Abstract
B cells are susceptible to Fas ligand (FasL)+ CD4+ Th1 cell–mediated apoptosis. We demonstrate that blocking the interactions between lymphocyte function associated (LFA)-1 and intercellular adhesion molecule(ICAM)-1 and ICAM-2 completely suppresses Fas-dependent B cell lysis. Antibodies to CD2 and CD48 partially suppress B cell apoptosis, whereas anti-B7.1 and anti-B7.2 antibodies have no effect. Also, B cells from ICAM-1–deficient mice are resistant to FasL+ T cell–mediated death. Our results suggest that LFA-1/ICAM interactions are crucial for Th1 cell–mediated B cell apoptosis and may contribute to the maintenance of B cell homeostasis in vivo.
Bcells are sensitive to apoptosis mediated by Fas (Apo-1/CD95; references 1–4). The importance of Fas in B cell apoptosis is supported by B cell accumulation and autoantibody production in mice with defective Fas or Fas ligand (FasL) genes or in humans with mutated Fas genes (5–8). Although Fas–FasL interactions are important for T cell apoptosis after TCR cross-linking, B cell apoptosis induced by cross-linking of the B cell antigen receptor does not involve Fas (9), suggesting that B and T cells differ in Fas-dependent apoptosis. Since FasL expression is undetectable in a variety of mouse and human B cell lines (9, 10), B cells are likely to depend on apoptotic signals through Fas that are generated by interactions with FasL-bearing T cells (11, 12).
Fas-mediated signaling in B cells appears to be regulated by the cell activation status. While resting B cells resist Fas cross-linking–induced death, mitogen-activated B cells are susceptible to Fas-mediated killing (13). Cross-linking of CD40 leads to upregulation of Fas expression and sensitivity to Fas-mediated apoptosis (14, 15). The roles of adhesion molecules or accessory molecules have not been characterized in CD4+ T cell–mediated B cell lysis by the Fas pathway (16). We examined the roles of accessory molecules in Fas-mediated B cell lysis. We found that the interactions of LFA-1 on CD4+ T cells and intercellular adhesion molecule-1 and ICAM-2 on B cells are essential for the T cell– mediated B cell lysis. Deficiency in ICAM-1 leads to decreased B cell apoptosis and the accumulation of B cells in vivo.
MATERIALS AND METHODS
Mice.
6–8-wk-old female C57BL/6, C57BL/6-ICAM-1−/−, B10.A, and BALB/c mice were obtained from the Jackson Laboratory (Bar Harbor, ME).
Antibodies, Fusion Proteins, and Peptides.
Anti–LFA-1 (M17.4), anti–ICAM-1 (3E2), anti–ICAM-2 (MIC2/4), anti-B7.1 (1G10), anti-B7.2 (GL1), anti-CD2 (RM2-5), anti-CD48 (HM48-1), anti-Fas (Jo2), anti-CD4 (GK1.5), anti-CD8 (53-6.7), anti–Thy-1.2 (30-H12), anti-NK1.1 (PK136), anti-CD40 (3/12), anti-FasL, anti-CD16/32, PE conjugated anti-Fas, PE-conjugated anti–ICAM-1, PE-conjugated anti–ICAM-2, FITC-conjugated anti–LFA-1, FITC- or PE-conjugated anti-CD4, and PE-conjugated anti-CD3 were purchased from PharMingen (San Diego, CA). FITC-conjugated F(ab′)2 goat anti–mouse IgM was obtained from CALTAG (South San Francisco, CA). Fas–Fc and TNFR–Fc fusion proteins were gifts from Dr. D. Lynch (Immunex, Seattle, WA). The pigeon cytochrome C peptide (amino acid residues 88–104: KAERADLIAYLKQATAK) was synthesized by the Peptide Synthesis Facility (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD).
Purification and Stimulation of B Cells.
