Abstract
We investigated the role of continuous thymus output in the shaping of mature T cell repertoires by studying in vivo the survival of a single clone of mature Rag2-deficient T cell receptor (TCR) transgenic cells at different stages of activation in the absence or presence of thymus export. In the absence of thymus export, TCR-transgenic lymphocytes survived indefinitely in the peripheral pools. When new lymphocytes were produced in the thymus and migrated to the periphery, resident memory T cells were maintained in constant numbers, whereas naive and self-reactive T cells were replaced by recent thymus migrants.
This T cell renewal ensured both the efficiency of recall responses to antigens as memory T cells persisted independently of thymus output, and the capacity of the immune system to respond to new antigen stimulation as the naive T cell pool was continuously renewed. Our results also indicate that thymus export is required to control the number of self-reactive peripheral T cells that may invade the peripheral pools if thymus output fails.
T lymphocytes are produced in the thymus, where T cell receptor gene rearrangements guarantee the continuous generation of a very diverse T cell repertoire. During this process of differentiation, the repertoire of developing thymocytes is submitted to both negative and positive selection events (see review in reference 1). Mature T cells leave the thymus and seed the peripheral pools. This process starts around birth, and continues throughout life.
Mature T lymphocytes can be produced by cell division in the periphery (2, 3). In adult euthymic mice, most mature T cells are produced this way (4). This peripheral expansion modifies clone sizes and relative clonal representation, and thus considerably alters the T cell repertoires generated in the thymus (5). It is required for the generation of efficient immune responses (by increasing the frequency of rare clones), but cannot generate new T cell specificities, a role restricted to the thymus.
It is not known how T cell repertoires shaped in the thymus are further modified in the peripheral pools. Not all self-antigens are expressed in the thymus, so that potentially autoreactive cells may be continuously produced; peripheral mechanisms rendering these cells tolerant have been reported (5–11). In addition, the peripheral immune system is under homeostatic control of cell numbers (4, 12). From early development until adult age, peripheral lymphocyte populations increase in size, and can accommodate thymus migrants. In adults, the total T cell number is constant. Within these constraints, T cells exported from the thymus or generated by cell division in the periphery can only survive upon the loss of other resident T cell populations.
In adult mice, it is not known how thymus migrants are incorporated into a “full” peripheral compartment. T cells produced in the thymus throughout life may be a continuous source of T cell diversity; on the other hand, their preferential incorporation in the peripheral pools may lead to the disappearance of resident T cells and the extinction of some memory responses. Conversely, successive antigen encounters may lead to a gradual reduction in repertoire diversity and the emergence of pauciclonal T cell repertoires, if thymus output is not maintained. The emergence of certain autoimmune diseases after thymectomy (13), as well as the age-related increase in self-reactive lymphocytes (13– 15), suggests also that thymus export may be involved in avoiding the emergence of self-reactive clones in the peripheral pools.
We have previously used TCR-transgenic (Tg)1 mice, bearing Tg α/β-TCR specific for the male antigen (1) to obtain the same clone of αTβT T cells (Tg cells) at different states of activation. Naive Tg cells can be recovered directly from female Tg mice as, in the absence of male antigen, Tg cells are CD44− and do not divide or produce lymphokine messenger RNAs (mRNAs; 16–19). Memory and tolerant Tg cells can be obtained after in vivo stimulation with male antigen. Depending on the priming conditions, Tg cells can maintain their capacity to recognize and respond to the male antigen (acquiring the properties of memory cells) or become tolerant to this antigen (18). In the work presented here, we devised experimental protocols to study the substitution of naive, memory, and tolerant Tg T cells by T lymphocytes newly generated in the thymus.
MATERIALS AND METHODS
Mice.
All mice were used at 6–8 wk of age: females expressing an α/β-TCR–Tg, specific for the male antigen (20; these mice were crossed into the Rag2-deficient background [21] to produce monoclonal mice); Ly51 C57Bl/6; females and males Ly51 and Ly52 CD3ε deficient (22) or Rag2 deficient. All animals were bred at the Centre de Developpement des Techniques Avancees pour l'experimentation animale, Orleans, France).
Bone Marrow Chimeras.
To obtain male/female bone marrow (BM) chimeras, Rag2-deficient male or female mice were irradiated (400 rads [R]) and injected 24 h later with 5 × 106 BM cells (90% female and 10% male). Male BM was from Ly52 CD3ε-deficient mice. Depending on the experiment, female cells were from Ly51 CD3ε-deficient or Ly51 C57Bl/6 mice. These mice were injected with 0.5 × 106 monoclonal TCR-Tg naive T cells 24 h after BM transfer.
To study the substitution of naive T cells, Tg mice were irradiated (400 R) and injected the day after with 5 × 106 BM cells from Ly51 C57Bl/6 female mice.
Antibodies and Immunofluorescence Analysis.
For surface staining (18), the following mAbs were used: H57-597(anti-β TCR); T3.70 (anti-α TCR Tg, 20); H35-172 (anti-CD8β chain); and A20-1-7 (anti-L51; a gift from Dr. Boyse). These antibodies were directly coupled to FITC or biotin, and revealed by streptavidin-Tricolor (Caltag, South San Francisco, CA). Anti-CD4 and anti-CD8 mAbs directly coupled to phycoerythrin were purchased from PharMingen, (San Diego, CA). Flow cytometric analysis was done on a FACScan® (Becton Dickinson, Mountain View, CA).
