.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hultgren B, Huang X, Dybal N, Stewart TA. Genetic absence of γ-interferon delays but does not prevent diabetes in NOD mice. Diabetes. 1996;45:812–817. doi: 10.2337/diab.45.6.812. [DOI] [PubMed] [Google Scholar]