Mouse spleen cells were suspended in RPMI 1640 medium containing 20% FCS (5 × 106 cells/ml) and 10 ml cells were added to each 100 mm tissue culture dish. After incubation at 37°C for 1 h, nonadherent cells were recovered and resuspended in HBSS medium (107/ml) containing 10 μg/ml of anti–Thy-1.2, anti-CD4, anti-CD8, and anti-NK1.1 antibodies, and then incubated on ice for 45 min. Next, 1:10 low-tox-M rabbit complement (Accurate Chemical and Science Corp., Westbury, NY) was added and the cells were incubated at 37°C for 1 h. Live cells were purified by Ficoll gradient separation and between 95 and 98% of the cells were positive for surface IgM when stained with a PE-conjugated F(ab′)2 goat anti–mouse IgM (data not shown). The cells (106 cells/ml) were resuspended in RPMI 1640 medium with 10% FCS, 0.2 mM glutamine, 5 × 10−4 M β-mercaptoethanol (RPMI complete medium) containing 100 μg/ml LPS, or 5 μg/ml anti-CD40 and cultured at 37°C for 2 or 3 d before use.
Stimulation of T Cells and 51Cr-release Assay.
A.E7 T cells were stimulated with antigen and IL-2 as previously described (17). To induce allo-specific CD4+ T cells, BALB/c spleen cells were suspended in RPMI complete medium containing 1 μg/ml FITC conjugated anti-CD4 (107 cells/ml) and incubated on ice for 30 min. CD4+ cells were then purified with magnetic beads conjugated with sheep antifluorescein antibody (PerSeptive Diagnostics, Cambridge, MA). The CD4+ T cells were mixed with 3,000 Rad-irradiated C57BL/6 mouse spleen cells at a ratio of ∼1:5 and suspended in RPMI complete medium (106 CD4+ T cells/ ml). After culture at 37°C for 5 d, live cells were purified and used as effector cells to assay B cell lysis. For 51Cr-release assay, B cells were incubated in RPMI medium containing 5% FCS (5 × 106/ml) and 200 μCi/ml Na[51Cr]O4 (New England Nuclear, Boston, MA) with or without 10 μm antigenic peptide at 37°C for 1 h followed by washing with HBSS three times. The labeled B cells (104/well) were incubated with T cells in a total volume of 200 μl in RPMI complete medium at various ratios in triplicates in 96-well U-bottomed plates (Nunc, Roskilde, Denmark). Various antibodies, isotype-matched antibody controls, Fas–Fc, or TNFR–Fc fusion proteins at 10 μg/ml were added as indicated. After 4 h incubation at 37°C, percentage cell loss was quantitated as described (2).
Induction of B Cell Apoptosis with Anti-Fas.
B cells were suspended in RPMI complete medium (0.5 × 106 cells/ml) containing 1 μg/ml of Jo2 (anti-Fas) or control hamster IgG. The cells (105/well) were cultured in 96-well flat-bottomed plates at 37°C for 24 h. Viable cells were quantitated and percentage of cell loss was calculated as described previously (6).
Staining of Cell Surface Markers.
AE.7 cells were cultured in RPMI complete medium (106/ml) with or without 3,000 Rad-irradiated B10A spleen cells (5 × 106/ml) and 10 μM pigeon cytochrome C peptide 88-104. A metalloproteinase inhibitor, KBR8301 (18), was added to 10 μg/ml and the cells were cultured at 37°C for 6 h. The cells were then stained with PE-conjugated anti-FasL and FITC-conjugated anti-CD4 followed by flow cytometry. CD4+ AE7 cells were gated to analyze FasL expression. To examine the expression of accessory molecules, B cells were blocked by incubating with 10 μg/ml anti-CD16/32, 1:100 normal rat serum, and 10 μg/ml normal hamster IgG on ice for 30 min. The cells were next stained with 1 μg/ml PE-conjugated anti-Fas, FITC-conjugated anti–LFA-1, PE-conjugated anti–ICAM-1, or PE-conjugated anti–ICAM-2. Isotype-matched FITC- or PE-conjugated rat anti-CD4 or PE-conjugated hamster anti-CD3 were used as negative controls. After incubation on ice for 30 min, the cells were analyzed by flow cytometry.
RESULTS AND DISCUSSION
Fas-dependent and Antigen-specific Lysis of B Cells by CD4+ T Cells.