Quantification of T Cell Populations in Host Mice.
A standard procedure was used to prepare single-cell suspensions from the thymus, spleen, and a pool of mesenteric, inguinal, and axillary LN. To determine the total number of cells recovered in each organ, two independent samples of each suspension were counted before washing. The total number of T cells of each phenotype in each organ was calculated from the frequency estimated by immunofluorescence analysis and the total number of cells recovered from the organ. The total number of cells recovered from the peripheral pools was considered equal to that obtained in the spleen plus twice that obtained in the LN pool.
RESULTS
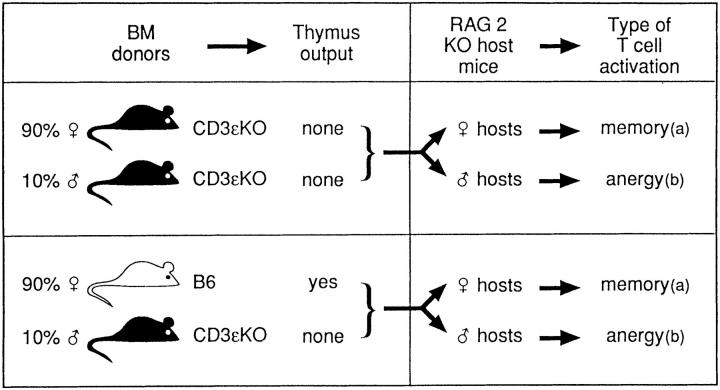
We devised protocols in which we could induce the same clone of T cells into different states of activation and then study the substitution of such clone by T cells migrating from the thymus. The protocols are summarized in Fig. 1.
Figure 1.
Generation of male/female chimeric mice, with our without thymus export. All male/female chimeras were injected with 0.5 × 106 Tg cells. (a) Tg cells remain functional. (b) Tg cells become tolerant.
In the Absence of Thymus Output, Memory and Tolerant T Cells Numbers Remain Constant in the Periphery.
To generate memory and tolerant T cells, naive monoclonal T cells from Rag2-deficient female mice bearing a α/β-TCR Tg specific for the male antigen (Tg cells) were injected into male/female BM chimeras expressing different amounts of male antigen.
We first produced chimeras in which thymus output was absent (Fig. 1), by injecting irradiated Rag2-deficient hosts with 90% female Ly52 and 10% Ly51 male BM cells from CD3ε-deficient mice. After BM reconstitution, 10% of BM-derived cells in these chimeras are of Ly51 male origin (19). The CD3ε-deficient BM cells are unable to repopulate the thymus (22).
In a first group of chimeras, host mice were female. Naive Tg cells transferred to these mice are stimulated by the male antigen presented by 10% of male CD3ε-deficient BM-derived cells (18, 19). Tg cells stimulated by this relatively small amount of antigen, acquire the properties of memory cells. Their phenotype (as evaluated by CD44, CD69, and CD25) (19) is that previously described in memory cells (23). These cells eliminate male cells in vivo, and proliferate in response to the male antigen in vitro and in vivo (18, 19).
To generate tolerant T cells, we used male irradiated Rag2-deficient hosts. Naive Tg cells transferred into these male mice are stimulated by antigen present in all tissues besides the BM-derived CD3ε-deficient male cells (Fig. 1). Tg cells recovered from male hosts are tolerant, as they are unable to eliminate male cells in vivo and to proliferate in response to the male antigen in vivo and in vitro (5).
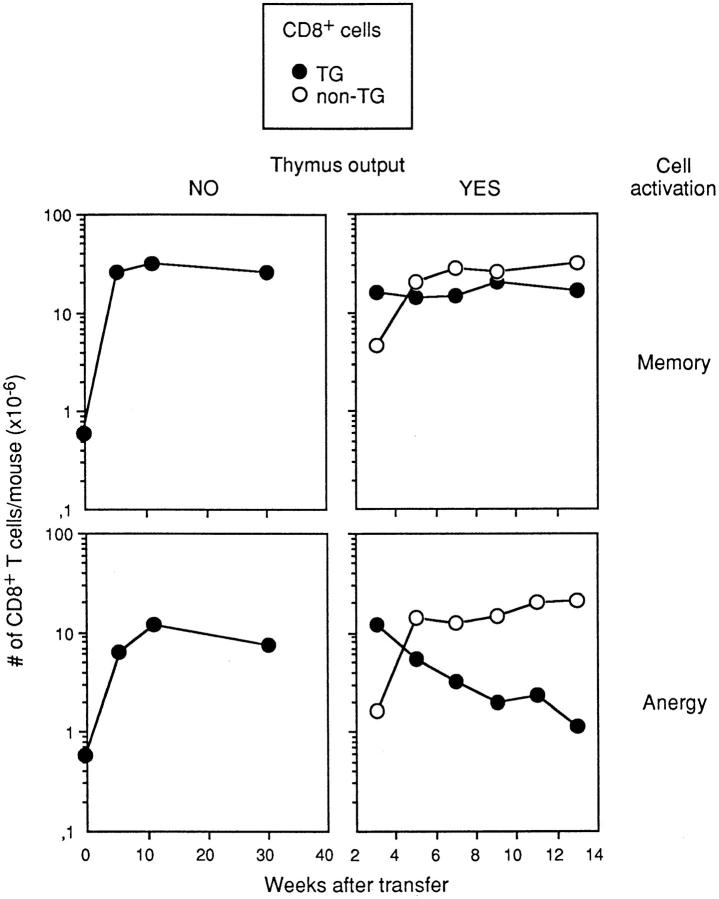
All naive Tg cells were activated and expanded after adoptive transfer into male/female chimeras (Fig. 2, left). In mice injected with 0.5 × 106 naive Tg cells, 25–30 × 106 Tg cells were recovered from female hosts and 15–20 × 106 cells from male hosts 3–4 wk after T cell transfer. These numbers remained constant for at least 8 mo (Fig. 2, left). These results demonstrate that once generated, memory and tolerant T cells can persist in constant numbers for long periods in the absence of thymus output.