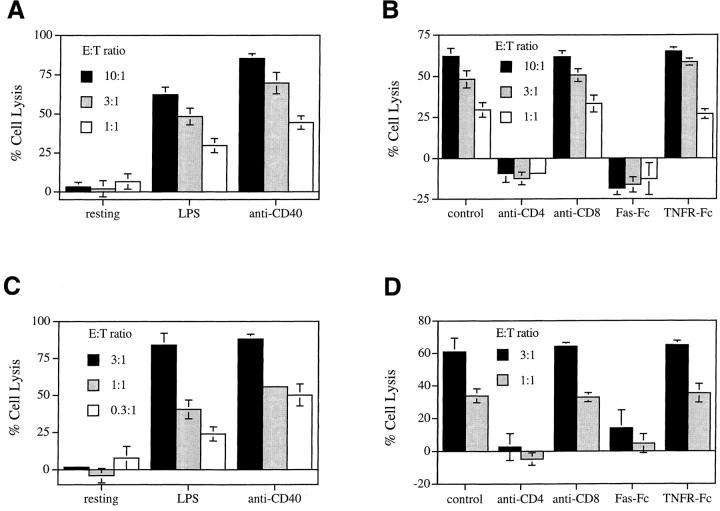
We first tested the lysis of B cells by T cells in a Th1-mediated cytotoxicity assay. We used either A.E7, a CD4+ Th1 clone that recognizes pigeon cytochrome C amino acids 88–104 presented by I-Ek (19), or allo-reactive CD4+ T cells generated from an MLR. Resting B cells or B cell blasts from either B10.A mice (I-Ek+) pulsed with the pigeon cytochrome C peptide or B cells from C57BL/ 6 mice were targets for A.E7 and the allo-reactive T cells, respectively. We found that A.E7 cells efficiently lyse B cell targets preactivated with either LPS or anti-CD40 but not untreated resting B cells (Fig. 1 A). Blocking of Fas–FasL interactions by Fas–Fc abolished the lysis of B cell targets by A.E7 cells (Fig. 1 B), whereas TNFR–Fc, a blocker for TNF action, did not interfere with B cell death (Fig. 1 B). Similar results were obtained with allo-reactive CD4+ T cells (Fig. 1, C and D). These data agree with previous findings that lysis of target B cells by CD4+ T cells is predominantly Fas dependent (2). Antigen-specific recognition by T cells is required because A.E7 cells failed to lyse target B cells that were not pulsed with antigen before the assay (reference 1 and data not shown). Thus, Th1-mediated cytotoxicity against B cells is Fas and antigen dependent.
Figure 1.
Lysis of B cells by CD4+ T cells. (A) Freshly isolated resting B cells, and LPS- or anti-CD40–activated B cells derived from B10.A mice were labeled with 51Cr and pulsed with antigen. A.E7 cells were mixed with the labeled B cells for a 4 h 51Cr-release assay. (B) B cells derived from B10.A mice were stimulated with LPS and labeled with 51Cr. A.E7 cells were mixed with the labeled B cells for a 4 h 51Cr-release assay in the absence or presence of 10 μg/ml anti-CD4, anti-CD8, Fas–Fc, or TNFR–Fc. (C) Freshly isolated resting B cells, LPS- or anti-CD40–activated B cells derived from C57BL/6 mice were labeled with 51Cr. CD4+ allo-reactive CTL were mixed with the labeled B cells for a 4 h 51Cr-release assay. (D) B cells derived from C57BL/6 mice were stimulated with LPS and labeled with 51Cr. MLR-derived CD4+ CTLs were mixed with the labeled B cells for a 4 h 51Cr-release assay in the absence or presence of 10 μg/ml anti-CD4, anti-CD8, Fas–Fc, or TNFR–Fc. The results are representatives of three experiments and the results were shown as average of triplicates with standard deviation.
Involvement of Accessory Molecules in Th1-mediated Lysis of B Cells.
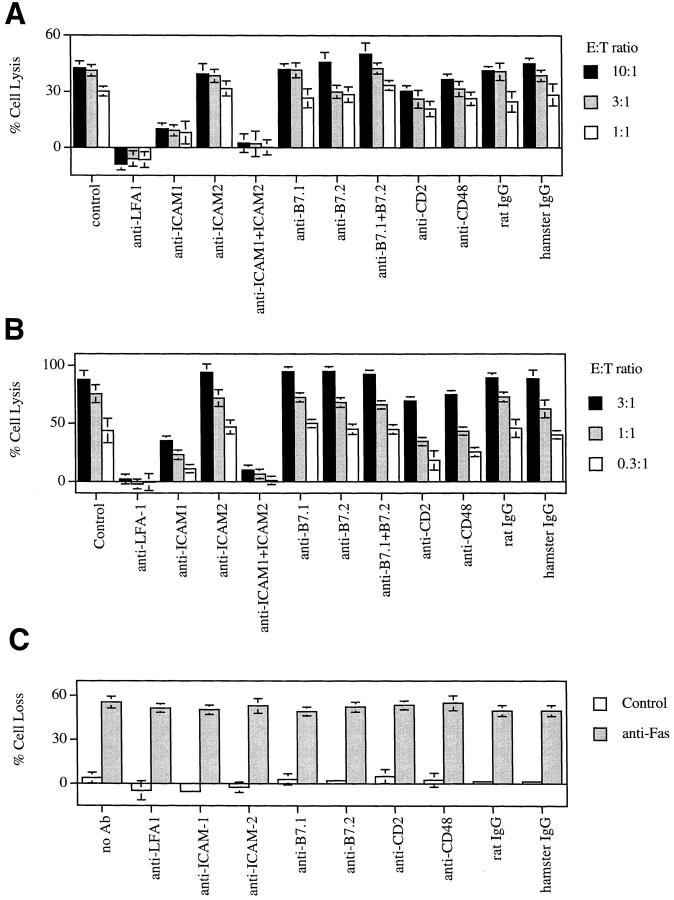
We next tested mAbs to block accessory molecules in the T cell–mediated, B cell cytotoxicity assays. We found that anti-LFA-1 completely blocked the killing of target B cells by A.E7 (Fig. 2 A). Similar results were observed with allo-reactive T cells. B cell lysis was also significantly suppressed by anti–ICAM-1 but not by anti– ICAM-2 alone (Fig. 2, A and B). Both anti–ICAM-1 and anti–ICAM-2 together nearly completely suppressed B cell apoptosis. Therefore, both ICAM-1 and ICAM-2 are involved in T cell–mediated B cell killing, but ICAM-1 appears to be more important.
Figure 2.
Inhibition of CD4+ T cell–dependent lysis of B cells. (A) B cells derived from B10.A mice were stimulated with LPS and labeled with 51Cr and pulsed with antigen. A.E7 cells were mixed with the labeled B cells for a 4 h 51Cr-release assay in the absence or presence of 10 μg/ml mAbs or control rat or hamster IgG. (B) B cells derived from C57BL/6 mice were stimulated with LPS and labeled with 51Cr. MLR-derived CD4+ CTLs were mixed with the labeled B cells for a 4 h 51Cr-release assay in the absence or presence of 10 μg/ml mAbs or control rat or hamster IgG. (C) LPS blasts prepared from C57BL/6 mice were resuspended in RPMI complete medium (106 cells/ml) containing 1 μg/ml either control hamster IgG or Jo2 antibody. The cells were added to 96-well plates (200 μl/well) and 10 μg/ml of various mAbs or control rat or hamster IgG were added to the cultures as indicated. After culture at 37°C for 18 h, the cells were harvested and the recovery of viable cells and the percentage of cell loss was calculated. The results presented are representatives of two experiments and the results were presented as average of triplicates with standard deviation.
In contrast, blocking mAbs to B7.1 and B7.2 had no effect on B cell lysis by CD4+ T cells (Fig. 2, A and B), although CD28 and B7 molecules are highly expressed on the T and B cells used in these assays (data not shown). This indicates that the CD28/B7 interaction is not involved in CD4+ T cell–mediated B cell killing. These results also suggest that the blocking of B cell lysis by other antibodies is not simply a steric effect due to antibodies bound to cell surface structures. We also examined CD2 on T cells and CD48 on B cells, another receptor–ligand pair important for T–B cell interactions in mice (20). We found that B cell lysis was inhibited by anti-CD2 and anti-CD48 (Fig. 2, A and B), but the effect was much weaker than by blocking of LFA-1–ICAM interactions.
Our results suggested that their physical interaction, possibly by promoting Th1-B cell adhesion, was involved in Th1-mediated B cell apoptosis. However, an alternative possibility is that those mAbs may deliver a negative signal into the cells and inhibit the killing process. We therefore examined whether these mAbs influenced anti–Fas antibody–mediated B cell apoptosis. We first established conditions in which anti–Fas antibody efficiently lysed the B cells (Fig. 2 C). We then added anti–LFA-1, anti–ICAM-1, anti–ICAM-2, anti-B7.1, anti-B7.2, anti-CD2, and anti-CD48 and found that none of these affected direct anti-Fas–mediated B cell lysis. Thus, these mAbs do not interfere with apoptotic signaling from Fas.