Figure 2.
Male/female chimeras were generated as described in Fig. 1, injected with 0.5 × 106 Tg cells and studied at different times after Tg cell transfer. Results represent the number of CD8+ Tg (closed circles) and non-Tg cells (open circles) recovered, and are the mean for 2–3 mice/time point. (Left) Chimeras received only CD3ε-deficient BM, and had no thymus output. (Right) Host mice received B6 BM that recolonized the thymus. (Top) Female hosts, where Tg cells acquire the properties of memory cells. (Bottom) Male hosts, where Tg cells become tolerant. The times scales on the left and right graphs are different.
In the Presence of Thymus Output, Memory Cells Persist in Constant Numbers in the Periphery, whereas Most Tolerant T Cells Disappear.
To study substitution of resident Tg cells by thymus migrants, the chimeras described above were slightly modified to permit repopulation of the thymus by BM-derived cells (Fig. 1). As above, Rag2-deficient irradiated hosts were reconstituted with 90% female and 10% male BM cells. Male BM were from CD3ε-deficient mice and were thus unable to recolonize the thymus and generate male T cells, avoiding variations of antigenic load in these chimeras. Female BM cells, however, were from normal B6 mice and were thus able to repopulate the thymus. These chimeras were injected with 0.5 × 106 Tg cells. In these mice, thymus reconstitution was such that thymus migrants only reached the periphery after maximal accumulation of Tg cells; thymus migrants could thus repopulate an already “full” peripheral compartment.
With respect to peripheral expansion, the kinetics of T cell accumulation was as found previously. All naive Tg cells were activated and expanded, and accumulation of Tg cells was maximal by 3–4 wk after transfer. At this time, the number of Tg cells recovered was the same as in previous chimeras (Fig. 2, compare right and left graphs). Thymus output started after maximal accumulation of Tg cells; B6 thymus migrants reached the periphery 3 wk after BM injection (Fig. 2, right; 24, 25). From wk 3–5, these migrants accumulated. During these 2 wk 1.5 × 107 B6 T cells were incorporated in the peripheral pools of both types of chimeras (Fig. 2, right), corresponding to an average rate of accumulation of 106 T cells/d. This thymic output was similar to that described previously in unmanipulated mice (26). Numbers of T cells of donor B6 BM origin reached steady state at wk 5 after BM injection.
The fate of resident Tg cells differed in male and female hosts. Memory cells in female hosts persisted in constant numbers, whereas tolerant T cells in male hosts gradually disappeared. Because tolerant T cells persisted in the absence of thymus output and their decay started only when thymus migrants reached the periphery, the decay of tolerant T cells appeared to be a consequence of peripheral repopulation by T cells originating in the thymus.
Substitution of Virgin T Cells by Recent Thymus Migrants.
In athymic or thymectomized mice, Tg female virgin T cells are able to persist in constant numbers for up to 2 yr (5, 16). As these naive Tg cells do not divide (5, 16) the generation of a full naive pool requires thymus output, and cannot be obtained after adoptive transfer. To study the survival of naive Tg cells in the presence of thymus export, we irradiated female Tg mice at 400 R, and injected female B6 BM cells the day after. These mice generated a full naive Tg peripheral compartment; the thymus was totally colonized by B6 BM-derived cells, and thymus export was that of a normal thymus. In addition, the output of B6 migrants started only after the reconstitution of naive Tg cells compartment.
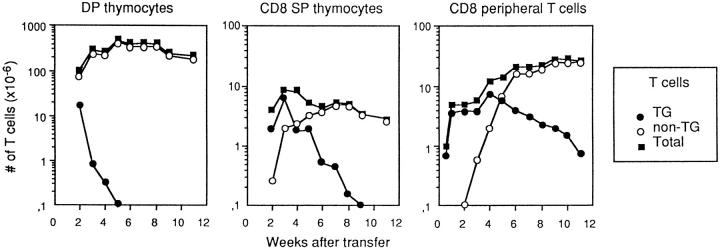
As shown in Fig. 3 (right), naive Tg cells were destroyed by irradiation, but radioresistant host thymocytes produced enough Tg cells to repopulate the periphery; 3 wk after irradiation, the number of peripheral Tg cells (∼ 4 × 106) was similar to that in nonirradiated female Tg mice (16).