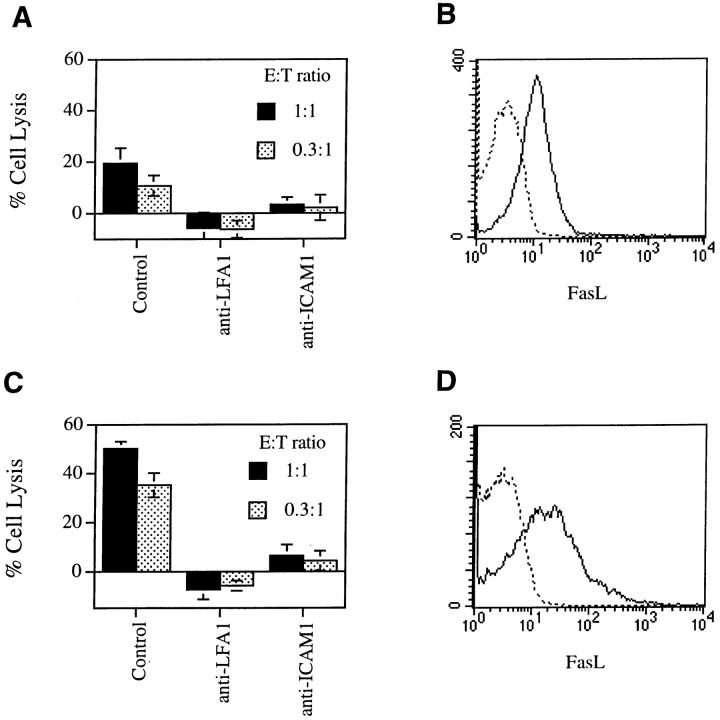
It remained possible that LFA-1–ICAM interactions in our assay simply promoted T cell activation and FasL expression rather than facilitate Fas–FasL interactions leading to death. To distinguish between these possibilities, we studied freshly activated A.E7 cells that express more FasL to see if the requirement for LFA-1–ICAM interactions was less. However, anti-LFA-1 and anti–ICAM-1 still blocked B cell lysis by freshly activated A.E7 cells (Fig. 3). Flow cytometry showed that FasL was upregulated on AE.7 cells immediately after antigen stimulation (Fig. 3). This supports the hypothesis that LFA-1 and ICAM function primarily to promote T–B cell adhesion for Fas-mediated B cell apoptosis. Although anti-Fas antibodies induce rapid formation of a death-inducing signaling complex linked to the cytoplasmic portion of Fas, proteolytic processing and activation of FLICE/caspase 8 requires up to 20 min of continuous Fas cross-linking (21). This suggests that the activation of caspases essential for apoptosis requires certain period of stabilized engagement of Fas receptor. It is therefore reasonable to postulate that LFA-1–ICAM binding promotes Fas signaling by enhancing FasL–Fas interactions between T and B cells.
Figure 3.
Suppression of activated AE.7-mediated lysis of B cells. AE.7 cells without (A) or with (C) further stimulation by B10A spleen cells plus antigen for 6 h were purified by Ficoll gradient separation. The AE.7 cells were then mixed with 51Cr-labeled B10A B cells pulsed with antigen for a 4 h 51Cr-release assay. For FasL staining, AE.7 cells were cultured in RPMI complete medium containing 10 μg/ml KBR8301 without (B) or with (D) the stimulation by B10A spleen cells plus antigen and were purified by Ficoll gradient separation. The cells were either stained with FITC-conjugated anti-CD4 with (solid line) or without (dotted line) PE-conjugated anti-FasL, and analyzed by flow cytometry. Live CD4+ AE.7 cells were gated for analysis. The results are representatives of two experiments.
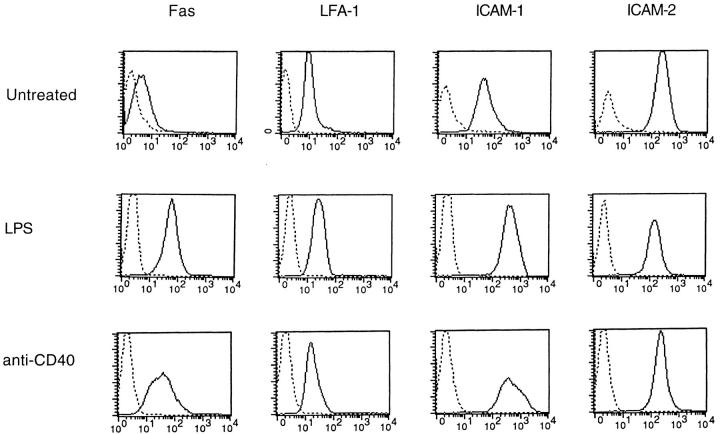
Stimulation of B cells with LPS or CD40 ligation sensitizes B cells for Fas-mediated apoptosis. This is at least partially due to Fas upregulation. Fig. 4 shows that both LPS and anti-CD40 upregulated Fas as they sensitized B cells for apoptosis. Concomitant with the sensitization for apoptosis, we found that LPS and anti-CD40 induced significant upregulation of ICAM-1 on B cells, whereas LFA-1 was slightly increased and ICAM-2 was not changed at all. Thus, the induction of both Fas and ICAM-1 on B cells by stimuli such as LPS and anti-CD40 may make B cells vulnerable to apoptosis mediated by FasL+ T cells.
Figure 4.
Staining of Fas, LFA-1, ICAM-1, and ICAM-2 on B cells. Freshly purified B cells and LPS- or anti-CD40– stimulated B cells were stained with 1 μg/ml PE-conjugated anti-Fas, FITC-conjugated anti– LFA-1, PE-conjugated anti– ICAM-1, or PE-conjugated anti–ICAM-2. Isotype-matched FITC- or PE-conjugated rat anti-CD4 or PE-conjugated hamster anti-CD3 was used as negative controls (dotted line). The results presented are representatives of four experiments.
Resistance of B Cells from ICAM-1 Knockout Mice to Th1-mediated Lysis.
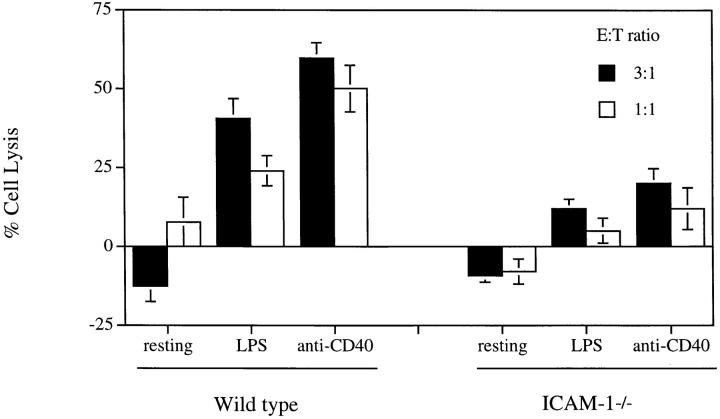
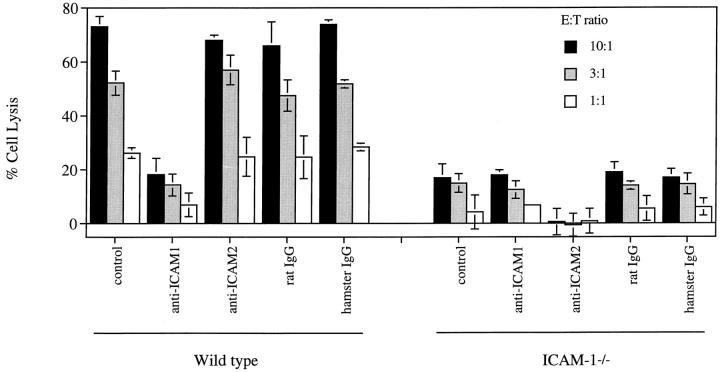
We also tested the cytotoxic effect of MLR-derived CD4+ T cells on B cells prepared from mice homozygously deficient for ICAM-1. Wild-type and ICAM-1−/− B cells express similar levels of Fas (data not shown). However, LPS- or anti-CD40–stimulated B cells from ICAM-1−/− mice are not efficiently lysed (Fig. 5). These data support the concept that it is LFA-1 on Th1 cells and ICAM-1 on B cells that are crucial for apoptosis. However, the deficiency of ICAM-1 on B cells did not completely block killing. This raised the possibility that ICAM-2 (22) could promote T–B adhesion in the absence of ICAM-1. Alternatively, the residual killing of ICAM-1−/− B cells could result from the binding of LFA-1 on B cells to ICAM-1 on T cells. Additional assays with ICAM-1–deficient B cells enabled us to examine these possibilities. The addition of anti–ICAM-1 did not further reduce the killing of ICAM-1−/− B cells (Fig. 6). By contrast, anti– ICAM-2 alone completely blocked B cell lysis. This argues that the interaction of LFA-1 on the B cells with ICAM-1 on the Th1 cells does not play a significant role in T–B cell interactions. Rather ICAM-1, and to some degree ICAM-2, on B cells interacting with LFA-1 on T cells appear to be crucial for Fas-mediated B cell killing. Why the interaction of LFA-1 on B cells and ICAM-1 on T cells is not required is unclear, although flow cytometry reveals their abundant expression. Interestingly, it has been shown that LFA-1 binding on T cells is transiently activated by TCR stimulation (23). It is possible that TCR engagement during T–B synapsis transiently upregulates LFA-1 adhesivity on T cells but LFA-1 on B cells remains inactive.