Figure 3.
Female TCR Tg mice were irradiated (400 R), injected with 5 × 106 B6 BM cells, and studied at different times after T cell transfer. Results are the number of CD4+CD8+ (left) and CD8+CD4− (middle) thymocytes and CD8+ T peripheral T cells (right), and are the mean for 2 mice/experimental point in one typical experiment out of three.
The thymus (Fig. 3, left and middle) was rapidly invaded by B6 non-Tg BM-derived cells. By 2 wk after irradiation most double positive (DP) thymocytes were non-Tg. By 3 wk, less than 106 Tg DP cells remained, and the total number of CD4+CD8+ (DP) and CD8+CD4− (CD8 single positive [SP]) thymocytes was similar to that in non-Tg mice. By 5 wk, de novo production of Tg cells (from Tg DP immature thymocytes) was totally abrogated; all DP cells were of B6 origin.
It has been reported that it takes a week for DP cells to generate CD8+ SP thymocytes (27, 28). We also found that CD8+ SP thymocyte production from DP cells took 1 wk. 3 wk after irradiation, when virtually all DP cells were non-Tg, most CD8 SP were still Tg cells (Fig. 3, middle). The number of CD8+ SP B6 non-Tg cells increased thereafter. By 6 wk after irradiation virtually all CD8 SP thymocytes were non-Tg.
Donor non-Tg cells were first detected in the peripheral pools 3 wk after BM transfer. These migrants accumulated exponentially during the next 2 wk, at an average rate of 106/day (Fig. 3, left); this output is similar to that found in RAG2-deficient mice reconstituted with B6 BM cells described above (Fig. 2, left). We also investigated the thymus output in our mice, in steady-state conditions. We found an average output of 106/d as described in unmanipulated mice (26). Therefore, thymus output appeared to be constant and independent of the number of peripheral T cells, suggesting the absence of regulatory feedback loops between the central and the peripheral T cell compartments.
Migration of non-Tg T cells into the peripheral pools was accompanied by a gradual decay of resident naive Tg lymphocytes. This decay continued well after the peripheral pools had reached steady-state numbers (by the wk 6 after BM transfer).
These results indicated that even in steady-state conditions when the peripheral T cell compartment was full, the pool of naive resident CD8+ cells was permissive of new thymus migrants.
Substitution of Resident T Cells by Recent Thymus Migrants Is Independent of Cell Age.
There are two possible explanations for the substitution of peripheral T cells; cell survival is either conditioned by age or is random, i.e., cell age independent.
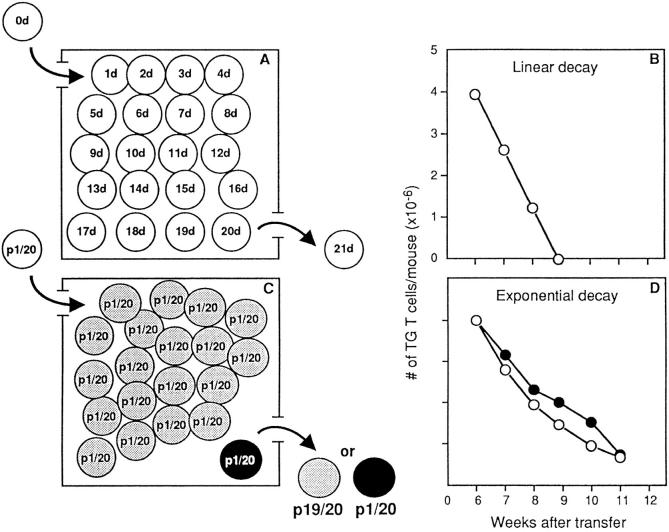
In the first hypothesis, T cells have a finite life span and only their age determines their survival; each day the younger cells (recent thymus migrants) are incorporated in the periphery, whereas the oldest resident cells die (“first in, first out” hypothesis, Fig. 4, top). Substitution of resident cells should be linear, and obey the formula. Xt = X0 (1 − at), where Xt is absolute number of resident Tg cells as a function of time, X0 is absolute number of resident Tg at time 0, a is the ratio between the number of thymus migrants and the total number of resident T cells, and t is time in days. This type of substitution would not be associated with T cell repertoire selection.
Figure 4.