Figure 5.
Resistance to CD4+ T cell–mediated lysis by B cells from ICAM-1−/− mice. Freshly isolated resting B cells and LPS- or anti-CD40–activated B cells derived from 8-wk-old C57BL/6 ICAM-1+/+ or C57BL/6 ICAM-1−/− mice were labeled with 51Cr. MLR-derived CD4+ CTLs were mixed with the labeled B cells for a 4 h 51Cr-release assay. The results are representatives of three experiments and were shown as average of triplicates with standard deviation.
Figure 6.
Inhibition of CD4+ CTL-mediated lysis of B cells from C57BL/6 ICAM-1−/− mice by anti–ICAM-2. B cells derived from C57BL/6 ICAM-1+/+ or ICAM-1−/− mice were stimulated with LPS and labeled with 51Cr. MLR-derived CD4+ CTLs were mixed with the labeled B cells for a 4 h 51Cr-release assay in the absence or presence of 5 μg/ml mAbs or control rat or hamster IgG. The results are representatives of two experiments and were shown as the average of triplicates with standard deviation.
Since the interactions of LFA-1 and ICAM-1 and ICAM-2 are important for the lysis of B cells in vitro, we asked whether they contribute to B cell homeostasis in vivo. We found no abnormality in the B cell compartment in relatively young ICAM-1−/− mice (references 24 and 25 and data not shown), but a 30% increase in IgM+ B cells was consistently observed in the spleens of ICAM-1−/− mice at 6 mo of age as compared with wild type mice (data not shown). This suggests that B cells accumulate in these mice with aging. This could be analogous to lpr mice which gradually develop abnormal B cell accumulation and autoimmunity depending on the background of the mouse strain (26). Although difficult to verify experimentally, our result is consistent with the hypothesis that ICAM-1 is important for B cell apoptosis mediated by T cells in vivo. However, no significant production of autoantibodies has yet been observed in older ICAM-1−/− mice (data not shown). This could be because autoreactive T cells have not accumulated in these mice since T cell apoptosis is not affected by ICAM-1 deficiency (data not shown). An alternative explanation is that the ICAM-1−/− mice used here are on C57BL/6 background which is not prone to autoimmunity. ICAM-1 deficiency on autoimmune mouse backgrounds, such as the MRL strain, might be more appropriate to address such questions.
Acknowledgments
We thank Dr. H. Kojima for technical support, Dr. J. Erikson for measuring autoantibodies, Drs. D. Lynch and H. Yagita for reagents, Dr. J. Puck for generous support and encouragement, and Drs. R. Germain, E. Dudley, L. D'Adamio, L. Zheng, and R. Siegel for critical reading of the manuscript.