The two hypotheses to explain the kinetics of substitution of naive T cells by thymus migrants. (Left) Boxes represent the periphery, where 20 different elements are pictured. Each element represents an individual cell. (Top) Substitution is conditioned by the age of the cell. (A) Each cell has a different age. Each day the younger cell (the thymus migrant) is incorporated in a peripheral pool, and the older cell dies. (B) Linear decay, calculated according to the formula Xt = X0 (1 − at). (Bottom) Substitution is cell age independent. (C) A large clone (19 elements), is shown in gray, and a small clone (1 element) in black. Each day, each cell has the same probability (1/20) of being replaced by a thymus migrant. The probability of replacing a gray cell will be 19 times higher than that of replacing the dark cell. (D) (Open circles) Exponential decay, calculated according to the formula X(t) = X0 (1 − a)t where Xt is absolute number of resident Tg cells as a function of time, X0 is absolute number of resident Tg cells at time 0, a is the ratio between the number of thymus migrants and the total number of resident T cells, and t is time in days. (Closed circles). Substitution of resident Tg cells by recent thymus migrants (experimental values). In B and D, the size of the T cell clones (y-axis) is shown on a linear scale.
In the second hypothesis, each cell in the peripheral pools has the same survival probability. T lymphocyte life spans can only be defined as this probability of survival; disappearance of residual cells should follow an exponential curve and obey to the formula: X(t) = X0 (1 − a)t (Fig. 4, bottom).
Random substitution of individual T cells may modify relative clone sizes. In Fig. 4 C, a large clone is represented by 19 gray elements and a small clone by one dark element. Random substitution will be equivalent to the blind removal of an element. Although each element has the same probability of being removed (1/20), the probability that the element removed belongs to a large clone (i.e., gray) will be 19 times higher than that of removing the dark element.
We applied the linear and exponential formulae to resident T cell substitution (Fig. 4, B and D, open circles), once the peripheral pool had reached steady state. In these conditions, the ratio of thymus output to the number of resident cells is constant (106/2.0 × 107 = 5%). To calculate the substitution of a particular clone of resident T cells, this value was multiplied by the size of the clone at time 0, i.e., 4 × 106 × 5% = 0.2 × 106 Tg cells/d. Linear regression would imply that all resident Tg cells (4 × 106) would be replaced in 20 d, i.e., by 8–9 wk after BM transfer (Fig. 4 B). Yet, we found that only 40% of Tg cells were replaced from wks 6 to 9, and that Tg cells persisted in the periphery of host mice for at least 12–14 wk. Comparison of theoretical curves of T cell regression (Fig. 4, B and D, open circles) and actual curves of T cell disappearance (Fig. 4 D, closed circles) showed that the decay of resident cells was exponential. The decay curve of tolerant T cells was also exponential (Fig. 2, lower right).
Our experiments permitted a long follow up of a single clone of T cells, but involved unphysiologic manipulation. Previous studies of naive Tg cell turnover in intact female Tg mice, however, are also strongly suggestive of nonlinear substitution; in these mice, only 40% of peripheral naive Tg cells are replaced by migrants during 3 wk (16). As thymus output of Tg lymphocytes averages 106 Tg cells/d (29, Tanchot, C., unpublished observations) and as the total number of Tg cells is around 4 × 106, linear substitution would imply that all Tg lymphocytes would be replaced after 3 wk.
Exponential Decay of Resident Cells Influences the Peripheral T Cell Repertoires.
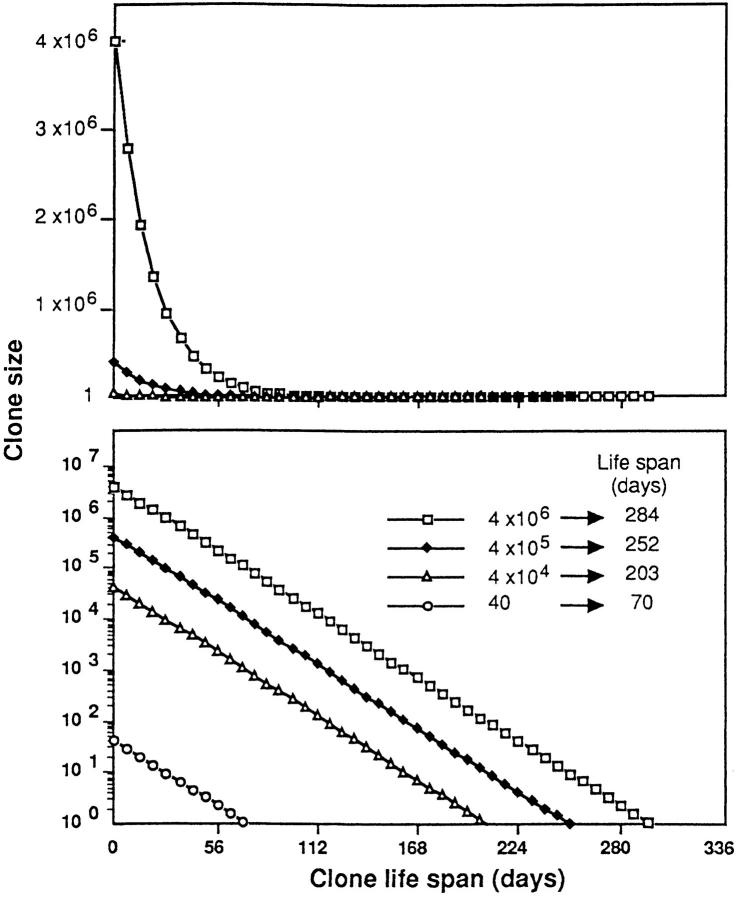
Using a thymus output of 106/d, taking into account the size of the peripheral pool and applying the formula X(t) = X0 (1 − a)t, we represent the putative exponential decay of clones of different sizes in Fig. 5. The curves in the upper and lower graphs are the same; in the upper graph, the size of each clone (y-axis) is shown on a linear scale, and in the lower graph, in a log scale. To determine the life span of each clone (the time taken for all members of a clone to disappear) we calculated the time required for each decay curve to intercept the x-axis, i.e., to fall below the value of one cell.
Figure 5.
Exponential decay of four T cell clones of different sizes, calculated according to the formula X(t) = X0 (1 − a)t. The clone size (y-axis) is shown on a linear scale (top) or a log scale (bottom). The life span of each clone (the number of days required for all members of the clone to disappear) was calculated as the time required for each decay curve to intercept the x-axis, i.e., to fall below one cell.
Decay curves following exponential laws would lead to rapid contraction of large clones. This is better visualized on the linear scale (top). The persistence of a clone, however, is not directly proportional to its size, but rather to the log of its size; as represented in Fig. 5, bottom, a clone composed of 4 × 104 cells will persist for 202 d, whereas a clone 1,000 times larger (4 × 106 cells) will persist for 284 d, i.e., only 1.3 times longer.
These graphs illustrate that random substitution of individual T cells by thymus migrants influences peripheral repertoires, as it is associated with modifications of the relative representation of T cell clones in the peripheral pools. This substitution will lead to rapid contraction of large clones, and to a relatively long survival of small clones.
DISCUSSION
We studied the persistence of a clone of T lymphocytes with a defined T cell specificity (imposed by the expression of a TCR-Tg) in the peripheral pools. We found that in the absence of newly generated T cells, Tg T lymphocytes survived indefinitely in the peripheral pools. When new lymphocytes were produced in the thymus and migrated to the periphery, survival of resident T cells could be modified. This modification was conditioned by their state of activation; naive and tolerant T cells decayed, whereas memory cells persisted in constant numbers in the presence of thymus output.
With regard to the mechanism of this decay, recent thymus B6 cells would not appear equipped to kill resident T cells, as they do not express mRNA for lymphokines, perforin, or FasL (19). The decay kinetics argued against a killing mechanism; when thymus production of B6 cells began, and the peripheral pools were full of Tg lymphocytes, substitution of resident Tg cells was very efficient; when Tg cells were rare, and the peripheral pools were full of B6 cells, this major cell population was unable to dislodge these few resident Tg cells. We found that substitution of peripheral resident T cells, was cell-age independent, with thymus migrants and resident T cells having the same survival probability. These results indicated that disappearance of resident T cells was due to random substitution when resident T cells and new thymus migrants were competing for the same niches in the peripheral T cell pools.
This kinetics of T cell renewal has important implications for the organization of T cell specificities in the peripheral pools. The exponential decay of resident T cells is associated with rapid contraction of large clones, and with relatively long survival of small clones. This will impose a peripheral T cell repertoire composed of many small T cell clones, i.e., guaranteeing repertoire diversity. The imposition of repertoire diversity by such a simple mechanism very likely already takes place during positive selection in the thymus. Indeed, it was shown that positive selection and survival of Tg cells increased when these cells represented a minority of immature thymocytes (27).
As in naive cells, decay of tolerant cells was exponential; the clone size was initially greatly reduced, but the efficiency of substitution decreased with time, and tolerant T cell clones persisted in small numbers for long periods. The long survival of a few tolerant T cells may have implications for recall responses to antigens to which the immune system was previously tolerized. Although tolerant male-specific Tg cells are unable to proliferate and eliminate the male antigen (18), they are not inert; they respond to male antigen by producing lymphokines (IL-10) that are never produced by memory cells and spontaneously secrete substantial amounts of γ-interferon (our manuscript in preparation). It is conceivable that these residual tolerant T cells have a major regulatory role in immune responses and are involved in the maintenance of tolerance to their specific antigen in vivo.
This decay of tolerant T cells in our experiments mimicked the “deletion” kinetics of tolerant T cells in many circumstances where antigen stimulation induces peripheral T cell tolerance; immediately after T cell expansion and tolerance induction, most tolerant T cells disappear rapidly, but a minority persist for long periods (5, 6, 9, 30). It was suggested that this deletion was a consequence of apoptosis induced by an antigenic overstimulation (9). Our results suggest that this apoptosis may not take place in the absence of other T cells. Indeed, we found no deletion when we tolerized a clone of Tg cells in the absence of other T cells, whereas deletion always occurs when other T lymphocytes are present (5); tolerant cells may become susceptible to substitution by other nontolerant T lymphocytes.
As naive and tolerant cells have such different characteristics, we were surprised that both populations were similarly substituted by recent thymus migrants. For the moment, we can only speculate that this may be due to the fact that both Tg populations do not cycle in the peripheral pools. Indeed, we have previously shown the naive male-specific Tg cells of female mice do not divide (16). Similarly, we previously showed that tolerance induction blocks the capacity of Tg cells to expand (31); in contrast, memory cells that resist substitution by recent migrants (see below) divide in the peripheral pools (19, 23). Regulation of lymphocyte numbers can involve the control of cell division and/or of cell death; since both naive and anergic Tg peripheral lymphocytes do not divide, the regulation of their number by recent thymus migrants is likely associated with an increased death rate of resident Tg cells in the presence of thymus output.