Footnotes
J. Wang was supported by a Fellowship from the Arthritis Foundation.
REFERENCES
- 1.Ju ST, Cui H, Panka DJ, Ettinger R, Marshak-Rothstein A. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc Natl Acad Sci USA. 1994;91:4185–4189. doi: 10.1073/pnas.91.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothstein TL, Wang JK, Panka DJ, Foote LC, Wang Z, Stanger B, Cui H, Ju ST, Marshak-Rothstein A. Protection against Fas-dependent Th1-mediated apoptosis by antigen receptor engagement in B cells. Nature (Lond) 1995;374:163–165. doi: 10.1038/374163a0. [DOI] [PubMed] [Google Scholar]
- 3.Nagata S, Golstein P. The Fas death factor. Science (Wash DC) 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 4.Krammer PH, Behrmann I, Daniel P, Dhein J, Debatin KM. Regulation of apoptosis in the immune system. Curr Opin Immunol. 1994;6:279–289. doi: 10.1016/0952-7915(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 5.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 6.Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, Strober W, Lenardo MJ, Puck JM. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 7.Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, de Villartay JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science (Wash DC) 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 8.Sneller MC, Wang J, Dale JK, Strober W, Middelton LA, Choi Y, Fleisher TA, Lim MS, Jaffe ES, Puck JM, et al. Clinical, immunologic, and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood. 1997;89:1341–1348. [PubMed] [Google Scholar]
- 9.Scott DW, Grdina T, Shi Y. T cells commit suicide, but B cells are murdered! . J Immunol. 1996;156:2352–2356. [PubMed] [Google Scholar]
- 10.Onel KB, Tucek-Szabo CL, Ashany D, Lacy E, Nikolic-Zugic J, Elkon KB. Expression and function of the murine CD95/FasR/APO-1 receptor in relation to B cell ontogeny. Eur J Immunol. 1995;25:2940–2947. doi: 10.1002/eji.1830251034. [DOI] [PubMed] [Google Scholar]
- 11.Rathmell JC, Cooke MP, Ho WY, Grein J, Townsend SE, Davis MM, Goodnow CC. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature (Lond) 1995;376:181–184. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- 12.Rathmell JC, Townsend SE, Xu JC, Flavell RA, Goodnow CC. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell. 1996;87:319–329. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- 13.Daniel PT, Krammer PH. Activation induces sensitivity toward APO-1 (CD95)–mediated apoptosis in human B cells. J Immunol. 1994;152:5624–5632. [PubMed] [Google Scholar]
- 14.Garrone P, Neidhardt EM, Garcia E, Galibert L, van Kooten C, Banchereau J. Fas ligation induces apoptosis of CD40-activated human B lymphocytes. J Exp Med. 1995;182:1265–1273. doi: 10.1084/jem.182.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schattner EJ, Elkon KB, Yoo DH, Tumang J, Krammer PH, Crow MK, Friedman SM. CD40 ligation induces Apo-1/Fas expression on human B lymphocytes and facilitates apoptosis through the Apo-1/Fas pathway. J Exp Med. 1995;182:1557–1565. doi: 10.1084/jem.182.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 17.Boehme SA, Lenardo MJ. Propriocidal apoptosis of mature T lymphocytes occurs at S phase of the cell cycle. Eur J Immunol. 1993;23:1552–1560. doi: 10.1002/eji.1830230724. [DOI] [PubMed] [Google Scholar]
- 18.Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, Yoshino K, Okumura K, Yagita H. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182:1777–1783. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht TT, Longo DL, Matis LA. The relationship between immune interferon production and proliferation in antigen-specific, MHC-restricted T cell lines and clones. J Immunol. 1983;131:1049–1055. [PubMed] [Google Scholar]
- 20.Kato K, Koyanagi M, Okada H, Takanashi T, Wong YW, Williams AF, Okumura K, Yagita H. CD48 is a counter-receptor for mouse CD2 and is involved in T cell activation. J Exp Med. 1992;176:1241–1249. doi: 10.1084/jem.176.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medema JP, Scaffidi C, Kischkel FC, Shevchenko A, Mann M, Krammer PH, Peter E. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO (Eur Mol Biol Organ) J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Fougerolles AR, Springer TA. Intercellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function–associated molecule 1 on resting lymphocytes. J Exp Med. 1992;175:185–190. doi: 10.1084/jem.175.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature (Lond) 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 24.Sligh JE, Jr, Ballantyne CM, Rich S, Hawkins HK, Smith CW, Bradley A, Beaudet AL. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecules 1. Proc Natl Acad Sci USA. 1993;90:8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Gonzalo JA, St. Pierre Y, Williams IR, Kupper TS, Cotran RS, Springer TA, Gutierrez-Ramos JC. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1–deficient mice. J Exp Med. 1994;180:95–109. doi: 10.1084/jem.180.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen PL, Creech E, Nakul-Aquaronne D, McDaniel R, Ackler S, Rapoport RG, Sobel ES, Eisenberg RA. Antigen nonspecific effect of major histocompatibility complex haplotype on autoantibody levels in systemic lupus erythematosus–prone lpr mice. J Clin Invest. 1993;91:2761–2768. doi: 10.1172/JCI116517. [DOI] [PMC free article] [PubMed] [Google Scholar]