In contrast to naive and tolerant T cells, memory cells were not substituted by recent thymus migrants; they persisted in constant numbers, independently of T cell production by the thymus. These memory cells might have a survival advantage over naive T cells generated in the thymus; if so, the presence of memory cells in the periphery should prevent the accumulation of recent migrants. However, on the contrary, we found that accumulation of recent migrants was unaffected by the presence of resident memory T cells. These results indicate that naive and memory cells do not compete for the same niches.
Our previous results on the homeostatic regulation of peripheral T cell pools also suggested that naive and memory cells had different niches. Indeed, pools of naive and memory cells are maintained by independent homeostatic mechanisms; the total number of naive cells is maintained independently of the total number of memory cells; similarly, the total number of memory cells is regulated independently of the presence of naive cells (17). The different niches to which memory and naive cells belong suggest that naive and memory T cell survival may not be dependent on the same type of environmental interactions. We have recently demonstrated that memory and naive T cells differ in the type of TCR–ligand interactions required for their survival (19).
We do not yet know if and how memory cell renewal takes place. It is possible that memory T cells are substituted only by other newly produced memory cells. Indeed, it has recently been reported that new antigen encounters may lead to a reduction in residual memory responses (32).
The present data clear up certain controversies over the evaluation and definition of lymphocyte life spans (see review in reference 12). We found that Tg peripheral T cells, when present alone, could survive indefinitely in the peripheral pools. When other cells were produced in the thymus, resident T cells could disappear. In the latter case, not all T lymphocytes belonging to the same clone disappeared simultaneously. Some members survived only a few days, whereas others survived for several months. These results clearly establish that the life span of individual T lymphocytes can no longer be considered as an intrinsic property of a cell depending only on the interaction of its TCR with its ligand. Rather, once T cells are generated, they have a certain probability of surviving. This probability is conditioned not only by their state of activation, but also by the presence of other T lymphocytes.
Since our model system used cells expressing a single TCR, the behavior of these cells might not be representative of all CD8+ lymphocytes. The properties of naive and memory Tg cells, however, appear largely shared by non-Tg CD8+ lymphocytes. Naive CD44−CD8+ T lymphocytes, like naive Tg cells, do not divide (17) or express lymphokine mRNA (19). CD44+CD8+ B6 cells and memory Tg cells divide at the same rate in the peripheral pools (17, 19, 23) and share the same pattern of lymphokine production (19). Besides, we also investigated the kinetics of generation of the CD44+ and CD44− peripheral pools in irradiated euthymic mice, Tg and non-Tg cells showing an identical behavior (17).
In conclusion, the dynamics of the turnover of resident peripheral T cell populations revealed different strategies in the organization of peripheral T cell repertoires. Memory cells, once generated, were maintained in constant numbers in the peripheral pools, guaranteeing recall responses. The potential to generate immune responses to new antigens is maintained by the naive T cell pool, which is organized in a way that ensures maximal diversity and continuous replacement by new T cells produced in the thymus. Continuous thymus output also ensures that self-reactive T cells are maintained at very low frequencies in the periphery. The absence of thymus migrants explains the emergence of certain autoimmune disorders in thymectomized mice (32), as well as the increased incidence of autoimmune conditions in aging individuals (14, 15) as thymus output gradually fails.
Acknowledgments
We are grateful to A. McLean and H. von Boehmer for helpful comments.
This work was supported by grants from the ANRS and DRET.
Footnotes
Abbreviations used in this paper: BM, bone marrow; DP, double positive; mRNA, messenger RNA; R, rads; SP, single positive; Tg, transgenic.
REFERENCES
- 1.Von Boehmer H. Developmental biology of T cells in T cell–receptor transgenic mice. Annu Rev Immunol. 1990;8:531–536. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]
- 2.Rocha B, Freitas AA, Coutinho AA. Population dynamics of T lymphocytes. Renewal rate and expansion in the peripheral lymphoid organs. J Immunol. 1983;131:2158–2164. [PubMed] [Google Scholar]
- 3.Stuttman O. Post-thymic T cell development. Immunol Rev. 1986;91:159–194. doi: 10.1111/j.1600-065x.1986.tb01488.x. [DOI] [PubMed] [Google Scholar]
- 4.Rocha B, Dautigny N, Pereira P. Peripheral T lymphocytes: expansion potential and homeostatic regulation of pool sizes and CD4/CD8 ratios in vivo. Eur J Immunol. 1989;19:905–911. doi: 10.1002/eji.1830190518. [DOI] [PubMed] [Google Scholar]
- 5.Rocha B, Von Boehmer H. Peripheral selection of the T cell repertoire. Science (Wash DC) 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 6.Webb SR, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 7.Morahan G, Allison J, Miller JFAP. Tolerance of class I histocompatibility antigens expressed extrathymically. Nature (Lond) 1989;339:622–624. doi: 10.1038/339622a0. [DOI] [PubMed] [Google Scholar]
- 8.Burkly LC, Lo D, Flavell RA. Tolerance in transgenic mice expressing major histocompatibility molecules extrathymically on pancreatic cells. Science (Wash DC) 1990;248:1364–1368. doi: 10.1126/science.1694042. [DOI] [PubMed] [Google Scholar]
- 9.Kawabe Y, Ochi A. Selective anergy of Vβ8+CD4+cells in staphylococcus enterotoxin B–primed mice. J Exp Med. 1990;172:1065–1070. doi: 10.1084/jem.172.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D. Transgenic mouse models of self-tolerance and autoreactivity by the immune system. Annu Rev Cell Biol. 1990;6:493–537. doi: 10.1146/annurev.cb.06.110190.002425. [DOI] [PubMed] [Google Scholar]
- 11.Wieties K, Hammer RE, Jones-Youngblood S, Forman J. Peripheral tolerance in mice expressing a liver-specific class I molecule: inactivation/deletion of a T cell subpopulation. Proc Natl Acad Sci USA. 1990;87:6604–6608. doi: 10.1073/pnas.87.17.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freitas AA, Rocha B. Lymphocyte lifespans: homeostasis selection and competition. Immunol Today. 1993;14:25–29. doi: 10.1016/0167-5699(93)90320-K. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Sakaguchi N. Thymus and autoimmunity: capacity of the normal thymus to produce pathogenic self-reactive T cells and conditions required for their induction of autoimmune disease. J Exp Med. 1990;172:537–545. doi: 10.1084/jem.172.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith H, Chen I-M, Kubo R, Tung KSK. Neonatal thymectomy results in a repertoire enriched in T cells deleted in adult thymus. Science (Wash DC) 1989;245:749–752. doi: 10.1126/science.2788921. [DOI] [PubMed] [Google Scholar]
- 15.Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 16.Von Boehmer H, Hafen K. The life span of naive α/β T cells in secondary lymphoid organs. J Exp Med. 1993;177:891–896. doi: 10.1084/jem.177.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanchot C, Rocha B. The peripheral T cell repertoire: independent homeostatic regulation of virgin and activated CD8+T cell pools. Eur J Immunol. 1995;25:2127–2136. doi: 10.1002/eji.1830250802. [DOI] [PubMed] [Google Scholar]
- 18.Rocha B, Grandien A, Freitas AA. Anergy and exhaustion are independent mechanisms of peripheral T cell tolerance. J Exp Med. 1995;181:993–1003. doi: 10.1084/jem.181.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanchot C, Lemmonier FA, Pérarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science (Wash DC) 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 20.Teh HS, Kisielow P, Scott B, Kishi H, Uematsu Y, Bluthmann H, Von Boehmer H. Thymic major histocompatibility complex antigens and the αβ T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature (Lond) 1988;335:229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- 21.Shinkai Y, Rathbun G, Lam K-P, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. RAG-2 deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 22.Malissen M, Gillet A, Ardouin L, Bouvier G, Trucy J, Ferrier P, Vivier E, Malissen B. Altered T cell development in mice with a targeted mutation of the CD3-ε gene. EMBO (Eur Mol Biol Organ) J. 1995;14:4641–4653. doi: 10.1002/j.1460-2075.1995.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science (Wash DC) 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 24.Pénit C, Ezine S. Cell proliferation and thymocyte subset reconstitution in sublethally irradiated mice: compared kinetics of endogenous and intrathymically transferred progenitors. Proc Natl Acad Sci USA. 1989;86:5547–5551. doi: 10.1073/pnas.86.14.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shortman K, Egerton M, Spangrude GJ, Scollay R. The generation and fate of thymocytes. Semin Immunol. 1990;2:3–12. [PubMed] [Google Scholar]
- 26.Scollay RG, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 27.Huesmann M, Scott B, Kisielow P, Von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 1991;66:533–540. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- 28.Lucas B, Vasseur F, Pénit C. Production, selection and maturation of thymocytes with high surface density of TCR. J Immunol. 1994;153:53–62. [PubMed] [Google Scholar]
- 29.Kelly KA, Pircher H, Von Boehmer H, Davis MM, Scollay R. Regulation of T cell production in T cell receptor transgenic mice. Eur J Immunol. 1993;23:1922–1928. doi: 10.1002/eji.1830230829. [DOI] [PubMed] [Google Scholar]
- 30.Dannecker G, Mecheri S, Staiao-Coico L, Hoffmann MK. A characteristic Mls-1a response precedes Mls-1aanergy in vivo. J Immunol. 1991;146:2083–2087. [PubMed] [Google Scholar]
- 31.Rocha B, Tanchot T, Von Boehmer H. Clonal anergy blocks in vivo growth of mature T cells and can be reversed in the absence of antigen. J Exp Med. 1993;177:1517–1521. doi: 10.1084/jem.177.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selin LK, Virgilis K, Welsh RM, Nahill SR. Reduction of otherwise remarkably stable virus-specific cytotoxic T lymphocyte memory by heterologous viral infections. J Exp Med. 1996;183:2489–2499. doi: 10.1084/jem.183.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]